Abstract
Background
Documenting the actions and effects of an antimicrobial stewardship program (ASP) is essential for quality improvement and support by hospital leadership. Thus, our ASP tallies the number of charts reviewed, types of recommendations, how and to whom they were communicated, whether they were followed, and any effects on antimicrobial days of therapy. Here we describe how we customized the electronic medical record at our institution to facilitate our workflow and data analysis, while highlighting principles that should be adaptable to other ASPs.
Methods
The documentation system involves the creation of a novel and intuitive ASP form in each chart reviewed and 2 mutually exclusive tracking systems: 1 for active forms to facilitate the daily ASP workflow and 1 for finalized forms to generate cumulative reports. The ASP form is created by the ASP pharmacist, edited by the ASP physician, reopened by the pharmacist to assess whether the recommendation was followed and to quantify any antimicrobial days avoided or added, then reviewed and finalized by the ASP physician. Active forms are visible on a real-time “MPage,” whereas all finalized forms are compiled nightly into 65 informative tables and associated graphs.
Results and Conclusions
This system and its underlying principles have automated much of the documentation, facilitated follow-up of interventions, improved the completeness and validity of recorded data and analysis, enabled our ASP to expand its activities, and been associated with decreased antimicrobial usage, drug resistance, and Clostridioides difficile infections.
Keywords: antimicrobial stewardship, electronic medical record, Cerner, PowerChart, technology
The first guidelines for developing an antimicrobial stewardship program (ASP) at acute care hospitals, published jointly in 2007 by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America, highlighted the importance of involving an information systems specialist [1]. Indeed, collection and analysis of relevant data are inherent in the 2014 recommendation by the Centers for Disease Control and Prevention that all hospitals implement an ASP that includes certain core elements [2, 3], the 2016 proposed rule from the Centers for Medicare and Medicaid Services that an ASP be a condition of participation [4], and the 2017 requirement by the Joint Commission that all hospitals have an ASP [5]. Moreover, documenting the actions and effects of an ASP is essential for quality improvement and continued support by hospital leadership [6].
ASPs may operate in varied settings and have distinct scopes, but each program must develop ways to identify situations that might require an intervention, review details to decide whether to intervene, communicate and follow up on recommendations, and track these efforts and their outcomes. On a typical weekday, 1 of our ASP pharmacists screens >150 computer-generated alerts from ~100 patients and selects ~50 charts for review, of which ~40 are opened with an ASP physician. Upon reviewing the notes and data in a patient electronic medical record (EMR), decisions are made as to whether an intervention is needed and—depending on the urgency or complexity—how and to whom any recommendation should be communicated.
From the inception of our ASP, we have appreciated the importance of tracking process measures (ie, what we did) and outcome measures (ie, the effects of those actions) [1, 7]. Thus, we tally the number of charts reviewed, the types of recommendations made, how and to whom the recommendations were communicated, whether they were followed up on, and any effects on antimicrobial days of therapy (DOT). Before 2014, we used a secure spreadsheet to track these data, but manually logging patient-specific information (EMR number, clinical information, details of any recommendations and outcomes, etc.), proofing entries for accuracy, and collating data for cumulative reports were tedious. To address these issues, our ASP collaborated with information systems analysts to leverage capabilities within Cerner PowerChart, the EMR at our institution, to record and track this information. The key aspects of this system (Figure 1) are the creation of a novel ASP form in each patient EMR reviewed by the ASP and 2 mutually exclusive tracking systems: 1 for active forms (to facilitate the daily ASP workflow) and 1 for finalized forms (to generate cumulative reports).
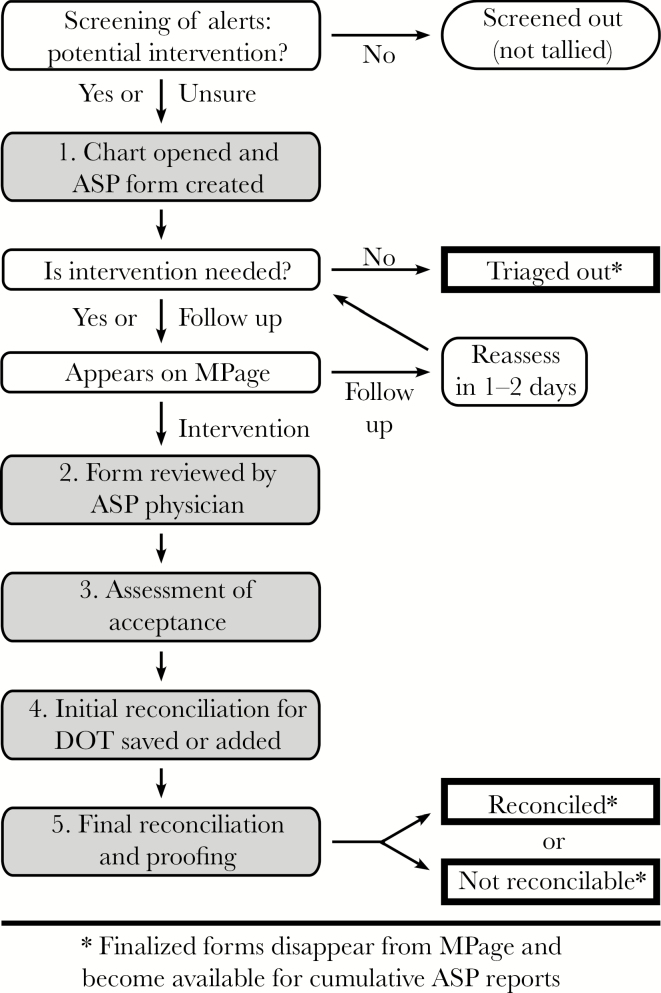
Figure 1.
