ABSTRACT
Background
Excess lipid availability has been associated with the development of anabolic resistance. As such, obesity may be accompanied by impairments in muscle protein metabolism.
Objective
We hypothesized that basal and postprandial muscle protein synthesis rates are lower in obese than in lean men.
Methods
Twelve obese men [mean ± SEM age: 48 ± 2 y; BMI (in kg/m2): 37.0 ± 1.5; body fat: 32 ± 2%] and 12 age-matched lean controls (age: 43 ± 3 y; BMI: 23.4 ± 0.4; body fat: 21 ± 1%) received primed continuous L-[ring-2H5]-phenylalanine and L-[ring-3,5-2H2]-tyrosine infusions and ingested 25 g intrinsically L-[1-13C]-phenylalanine labeled whey protein. Repeated blood and muscle samples were obtained to assess protein digestion and amino acid absorption kinetics, and basal and postprandial myofibrillar protein synthesis rates.
Results
Exogenous phenylalanine appearance rates increased after protein ingestion in both groups (P < 0.001), with a total of 53 ± 1% and 53 ± 2% of dietary protein–derived phenylalanine appearing in the circulation over the 5-h postprandial period in lean and obese men, respectively (P = 0.82). After protein ingestion, whole-body protein synthesis and oxidation rates increased to a greater extent in lean men than in the obese (P-interaction < 0.05), resulting in a higher whole-body protein net balance in the lean than in the obese (7.1 ± 0.2 and 4.6 ± 0.4 µmol phenylalanine · h−1 · kg−1, respectively; P-interaction < 0.001). Myofibrillar protein synthesis rates increased from 0.030 ± 0.002 and 0.028 ± 0.003%/h in the postabsorptive period to 0.034 ± 0.002 and 0.035 ± 0.003%.h−1 in the 5-h postprandial period (P = 0.03) in lean and obese men, respectively, with no differences between groups (P-interaction = 0.58).
Conclusions
Basal, postabsorptive myofibrillar protein synthesis rates do not differ between lean and obese middle-aged men. Postprandial protein handling, including protein digestion and amino acid absorption, and the postprandial muscle protein synthetic response after the ingestion of 25 g whey protein are not impaired in obese men. This trial was registered at www.trialregister.nl as NTR4060.
Keywords: obesity, protein ingestion, metabolism, anabolic resistance, postprandial protein handling, muscle protein synthesis
Introduction
Skeletal muscle mass maintenance is largely regulated by diurnal rates of muscle protein synthesis and breakdown. It has been well established that the postprandial rise in amino acid concentrations after the ingestion of dietary protein stimulates muscle protein synthesis (1–3). More recent work shows that aging, inactivity, and/or insulin resistance are associated with a reduced responsiveness to the anabolic properties of amino acid and protein ingestion (4–6). This anabolic resistance to protein intake is now believed to represent a key factor responsible for accelerated (age-related) muscle loss (5, 7, 8). Skeletal muscle insulin resistance may play an important role in the development of this age-related anabolic resistance (9–11). Recently, we showed that the muscle protein synthetic response to amino acid administration is blunted under conditions of lipid oversupply (12). Lipid accumulation in skeletal muscle occurs in response to prolonged obesity and is associated with metabolic dysfunction including reduced insulin sensitivity (13, 14). Consequently, it is hypothesized that obesity is accompanied by impairments in muscle protein metabolism (15–17).
