Abstract
Connective tissue growth factor (also known as CTGF or CCN2) is a secreted matricellular protein that belongs to the CCN family. With wide-ranging biological activities and tissue expression patterns, CTGF plays a critical role in regulating various cellular functions. In the female reproductive system, CTGF is highly expressed in granulosa cells in growing ovarian follicles and is involved in the regulation of follicular development, ovulation, and luteal function. In the mammalian ovary, bone morphogenetic protein 6 (BMP6) is an important intraovarian modulator of follicular development. In this study, we demonstrated that BMP6 treatment significantly increased the expression of CTGF in both primary and immortalized human granulosa cells. Using both pharmacological inhibitors and Small interfering RNA-mediated knockdown approaches, we showed that ALK2 and ALK3 type I receptors are required for BMP6-induced cellular activities. Furthermore, this effect is most likely mediated by a Sma- and Mad-related protein (SMAD)-dependent pathway. Our studies provide novel insight into the molecular mechanisms by which an intraovarian growth factor affects the production of another factor via a paracrine effect in human granulosa cells.
Keywords: BMP6, CTGF, SMAD signaling, human granulosa cells
BMP6 induces the expression of CTGF, which is most likely mediated by an ALK2/ALK3-SMAD dependent signaling pathway in human granulosa cells.
Introduction
Initially identified as immediate early response gene products, cysteine rich angiogenic inducer 61 (CYR61), connective tissue growth factor (CTGF), and nephroblastoma overexpressed gene product (NOV) (also known as CCN1, CCN2, and CCN3, respectively) belong to the CCN family [1]. These matricellular proteins are critical signal modifiers that can modulate multiple signaling pathways, including Wnt, Notch, and transforming growth factor-β (TGF-β) [2]. Due to its wide-ranging biological activities and tissue expression patterns, CTGF has been shown to participate in various essential processes, including embryogenesis, organogenesis, cell proliferation, tissue differentiation, and wound healing [3]. Additionally, the dysregulation of CTGF has been reported to be associated with several pathological conditions, such as inflammation, fibrosis, and tumor development [4, 5]. In the female reproductive system, CTGF is highly expressed in granulosa and theca cells in mammalian growing follicles and the corpus luteum [6, 7]. The results obtained from animal studies demonstrated that CTGF is a downstream molecule that mediates TGF-β signaling and is required for normal folliculogenesis, ovulation, and luteolysis [7, 8]. Indeed, granulosa cell-specific knockouts of Ctgf in mice exhibit multiple defects in the reproductive system and severe subfertility [8]. In rat antral follicles, CTGF is positively regulated by TGF-β and negatively regulated by follicle stimulating hormone (FSH) both in vivo and in vitro [9]. Currently, the paracrine regulation and reproductive functions of CTGF in humans remain elusive.
As a member of the TGF-β superfamily, bone morphogenetic protein 6 (BMP6) is an intraovarian paracrine factor that is expressed in the oocyte and granulosa cells in mammalian follicles [10, 11]. Animal studies have shown that the depletion of Bmp6 in mice resulted in a subfertile phenotype with a reduced ovulation rate (ovulate 24% fewer eggs), oocytes and embryos with an impaired quality (preimplantation decreased by 50%), and a decreased litter size (reduced by 24%) [12]. The essential role of BMP signaling pathway in follicular function was further demonstrated by conditional knockout mouse models for BMP type I receptors (BMPR1A and BMPR1B) or SMAD1/5/8 in granulosa cells of growing follicles, as the depletion of these genes led to the oncogenic transformation of the related cells [13, 14]. Moreover, targeted depletion of Smad4 in granulosa cells was associated with premature luteinisation, cumulus cell defect, and subsequent ovulation failure [15]. These results suggest that BMP6 is part of the complex genetic network that modulates female fertility. Additionally, in vitro studies have shown that BMP6 significantly attenuated the stimulatory effect of FSH on the production of progesterone by downregulating several steroidogenic enzymes in rats [16]. In the human ovary, BMP6 suppressed luteinization and as a result the production of progesterone was decreased. [17]. Indeed, the expression of BMP6 is increased in the human corpus luteum and acts as a critical luteolysis mediator [17]. Collectively, the results obtained from animal studies and clinical data suggest that BMP6 is an important intraovarian modulator of follicular development.
Given its essential role in the regulation of follicular function, the regulation and underlying molecular mechanism of intraovarian CTGF have been the subjects of considerable studies investigating reproductive biology. The dramatic spatiotemporal expression pattern of CTGF in the ovarian follicles is accompanied by the intrafollicular expression of BMP6. Based on these findings, we propose that the intraovarian paracrine factor BMP6 exerts its regulatory function by promoting the expression of CTGF in granulosa cells in periovulatory follicles. In this study, we aimed to examine the regulatory effect of BMP6 on the expression of CTGF and the molecular mechanisms underlying this regulation in human granulosa-lutein (hGL) cells.
Materials and methods
Preparation and culture of primary and immortalized hGL cells
This research was conducted in accordance with SSR's specific guidance and standards. The primary hGL cells used in this study were obtained from patients who provided informed consent after obtaining approval from the University of British Columbia Research Ethics Board. The controlled ovarian stimulation protocol applied to the in vitro fertilization (IVF) patients was performed as previous described [18]. The collected hGL cells were purified via density centrifugation from follicular aspirates collected from women undergoing oocyte retrieval as previously described [19, 20]. The individual primary cell cultures were composed of cells from one individual patient. The purified granulosa cells were seeded (2 × 105 cells/well in 12-well plates) and cultured at 37°C in a humidified atmosphere with 5% CO2 in 95% air in Dulbecco's Modified Eagle Medium/nutrient mixture F-12 Ham (DMEM/F-12; Sigma-Aldrich Corp, Oakville, ON) supplemented with 10% charcoal/dextran-treated fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin (Life Technologies, Inc/BRL, Grand Island, NY), 100 μg/ml streptomycin sulfate (Life Technologies, Inc/BRL), and 1X GlutaMAX (Invitrogen, Life Technologies). The culture medium was changed every other day in all experiments. Alternatively, the nontumorigenic immortalized human granulosa cell line SVOG (passage number 8–20), which was previously produced by Simian virus 40 large T antigen transfection of early luteal phase human granulosa cells obtained from patients undergoing IVF [21], was used in this study. Because the immortalized SVOG cells were generated from primary hGL cells, these cells display biological responses to many different treatments that are similar to the responses of hGL cells [22–26].
