Abstract
Background
Historically, Nigeria has experienced large bacterial meningitis outbreaks with high mortality in children. Streptococcus pneumoniae (pneumococcus), Neisseria meningitidis (meningococcus), and Haemophilus influenzae are major causes of this invasive disease. In collaboration with the World Health Organization, we conducted longitudinal surveillance in sentinel hospitals within Nigeria to establish the burden of pediatric bacterial meningitis (PBM).
Methods
From 2010 to 2016, cerebrospinal fluid was collected from children <5 years of age, admitted to 5 sentinel hospitals in 5 Nigerian states. Microbiological and latex agglutination techniques were performed to detect the presence of pneumococcus, meningococcus, and H. influenzae. Species-specific polymerase chain reaction and serotyping/grouping were conducted to determine specific causative agents of PBM.
Results
A total of 5134 children with suspected meningitis were enrolled at the participating hospitals; of these 153 (2.9%) were confirmed PBM cases. The mortality rate for those infected was 15.0% (23/153). The dominant pathogen was pneumococcus (46.4%: 71/153) followed by meningococcus (34.6%: 53/153) and H. influenzae (19.0%: 29/153). Nearly half the pneumococcal meningitis cases successfully serotyped (46.4%: 13/28) were caused by serotypes that are included in the 10-valent pneumococcal conjugate vaccine. The most prevalent meningococcal and H. influenzae strains were serogroup W and serotype b, respectively.
Conclusions
Vaccine-type bacterial meningitis continues to be common among children <5 years in Nigeria. Challenges with vaccine introduction and coverage may explain some of these finding. Continued surveillance is needed to determine the distribution of serotypes/groups of meningeal pathogens across Nigeria and help inform and sustain vaccination policies in the country.
Keywords: pediatric, meningitis, Nigeria, neumococcus, meningococcus
Streptococcus pneumoniae was the predominant pathogen responsible pediatric bacterial meningitis (46.4%: 71/153) during longitudinal sentinel surveillance within Nigeria, from 2010 to 2016. However, with the introduction of PCV10 across Nigeria, it is hoped that rates of pneumococcal meningitis will decline.
Acute bacterial meningitis is a much-dreaded infectious disease in children and a major cause of morbidity and mortality [1, 2]. The incidence and case-fatality rates for this invasive bacterial disease vary by region, country, causative-agent, and age-group affected, but without adequate treatment, fatalities can be as high as 70% [1, 3]. The African meningitis belt is the most significantly affected area worldwide, with an estimated 400 million people at-risk annually. Bacterial meningitis epidemics have occurred frequently across this region for over a century: one of the largest occurred in Nigeria during 1996, with over 109 000 cases and 11 000 deaths [4]. Subsequently, there have been several major outbreaks within Nigeria, over wide geographical areas, most notably in 2009 and 2015 [4, 5]. In December 2016, the Nigeria Centre for Disease Control (NCDC) received reports of a meningitis outbreak. By May 2017, there were 13 420 suspected cases and 1069 deaths (8%), of which 47% were children in the 5–14 year age group [6].
The leading causes of bacterial meningitis are Neisseria meningitidis (meningococcus), Streptococcus pneumoniae (pneumococcus), and Haemophilus influenzae; which are usually carried asymptomatically in the nasopharynx and transmitted through respiratory droplets [1]. Normally, the pathogen responsible for epidemics is the Neisseria species, whereas pneumococcus and H. influenzae are endemic throughout the year [4, 7, 8]. In 2008, the World Health Organization (WHO) established the Global Invasive Bacterial-Vaccine Preventable Diseases (IB-VPD) network building on regional surveillance networks. This global surveillance network encompasses 100 sentinel hospital laboratories, aiming to estimate the burden of bacterial meningitis and characterize circulating bacterium within member countries [2, 9]. As part of this, the Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine (MRCG at LSHTM), a WHO Collaborating Center for New Vaccines Surveillance (WHO CC NVs), provides laboratory support for pediatric bacterial meningitis (PBM) surveillance in 10 countries across West Africa, including Nigeria.
Nigeria is the most populous country within Africa, with an estimated 193 million people living in 36 states [10]. The national human immunodeficiency virus (HIV) prevalence among adults aged 15–49 year in Nigeria is 1.4%; however, this varies between states with 5.6% estimated in the southern Akwa Ibom State and 0.3% in the northwestern Katsina State [11, 12]. In 2012, the Nigerian Expanded Program on Immunization (EPI) replaced the diphtheria-tetanus-pertussis and hepatitis B vaccines with a pentavalent vaccine that included H. influenzae type b (Hib) and underwent a phased introduction [13]. Additionally, meningococcal A conjugate vaccine (MenAfriVac) campaigns were carried out from 2011 to 2014 in 19 states in Northern Nigeria. The 2017 outbreak was caused by meningococcal serogroup C; thus, a vaccine with longevity against this strain is required [14]. A phased introduction of the 10-valent pneumococcal conjugate vaccine (PCV10) throughout Nigeria, targeting 10 invasive pneumococcal serotypes, commenced in December 2014 and concluded in October 2016. However, the 2017 WHO and United Nations Children’s Fund (UNICEF) national immunization coverage estimates suggest that coverage rates for children who received 3 doses of PCV10 and Hib-containing vaccines remain low, at 36% and 42%, respectively [15]. As part of the IB-VPD network, longitudinal surveillance was established in 2010 within 5 sentinel hospitals in 5 Nigerian states. We have conducted analysis to estimate the prevalence of bacterial meningitis in children <5 years of age and determine the distribution of the causative pathogens from 2010 to 2016.