Only a few studies have examined basal and/or postprandial muscle protein synthesis rates in obese individuals (17–22) and even fewer have made direct comparisons between overweight or obese participants and lean controls (22–26). Data obtained from these studies demonstrate that obese individuals experience either impairments (16, 23) or no differences (18, 20, 22, 24, 25) in basal, postabsorptive muscle protein synthesis rates when compared with lean controls. Studies examining the postprandial period after protein administration have shown similar (16) or impaired (16, 24) muscle protein synthesis rates in obese individuals when compared with lean controls. However, previous studies have typically been performed under hyperinsulinemic-hyperaminoacidemic clamp conditions, which do not represent a normal physiological response to the ingestion of a single meal-like amount of dietary protein. Recently, Beals et al. (25) compared the postprandial muscle protein synthetic response after the ingestion of 170 g pork meat (∼36 g protein) between young lean, overweight, and obese men. In this study, the muscle protein synthetic response to protein ingestion was lower in both overweight and obese young men than in healthy-weight controls (25). Smeuninx et al. (22) showed that the presence of obesity in older men and women does not further impair the anabolic response to the ingestion of a moderate 15-g protein dose when compared with lean controls. It remains unclear whether the muscle protein synthetic response is impaired in middle-aged obese individuals after the ingestion of a meal-like amount of dietary protein and whether this could be attributed to impairments in protein digestion and amino acid absorption kinetics and/or an attenuated rise in postprandial muscle protein synthesis rates when compared with lean controls.
In the present study, we combined contemporary stable isotope amino acid infusions with the ingestion of intrinsically labeled protein to compare basal, postabsorptive muscle protein synthesis rates, dietary protein digestion and amino acid absorption kinetics, and subsequent muscle protein synthesis rates after the ingestion of 25 g whey protein between middle-aged lean and obese men. We hypothesized that basal and postprandial muscle protein synthesis rates would be lower in obese men than in age-matched lean controls.
Methods
Study design
Healthy, recreationally active, middle-aged (age: 30–55 y) lean [BMI (in kg/m2): 19–25] and obese (BMI > 30) men were recruited by advertisements in local newspapers and through the Obesity Center (Máxima Medical Center, Eindhoven, Netherlands) to participate in a single experimental stable isotope tracer infusion trial. Participants were informed of the nature and possible risks of the experimental procedures before providing written informed consent. The participants were deemed healthy based on their responses to a medical questionnaire and screening results. The trial (NTR4060) was conducted between October, 2013 and June, 2015 at Maastricht University Medical Centre+, Netherlands. The study was part of a larger project on muscle protein metabolism in middle-aged individuals (27). This study was approved by the Medical Ethical Committee of the Maastricht University Medical Centre+ and conformed to the standards for the use of human subjects in research as outlined in the latest version of the Declaration of Helsinki.
Pretesting
All men participated in a screening session to assess their glycated hemoglobin, glucose tolerance [2-h oral-glucose-tolerance test (28)], blood pressure, weight, height, and body composition. Fat mass, fat-free mass (FFM), and bone mineral content were determined by DXA scan (Discovery A, QDR series, Hologic Inc.). Whole-body and regional lean mass, percentage body fat, and visceral adipose tissue (VAT) were determined using the software package Apex (en-CORE 2005, version 4.0.2; Hologic) and reference values from the NHANES population-based data set (29). A single-slice computed tomography scan (Philips Brilliance 64, Philips Medical Systems) was performed to assess upper leg muscle cross-sectional area (CSA). The scanning characteristics were as follows: 120 kV, 300 mA, rotation time of 0.75 s, and field of view of 500 mm. With participants lying supine with their legs extended and feet secured, a 3-mm-thick axial image was taken 15 cm proximal to the top of the patella. Intermuscular adipose tissue was defined using radiation attenuation ranges from −190 to −30 Hounsfield units (HU); muscle area of the legs was selected between −29 and +150 HU (30); after which the thigh muscles, quadriceps muscle, and femoral bone were selected by manual tracing using ImageJ software (version 1.45d, NIH) (31, 32). All participants were instructed to refrain from strenuous physical activity and to maintain their diet as constant as possible for 2 d before the experimental infusion. On the evening before the trial, a standardized meal was consumed [2.3 MJ; composed of 19% energy (En%) protein, 40 En% carbohydrate, and 41 En% fat]. Before the experimental test, habitual physical activity was assessed using a validated triaxial accelerometer (Actigraph GTX3, Actigraph Inc.) worn in a belt around the waist over a 5-d period. Habitual physical activity was determined by the sum of accelerometer counts over a 24-h period. Analysis was carried out using Actilife software version 6 (Actigraph Inc.). The collected data were analyzed for total time spent in different activity levels (sedentary, light, moderate, vigorous, and very vigorous activities) and step counts. Participants’ habitual dietary intakes were calculated from 3-day dietary records prior to the experimental trial. The days of recording included two weekdays and one weekend day. Energy and macronutrient intakes were calculated with the use of the Dutch Nutrients Database (NEVO-online version 2016/ 5.0; http://nevo-online.rivm.nl/).