Workflow for documentation, tracking, and reports. Computer-generated alerts are screened to identify charts to review for potential intervention (the number of alerts and charts screened out are not tallied). An antimicrobial stewardship program (ASP) form is created for any chart reviewed, and this form is potentially accessed 5 times (shaded and numbered boxes): (1) creation by the ASP pharmacist, (2) review by the ASP physician, (3) assessment by the pharmacist for acceptance of recommendations, (4) initial reconciliation by the pharmacist to record any antimicrobial days of therapy saved or added as a result of the recommendations, and (5) final reconciliation by the ASP physician to proof and finalize the form. Thick-bordered rectangles with an asterisk indicate 3 ways a form can reach final status.
Implementation of this system has streamlined the daily work of our ASP, which has facilitated review of an increasing number of charts and attention to other aspects of antimicrobial stewardship. This report describes the features and principles underlying this system, many of which should be generalizable to ASPs in other settings.
METHODS
Description of ASP
Penn State Health Milton S. Hershey Medical Center is an ~550-bed academic health center, including an ~125-bed children’s hospital. Our adult ASP began operation in April 2011 [7], pediatrics was added in September 2014, and in 2018 our ASP was 1 of just 25 programs to be designated an Antimicrobial Stewardship Center of Excellence by the IDSA [8]. On a typical weekday, the same ASP pharmacist completes rounds with the adult ASP physician in the morning and the pediatric ASP physician in the afternoon. Two strategies commonly employed by ASPs are used [1, 9–11]: (1) pre-authorization (or prior approval) of certain antimicrobials that are considered “protected” [12] and (2) prospective audit (or concurrent review) with feedback. Our efforts focus particularly on the latter strategy, which involves reviewing patient charts in real time and contacting the clinical team with any recommendations [7]. The effort committed by personnel for this program and details about the computer-generated alerts and quantifying institutional antibiotic usage are described in the Supplementary Data.
Documentation and Tracking System
Technical aspects and features of the documentation system are described in more detail in the Supplementary Data, but the rationale and timing are described here or in the “Results.” There are 3 components to this system:
The ASP Form
This form (Supplementary Figure 1), which is created in any EMR opened for review, utilizes autopopulated fields, selectable options, concise text entry, and built-in logic. The form has 3 main sections: (1) Details and Recommendations, for information about the ASP review; (2) Acceptance by Primary Team?, to document whether the recommendation was followed; and (3) Reconciliation, to record effects on antimicrobial DOT. ASP forms that have not reached final status appear on the MPage of active forms, and forms that have been finalized provide the data for cumulative reports.
The MPage for Active ASP Forms
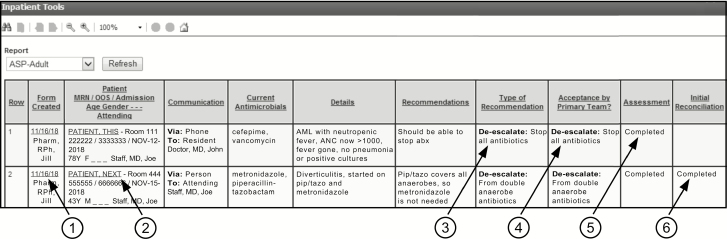
Information from each ASP form that has not been finalized is displayed in a single row on an adult or pediatric MPage (“MPages” refers to Cerner Millennium Pages, which are customized views in PowerChart) (Figure 2). Each row also contains hyperlinks (indicated by the circled 1 and 2) to open the ASP form for updating and to access the chart to review details, which facilitates timely reassessment of any recommendation not followed but still considered significant. Additionally, the Assessment and the Initial Reconciliation columns (indicated by 5 and 6) permit the ASP pharmacist to identify uncompleted tasks and the physician to identify forms that are ready for final review.
Figure 2.