Antibodies and reagents
Recombinant human BMP6 (#507-BP-020), recombinant human TGF-β1 (#240-B-010), dorsomorphin dihydrochloride (dorsomorphin) (#3093/10), DMH-1 (#4126/10), and SB431542 (#1614/10) were obtained from R&D Systems (Minneapolis, MN). Recombinant human BMP6 and TGF-β1 were supplied lyophilized from a 0.2 μm filtered solution of 4 mM HCL with 0.1% BSA as a carrier protein. The polyclonal goat anti-CTGF antibody (sc-34 772, RRID:AB_2087481, diluted at 1:1000), monoclonal mouse anti-CYR61 antibody (sc-271 217, RRID:AB_10608730, diluted at 1:1000), polyclonal rabbit anti-SMAD1/5/8 antibody (N-18; sc-6031-R, RRID:AB_785721, diluted at 1:1000), and polyclonal anti-actin antibody (C-11) (sc-1615, RRID:AB_630835, diluted at 1:2000) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal rabbit anti-phospho-SMAD1 (Ser463/465)/SMAD5 (Ser463/465)/SMAD8 (Ser426/428) (#13 820, RRID: AB_2 493 181, diluted at 1:1000) antibody, monoclonal rabbit anti-phospho-SMAD2 (#3108, RRID:_490941, diluted at 1:1000) antibody, monoclonal mouse anti-SMAD2 (#3103, RRID:_490816, diluted at 1:1000) antibody, monoclonal rabbit anti-phospho-SMAD3 (#9520, RRID:_2193207, diluted at 1:1000) antibody, monoclonal rabbit anti-SMAD3 (#9523, RRID:_2193182, diluted at 1:1000) antibody, and polyclonal rabbit anti-SMAD4 (#9515, RRID:AB_2193344, diluted at 1:1000) antibody were obtained from Cell Signaling Technology (Beverly, MA). The horseradish peroxidase-conjugated goat anti-rabbit (diluted at 1:5000) and goat anti-mouse IgGs (diluted at 1:5000) were obtained from Bio-Rad Laboratories (Hercules, CA). The horseradish peroxidase-conjugated donkey anti-goat IgG was obtained from Santa Cruz Biotechnology.
Reverse transcription quantitative real-time PCR
The total RNA was extracted using TRIzol reagent (Invitrogen, Life Technologies) according to the manufacturer's instructions. Reverse transcription into first-strand cDNA was performed with 2 μg of RNA, random primers, and Moloney murine leukemia virus reverse transcriptase (Promega, Medison WI). Each 20-μl qPCR reaction contained 1X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 20 ng of cDNA and 250 nM of each specific primer. RT-qPCR was performed using an Applied Biosystems 7300 real-time PCR (RT-qPCR) system equipped with a 96-well optical reaction plate. The specificity of each assay was validated based on a dissociation curve analysis and agarose gel electrophoresis of the PCR products. Three independent experiments were performed using different cultures, and each sample used in the RT-qPCR experiment was assayed in triplicate. The mRNA levels were determined by obtaining the mean value of triplicate measurements. The relative quantification of the mRNA levels was performed using the comparative cycle threshold (Ct) method with the 2ΔΔCt formula, and GAPDH was used as a reference gene.
Western blot analysis
After the treatment, the cells were lysed in lysis buffer (Cell Signaling Technology) containing a protease inhibitor cocktail (Sigma-Aldrich). The extracts were centrifuged at 14 000 rpm for 15 min at 4°C to remove the cellular debris. The protein concentrations were quantified using a DC Protein Assay (Bio-Rad Laboratories). Equal amounts (50 μg) of protein were separated via 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 for 1 h and incubated overnight at 4°C with the primary antibodies. After washing with 1X Tris-buffered saline, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 h. The immunoreactive bands were detected via an enhanced chemiluminescent substrate or a SuperSignal West Femto Chemiluminescence Substrate (Pierce, Rockford, IL). The membranes were stripped with stripping buffer at 50°C for 30 min and reprobed with the goat anti-actin antibody as a loading control.
Immunofluorescence staining
Cells were plated on glass cover slips, fixed with 4% paraformaldehyde in PBS for 20 min, and then permeabilized with 0.1% Triton X-100 in PBS for 5 min. After the cells were washed with PBS, the cover slips were mounted on microscope slides and blocked with Dako Protein Block (Dako, Mississauga, ON, Canada) for 1 h followed by an overnight incubation with phosphor-SMAD1/5/8 antibodies (1:50 diluted in Dako Protein Block). Alexa Fluor 555 donkey antirat IgG (Life Technologies) was used as a secondary antibody. Finally, the cells were counterstained with the chromosomal dye DAPI (Sigma-Aldrich), rinsed with PBS, mounted in Gelvatol and imaged under a Zeiss Axiophot fluorescence microscope equipped with a digital camera (Q Imaging, Burnaby, BC, Canada).
Small interfering RNA transfection
To knock down endogenous gene expression, the cells were transfected with 25 nM or 50 nM ON-TARGET plus SMART pool small interfering RNA (siRNA) targeting specific genes, ALK2 (ACVR1, #L-0 04924–00-0010), ALK3 (BMRP1A, #L-0 04933–00-0010), SMAD4 (#L-0 03902–00-0010), or CTGF (#L-01 2633–01-0010) (Dharmacon, Lafayette, CO) using Lipofectamine RNAiMAX (Invitrogen, Life Technologies). siCONTROL Non-Targeting pool siRNA (#D-0 01810–10-20) (Dharmacon) was used as the transfection control. The knockdown efficiency was confirmed via RT-PCR or western blotting.
Statistical analysis
The results are presented as the mean ± SEM of at least three independent experiments. Multiple comparisons were analyzed via a one-way analysis of variance followed by Tukey's multiple comparison tests using PRISM software (GraphPad Software, Inc., San Diego, CA). Statistical significance was defined as P < 0.05.