METHODS
Surveillance Sites
Surveillance of children <5 years admitted to sentinel hospitals with suspected meningitis, was carried out for 7 years. PBM surveillance started in Lagos University Teaching Hospital (Lagos State) in 2010, followed by University of Nigeria Teaching Hospital (Enugu State) in 2011. In the following year, 2 more sites were included: Abubakar Tafawa Balewa University Teaching Hospital (Bauchi State) and University of Ilorin Teaching Hospital (Kwara State). Finally, in 2013, University of Benin Teaching Hospital (Edo State) joined surveillance. Therefore, surveillance was conducted in 5 states with a combined estimated population of approximately 32 million people.
Case Enrollment
Hospitalized children aged 0–59 months (<5 years) with features of suspected meningitis: rapid onset of fever with axillary or rectal temperatures of >38°C and >38.5°C, combined with any of the following symptoms: impaired consciousness, meningismus (stiff neck), photophobia, bulging fontanelle (infants), and convulsions were enrolled in the surveillance [16]. A lumbar puncture (LP) was performed for routine diagnostic tests, and cerebrospinal fluid (CSF) was collected.
CSF Analysis
CSF samples were processed following the WHO standard operating procedure [16]. An aliquot of CSF was centrifuged, and the deposit inoculated onto Columbia blood and chocolate agar plates and incubated overnight. Isolates of the target pathogens were identified using the optochin test (5 μg optochin disk; Oxoid, Basingstoke, UK) for pneumococcus and analytical profile index (API NH; Biomerieux, Basingstoke, UK) for meningococcus and H. influenzae. The remaining centrifuged pellet was used to prepare smears for Gram staining. Using the supernatant, latex agglutination was performed with the Pastorex meningitis kit (Biorad, Watford, UK), for the detection of pneumococcus, Hib and meningococcus groups A, B, C, Y, and W antigens. When possible, CSF was used to detect the presence of pneumococcus using the BINAX® NOW kit (Alere Inc., Waltham, MA, USA). A white blood cell (WBC) count was conducted along with CSF protein and glucose analysis using trichloroacetic acid turbidimetric and glucose oxidase methods [17, 18].
Molecular Analysis
CSF samples were transported to the MRCG at LSHTM according to International Air Transport Association (IATA) regulations [19]. Species-specific quantitative polymerase chain reaction (qPCR) assays for detection of pneumococcus, meningococcus, and H. influenzae were performed, using the autolysin gene (lytA), Cu, Zn superoxide dismutase gene (sodC) and protein D encoding gene (hpd), respectively, as described elsewhere [20]. For amplification, samples were heated at 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. Cycle threshold (CT) values of ≤36 were considered positive results.
Serotyping and Serogrouping
Meningococcus and H. influenzae serogrouping/typing was conducted using direct qPCR. Meningococcal gene targets were: sacB, synD, synE, synG, xcbB, synF for serogroups A, B, C, W, X, and Y, respectively. Additionally, for H. influenzae gene targets included acsB, bcsB, ccsD, dscE, ecsH, bexD for serotypes Hia, Hib, Hic, Hid, Hie, and Hif, respectively. CT values of ≤32 were considered positive [21]. For pneumococcus, nucleic acid extraction using Qiagen DNA Mini-kit was performed. Purified DNA underwent sequential triplex qPCR for detection of 21 capsular serotypes as described elsewhere [22]. Nontypeable pneumococci, with CT values ≤32 by qPCR, were further subjected to conventional multiplex serotyping PCR assays.
Statistical Analysis
Data were collected at the sentinel hospitals using a standardized WHO Regional Office for Africa (Afro) PBM network case report form. Information recorded included, patient demographics, clinical symptoms, vaccination history, laboratory information (CSF microscopy, bacteriological tests, genotyping), and outcome at discharge. Data were subsequently put into a WHO Epi Info-based customized new-vaccine surveillance data module. Data cleaning and analysis were first performed at the sentinel site level before being sent to the national and regional WHO data managers. At the national level, data from sites were merged, cleaned, analyzed, and interpreted. Merged data were then sent to WHO Afro data managers, and feedback was provided to sites every 3–6 months. WHO Afro also sent regional data to WHO Global data managers. For presentation here, data were analyzed using GraphPad Prism 8.1.1; percentages, proportion, means, and standard deviations were calculated as appropriate and presented as prose, tables, and figures.
Ethical approval was not a requirement in Nigeria for routine meningitis surveillance including drug susceptibility testing of collected isolates as this is approved within the routine diagnostic algorithm at the Ministry of Health. However, informed consent was sought from caregivers of the surveillance participants. Additionally, the surveillance received overarching ethical approval (SCC1188) by the joint MRC/The Gambia Government ethics board that allowed the analysis of collected West African isolates at MRC Unit, The Gambia.
RESULTS
Demographic and Clinical Characteristics
Details of the demographic characteristics of the children enrolled in surveillance are shown in Table 1. A total of 5134 children <5 years of age with suspected bacterial meningitis were enrolled at the sentinel hospitals from 2010 to 2016. Of these, 5008 (97.5%: 5008/5134) children had CSF samples collected, which then underwent diagnostic testing. Overall, 57.8% (2969/5134) of patients were male and the median age of patients was 22 months (interquartile range: 1–23). The largest proportion of meningitis cases were reported in children from the youngest age group, 0–11 months, with 3014 (58.7%: 3014/5134) suspected cases. Unfortunately, 278 patients with suspected meningitis died in hospital resulting in a case fatality of 5.4% (278/5134).
Table 1.