The MPage. A mock-up of 2 rows from fictitious patients is shown. All information from each active ASP form is displayed in a single row. Underlined entries provide hyperlinks to the ASP form (1) and its associated patient chart (2). The category and the specific type of recommendation appear under Type of Recommendation (3), and if accepted also under Acceptance by Primary Team? (4). Rows that show “Completed” in the final 2 columns (5 and 6) are ready for final review by the ASP physician, which will remove that ASP form from this page and make its data available for cumulative reports. To enhance this reproduction, the Notes for ASP Team column is not shown and some text has been overwritten. Abbreviations: abx, antibiotics; AML, acute myeloid leukemia; ANC, absolute neutrophil count; ASP, antimicrobial stewardship program; F, female; M, male; MRN, medical record number; OOS, occasion-of-service financial number; pip/tazo, piperacillin-tazobactam.
Cumulative Reports From Finalized ASP Forms
Reports that compile data from all ASP forms that have reached final status are generated nightly in an Excel file (Microsoft Corp., Redmond, WA) with 65 tables and associated graphs that are displayed in 7 sets of related items (described in the “Results”). Separate reports are created for adults, pediatrics, and all patients combined, and the data within each report can be viewed by month, quarter, half-year, or year.
Statistical Analyses
Poisson regression was performed to assess yearly trends (increase or decrease) of rates, and statistical significance was evaluated using 2-sided P values (<.05) from Wald chi-square tests.
RESULTS
Overview of ASP Workflow
The first decision ASP personnel must make upon reviewing an EMR is whether the chart should be triaged out because no intervention is needed, an intervention is deemed necessary, or a decision to contact the clinical team should be delayed pending more information (Figure 1). All 3 options are accommodated by the ASP form (Supplementary Figure 1). There also are boxes for free-text entry of concise clinical details and for specific recommendations (examples are in Figure 2), as well as a field for any other notes, all of which are useful when initially contacting a clinician and during any follow-up on subsequent days or by different ASP pharmacists or physicians. Characterizing the type of recommendation is particularly important. Soon after our ASP began, we realized we were making 16 types of recommendations that could be grouped into 4 categories, as listed with examples in Table 1: 4 recommendations relate to pharmacokinetics (ie, dosing or route of administration), 8 involve de-escalation, 3 are grouped as miscellaneous, and 1 is a suggestion to obtain an infectious diseases consultation.
Table 1.
Types of Recommendations
| Category and Recommendationa | Examplesb |
|---|---|
| Pharmacokinetics | |
| 1. Vancomycin dosing | “Should change from 1000 mg to 1500 mg at the same dosing interval” |
| 2. Aminoglycoside dosing | “Should change from extended-interval dosing to low-dose synergistic dosing” |
| 3. Other dosing | “Should increase the dose of cefepime for a central nervous system infection” |
| 4. IV to PO | Often for metronidazole, fluoroquinolones, or fluconazole |
| De-escalate | |
| 1. Stop all antibiotics | “No longer neutropenic or febrile and no evidence of infection” |
| 2. From double anaerobe antibiotics | “Should stop metronidazole if changing cefepime to piperacillin-tazobactam” |
| 3. From double GNR for positive culture | “Can stop ciprofloxacin and continue cefepime alone for the cultured Serratia” |
| 4. From double Gram-positive for positive culture | “Can stop vancomycin and continue ceftriaxone alone for the cultured Strep” |
| 5. From empiric and no positive culture | “Can change to moxifloxacin to complete the course for improving pneumonia” |
| 6. To narrower antibiotics for a positive culture | “Can change from cefepime to ceftriaxone for the cultured Escherichia coli” |
| 7. Drug without indication or de-esc miscellaneous | “Can change piperacillin-tazobactam to cefazolin for nonpurulent cellulitis” |
| 8. From echinocandin to fluconazole | “Can change caspofungin to fluconazole for the cultured Candida albicans” |
| Miscellaneous | |
| 1. Indication without drug | An organism cultured from a normally sterile site is not currently covered |
| 2. Better treatment | Changing to a carbapenem for an ESBL-producing Klebsiella |
| 3. Other | Request to document a fact or thought in a progress note |
| Suggest ID consult | |
| 1. Yes | “An infectious diseases consultation would help with this complex situation” |
Abbreviations: de-esc, de-escalate; ESBL, extended-spectrum beta-lactamase; GNR, Gram-negative rod; IV, intravenous; PO, per os (oral); Strep, Streptococcus.
aAs they appear on the Antimicrobial Stewardship Program form.
bPresented in quotes for examples of wording that might be used, or without quotes for comments about typical situations.
As shown schematically in Figure 1, the ASP form is started by the pharmacist when reviewing a chart and—unless it gets triaged out—becomes available to the second component of the documentation system, the MPage (Figure 2). The ASP physician subsequently uses hyperlinks on the MPage to review each ASP form created that day to ensure accuracy. The form is accessed again by the pharmacist to assess whether the recommendation was followed by the patient’s clinical team, and if it was followed the form is reopened at a later time to record any antimicrobial DOT avoided or added. Finally, the ASP physician reviews every form to ensure the validity of the data (see Figure 2 legend) and brings the form to a final status as either “Reconciled” or, occasionally, “Not Reconcilable” (if, for example, the recommendation was not followed due to sudden changes in the patient’s status that day or additional information from discussion with the clinician, as documented on the ASP form). Thus, there are 3 ways an ASP form can reach final status: by the ASP pharmacist indicating the form should be “Triaged Out” early in the process or by the ASP physician marking the form as “Reconciled” or “Not Reconcilable” after final review (Figure 1). Once finalized, the form is no longer captured by the MPage, and its data become available to the cumulative reports program (Table 2, which displays the large range of information that can be monitored for quality purposes).