Results
Regulation of CTGF by BMP6 in hGL cells
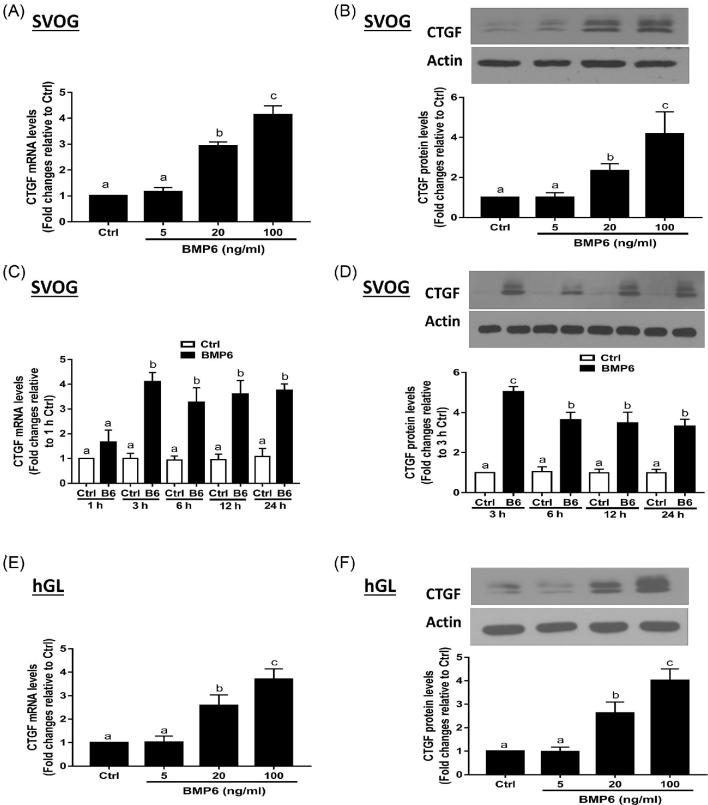
The serum levels of BMP6 in mice, bovine and humans are 55.46–128.7 pg/ml, 2.75–8.14 ng/ml, and 0.5–2.75 ng/ml, respectively [27, 28]. Based on most granulosa cell culture-related studies, we chose the concentration levels of 5–100 ng/ml to conduct our in vitro study [11, 17, 29]. To investigate whether BMP6 might mediate the regulation of CTGF expression, we treated SVOG cells with various concentrations (5, 20, and 100 ng/ml) of recombinant human BMP6 (BMP6). The expression of CTGF was analyzed using RT-qPCR and western blotting. As shown in Figure 1A, the 3-h BMP6 treatment increased the mRNA levels of CTGF in a concentration-dependent manner. Additionally, the results obtained from western blot analysis showed that the 6-h BMP6 treatment increased the protein levels of CTGF in a concentration-dependent manner (Figure 1B). Then, we examined the time course effects of BMP6 on CTGF expression. Because the concentration of BMP6 that had biological effect started at 20 ng/ml, we chose 30 ng/ml BMP6 to conduct the time course study. The SVOG cells were treated with 30 ng/ml BMP6 for various durations (1, 3, 6, 12, and 24 h). The results showed that the mRNA levels of CTGF increased at 1 h of the BMP6 treatment, reached its peak at 3 h, and remained elevated at 24 h (Figure 1C). Moreover, the protein levels of CTGF were increased at 3 h and remained elevated at 24 h after the BMP6 treatment (Figure 1D). The primary hGL cells obtained from patients undergoing the IVF procedure were used to further confirm the functional role of BMP6 in the regulation of CTGF expression. Consistent with the results obtained using the SVOG cells, the BMP6 treatment increased the mRNA and protein levels of CTGF in a concentration-dependent manner (Figures 1E and F).
Figure 1.
BMP6 upregulates the expression of CTGF in hGL cells. A and B, SVOG cells were treated with a vehicle control or increasing concentrations (5, 20, or 100 ng/ml) of BMP6 for 3 h (A) or 6 h (B), and the mRNA (A) and protein (B) levels of CTGF were examined using RT-qPCR and western blotting, respectively. C and D, SVOG cells were treated with vehicle control or 30 ng/ml of BMP6 for 1, 3, 6, 12, or 24 h, and the mRNA (C) and protein (D) levels of CTGF were examined using RT-qPCR and western blotting, respectively. E and F, Primary hGL cells were treated with a vehicle control or increasing concentrations (5, 20, or 100 ng/ml) of BMP6 for 3 h (E) or 6 h (F), and the mRNA (E) and protein (F) levels of CTGF were examined using RT-qPCR and Western blot, respectively. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05). B6, BMP6; Ctrl, control; hGL, primary human granulosa lutein.
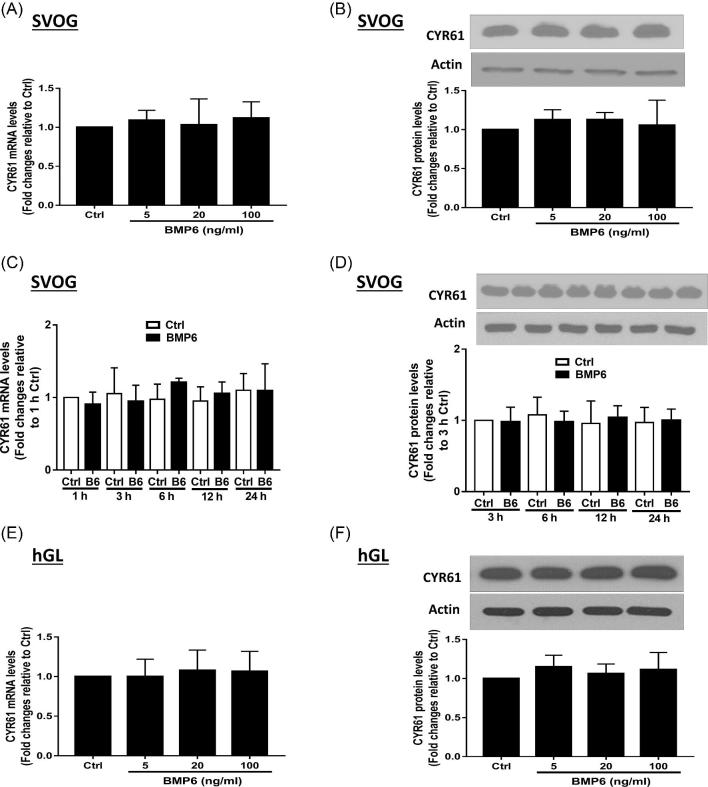
BMP6 did not affect the expression of CYR61 in hGL cells
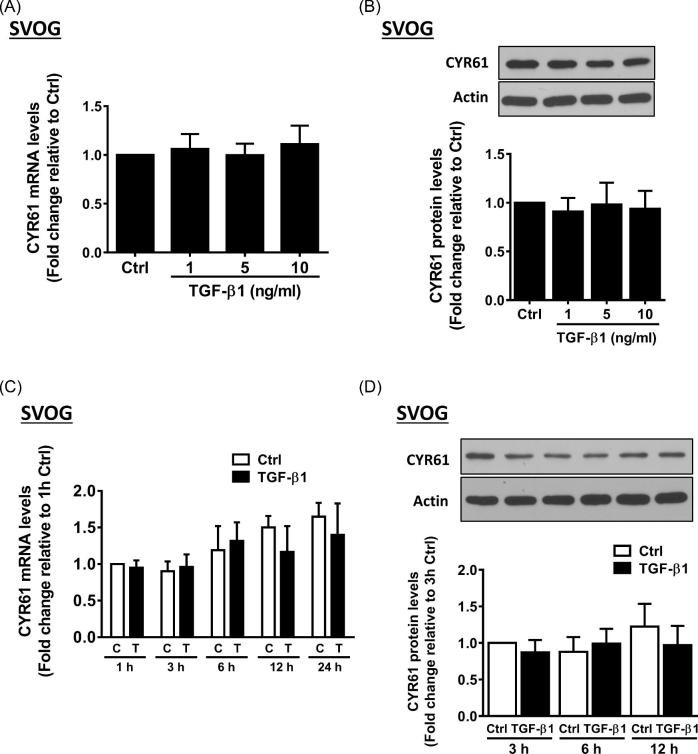
CYR61 and CTGF represent the two first identified proteins in the CCN family that play critical roles in the female reproductive system [1, 8, 30]. Subsequently, we investigated the effect of BMP6 on the expression of CYR61 in hGL cells. As shown in Figures 2A and B, the treatment with various concentrations (5, 20, and 100 ng/ml) of BMP6 for 3 h did not alter the mRNA (Figure 2A) and protein (Figure 2B) levels of CYR61 in SVOG cells. Additionally, the time course studies revealed that neither the mRNA (Figure 2C) nor the protein (Figure 2D) levels were altered by the treatment with 30 ng/ml BMP6 at any time point examined in SVOG cells. Similarly, the treatment with various concentrations (5, 20, and 100 ng/ml) of BMP6 for 3 h did not alter the mRNA (Figure 2E) and protein (Figure 2F) levels of CYR61 in primary hGL cells. To investigate the functional role of intraovarian factor in the regulation of CYR61 in hGL cells, we used another TGF-β superfamily member TGF-β1 to examine the expression of CYR61. As shown in Figures 3, the treatment with TGF-β1 did not alter the expression levels (mRNA and protein) of CYR61 in any concentration used (Figures 3A and B) or at any time point examined (Figures 3C and D).