Experimental protocol
At 08:00, after an overnight fast, participants arrived at the laboratory by car or public transport. A Teflon catheter was inserted into an antecubital vein for infusion and a second cannula into the dorsal hand vein of the contralateral arm for arterialized blood sampling (33). After baseline blood collection (t = −210 min), the plasma phenylalanine and tyrosine pools were primed with a single intravenous dose (priming dose) of L-[ring-2H5]-phenylalanine (3.6 μmol/kg FFM) and L-[ring-3,5-2H2]-tyrosine (1.1 µmol/kg FFM). Subsequently, the continuous infusion was initiated (infusion rate: 0.060 µmol L-[ring-2H5]-phenylalanine · kg FFM−1 · min−1 and 0.018 µmol L-[ring-2H2]-tyrosine · kg FFM−1 · min−1) and maintained for 8.5 h. Multiple blood samples were collected during the infusion at t = −120, −90, −60, −30, 0, 20, 40, 60, 90, 120, 150, 180, 240, and 300 min for the analysis of plasma amino acid concentrations and enrichments, glucose and insulin concentrations, and nonesterified fatty acids (NEFAs). Postabsorptive triacylglycerol (TG) concentrations and plasma IL-6 concentrations were analyzed at t = −210 min. Blood samples were collected in EDTA-containing tubes and centrifuged at 1000 × g at 4°C for 10 min. Aliquots of plasma were then immediately frozen in liquid nitrogen and stored at −80°C. Muscle biopsies were collected at t = −120 and 0 min for the determination of postabsorptive muscle protein synthesis rates. Immediately after the second biopsy (t = 0 min, from the contralateral leg), participants ingested a single bolus of 25 g intrinsically L-[1-13C]-phenylalanine labeled whey protein dissolved in 350 mL water, which was flavored with 1.5 mL vanilla (IMCD Benelux N.V.). Additional biopsies were then collected at t = 120 and 300 min (alternating between legs) relative to the ingestion of the drink for the measurement of postprandial muscle protein synthesis rates. Muscle biopsies were randomized between legs and collected from the middle region of the vastus lateralis (15 cm above the patella) using the Bergström needle technique (34). All biopsy samples were freed from any visible adipose tissue and blood, immediately frozen in liquid nitrogen, and stored at −80°C until subsequent analysis.
Production of intrinsically labeled protein
Intrinsically L-[1-13C]-phenylalanine labeled whey protein was obtained by infusing a lactating Holstein cow with L-[1-13C]-phenylalanine (455 µmol/min), collecting milk, and purifying the whey fraction, as described previously (35–37). The enrichment of L-[1-13C]-phenylalanine in the whey protein was 7.6 mole percent excess (MPE). The whey protein met all chemical and bacteriological specifications for human consumption.
Plasma analyses
Plasma glucose, NEFA, and TG concentrations were analyzed using enzymatic assays on an automated spectrophotometer (ABX Pentra 400 autoanalyzer, Horiba ANX). Plasma insulin concentrations were determined with RIA kits (Human Insulin specific RIA, Millipore Corporation). Plasma IL-6 concentrations were measured using an ELISA kit (Human ProInflammatory IL-6, Thermofischer, Bender MedSystems GmbG). Plasma amino acid concentrations and enrichments were determined by GC-MS analysis (Agilent 7890A GC/5975C; MSD) as described in detail previously (27).