Table 2.
Tables and Associated Graphs in the Cumulative Reports (65 Total)
| Groups/Individual Tables and Graphsa | Data Displayed |
|---|---|
| Activities and outcomes | |
| 1. Chart reviews | Number of triaged out, not reconcilable, reconciled, and total charts; rate of charts with a rec to total charts reviewed; rate of nonreconcilable charts to charts with a rec |
| 2. Interventions | Number of recs and charts with a rec; rate of recs to charts with a rec |
| 3. Net antibiotic days saved | Antimicrobial days of therapy avoided or added and net days saved |
| Distribution of recommendation categories | |
| 1. Pharmacokinetics recs to total recs | Number and rate of pharmacokinetics recs to total recs |
| 2. Non-PK recs to total recs | Number and rate of non-PK recs to total recs |
| 3. De-escalate recs to total recs | Number and rate of de-escalate recs to total recs |
| 4. Miscellaneous recs to total recs | Number and rate of miscellaneous recs to total recs |
| 5. Suggest ID consult recs to total recs | Number and rate of suggest ID consult recs to total recs |
| Acceptance rates—overall and by category | |
| 1. Overall acceptance of recs | Number and rate of recs accepted to recs made |
| 2. Acceptance of PK recs | Number and rate of PK recs accepted to PK recs made |
| 3. Acceptance of non-PK recs | Number and rate of non-PK recs accepted to non-PK recs made |
| 4. Acceptance of de-escalate recs | Number and rate of de-escalate recs accepted to de-escalate recs made |
| 5. Acceptance of miscellaneous recs | Number and rate of miscellaneous recs accepted to miscellaneous recs made |
| 6. Acceptance of suggest ID consult recs | Number and rate of suggest ID consult recs accepted to suggest ID consult recs made |
| Communication of recommendations | |
| 1. Communicated to (absolute numbers) | Number of communications to attending, resident, APC, and pharmacist |
| 2. Communicated via (absolute numbers) | Number of communications made in person, by phone, or electronically |
| 3. Communicated to (relative numbers) | Percentage of communications to attending, resident, APC, or pharmacist |
| 4. Communicated via (relative numbers) | Percentage of communications made in person, by phone, or electronically |
| Acceptance rates for 16 types of recommendations | |
| 16 individual tables and graphs | Number and rate of acceptance for each of the 16 types of recs |
| Distribution of recommendations within their category | |
| 15 individual tables and graphsb | Number and rate of each type of rec to total recs in its category |
| Distribution of 16 types of recommendations globally | |
| 16 individual tables and graphs | Number and rate of each type of rec to total recs |
Abbreviations: APC, advanced practice clinician; ID, infectious diseases; PK, pharmacokinetics; recs, recommendations.
aExcept for the first item (chart reviews), does not include data from charts with a nonreconcilable recommendation.
bNot necessary for suggest ID consult recommendation (it is the only recommendation in its category and would always be 100%).
Chart Reviews
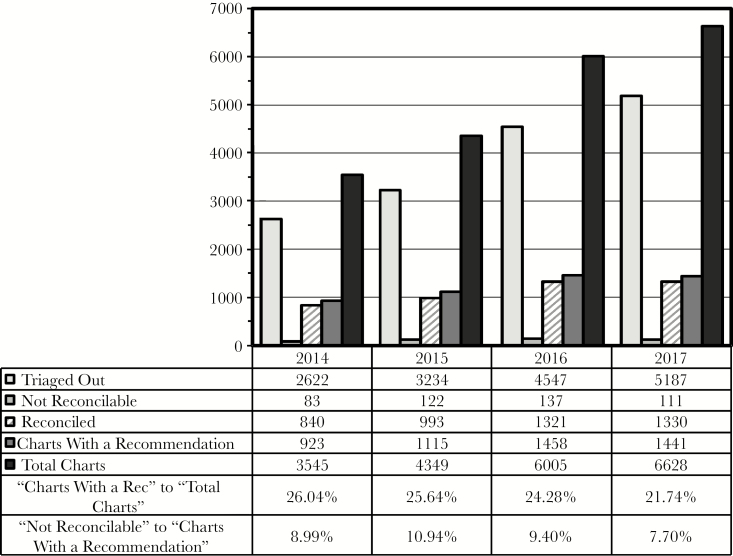
Since implementation of this documentation system, there has been a steady increase in the total number of charts reviewed, from approximately 3500 in 2014 to 6600 in 2017 (the last year with complete data), and the number of charts leading to an ASP recommendation has similarly grown from approximately 900 to >1400 (Figure 3). Approximately one-fourth of all charts opened led to an ASP intervention (Figure 3, second-to-bottom row). The decrease in this rate from 26% to 22% was statistically significant (P < .0001). Approximately 10% of recommendations ultimately are deemed not reconcilable (Figure 3, bottom row). Pediatric patients contributed approximately 25%–30% to the more recent absolute numbers in Figure 3, with similar rates of intervention and nonreconcilable recommendations for adult vs pediatric patients (data not shown). The number of recommendations made per chart with a reconcilable recommendation also was similar for adult and pediatric patients, at ~1.1 (data not shown).