Figure 2.
BMP6 does not affect the expression of CYR61 in hGL cells. (A and B) SVOG cells were treated with a vehicle control or increasing concentrations (5, 20, or 100 ng/ml) of BMP6 for 3 h (A) or 6 h (B), and the mRNA (A) and protein (B) levels of CYR61 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells were treated with vehicle control or 30 ng/ml of BMP6 for 1, 3, 6, 12, or 24 h, and the mRNA (C) and protein (D) levels of CYR61 were examined using RT-qPCR and western blotting, respectively. (E and F) Primary hGL cells were treated with a vehicle control or increasing concentrations (5, 20, or 100 ng/ml) of BMP6 for 3 h (E) or 6 h (F), and the mRNA (E) and protein (F) levels of CYR61 were examined using RT-qPCR and western blotting, respectively. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05).
Figure 3.
TGF-β1 does not affect the expression of CYR61 in SVOG cells. (A and B) SVOG cells were treated with a vehicle control or increasing concentrations (1, 5, or 10 ng/ml) of TGF-β1 for 3 h (A) or 6 h (B), and the mRNA (A) and protein (B) levels of CYR61 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells were treated with vehicle control or 5 ng/ml of TGF-β1 for 1, 3, 6, 12 or 24 h, and the mRNA (C) and protein (D) levels of CYR61 were examined using RT-qPCR and western blotting, respectively. The results are expressed as the mean ± SEM of at least three independent experiments.
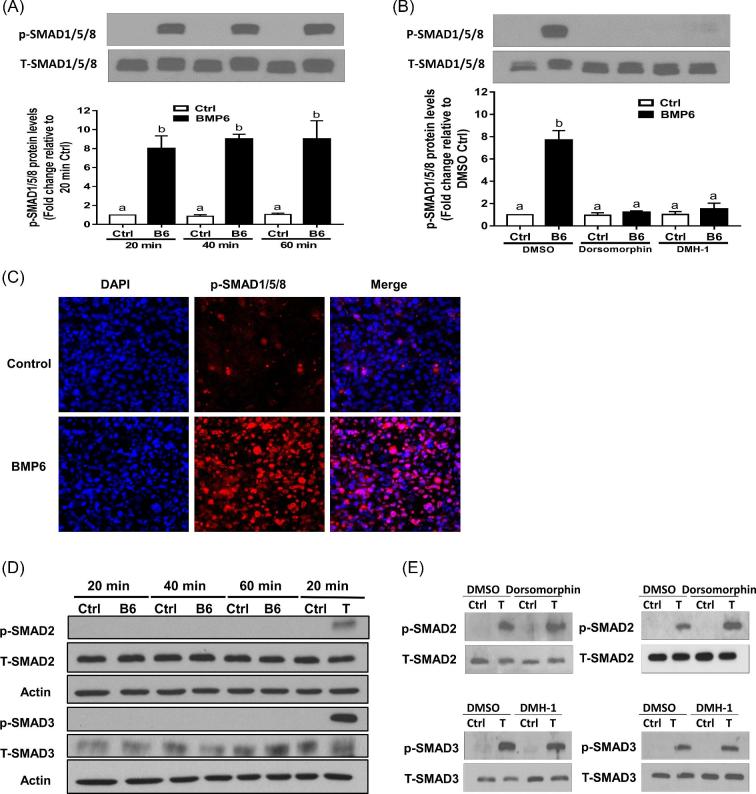
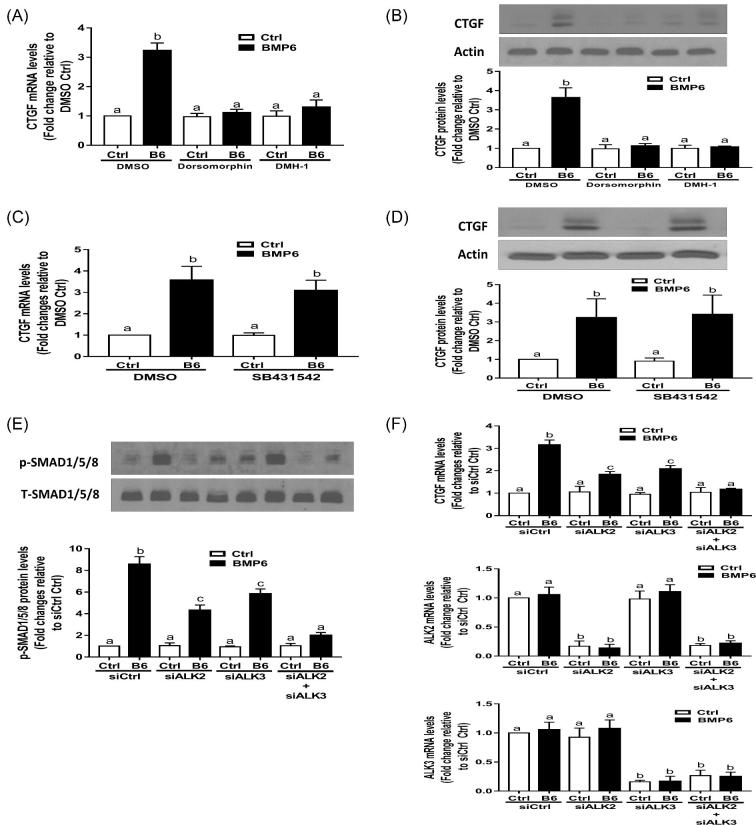
ALK2 and ALK3 mediated the BMP6-induced activation of SMAD1/5/8 and expression of CTGF
To further investigate the molecular mechanisms underlying the BMP6-induced increase in CTGF expression, we treated SVOG cells with 30 ng/ml BMP6 for different durations. The results showed that the BMP6 treatment promptly increased the p-SMAD1/5/8 protein levels at all time points examined (20, 40, and 60 min) (Figure 4A). We further used two BMP type I receptor inhibitors, i.e. dorsomorphin (inhibitor of ALK2/3/6) and DMH-1 (inhibitor of ALK2/3), to investigate the involvement of type I receptor in this effect. As shown in Figure 4B, the pretreatment with either dorsomorphin (5 μM) or DMH-1 (5 μM) inhibited the response that BMP6 increased the p-SMAD1/5/8 protein. To determine the subcellular localization of the phosphorylated SMAD1/5/8 protein, we immunolabelled and probed SVOG cells with phosphor-SMAD1/5/8 antibodies. The immunofluorescence staining in SVOG cells showed that there was a significant increase in cytoplasmic immunoreactivity for phosphorylated SMAD1/5/8 protein (Figure 4C). However, the treatment with 30 ng/ml BMP6 for 20, 40 or 60 min did not induce the phosphorylated SMAD2 and SMAD3, which are the canonical downstream signaling mediators for TGF-β1 (Figure 4D). Similarly, the addition of BMP type I inhibitors dorsomorphin and DMH-1 did not alter the TGF-β1-induced phosphorylation of SMAD2 or SMAD3 (Figure 4E). Notably, the pretreatment with either dorsomorphin (5 μM) or DMH-1 (5 μM) reversed the BMP6-induced