Summary of Demographic Characteristics of Study Population
| Total | Bauchia | Lagosb | Edoc | Kwarad | Enugue | ||
|---|---|---|---|---|---|---|---|
| Characteristic | Category | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Age | 0–11 m | 3014 (58.7) | 230 (32.4) | 1473 (72.3) | 588 (67.1) | 222 (37.1) | 501 (54.9) |
| 12–23 m | 798 (15.5) | 120 (16.9) | 222 (10.9) | 120 (13.7) | 127 (21.2) | 209 (22.9) | |
| 24–59 m | 1234 (24.0) | 358 (50.4) | 264 (13.0) | 164 (18.7) | 247 (41.3) | 201 (22.0) | |
| Unknown | 88 (1.7) | 2 (0.3) | 79 (3.9) | 4 (0.5) | 2 (0.3) | 1 (0.1) | |
| Sex | Female | 2147 (41.8) | 300 (42.3) | 843 (41.4) | 356 (40.6) | 269 (45.0) | 379 (41.6) |
| Male | 2969 (57.8) | 410 (57.7) | 1181 (57.9) | 516 (58.9) | 329 (55.0) | 533 (58.4) | |
| Unknown | 18 (0.4) | 0 (0.0) | 14 (0.7) | 4 (0.5) | 0 (0.0) | 0 (0.0) | |
| Antibiotic before admission | Yes | 743 (14.5) | 62 (8.7) | 313 (15.4) | 24 (2.7) | 85 (14.2) | 259 (28.4) |
| No | 2972 (57.9) | 581 (81.8) | 622 (30.5) | 834 (95.2) | 329 (55.0) | 606 (66.4) | |
| Unknown | 1419 (27.6) | 67 (9.4) | 1103 (54.1) | 18 (2.1) | 184 (30.8) | 47 (5.2) | |
| Outcome diagnosis | Meningitis | 363 (7.1) | 107 (15.1) | 87 (4.3) | 42 (4.8) | 22 (3.7) | 105 (11.5) |
| Pneumonia | 72 (1.4) | 37 (5.2) | 11 (0.5) | 4 (0.5) | 12 (2.0) | 8 (0.9) | |
| Septicemia | 235 (4.6) | 26 (3.7) | 101 (5.0) | 4 (0.5) | 34 (5.7) | 70 (7.7) | |
| Other/multiple | 1022 (19.9) | 301 (42.4) | 120 (5.9) | 173 (19.7) | 255 (42.6) | 173 (19.0) | |
| Unknown | 3442 (67.0) | 239 (33.7) | 1719 (84.3) | 653 (74.5) | 275 (46.0) | 556 (61.0) | |
| Outcome | Discharged Alive | 3107 (60.5) | 561 (79.0) | 935 (45.9) | 308 (35.2) | 456 (76.3) | 847 (92.9) |
| Died | 278 (5.4) | 93 (13.1) | 49 (2.4) | 52 (5.9) | 39 (6.5) | 45 (4.9) | |
| Unknown | 1749 (34.1) | 56 (7.9) | 1054 (51.7) | 516 (58.9) | 103 (17.2) | 20 (2.2) | |
| Total no. of suspected cases recruitedf | 5134 (100.0) | 710 (13.8) | 2038 (39.7) | 876 (17.1) | 598 (11.6) | 912 (17.8) |
aAbubakar Tafawa Balewa University Teaching Hospital.
bLagos University Teaching Hospital.
cUniversity of Benin Teaching Hospital.
dUniversity of Ilorin Teaching Hospital.
eUniversity of Nigeria Teaching Hospital.
fSuspected cases include cases that were defined as probable per World Health Organization case definition guidelines [16].
The clinical characteristics of the children enrolled are detailed in Table 2. In summary, 75 (2.2%: 75/3338) children who presented with clear CSF samples had confirmed bacterial meningitis. However, a higher proportion of patients (4.5%: 75/1667) with turbid, xanthrochromic and blood-stained CSF samples had confirmed bacterial meningitis. The percentage of patients with PBM increased with WBC count; 1.7% (67/3888) of patients with a low WBC count (≤10 cells/mm3) had bacterial meningitis, whereas 3.4% (12/352) and 14.8% (29/196) of children with >10 to 100 cells/mm3 and >100 cells/mm3, respectively, were infected. More patients with PBM had high levels of protein (>100 mg/dL) in their CSF (9.5%: 42/441) compared to those with low protein levels ≤100 mg/dL (2.7%: 81/3010). However, more children with CSF glucose levels of ≤40 g/dL had PBM (4.5%: 57/1279) compared to 2.9% (64/2193) of patients with lower glucose levels (>40 g/dL).
Table 2.
Summary of Clinical Characteristics of Patients in Relation to Causative
| Recruited | Tested | Pneumococcus | Meningococcus | Haemophilus influenzae | ||
|---|---|---|---|---|---|---|
| Characteristic | n | n (%) | n (%) | n (%) | n (%) | |
| Cerebrospinal fluid appearance | Clear | 3338 | 3281 (98.3) | 39 (1.2) | 18 (0.5) | 18 (0.5) |
| Turbid | 418 | 411 (98.3) | 20 (4.9) | 18 (4.4) | 6 (1.5) | |
| Xanthrochromic | 635 | 624 (98.3) | 6 (1.0) | 9 (1.4) | 1 (0.2) | |
| Blood stained | 614 | 607 (98.9) | 4 (0.7) | 8 (1.3) | 3 (0.5) | |
| Unknown | 129 | 85 (65.9) | 2 (2.4) | 0 | 1 (1.2) | |
| White blood cell count (cells/mm3) | ≤10 | 3888 | 3840 (98.8) | 33 (0.9) | 20 (0.5) | 14 (0.4) |
| >10 to 100 | 352 | 348 (98.3) | 4 (1.1) | 6 (1.7) | 2 (0.6) | |
| >100 | 196 | 195 (99.5) | 15 (7.7) | 11 (5.6) | 3 (1.5) | |
| Unknown/not done | 698 | 625 (90.0) | 19 (3.0) | 16 (2.6) | 10 (1.6) | |
| Protein (mg/dL) | ≤100 | 3010 | 2952 (98.1) | 41 (1.4) | 20 (0.7) | 20 (0.7) |
| >100 | 441 | 432 (98.0) | 15 (3.5) | 23 (5.3) | 4 (0.9) | |
| Unknown/not done | 1683 | 1624 (96.5) | 15 (0.9) | 10 (0.6) | 5 (0.3) | |
| Glucose (g/dL) | ≤40 | 1279 | 1261 (98.6) | 21 (1.7) | 23 (1.8) | 13 (1.0) |
| ≥40 | 2193 | 2146 (97.9) | 35 (1.6) | 19 (0.9) | 10 (0.5) | |
| Unknown/not done | 1662 | 1601 (96.3) | 15 (0.9) | 11 (0.7) | 6 (0.4) | |
| Total no. of suspected cases recruiteda | 5134 | 5008 (97.5) | 71 (1.4) | 53 (1.1) | 29 (0.6) |
aSuspected cases include cases that were defined as probable as per World Health Organization case definition guidelines [16].