Figure 3.
Chart reviews. Each chart opened for review reaches final status when it is characterized as triaged out without a recommendation, deemed not reconcilable, or its recommendations are reconciled (the first 3 data rows, as explained in the text). “Charts With a Recommendation” is the sum of “Not Reconcilable” and “Reconciled” charts, and “Total Charts” is the sum of all 3 categories. Data refer to the numbers of charts, not individual recommendations, and are shown for the years 2014–2017. The bottom 2 rows are ratios of the indicated data. Abbreviation: rec, recommendation.
Communication of Recommendations
As could have been expected for a teaching hospital, recommendations were communicated to a resident physician 58% of the time and to the attending physician 19% of the time using the most recent data, with the remaining recommendations going to others on the clinical team (data not shown). However, communication was more often with the attending physician for adult patients (23%) than for pediatric patients (10%). We also found that the percentage of face-to-face interventions decreased from 14% in 2014 to 9% in 2017 (4% for adults and 23% for pediatrics in the most recent year), whereas telephone interactions increased from 58% to 79% (81% for adults and 76% for pediatrics in the most recent year).
Types of Recommendations
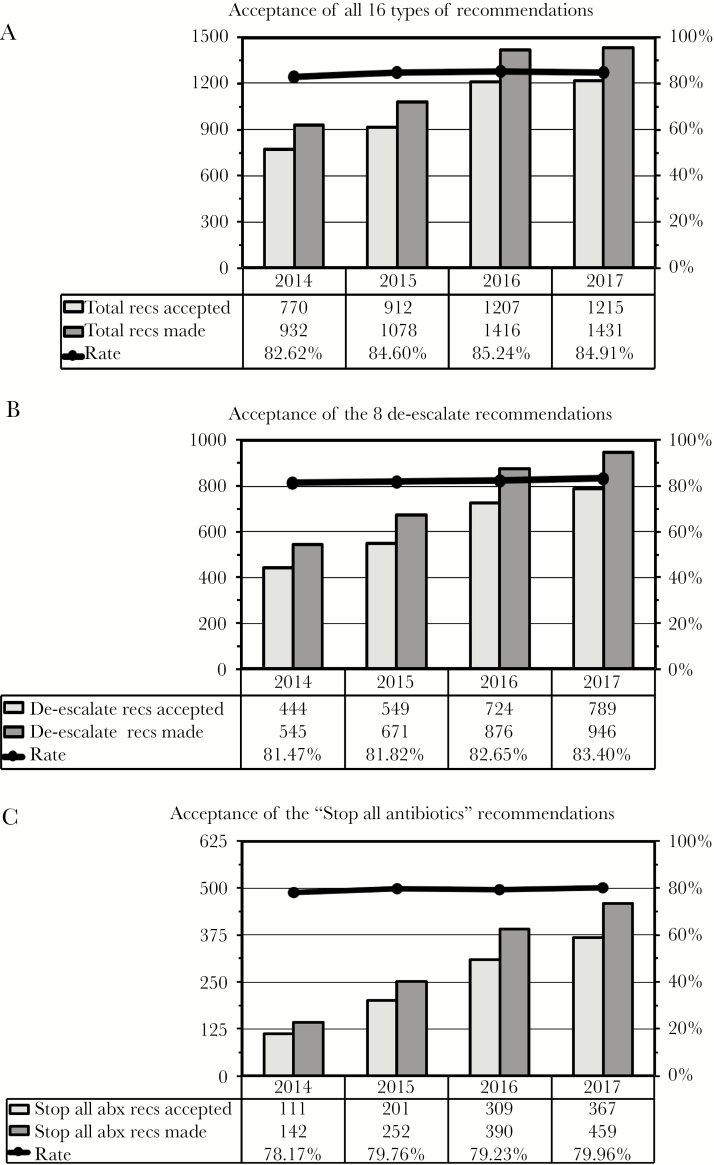
It is noteworthy that as the number of individual reconcilable recommendations increased over time from 932 to 1431 (Figure 4A, “Total Recs Made”), the percentage of pharmacokinetics recommendations decreased from 21% to 11% (data not shown), as many of these interventions are now delegated to unit-based pharmacists. In contrast, recommendations in the de-escalate category (the majority of interventions) increased from 58% to 66%. Moreover, 32% of all recommendations (and 49% of the de-escalate recommendations) in the most recent year were to stop all antimicrobials. Another 14% of all recommendations were to narrow therapy based on positive culture results, and 8% were to de-escalate in the absence of positive cultures. On the other hand, 9% of recent recommendations were categorized as “Indication Without Drug” or “Better Treatment,” and 5% were to obtain an infectious diseases consultation (not shown). These data all were similar for adult and pediatric patients.