increases in the mRNA (Figure 5A) and protein (Figure 5B) levels of CTGF. In contrast, the pretreatment with TGF-β type I receptor inhibitor SB431542 did not BMP6-induced up-regulation of CTGF (Figures 5C and D). The siRNA-based approach was used to confirm that specific type I receptor mediates the effects of BMP6. As shown in Figure 5E, the knockdown of either ALK2 or ALK3 only partially reversed the increases in p-SMAD1/5/8 in response to BMP6. However, the concomitant knockdown of ALK2 and ALK3 completely reversed the BMP6-induced increases in the CTGF mRNA levels (Figure 5F).
Figure 4.
BMP6 induces the phosphorylation of SMAD1/5/8 in SVOG cells. (A) SVOG cells were treated with a vehicle control or 30 ng/ml of BMP6 for 20, 40, or 60 min, and the phosphorylated protein levels of SMAD1/5/8 were examined using western blotting. (B) SVOG cells were pretreated with a vehicle control (DMSO), 5 μM dorsomorphin, or 5 μM DMH-1 for 60 min, and then treated with 30 ng/ml BMP6 for an additional 40 min. The phosphorylated protein levels of SMAD1/5/8 were examined using western blotting. (C) SVOG cells were treated with a vehicle control or 30 ng/ml BMP6 for 40 min, fixed in 4% paraformaldehyde in PBS, and examined for phosphorylated SMAD1/5/8 (red) and nuclear (DAPI in blue) immunofluorescence. (D) SVOG cells were treated with a vehicle control, 30 ng/ml of BMP6 (for 20, 40, or 60 min) or 5 ng/ml of TGF-β1 (for 20 min as a positive control) and the phosphorylated protein levels of SMAD2 and SMAD3 were examined using western blotting. (E) SVOG cells were pretreated with a vehicle control (DMSO), 5 μM dorsomorphin, or 5 μM DMH-1 for 60 min, and then treated with 30 ng/ml BMP6 for an additional 40 min. The phosphorylated protein levels of SMAD2 and SMAD3 were examined using western blotting. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05).
Figure 5.
ALK2 or ALK3 is required for the BMP6-induced upregulation of CTGF expression in SVOG cells. (A and B) SVOG cells were pretreated with a vehicle control (DMSO), 5 μM dorsomorphin, or 5 μM DMH-1 for 60 min, and then treated with 30 ng/ml BMP6 for an additional 3 h (A) and 6 h (B), and the mRNA (A) and protein (B) levels of CTGF were examined using RT-qPCR and western blot, respectively. (C and D) SVOG cells were pretreated with a vehicle control (DMSO) or 5 μM SB431542 for 60 min, and then treated with 30 ng/ml BMP6 for an additional 3 h (C) and 6 h (D), and the mRNA (A) and protein (B) levels of CTGF were examined using RT-qPCR and western blot, respectively. (E and F) SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl), 25 nM of ALK2 siRNA (siALK2), 25 nM of ALK3 siRNA (siALK3), or 25 nM of siALK2 combined with 25 nM of siALK3, followed by treatment with vehicle control or 30 ng/ml of BMP6 for 40 min (E) and 3 h (F). The phosphorylated protein levels of SMAD1/5/8 (E) and mRNA levels of CTGF were examined using western blotting and RT-qPCR, respectively. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05). ALK, activin receptor-like kinase.