Muscle analyses
Myofibrillar protein-enriched fractions were extracted from an ∼60-mg piece of wet muscle tissue by hand-homogenizing on ice using a pestle (Teflon) in a standard extraction buffer (7 μL/mg) (38). Myofibrillar protein–bound L-[ring-2H5]-phenylalanine enrichments were determined by GC-MS as described in detail previously (27).
Calculations
Whole-body amino acid kinetics in non–steady state conditions were calculated from the ingestion of L-[1-13C]-phenylalanine labeled whey protein, intravenous infusion of L-[ring-2H5]-phenylalanine and L-[ring-3,5-2H2]-tyrosine, and arterialized venous blood sampling. Total, exogenous, and endogenous phenylalanine appearance rates (Ra in micromoles Phe per kilogram per minute), and plasma phenylalanine availability (i.e., the fraction of dietary protein–derived phenylalanine that appeared in the systemic circulation, Pheplasma) were calculated using modified Steele's equations (39–41). Furthermore, total rate of phenylalanine disappearance, utilization of phenylalanine for protein synthesis, and phenylalanine hydroxylation (the first step of conversion of phenylalanine to tyrosine) were calculated. Whole-body protein net balance was calculated by whole-body protein synthesis minus endogenous Ra. Whole-body protein turnover rates (synthesis, breakdown, oxidation, and net balance) were calculated as AUC over the 2 h (t = −120 to 0 min) postabsorptive and 5 h (t = 0–300 min) postprandial phases. The fractional synthetic rate (FSR) of myofibrillar protein was calculated using the standard precursor–product equation for the postabsorptive and postprandial (early: 0–2 h, late: 2–5 h, and cumulative: 0–5 h) periods. For complete details see the previously described methodology (27).
Statistics
All data are expressed as mean ± SEM. Differences in baseline values, body composition, nutritional intake, and physical activity levels were determined using unpaired 2-tailed Student's t tests. Peak values and time to peak were calculated for plasma time curves and differences were determined using an unpaired 2-tailed Student's t test. Two-factor ANOVA with time as the within-group factor and condition as the between-group factor was used to compare differences over time in plasma glucose, insulin, NEFA, and amino acid concentrations and enrichments, whole-body phenylalanine appearance rates, and FSR (postabsorptive to the 0–2 h and 2–5 h postprandial periods, and postabsorptive to the cumulative 0–5 h postprandial period). In case of significant time × group interactions, separate analyses were performed to determine time effects for each group (1-factor repeated-measures ANOVA with Bonferroni post hoc tests to identify time differences) and between-group effects for each time point (2-tailed Student's t test). Based upon previous studies (2, 42), a sample size of 12 subjects per group including a 10% dropout rate was calculated, using a 2-sided statistical test (P < 0.05, 80% power), to detect differences in FSRs between groups. For all analyses, statistical significance was set at P < 0.05. All calculations were performed using SPSS version 24.0 (IBM Statistics, IBM Corp.).
Results
Participants’ characteristics and body composition
Participants’ characteristics are presented in Table 1 and body composition data are presented in Table 2. Whole-body and appendicular lean body mass were greater in obese men than in lean controls (P < 0.05). Body fat percentage was higher in obese men than in lean controls (P < 0.05). Fat mass distribution was predominantly around the upper body and VAT mass was higher in obese than in lean men (both P < 0.001). Upper leg muscle and quadriceps CSA were greater in obese men than in lean controls (both P < 0.05).
TABLE 1.