There were a total of 153 (3.0%: 153/5134) cases of PBM observed at the 5 sentinel hospitals during the surveillance period in Nigeria. Pneumococcus was responsible for 71 cases (46.4%: 71/153) with a mortality rate of 14.1% (10/71). Meningococcal meningitis was confirmed in 53 (34.6%:53/153) pediatric patients with a case fatality rate of 20.7% (11/53). A total of 29 (19.0%: 29/153) children had meningitis caused by H. influenzae, among these 2 patients died; mortality rate of 3.4% (2/29). The overall mortality rate for confirmed bacterial meningitis cases was 15% (23/153).
Distribution of Bacterial Pathogens
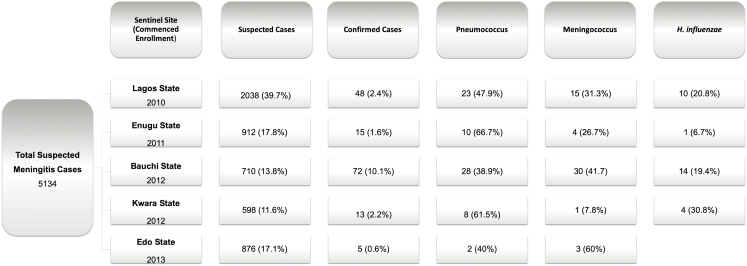
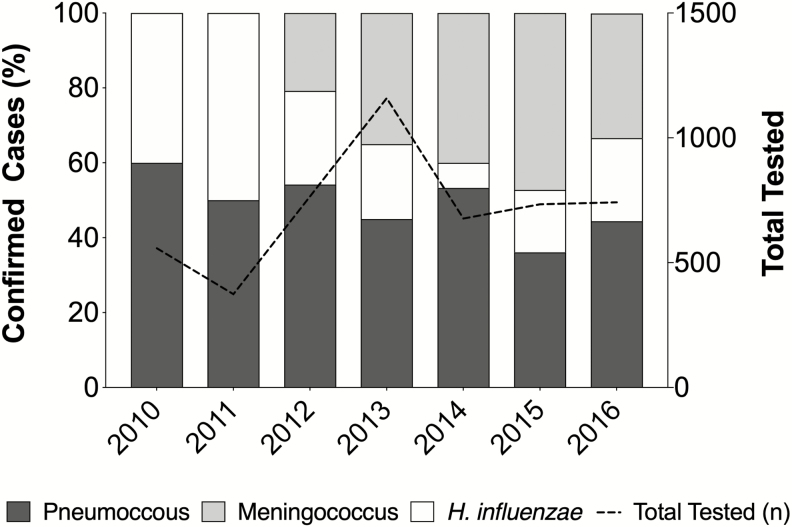
The number of suspected PBM cases observed in each of the 5 sentinel hospitals varied annually with an average of 715.4 cases per year (Figure 1). In 2013, with 5 sentinel hospitals enrolled, the total number of suspected cases peaked at 1204. Lagos State had the highest proportion of suspected meningitis over the study period (39.7%: 2038/5134) and Kwara State the least (11.6%: 598/5134). Additionally, the prevalence of meningitis caused by each of the 3 bacterial pathogens varied annually (Figure 2).
Figure 1.
Distribution of suspected pediatric bacterial meningitis cases from 2010 to 2016 within 5 sentinel hospitals in 5 Nigerian states. A total of 5134 suspected pediatric bacterial meningitis cases were observed at 5 sentinel hospitals: Lagos University Teaching Hospital (Lagos State), University of Nigeria Teaching Hospital (Enugu State), Abubakar Tafawa Balewa University Teaching Hospital (Bauchi State), University of Ilorin Teaching Hospital (Kwara State), and University of Benin Teaching Hospital (Edo State). Each hospital commenced surveillance at different time points; however, surveillance continued for all hospitals until 2016. The number of suspected cases of bacterial meningitis and the number of confirmed cases of meningitis (World Health Organization definitions [16]) varied per hospital. The main causative agents for bacterial meningitis were Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae.
Figure 2.
Proportion of confirmed pediatric bacterial meningitis cases and the pathogens responsible from 2010 to 2016 in 5 Nigerian states. The percentage of confirmed bacterial meningitis cases caused by Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae in children <5 years across 5 Nigerian states. A dashed black line indicates the total number of cerebrospinal fluid samples that were tested each year of surveillance.