Figure 4.
Acceptance of recommendations. The number of recommendations accepted and made and the rates of acceptance (the ratio of the 2 columns) are shown for all 16 types of recommendations (A), the 8 recommendations in the de-escalate category (B), and the single de-escalate recommendation to stop all antibiotics (C). Data refer to individual reconcilable recommendations and are shown for the years 2014–2017. Abbreviations: abx, antibiotics; recs, recommendations.
Acceptance of Recommendations
Monitoring how often clinicians accept recommendations from an ASP is a key indicator of the appropriateness of those recommendations and the effectiveness of how they are communicated. Our data show that even while the number of recommendations has been increasing, the rate of acceptance has consistently been ~80% or higher. Figure 4 displays the number of recommendations made, the number of recommendations followed, and the rate of acceptance for all 16 recommendations (Figure 4A), the 8 recommendations in the de-escalate category (Figure 4B), and the single de-escalation recommendation to stop all antibiotics (Figure 4C). In fact, the rate of acceptance for each of the individual 16 types of recommendations has been similarly high, and comparable for adults and pediatrics (data not shown).
Antibiotic Usage
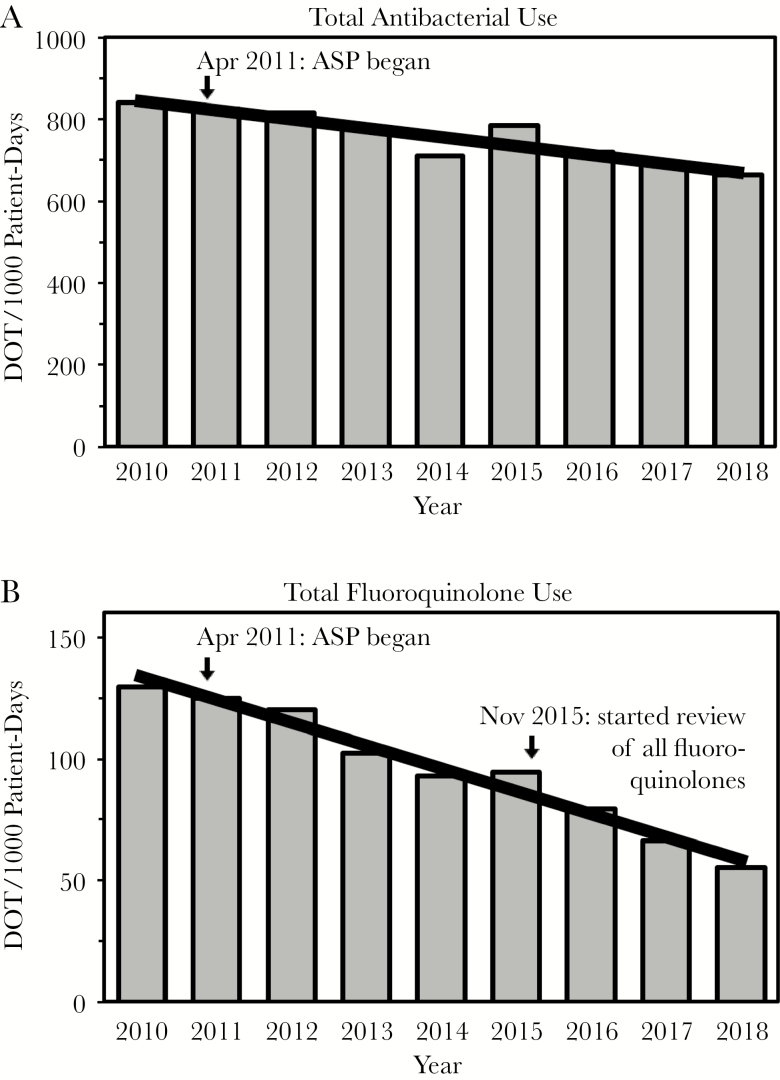
The direct effect of our ASP interventions in the most recent year was a net of 2054 inpatient antimicrobial DOT avoided (3933 DOT avoided minus 1879 DOT added). Thus, the prospective audit and feedback component of our ASP directly reduced the total antimicrobial usage by 9.3 DOT/1000 patient-days for our hospital (which had 220 916 patient-days in 2017). Overall, there has been a 21% decrease in total antibiotic DOT/1000 patient-days since the ASP began, from 843 in 2010 to 665 in 2018 (P < .0001) (Figure 5A). An even more profound effect for fluoroquinolones was seen, with a 58% decrease from 130 to 55 DOT/1000 patient-days over this time (P < .0001) (Figure 5B), which is not explained by any compensatory increase in other antimicrobials that cover Gram-negative bacteria (data not shown). Moreover, the decision in 2015 to review all fluoroquinolone usage on a daily basis was associated with further declines in fluoroquinolone (and total antibiotic) usage following temporary rises (Figure 5A, B).