Subjects’ characteristics1
| Lean | Obese | |
|---|---|---|
| Age, y | 43 ± 3 | 48 ± 2 |
| Weight, kg | 76.5 ± 1.6 | 117.9 ± 6.5* |
| BMI, kg/m2 | 23.4 ± 0.4 | 37.0 ± 1.5* |
| Plasma glucose OGTT t = 0 min, mmol/L | 5.2 ± 0.1 | 6.1 ± 0.2* |
| Plasma glucose OGTT t = 120 min, mmol/L | 4.4 ± 0.3 | 6.8 ± 0.7* |
| Plasma insulin OGTT t = 0 min, pmol/L | 61 ± 6 | 215 ± 19* |
| Plasma insulin OGTT t = 120 min, pmol/L | 121 ± 17 | 756 ± 171* |
| Glycated hemoglobin, mmol/mol | 33.7 ± 1.0 | 36.8 ± 1.0** |
| Oral glucose insulin sensitivity,2 mL · min−1 · m−2 | 451 ± 12 | 277 ± 19* |
| HOMA-IR2 | 1.14 ± 0.12 | 4.03 ± 0.34* |
| Postabsorptive plasma IL-6,3 pg/mL | 0.68 ± 0.12 | 1.19 ± 0.20** |
| Postabsorptive plasma NEFA,3 μmol/L | 390 ± 39 | 451 ± 45 |
| Postabsorptive plasma TG,3 mmol/L | 0.98 ± 0.16 | 1.81 ± 0.29* |
Values are mean ± SEM, n = 12/group. Data were analyzed by unpaired Student's t test. *, **Different from lean: *P < 0.05, **P < 0.1. NEFA, nonesterified fatty acid; OGTT, oral-glucose-tolerance test; TG, triacylglycerol.
Assessed by 2-h OGTT after consumption of 75 g glucose (28).
Postabsorptive plasma IL-6, NEFA, and TG were analyzed during the experimental trial at t = −210 min.
TABLE 2.
Body composition before the experimental infusion trial in lean and obese middle-aged men1
| Lean | Obese | |
|---|---|---|
| Lean body mass, kg (%) | 57.7 ± 1.6 (75 ± 1) | 76.8 ± 2.7 (66 ± 2)* |
| Appendicular lean mass, kg (%) | 26.1 ± 0.9 (34 ± 1) | 34.5 ± 1.4 (30 ± 1)* |
| Fat mass, kg (%) | 16.4 ± 0.7 (21 ± 1) | 38.8 ± 3.8 (32 ± 2)* |
| Trunk:limb fat mass ratio | 1.1 ± 0.1 | 1.5 ± 0.1* |
| Android fat mass, kg (%) | 1.4 ± 0.1 (25 ± 2) | 4.6 ± 0.5 (40 ± 2)* |
| Gynoid fat mass, kg (%) | 2.7 ± 0.1 (23 ± 1) | 5.5 ± 0.7 (30 ± 2)* |
| Android:gynoid fat ratio | 1.1 ± 0.1 | 1.4 ± 0.04* |
| Visceral adipose tissue, g | 363 ± 28 | 917 ± 59* |
| Left leg thigh CSA, cm2 | 144 ± 4 | 178 ± 5* |
| Right leg thigh CSA, cm2 | 146 ± 5 | 181 ± 5* |
| Left quadriceps CSA, cm2 | 77 ± 2 | 88 ± 3* |
| Right quadriceps CSA, cm2 | 79 ± 3 | 91 ± 4** |
| Left fat CSA, cm2 | 38 ± 4 | 95 ± 13* |
| Right fat CSA, cm2 | 38 ± 4 | 95 ± 14* |
| Left leg thigh, HU | 51 ± 1 | 45 ± 1* |
| Right leg thigh, HU | 52 ± 1 | 46 ± 1* |
| Left leg quadriceps, HU | 55 ± 1 | 51 ± 1* |
| Right leg quadriceps, HU | 55 ± 1 | 51 ± 1* |
Values are mean ± SEM, n = 12/group. Data were analyzed by unpaired Student's t test. *,**Different from lean: *P < 0.05, **P < 0.1. CSA, cross-sectional area; HU, Hounsfield Units.