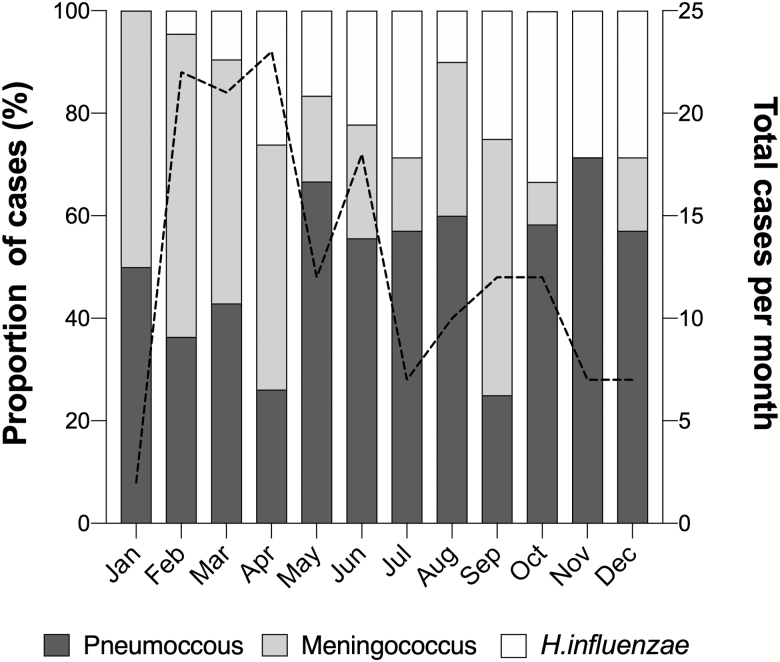
The frequency of confirmed meningitis cases observed fluctuated between months of the year (Figure 3). Presentation of PBM was highest from February to June throughout the surveillance period, and the highest number of cases was recorded in April. The prevalence of each causative agent varied from month to month. For instance, from February to April, most cases were caused by meningococcus 34 (51.5%: 34/66) and from May to August the dominant pathogen switched to pneumococcus with 28 (59.6%: 28/47) cases.
Figure 3.
Monthly distribution of suspected pediatric bacterial meningitis cases for the period 2010 to 2016 within 5 sentinel hospitals across Nigeria. The percentage of pediatric bacterial meningitis (PBM) cases seen across 5 hospitals in 5 Nigerian states, caused by Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae per month. A black dashed line indicates the total number of PBM cases per month throughout the surveillance period.
Serotype and Serogroup Distribution
Serotype analysis was attempted on 31 (43.7%: 31/71) pneumococcal positive CSF samples, however, 3 samples had a low concentration of DNA with CT values of >32 so could not be serotyped. Of the 28 isolates that were successfully serotyped, a variety of pneumococcal serotypes were responsible for PBM from year to year. For instance, the pneumococcal meningitis cases in 2011 and 2012 were caused by serotype 23F and 14, respectively, both of which are targeted by the PCV10 vaccine. We successfully serotyped 2 cases in 2013, which were caused by serotype 23F and 19A, which is a non-PCV10 serotype. In 2014, 10 pneumococcal meningitis cases were serotyped; of these, 1 case was caused by serotype 4, a PCV10 serotype. However, the remaining 9 (90%: 9/10) were caused by pneumococcal strains that were nontypeable by PCR and, thus, not covered by the current formulations of PCV. The 3 serotyped cases in 2015 were all caused by PCV10 serotypes, serotype’s 1, 5, and 18C. A further 11 cases were serotyped in 2016: 6 cases were caused by 4 PCV10 serotypes (4, 6B, 19F, and 23F), 4 cases were caused by pneumococci that were nontypeable by PCR, and 1 case was caused by serotype 23A, a non-PCV10 pathogen. Overall, half (50.0%: 14/28) of the pneumococcal meningitis cases that were successfully serotyped were caused by serotypes that are not targeted by PCV10 vaccine.
Furthermore, most of the meningococcal meningitis cases were reported from 2014 to 2016, with 41 (77.4%: 41/53) patients across the 3 years and a peak of 17 cases in 2015. In total, 20 (37.7%: 20/53) meningococcal isolates underwent serogrouping analysis. Four serogroups were observed within the surveillance period, the most prevalent was serogroup W, with 8 cases (40%: 8/20) reported in 2015, followed by a nongroupable strain causing 1 case in 2015 and 5 cases in 2016 (30%: 6/20). Serogroup B meningococcus was responsible for 5 case, 1 each in 2013 and 2015 and a further 3 in 2016. However, serogroup C was identified in 1 case of meningococcal meningitis in 2014.
Finally, of the 29 H. influenzae positive CSF samples, serotype analysis was successfully performed on 16 (55.2%: 16/29) of these. The most prevalent serotype was Hib, responsible for 1 case in 2012, 2 cases in 2013, and 8 cases in 2016 (69%:11/16). Hia caused 3 infections in 2015, and Hic was responsible for 2 infection in 2015. Interestingly, 2 of the patients with H. influenzae meningitis reported receiving at least 1 dose of the Hib vaccine, 1 case was caused by serotype Hia, and the other was not serotyped.
DISCUSSION
We conducted detailed analysis of longitudinal PBM surveillance data from 5 hospitals in 5 states of Nigeria. We found the highest number of suspected bacterial meningitis cases in children from the youngest age group and a lower number of cases reported in children aged 12–23 months but rising in the eldest cohort of children aged 24–59 months (Table 1). This was unexpected, as previous studies in The Gambia, Oman, and Turkey have shown that the incidence of bacterial meningitis decreases with age, due to maturation of the immune response [23–25]. The mortality rate of suspected meningitis was 5.4% (278/5134). However, when excluding data for patients where the outcome at discharge was not recorded (34.1%: 1749/5134), the mortality rate increased to 8.2% (278/3385), suggesting that suspected meningitis patients were severely unwell. Sequelae was only reported for 2% (102/5134) of patients with suspected meningitis; however, this prevalence is likely to be higher given the large number of patients (62.3%: 3245/5134) without follow-up after discharge.