Figure 5.
Antibacterial usage. Total antibacterial (A) and fluoroquinolone (B) usage are shown as days of therapy per 1000 patient-days. The 2010 year serves as the baseline before the antimicrobial stewardship program began in April 2011. Daily review of all fluoroquinolone use began in November 2015, as indicated.
Other Outcomes
Our hospital inpatient antibiograms show that from 2011 to 2018, the susceptibility to ciprofloxacin increased from 72% to 78% for Escherichia coli and from 78% to 83% for Pseudomonas aeruginosa, and the rate of vancomycin resistance for Enterococcus isolates decreased from 23% to 15%. Moreover, since 2014, the standardized infection ratio of observed to expected cases of Clostridioides (formerly Clostridium) difficile infection has been <1, and in 2018 it fell to 0.62 (data not shown).
DISCUSSION
All ASPs share the synergistic goals of improving patient outcomes, limiting unintended consequences of drug resistance and superinfections, and reducing health care expenditures. From the start of our ASP in 2011, we realized the importance of documentation and that validity of the data would be critical for meaningful assessment of what we were doing and how well we were doing it. Thus, we developed a process involving initial documentation by the ASP pharmacist, review by the ASP physician, assessment by the pharmacist for acceptance of the recommendation, initial reconciliation by the pharmacist for effects on DOT, and final review by the physician (Figure 1). The current report describes how we harnessed features of Cerner PowerChart (customized forms, MPages, etc.) to document and track this information. However, even before partially automating this system, we applied an Excel spreadsheet to the same principles of data entry, review, assessment, reconciliation, and final review. Thus, we believe a similar sequence can be adapted by any ASP, no matter the setting or resources.
With this system in place, we can confidently state that during the last year for which data are complete, we reviewed more than 6600 charts and contacted clinicians on more than 1400 of these patients (22% of all charts opened). The decreasing rate of interventions per charts reviewed (Figure 3, second-to-bottom row) suggests that as stewardship rounds have become more efficient, there has been a lowering of the threshold for selecting charts to open from the initial screen, resulting in more interventions but a reduced rate of intervening. An enduring salutary effect from stewardship efforts also may have contributed to the diminishing rate of interventions per charts reviewed. Importantly, 946 (66%) of 1431 reconcilable recommendations involved de-escalation of antimicrobials (Figure 4A, B). Furthermore, 85% of all recommendations were followed, including 80% of 459 recommendations to stop all antibiotics (Figure 4A, C).
Although the above process measures are gratifying, we recognize the importance of assessing relevant outcome measures. For example, global antimicrobial DOT across an institution should reflect direct and indirect effects of all stewardship efforts (chart reviews, antibiograms, guidelines, formulary decisions, educational efforts, etc.) in changing the culture of antimicrobial use. Thus, the continuing decrease in antibiotic usage indicates the success of our ASP (Figure 5). In particular, fluoroquinolone usage decreased by 58% during the first 8 years of our program compared with the baseline in 2010. This decrease is much larger than the ~20% nationwide decrease in inpatient fluoroquinolone use reported for the 6-year period from 2006 to 2012 [13] and is similar to national trends in recent years (data not shown, obtained from BD MedMined, as described in the Supplementary Data). Although the first year of operation of our ASP was associated with a decrease of almost $600 000 in antimicrobial drug acquisition costs for our medical center (data not shown), ASPs optimally should be viewed as quality improvement initiatives [14, 15], especially as such programs cannot continue to decrease costs indefinitely [16].
Increasingly, improved patient-centered outcomes and institutional rates of drug resistance and C. difficile infection will become important (though difficult) to assess as attributable outcomes of a stewardship program [11, 14, 15]. It is therefore reassuring that our hospital inpatient antibiograms show improved susceptibility to ciprofloxacin for important Gram-negative bacilli (E. coli and P. aeruginosa) and to vancomycin for Enterococcus isolates. The decreased rate of C. difficile infection also indicates the success of our stewardship efforts.
Others have described adapting EMRs to assist stewardship programs (reviewed by Forrest et al. [17]). In particular, Schulz et al. developed a best practice alert to communicate ASP recommendations as a progress note within Epic (Verona, WI) [18]. Similarly, Kuller et al. reported using features of Epic to record and communicate ASP recommendations [19]. Of note, our ASP form is not used to communicate recommendations via the EMR. Rather, our early decision that personal interactions would underscore the collegiality of our approach and minimize resistance by clinicians, while providing opportunities for education, has been substantiated by the high acceptance rate of our interventions [7]. In fact, this rate has been maintained (Figure 4), despite less frequent face-to-face interventions and more telephone interactions due to increasing numbers of chart reviews. These data suggest that direct interaction with a clinician is the critical aspect, but it does not need be in person.