Dietary intake and physical activity
Dietary intake and physical activity data are presented in Table 3. Dietary intake did not differ between groups (P = 0.15). Absolute habitual dietary protein intake did not differ between lean and obese men (92 ± 8 and 102 ± 6 g, respectively, P = 0.33), whereas habitual protein intake expressed per kilogram of bodyweight was lower in obese men (0.9 ± 0.1 g · kg−1 · d−1) than in lean controls (1.2 ± 0.1 g · kg−1 · d−1, P = 0.02). Daily step counts were ∼41% lower in obese than in lean men (P = 0.03). The time spent in sedentary and light-intensity activities per day did not differ between lean and obese individuals (both P > 0.05), whereas the amount of time spent in moderate-to-vigorous physical activities per day was ∼58% lower in obese men than in lean controls (P = 0.005).
TABLE 3.
Dietary intake and physical activity before experimental infusion trial in lean and obese middle-aged men1
| Lean | Obese | |
|---|---|---|
| Habitual energy intake, MJ/d | 10.8 ± 0.8 | 9.3 ± 0.6 |
| Macronutrient intake (carbohydrate/protein/fat), En% | 47/15/33 | 45/19/32 |
| Habitual protein intake, g · kg−1 · d−1 | 1.2 ± 0.1 | 0.9 ± 0.1* |
| Time accelerometer worn, min/d | 890 ± 21 | 845 ± 30 |
| Total steps per day | 12,574 ± 1783 | 7436 ± 1178* |
| Sedentary time, min/d | 544 ± 28 | 554 ± 37 |
| Sedentary time, %/d | 61 ± 3 | 65 ± 3 |
| Light-intensity activity time, min/d | 288 ± 23 | 267 ± 19 |
| Light-intensity activity time, %/d | 32 ± 2 | 32 ± 2 |
| MVPA time, min/d | 58 ± 9 | 24 ± 6* |
| MVPA time, %/d | 7 ± 1 | 3 ± 1* |
Values are mean ± SEM, n = 12/group. Habitual dietary intake was assessed by 3-d food records and physical activity by 5-d accelerometers before the experimental trial. Data were analyzed by unpaired Student's t test. *Different from lean, P < 0.05. En%, energy percentage; MVPA, moderate-to-vigorous physical activity.
Plasma glucose, insulin, and free fatty acids
Plasma glucose, insulin, and NEFA concentrations are presented in Figure 1. Postabsorptive plasma glucose concentrations at the start of the experimental trial were higher in obese subjects than in lean controls (6.1 ± 0.2 and 5.1 ± 0.1 mmol/L in obese and lean subjects, respectively, P < 0.001; Figure 1A). Plasma glucose concentrations in the postprandial period decreased over time in both groups (both P < 0.001), with higher concentrations in obese than in lean subjects (during t = 0–180 min, P < 0.05). Postabsorptive plasma insulin concentrations at t = −210 min were higher in obese subjects than in lean subjects (187 ± 75 and 57 ± 29 pmol/L, respectively, P < 0.001; Figure 1B). After protein ingestion, plasma insulin concentrations increased in both groups (both P < 0.001), although the postprandial increment was substantially greater in the obese, reaching higher peak values (636 ± 78 pmol/L) in obese than in lean subjects (155 ± 18 pmol/L; P < 0.001), and remained higher throughout the postprandial period in obese subjects (during t = 0–300 min, P < 0.001). Postabsorptive plasma TG concentrations (Table 1) were higher in obese subjects than in lean subjects (P = 0.02). NEFA concentrations at t = −210 min did not differ between groups (P = 0.35; Table 1) and decreased during the postabsorptive period (P < 0.05) to a similar extent in both groups (P-interaction = 0.41; Figure 1C). NEFAs in the postabsorptive period tended to be higher in the obese subjects than in the lean subjects (main group effect, P = 0.06). After protein ingestion, NEFA concentrations decreased (P-interaction < 0.001) and were higher during t = 20–90 min in the obese subjects than in the lean controls (all P < 0.05).