Diagnosis of acute bacterial meningitis can be difficult in sub-Saharan Africa where resources are limited and clinical features are similar to malarial infections [26]. Analysis of CSF through microbiology culture is the gold standard technique used to detect PBM; however, studies have found low bacterial recovery rates in West Africa [27]. Additionally, 14.5% (743/5134) of patients with suspected meningitis in Nigeria received antibiotics before admission, which may have contributed to low bacteriological recovery. Moreover, without the sophisticated laboratory techniques used, diagnosis of bacterial meningitis would have relied on clinical characteristics. Thus, the 44 (28.8%: 44/153) PBM patients presenting with clear CSF and low WBC count may have been misdiagnosed. A history of fever (84.3%: 129 /153) and presence of seizures (71.2%: 109/153) were the most common symptoms associated with confirmed PBM cases in Nigeria. However, an altered consciousness is a more accurate indicator of bacterial meningitis when combined with other common symptoms [28]. During surveillance, only 22% (34/153) of meningitis patients had an altered consciousness. Additionally, meningismus (10.5%: 16/153) and bulging fontanelle (8.5%: 13/153) were observed less frequently, highlighting the importance of considering all clinical symptoms when diagnosing PBM as outlined in the WHO guidelines [16].
West Africa has a bimodal climate: wet season occurs from mid-April until mid-October, and the remaining months are characterized by dry season. Available data suggest the intensity of bacterial meningitis epidemics are associated with the Harmattan winds during dry season; thus, epidemics are rare during rainy season [29, 30]. The seasonal distribution of PBM within Nigeria from 2010 to 2016 is in line with this trend (Figure 3). Previous studies have shown that respiratory syncytial virus (RSV) is also seasonal in Nigeria, with cases mainly observed during the dry season and often peaking in November [31, 32]. Conversely, influenza has been shown to have year-round activity within Nigeria [33, 34]. Moreover, the seasonality of malaria transmission within Nigeria differs across the country based on the various ecological regions. Thus, malaria transmission is often year-round in southern Nigeria where there are high levels of mangrove swamps but lasts 3 months or less in northern regions such as the Sahel-savannah [35, 36].
Vaccines targeting the main bacterial pathogens causing meningitis are effective at reducing morbidity and mortality [37, 38]. The Hib vaccine was introduced in phases across Nigeria from May 2012 to May 2014, and 41.4% (12/29) of the H. influenzae associated meningitis cases occurred during this period, with a further 48.3% (14/29) cases postvaccine introduction. We identified 11 cases of Hib meningitis; of these, 7 patients had not received the Hib vaccine, and the Hib vaccination status for the remaining 4 patients was unknown. The WHO and UNICEF 2017 national immunization coverage estimates for the Hib containing vaccine in Nigeria were <50% [15]. Additionally, in 2017, Nigeria’s National Primary Health Care Development Agency (NPHCDA) found that only Bauchi and Kwara of the 5 states participating in PBM surveillance reported >50% coverage of the Hib vaccine, indicating that coverage across Nigeria needs to be improved [39].
MenAfriVac against serogroup A has not been introduced into routine immunization programs in Nigeria but was given to at risk populations during mass campaigns from 2011 to 2014 in high-risk states. Therefore, the recording of MenAfriVac vaccination status of patients during surveillance was limited, with 4 patients reporting they had received the vaccines, 650 patients reporting they had not, and 4480 patients where this information was unknown or not recorded. However, of the 20 isolates we serogrouped, none were serogroup A; instead, serogroup W was the predominant strain, followed by nongroupable strains. Expansion of nonvaccine serogroups due to vaccine selection pressures could be responsible for the increased incidence of nonserogroup A meningococcus, but, as limited serogrouping data were obtained, further work is required to conclude serotype replacement is occurring [40].
Pneumococcus was the predominant pathogen causing 71 (46.4%: 71/153) PBM cases during surveillance in Nigeria. A high incidence of pneumococcal meningitis is also common in The Gambia [25]. Of the 5 states where surveillance occurred, Edo state commenced PCV10 vaccinations in 2014, and only 2 pneumococcal meningitis cases were reported here afterward. The remaining 4 states received PCV10 in 2016; thus, pre- and postvaccine comparisons are not possible, and we were unable to record the PCV10 vaccination status of patients throughout our surveillance period. However, 46.4% (13/28) of the isolates serotyped were strains that are included in PCV10 and thus could have been prevented by immunization. The 2017 WHO and UNICEF national immunization coverage estimates suggest that coverage rates for PCV10 are <50% in Nigeria [15]. For each of the 5 states enrolled in surveillance, coverage of 3 PCV10 doses from January to November 2017 was >50% in Bauchi and Kwara only [39]. We found 1 case of pneumococcal meningitis caused by serotype 19A, targeted by PCV13 but not PCV10, and 13 cases caused by pneumococcal serotypes that were nontypeable and thus not covered by current PCV formulations. Serotype replacement of vaccine serotypes with nonvaccine serotypes is a phenomenon that has been widely reported since the introduction of PCVs [41, 42]. However, due to the limited pneumococcal serotype data reported and the recent introduction of PCV10, surveillance should be continued in Nigeria to monitor the burden of vaccine preventable bacterial meningitis and any potential changes in serotype distribution over time.
Limitations
During this surveillance, children with suspected meningitis were not followed up after hospital discharge. There were a total of 278 (5.4%: 278/5134) in-hospital deaths; of these, 23 (8.2%: 23/278) were patients with confirmed bacterial meningitis. However, the outcome for 1749 patients (34.1% 1749/5134) was unknown or not recorded, and of these, 26 (1.5%: 26/1749) were patients with confirmed bacterial meningitis. Therefore, the case fatality of 5.4% (278/5134) among children with suspected meningitis may have been higher, but this cannot be confirmed. Additionally, 102 (2%: 102/5134) patients were reported to have long-term sequelae; however, 3245 (63.2%: 3245/5134) were not followed up after discharge, and the HIV status was not routinely recorded during the surveillance. Moreover, there were discrepancies between the level of reporting from each sentinel hospital. In future studies, it would be beneficial to record information regarding long-term sequelae, outcome at discharge, and HIV status for all patients with suspected meningitis and to do this in a standardized manner across all sites. This would allow more accurate rates of morbidity and mortality associated with bacterial meningitis within the surveillance populations to be estimated.