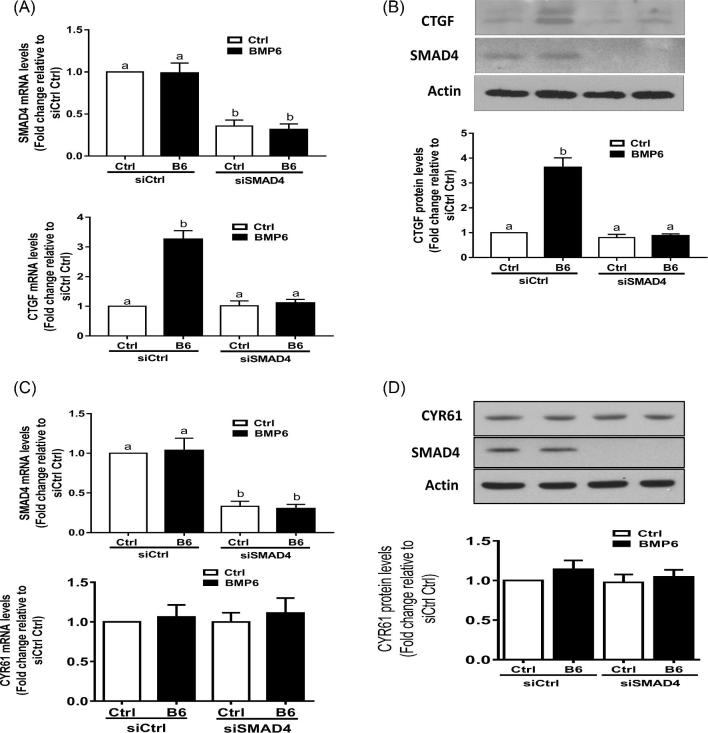
SMAD-dependent signaling mediates BMP6-induced stimulation of CTGF in SVOG cells
To further confirm the role of the SMAD signaling pathway in the BMP6-induced stimulation of CTGF expression, we knocked down the common signaling transducer SMAD4 using specific siRNA. As shown in Figure 6A, the knockdown of SMAD4 significantly reversed the stimulatory effect of BMP6 on the CTGF mRNA level. Consistent with the results obtained from RT-qPCR, the western blot analysis showed that the knockdown of SMAD4 significantly reversed the stimulatory effect of BMP6 on the CTGF protein level (Figure 6B). However, the knockdown of SMAD4 did not alter the basal level or BMP6-induced level of CYR61 expression in SVOG cells (Figures 6C and D).
Figure 6.
SMAD-dependent signaling pathway is required for the BMP6-induced stimulation of CTGF in SVOG cells. (A) SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of SMAD4 siRNA (siSMAD4), followed by treatment with vehicle control or 30 ng/ml of BMP6 for 3 h. The mRNA levels of SMAD4 and CTGF were examined using RT-qPCR. (B) SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of SMAD4 siRNA (siSMAD4) followed by treatment with vehicle control or 30 ng/ml of BMP6 for 6 h. The protein levels of CTGF and SMAD4 were examined using western blotting. (C) SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of SMAD4 siRNA (siSMAD4), followed by treatment with vehicle control or 30 ng/ml of BMP6 for 3 h. The mRNA levels of SMAD4 and CYR61 were examined using RT-qPCR. (D) SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of SMAD4 siRNA (siSMAD4) followed by treatment with vehicle control or 30 ng/ml of BMP6 for 6 h. The protein levels of CYR61 and SMAD4 were examined using western blotting. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05).
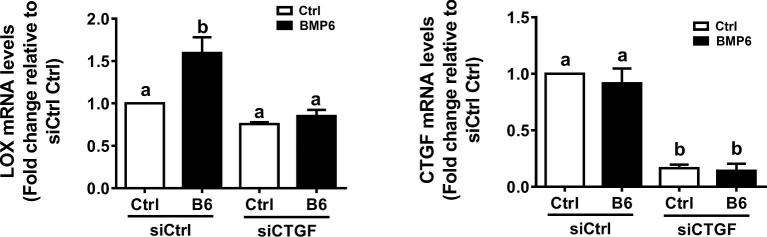
CTGF mediates BMP6-induced up-regulation of lysyl oxidase expression in SVOG cells
To demonstrate the biological response of the increase in CTGF expression induced by BMP6 in immortalized hGL cells, we then used a target gene lysyl oxidase (LOX) that is the downstream of CTGF [22, 23]. As shown in Figure 7, the treatment with BMP6 (30 ng/ml) up-regulated the expression of LOX, and this stimulatory effect was reversed by knocking down of CTGF in SVOG cells.
Figure 7.
CTGF mediates the BMP6-induced up-regulation of LOX expression in SVOG cells. SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of CTGF siRNA (siCTGF), followed by treatment with vehicle control or 30 ng/ml of BMP6 for 3 h. The mRNA levels of CTGF and LOX were examined using RT-qPCR. The results are expressed as the mean ± SEM of at least three independent experiments. Values marked by different letters are significantly different (P < 0.05). LOX, lysyl oxidase.
Discussion
In the present study, we demonstrated that CTGF is an intrafollicular molecule that is expressed in both immortalized and primary hGL cells. Consistent with our results, previous studies have shown that CTGF was highly expressed in hGL cells in growing follicles [31]. It has been well-documented that the expression of intraovarian CTGF is mainly controlled by pituitary-derived endocrine effects. Specifically, FSH and human chorionic gonadotropin negatively regulate CTGF expression [9, 32]. However, the intrafollicular paracrine regulation of this factor in human granulosa cells is largely unknown. Using both SVOG and primary hGL cells as study models, we showed that the expression of CTGF was induced by BMP6. Similarly, our previous studies have shown that CTGF can be upregulated by several TGF-β superfamily members, including TGF-β1, activin A, and growth differentiation factor (GDF) 8 [23, 33, 34]. Ligands of the TGF-β superfamily exert their functions by binding to two serine/Threonine (type I and type II) receptors, which further regulate downstream target gene expression by phosphorylating the receptor-regulatory SMAD (R-SMAD) transcription factors. Specifically, two distinct models of the ligand-receptor binding exist. BMPs and AMH activate SMAD1/5/8 via ALK2, ALK3, or ALK6, whereas TGF-βs, activins and some GDFs activate SMAD2/3 via ALK4, ALK5, or ALK7 [35, 36]. In most tissues, after the phosphorylation of type I receptors, the phosphorylated R-SMADs then associate with the common SMAD (SMAD4) and translocate into the nucleus to regulate target gene expression. In this regard, both activated SMAD2/3 and SMAD1/5/8 act to modulate the expression of CTGF in a similar manner, indicating that the human CTGF promoter may have SMAD binding sites for either phosphorylated SMAD2/3 or SMAD1/5/8. Taken together, these findings suggest that the regulation of CTGF in hGL cells is multifactorial and modulated in both endocrine and paracrine manners. Future studies are required to investigate the interaction between the action exerted by these endocrine and paracrine factors in the regulation of CTGF expression and the mechanism by which CTGF mediates these intraovarian growth factors to perform various follicular and luteal functions.
In the present study, our results showing that the intraovarian BMP6 can regulate the expression of CTGF but not CYR61, indicating that these two structure-related molecules are differentially regulated in hGL cells. Accumulating evidence has supported functional roles for these two factors in endocrine pathways and endocrine-related processes [37]. However, study have shown that there is an aberrant expression of these CCN family members in normal and pathological tissues in humans [2, 38]. In addition to the clinical implications of aberrant expression, studies have demonstrated a contrasting and differential expression of CTGF and CYR61, which are differentially regulated and may play important but contrasting roles in certain human diseases [38].