Pogue et al. previously described features of Cerner PowerChart that were used by various ASPs, including preauthorization for restricted antimicrobials, MPages to gather patient-level information, requiring an indication at the time of antibiotic ordering, and the use of order sets and PowerPlans [20]. However, the tracking and reporting system we describe in this report is distinctly different. Indeed, our documentation system has streamlined much of the regular work of our ASP, so that even with an increasing number of chart reviews, our ASP has been able to incorporate features described by Pogue et al. while also addressing other important aspects of stewardship. For example, we made a major effort in the last year to change the way C. difficile is diagnosed and treated at our institution [21]. Interestingly, Pettit et al. recently reported a correlation between their use of the Epic Antimicrobial Stewardship Module and an increased number of stewardship interventions, which they also attributed to a streamlined and consistent process for documentation, tracking, and reporting [22]. Thus, our report extends that observation to the Cerner system, while highlighting important underlying principles required for effective documentation and tracking of an ASP.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank David Craft and Debra Myers for the antibiotic resistance data, Patricia Hnatuck for the C. difficile data, and Ping Du for assistance with the statistical analyses. We also thank Thomas Abendroth, Susan Craft, Keri Donaldson, Gregory Caputo, and Carol Freer for their support of this project.
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dellit TH, Owens RC, McGowan JE Jr, et al. ; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 2. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59(Suppl 3):S97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fridkin S, Baggs J, Fagan R, et al. ; Centers for Disease Control and Prevention (CDC) Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services; Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care. Federal Register website. http://federalregister.gov/a/2016-13925 . Published 16 June 2016. Accessed 24 January 2019. [Google Scholar]
- 5. The Joint Commission. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 2016; 36(7):1, 3–4, 8. [PubMed] [Google Scholar]
- 6. Nagel JL, Stevenson JG, Eiland EH III, Kaye KS. Demonstrating the value of antimicrobial stewardship programs to hospital administrators. Clin Infect Dis 2014; 59(Suppl 3):S146–53. [DOI] [PubMed] [Google Scholar]
- 7. Kim J, Craft DW, Katzman M. Building an antimicrobial stewardship program: cooperative roles for pharmacists, infectious diseases specialists, and clinical microbiologists. Lab Med 2015; 46:e65–71. [DOI] [PubMed] [Google Scholar]
- 8. Infectious Diseases Society of America. 25 institutions receive the IDSA Antimicrobial Stewardship Centers of Excellence designation Available at: https://www.idsociety.org/news--publications-new/articles/2018/25-institutions-receive-the-idsa-antimicrobial-stewardship-centers-of-excellence-designation/. Accessed 24 January 2019.
- 9. Chung GW, Wu JE, Yeo CL, et al. Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence 2013; 4:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Schooneveld TC, Rupp ME. Antimicrobial stewardship strategies: preauthorization or postprescription audit and feedback? Infect Control Hosp Epidemiol 2014; 35:1100–2. [DOI] [PubMed] [Google Scholar]
- 11. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstein EJ, Goff DA, Reeve W, et al. Approaches to modifying the behavior of clinicians who are noncompliant with antimicrobial stewardship program guidelines. Clin Infect Dis 2016; 63:532–8. [DOI] [PubMed] [Google Scholar]
- 13. Baggs J, Fridkin SK, Pollack LA, et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. File TM Jr, Srinivasan A, Bartlett JG. Antimicrobial stewardship: importance for patient and public health. Clin Infect Dis 2014; 59(Suppl 3):S93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodds Ashley ES, Kaye KS, DePestel DD, Hermsen ED. Antimicrobial stewardship: philosophy versus practice. Clin Infect Dis 2014; 59(Suppl 3):S112–21. [DOI] [PubMed] [Google Scholar]
- 16. Standiford HC, Chan S, Tripoli M, et al. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol 2012; 33:338–45. [DOI] [PubMed] [Google Scholar]
- 17. Forrest GN, Van Schooneveld TC, Kullar R, et al. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin Infect Dis 2014; 59(Suppl 3):S122–33. [DOI] [PubMed] [Google Scholar]
- 18. Schulz L, Osterby K, Fox B. The use of best practice alerts with the development of an antimicrobial stewardship navigator to promote antibiotic de-escalation in the electronic medical record. Infect Control Hosp Epidemiol 2013; 34:1259–65. [DOI] [PubMed] [Google Scholar]
- 19. Kullar R, Goff DA, Schulz LT, et al. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis 2013; 57:1005–13. [DOI] [PubMed] [Google Scholar]
- 20. Pogue JM, Potoski BA, Postelnick M, et al. Bringing the “power” to Cerner’s PowerChart for antimicrobial stewardship. Clin Infect Dis 2014; 59:416–24. [DOI] [PubMed] [Google Scholar]
- 21. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 22. Pettit NN, Han Z, Choksi AR, et al. Improved rates of antimicrobial stewardship interventions following implementation of the Epic Antimicrobial Stewardship Module. Infect Control Hosp Epidemiol 2018; 39:980–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.