CONCLUSIONS
Pneumococcus was responsible for the majority of PBM cases in Nigeria; however, PCV10 has now been introduced within all states included in surveillance, and with improved vaccine coverage it is expected that pneumococcal meningitis rates will start to decline. Hib remains responsible for a significant proportion of meningitis despite vaccine introduction. Therefore, our findings emphasize the need for further monitoring to establish the impact of conjugate vaccines on reducing the prevalence of bacterial meningitis within Nigeria. Further serotype/group data for PBM cases is required to understand the distribution of specific pathogen strains across Nigeria and to enhance efficacy of target vaccines.
Notes
Invasive Bacterial Disease Writing Group members. Brenda Kwambana Adams, Senghore Madikay, Effua Usuf, Archibald Worwui, Uzochukwu Egere, Akram Zaman, Catherine Okoi, Florian Gehre, Leopold Tientcheu, Nuredin Ibrahim Mohammed, Felix Dube, Peter Ndow, Sambou M. Suso, Sheikh Jarju, Dam Khan, Chinelo Ebruke, Rowan Bancroft, Jason M. Mwenda, and Martin Antonio.
Author contributions. M. A. and J. M. M. established the World Health Organization Regional Office for Africa–supported Paediatric Bacterial Meningitis Surveillance Network in West Africa. M. A. supervised the overall network including setting up the sentinel surveillance system. B. N. T., I. F., M. B. A., M. F. B., O. P. O., A. H. I., N. M. L., B. O. E., N. O., C. J. I., F. N., U. C. O., F. U., A. O. S., A. F., S. A. A., J. A., A. G. Y., P. O., C. K., V. E., and O. J. M. clinically investigated and recruited the patients at the sentinel sites, collected demographic data, performed microbiological testing, and shipped cerebrospinal fluid and bacterial isolates to the Medical Research Council (MRC) Unit The Gambia (MRCG) for confirmatory testing and molecular analysis supervised by B. K. A. and M. A. B. K. A. and M. A. developed the analysis plan and contributed to analysis and interpretation of data along with the IBD writing group. R. E. B., B. K. A., and M. A. drafted the article along with B. N. T. All authors contributed to the interpretation of the findings and the writing of the final article.
Acknowledgments. The authors thank the WHO Country Office of Nigeria and WHO Inter-country Support Team for coordination, advice and support throughout the project. They thank the surveillance team members as well as patients and their families in Nigeria; the staff and students at the MRCG at London School of Hygiene and Tropical Medicine [LSHTM], as well as the IBD writing group for their advice and input.
Disclaimer. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions the World Health Organization, the Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine or the authors’ affiliated institutions.
Financial support. Financial support for sentinel site surveillance was provided by the Federal Ministry of Health Nigeria, GAVI - the Vaccine Alliance, through a grant to the WHO for the African Paediatric Bacterial Meningitis Surveillance Network.
Supplement sponsorship. This supplement was supported with funds from Gavi, the Vaccine Alliance through The World Health Organization and the CDC Foundation, and The Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray J, Agócs M, Serhan F, et al. ; Centers for Disease Control and Prevention (CDC) Global invasive bacterial vaccine-preventable diseases surveillance—2008–2014. MMWR Morb Mortal Wkly Rep 2014; 63:1159–62. [PMC free article] [PubMed] [Google Scholar]
- 3. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Omeh DJOB, Omeh CK. Reccuring epidemics of meningococcal meningitis in African meningitis belt: a review of challenges and prospects. JAMMR 2017; 22:1–12 [Google Scholar]
- 5. Chow J, Uadiale K, Bestman A, et al. Invasive meningococcal meningitis serogroup c outbreak in northwest Nigeria, 2015: third consecutive outbreak of a new strain. PLoS Currents 2016; 8:ecurrents.outbreaks.06d10b6b4e690917d8b0a04268906143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCDC. Cerebrospinal meningitis outbreak in Nigeria: situation report. Available at: http://ncdc.gov.ng/themes/common/files/sitreps/153667690b5c184d9fab417f65a25b1e.pdf. Accessed 30 October 2017. [Google Scholar]
- 7. Iwalokun BA, Fowora M, Akinloye O, Oluwadun A, Antonio M, Adegbola RA. A retrospective study of clinical Streptococcus pneumoniae isolates from four health facilities in South-West Nigeria. Int J Med Sci Public Health 2012; 4:160–70. [Google Scholar]
- 8. Thairu Y, Egah D, Banwat E, Dd S, Oh K. Prevalence of H. influenzae among under-five children presenting at the emergency paediatric unit (Epu) of two teaching hospitals in Jos, Plateau State, Nigeria. J Dent Med Sci 2014; 13:73–9. [Google Scholar]
- 9. World Health Organization. Immunization, vaccines and biologicals: invasive bacterial vaccine preventable diseases laboratory network. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/laboratory/IBVPD/en/. Accessed 24 October 2017.
- 10. Nigeria National Bureau of Statistics. National population estimates. Available at: https://nigerianstat.gov.ng/elibrary?queries[search]=population. Accessed 12 May 2019.
- 11. UNAIDS. Press release: new survey results indicate that Nigeria has an HIV prevalence of 1.4%. Available at: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2019/march/20190314_nigeria. Accessed 14 May 2019.
- 12. NACA Nigeria. Available at: https://naca.gov.ng/nigeria-prevalence-rate/. Accessed 14 May 2019.
- 13. Sadoh AE, Nwaneri DU, Ogboghodo BC, Sadoh WE. Effect of introduction of pentavalent vaccine as replacement for diphtheria-tetanus-pertussis and hepatitis B vaccines on vaccination uptake in a health facility in Nigeria. Vaccine 2016; 34:2722–8. [DOI] [PubMed] [Google Scholar]
- 14. NCDC. Meningitis outbreak in Nigeria affects five states. Available at: http://www.ncdc.gov.ng/news/67/meningitis-outbreak-in-nigeria-affects-five-states. Accessed 30 October 2017.