A comprehensive understanding of the molecular mechanisms and related cellular components mediating the responses to BMP6 is highly important for the development of treatment strategies for patients with ovarian pathology and diseases. Despite the critical role of BMP6 in ovarian physiology and fertility, the cellular receptors mediating the biological activities of BMP6 are largely unknown. In many mammalian cells, BMP-induced activation of SMAD1/5/8 is mainly mediated by ALK2, ALK3, or ALK6 [36]. Only a few studies have attempted to identify the ALKs required for cellular activities in response to BMP6 in human granulosa cells. Using two BMP type I receptor inhibitors (DMH-1 and dorsomorphin), our results showed that pretreatment with either DMH-1 (ALK2/3 inhibitor) or dorsomorphin (ALK2/3/6 inhibitor) completely reversed the effects of BMP6 on the activation of SMAD1/5/8 and upregulation of CTGF, indicating that either ALK2 or ALK3 is required for the cellular activities of BMP6. To further determine which ALK is involved in the BMP6-induced cellular activities, we subsequently used specific siRNA to knock down these type I receptors. The results obtained from our siRNA-based experiments clearly showed that ALK2 and ALK3 are the principal physiologic receptors for BMP6 in hGL cells, providing convincing evidence for the development of translational approaches in clinical applications. Our previous studies have demonstrated that CTGF expression can be regulated by another TGF-β superfamily member TGF-β1, which is mediated by ALK5 (TGF-β type I receptor) [33]. All these finding suggest that this critical endocrine regulator (CTGF) is differentially regulated by different intrafollicular factors through a receptor specific manner, which sheds light into detailed molecular mechanisms for therapeutic implications.
Data obtained from studies using conditional knockout models demonstrated that the ovarian-specific depletion of Smad4 in mice resulted in subfertility with multiple defects in follicular development, including cumulus cell defects, disruption of steroidogenesis, and premature luteinization of granulosa cells, leading to premature ovarian failure [15]. These results highlight the essential role of the SMAD4-mediated signaling pathway in the development of ovarian follicles, especially during the differentiation process of granulosa cells. Using the specific siRNA knock down technique, our results showed that a SMAD4-driven signaling pathway is required for the BMP6-induced stimulation of CTGF expression. Although previous studies have demonstrated that members of the BMP subfamily can activate both SMAD-dependent and SMAD-independent pathways [39], our results indicate that BMP6 positively regulates the expression of CTGF most likely via the SMAD-dependent pathway. A limitation of the present study is that we did not provide data regarding how SMAD1/5/8-SMAD4 translocates into the nucleus and interacts with CTGF promoter sequences following the response to BMP6. However, our previous studies have identified a specific SMAD binding site in the human CTGF promoter of SVOG cells after exposure to GDF 8 [22].
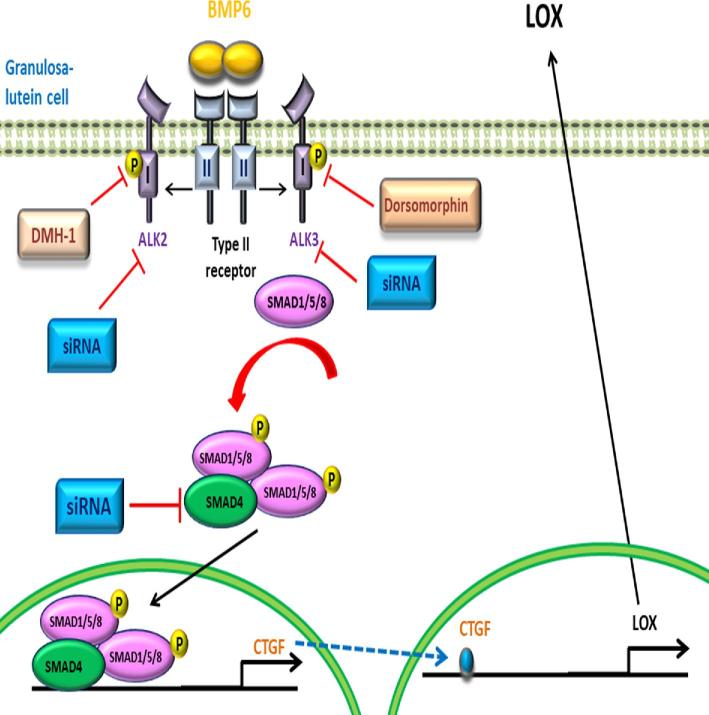
In summary, we demonstrated that BMP6 stimulates the expression of CFGF at both the transcriptional and translational levels in hGL cells. Additionally, our results indicate that ALK2 and ALK3 type I receptors are required for BMP6-induced cellular activities. Furthermore, this effect is most likely mediated by a SMAD-dependent pathway. Notably, functional study demonstrated that the increased CTGF expression mediates the BMP6-induced up-regulation of LOX in hGL cells (Figure 8). Our in vitro studies provided novel insight into the molecular mechanisms by which an intraovarian growth factor affects the production of another factor via a paracrine mechanism in hGL cells.
Figure 8.
Proposed model for the effect of BMP6 on the expression levels CTGF and LOX in human granulosa-lutein cells. BMP6 binds to a complex containing type I (ALK2 and ALK3) and II receptors leading to the phosphorylation/activation of receptor-regulated SMAD (SMAD1/5/8), which binds to the common SMAD (SMAD4). This complex then translocates into the nucleus to promote the transcription of CTGF. The up-regulation of CTGF subsequently contributes to the increase in LOX expression in human granulosa-lutein cells.
Supplementary data
Supplementary Figure. The specificity of the antibodies used in the present study. SVOG cells were transfected for 24 h with 25 nM of control siRNA (siCtrl) or 25 nM of specific siRNA targeting CTGF (siCTGF), CYR61 (siCYR61), SMAD2 (siSMAD2), SMAD3 (siSMAD3), or SMAD4 (siSMAD4). The protein levels of CTGF, CYR61, SMAD2, SMAD3, or SMAD4 were examined using western blotting.
Notes
Edited by Dr. T. Rajendra Kumar, PhD, University of Colorado Anschutz Medical Campus
Footnotes
Grant support: This research was supported by an operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L.
Disclosure
The authors have nothing to disclose.