- 15. World Health Organization. Nigeria: WHO and UNICEF estimates of immunization coverage: 2016 revision. Available at: http://www.who.int/immunization/monitoring_surveillance/data/nga.pdf. Accessed 04 July 2018.
- 16. World Health Organization. Standard operating procedures for enhanced meningitis surveillance in Africa. August, 2009 Available at: http://apps.who.int/iris/bitstream/handle/10665/1906/SOP_2009.pdf?sequence=1&isAllowed=y. Accessed 21 October 2017.
- 17. Shahangian S, Brown PI, Ash KO. Turbidimetric measurement of total urinary proteins: a revised method. Am J Clin Pathol 1984; 81:651–4. [DOI] [PubMed] [Google Scholar]
- 18. McMillin JM. Blood glucose. In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Oxford: Butterworth-Heinemann Ltd, 1990. [PubMed] [Google Scholar]
- 19. World Health Organization. Guidance on regulations for the transport of infectious substances 2015–2016. Available at: http://apps.who.int/iris/bitstream/10665/149288/1/WHO_HSE_GCR_2015.2_eng.pdf. Accessed 24 October 2017.
- 20. Messmer TO, Sampson JS, Stinson A, Wong B, Carlone GM, Facklam RR. Comparison of four polymerase chain reaction assays for specificity in the identification of Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2004; 49:249–54. [DOI] [PubMed] [Google Scholar]
- 21. Vuong J, Collard JM, Whaley MJ, et al. Development of real-time PCR methods for the detection of bacterial meningitis pathogens without DNA extraction. PLoS One 2016; 11:e0147765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pimenta FC, Roundtree A, Soysal A, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 2013; 51:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceyhan M, Yildirim I, Balmer P, et al. A prospective study of etiology of childhood acute bacterial meningitis, Turkey. Emerg Infect Dis 2008; 14:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dash N, Panigrahi D, Al Khusaiby S, Al Awaidy S, Bawikar S. Acute bacterial meningitis among children <5 years of age in Oman: a retrospective study during 2000–2005. J Infect Dev Ctries 2008; 2:112–5. [PubMed] [Google Scholar]
- 25. Goetghebuer T, West TE, Wermenbol V, et al. Outcome of meningitis caused by Streptococcus pneumoniae and Haemophilus influenzae type b in children in The Gambia. Trop Med Int Health 2000; 5:207–13. [DOI] [PubMed] [Google Scholar]
- 26. Wright PW, Avery WG, Ardill WD, McLarty JW. Initial clinical assessment of the comatose patient: cerebral malaria vs. meningitis. Pediatr Infect Dis J 1993; 12:37–41. [DOI] [PubMed] [Google Scholar]
- 27. Wu HM, Cordeiro SM, Harcourt BH, et al. Accuracy of real-time PCR, Gram stain and culture for Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae meningitis diagnosis. BMC Infect Dis 2013; 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Best JHS. Evidence behind the WHO guidelines: hospital care for children—what are the useful clinical features of bacterial meningitis found in infants and children?. J Trop Ped 2008; 54:83–6. [DOI] [PubMed] [Google Scholar]
- 29. Agier L, Deroubaix A, Martiny N, Yaka P, Djibo A, Broutin H. Seasonality of meningitis in Africa and climate forcing: aerosols stand out. J R Soc Interface 2013; 10:20120814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yaka P, Sultan B, Broutin H, Janicot S, Philippon S, Fourquet N. Relationships between climate and year-to-year variability in meningitis outbreaks: a case study in Burkina Faso and Niger. Int J Health Geogr 2008; 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ 2004; 82:914–22. [PMC free article] [PubMed] [Google Scholar]
- 32. Sricharoenchai S, Palla E, Sanicas M. Seasonality of respiratory syncytial virus - lower respiratory tract infection (RSV-LRTI) in children in developing countries. J Hum Virol Retrovirol 2016; 3: 00076. doi: 10.15406/jhvrv.2016.03.00076 [DOI] [Google Scholar]
- 33. Hirve S, Newman LP, Paget J, et al. Influenza seasonality in the tropics and subtropics: when to vaccinate? PLoS One 2016; 11:e0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newman LP, Bhat N, Fleming JA, Neuzil KM. Global influenza seasonality to inform country-level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS ONE 2018; 13:e0193263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ayansina A, Nathaniel Olugbade A, Oyekanmi B. Intra-annual climate variability and malaria transmission in Nigeria. Bulletin of Geography 2013; 21:7–19. [Google Scholar]
- 36. Malaria Consortium. Seasonal malaria chemoprevention. Available at: https://www.malariaconsortium.org/media-downloads/197/Seasonal%20Malaria%20Chemoprevention%20(SMC)%20in%20Nigeria. Accessed 14 May 2019.
- 37. Mackenzie GA, Hill PC, Jeffries DJ, et al. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect Dis 2016; 16:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wenger J. Hib vaccine introduced in The Gambia. Africa Health 1997; 20:13, 5. [PubMed] [Google Scholar]
- 39. NPHCDA. National routine immunization performance monitoring. Available at: http://www.nphcda.gov.ng/wp-content/uploads/2017/06/April-2017-National-RI-Feedback_.pdf. Accessed 01 November 2017.
- 40. Meyer SA, Novak RT. Effect of a vaccine to prevent serogroup A N meningitidis epidemics in Africa. Lancet Infect Dis 2017; 17:789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwambana-Adams B, Hanson B, Worwui A, et al. Rapid replacement by non-vaccine pneumococcal serotypes may mitigate the impact of the pneumococcal conjugate vaccine on nasopharyngeal bacterial ecology. Sci Rep 2017; 7:8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]