References
- 1. Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 1993; 327:125–130. [DOI] [PubMed] [Google Scholar]
- 2. Malik AR, Liszewska E, Jaworski J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front Cell Neurosci 2015; 9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. MBoC 1993; 4:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 1999; 20:189–206. [DOI] [PubMed] [Google Scholar]
- 5. Jacobson A, Cunningham JL. Connective tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue Repair 2012; 5:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harlow CR, Hillier SG. Connective tissue growth factor in the ovarian paracrine system. Mol Cell Endocrinol 2002; 187:23–27. [DOI] [PubMed] [Google Scholar]
- 7. Duncan WC, Hillier SG, Gay E, Bell J, Fraser HM. Connective tissue growth factor expression in the human corpus luteum: Paracrine regulation by human chorionic gonadotropin. J Clin Endocrinol Metab 2005; 90:5366–5376. [DOI] [PubMed] [Google Scholar]
- 8. Nagashima T, Kim J, Li Q, Lydon JP, DeMayo FJ, Lyons KM, Matzuk MM. Connective tissue growth factor is required for normal follicle development and ovulation. Mol Endocrinol 2011; 25:1740–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harlow CR, Davidson L, Burns KH, Yan C, Matzuk MM, Hillier SG. FSH and TGF-beta superfamily members regulate granulosa cell connective tissue growth factor gene expression in vitro and in vivo. Endocrinology 2002; 143:3316–3325. [DOI] [PubMed] [Google Scholar]
- 10. Emori C, Sugiura K. Role of oocyte-derived paracrine factors in follicular development. Anim Sci J 2014; 85:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khalaf M, Morera J, Bourret A, Reznik Y, Denoual C, Herlicoviez M, Mittre H, Benhaim A. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J Endocrinol 2013; 168:437–444. [DOI] [PubMed] [Google Scholar]
- 12. Sugiura K, Su YQ, Eppig JJ. Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse? Biol Reprod 2010; 83:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol 2010; 24:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 2008; 28:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific smad4 knockout mice. Mol Endocrinol 2006; 20:1406–1422. [DOI] [PubMed] [Google Scholar]
- 16. Otsuka F, Moore RK, Shimasaki S. Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem 2001; 276:32889–32895. [DOI] [PubMed] [Google Scholar]
- 17. Nio-Kobayashi J, Trendell J, Giakoumelou S, Boswell L, Nicol L, Kudo M, Sakuragi N, Iwanaga T, Duncan WC. Bone morphogenetic proteins are mediators of luteolysis in the human corpus luteum. Endocrinology 2015; 156:1494–1503. [DOI] [PubMed] [Google Scholar]
- 18. Chang HM, Pan HH, Cheng JC, Zhu YM, Leung PC. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol Cell Endocrinol 2016; 422:9–17. [DOI] [PubMed] [Google Scholar]
- 19. Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril 2016; 105:520–528. [DOI] [PubMed] [Google Scholar]
- 20. Chang HM, Cheng JC, Klausen C, Taylor EL, Leung PC. Effects of recombinant activins on steroidogenesis in human granulosa-lutein cells. J Clin Endocrinol Metab 2014; 99:E1922–E1932. [DOI] [PubMed] [Google Scholar]
- 21. Lie BL, Leung E, Leung PC, Auersperg N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol Cell Endocrinol 1996; 120:169–176. [DOI] [PubMed] [Google Scholar]
- 22. Chang HM, Fang Y, Liu PP, Cheng JC, Yang X, Leung PC. Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells. Mol Cell Endocrinol 2016; 434:186–198. [DOI] [PubMed] [Google Scholar]
- 23. Chang HM, Cheng JC, Liu Y, Klausen C, Xu C, Leung PC. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction 2016; 152:293–301. [DOI] [PubMed] [Google Scholar]
- 24. Bai L, Chang HM, Cheng JC, Klausen C, Chu G, Leung PCK, Yang G. SMAD1/5 mediates bone morphogenetic protein 2-induced up-regulation of BAMBI expression in human granulosa-lutein cells. Cell Signal 2017; 37:52–61. [DOI] [PubMed] [Google Scholar]
- 25. Wu YT, Chang HM, Huang HF, Sheng JZ, Leung PC. Bone morphogenetic protein 2 regulates cell-cell communication by down-regulating connexin43 expression in luteinized human granulosa cells. Mol Hum Reprod 2017; 23:155–165. [DOI] [PubMed] [Google Scholar]
- 26. Bai L, Chang HM, Cheng JC, Chu G, Leung PCK, Yang G. ALK2/ALK3-BMPR2/ACVR2A mediate BMP2-induced downregulation of pentraxin 3 expression in human granulosa-lutein cells. Endocrinology 2017; 158:3501–3511. [DOI] [PubMed] [Google Scholar]
- 27. Pauk M, Grgurevic L, Brkljacic J, Kufner V, Bordukalo-Niksic T, Grabusic K, Razdorov G, Rogic D, Zuvic M, Oppermann H, Babitt JL, Lin HY et al.. Exogenous BMP7 corrects plasma iron overload and bone loss in Bmp6-/- mice. Int Orthop (SICOT) 2015; 39:161–172. [DOI] [PubMed] [Google Scholar]
- 28. Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol 2009; 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi J, Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Yano T, Nishii O, Taketani Y. Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin beta subunits, and anti-Müllerian hormone in human granulosa cells. Fertil Steril 2009; 92:1794–1798. [DOI] [PubMed] [Google Scholar]
- 30. Yang R, Chen Y, Chen D. Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review). Mol Med Rep 2018; 17:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phan B, Rakenius A, Pietrowski D, Bettendorf H, Keck C, Herr D. hCG-dependent regulation of angiogenic factors in human granulosa lutein cells. Mol Reprod Dev 2006; 73:878–884. [DOI] [PubMed] [Google Scholar]
- 32. Slee RB, Hillier SG, Largue P, Harlow CR, Miele G, Clinton M. Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology 2001; 142:1082–1089. [DOI] [PubMed] [Google Scholar]
- 33. Cheng JC, Chang HM, Fang L, Sun YP, Leung PC. TGF-β1 up-regulates connective tissue growth factor expression in human granulosa cells through smad and ERK1/2 signaling pathways. PLoS One 2015; 10:e0126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang HM, Pan HH, Cheng JC, Zhu YM, Leung PCK. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol Cell Endocrinol 2016; 422:9–17. [DOI] [PubMed] [Google Scholar]
- 35. Drummond AE. TGFbeta signalling in the development of ovarian function. Cell Tissue Res 2005; 322:107–115. [DOI] [PubMed] [Google Scholar]
- 36. Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett 2012; 586:1846–1859. [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology 2008; 149:1666–1677. [DOI] [PubMed] [Google Scholar]
- 38. Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, Mansel RE. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocr Relat Cancer 2004; 11:781–791. [DOI] [PubMed] [Google Scholar]
- 39. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425:577–584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.