FIGURE 1.
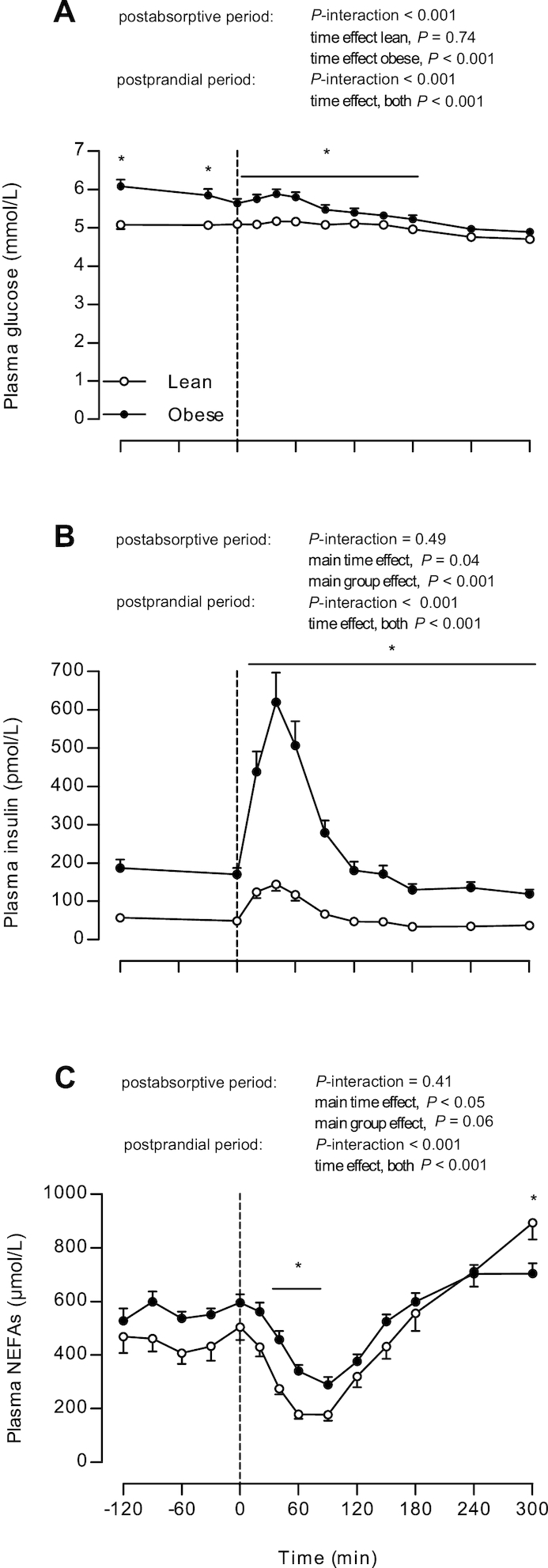
Plasma glucose (A), insulin (B), and NEFA (C) concentrations in lean and obese middle-aged men under postabsorptive conditions and after the ingestion of 25 g whey protein. Values are means ± SEMs, n = 12/group. The dotted line indicates the ingestion of the protein beverage. Data were analyzed by repeated-measures (time × group) ANOVA. Bonferroni post hoc test was used to locate differences over time and unpaired Student's t tests were used to locate differences between groups for each time point. *Different from lean, P < 0.05. NEFA, nonesterified fatty acid.
Plasma amino acid concentrations
Plasma amino acid concentrations are presented in Figure 2. Postabsorptive phenylalanine (Figure 2A), leucine (Figure 2B), and tyrosine (Figure 2C) concentrations were higher in obese subjects than in lean controls (group effect, all P < 0.05). The increments in plasma amino acid concentrations after protein ingestion were comparable between lean and obese men (for all amino acids, P-interaction > 0.05). Postprandial phenylalanine concentrations were higher in obese subjects than in lean controls (main group effect, P = 0.03).
FIGURE 2.
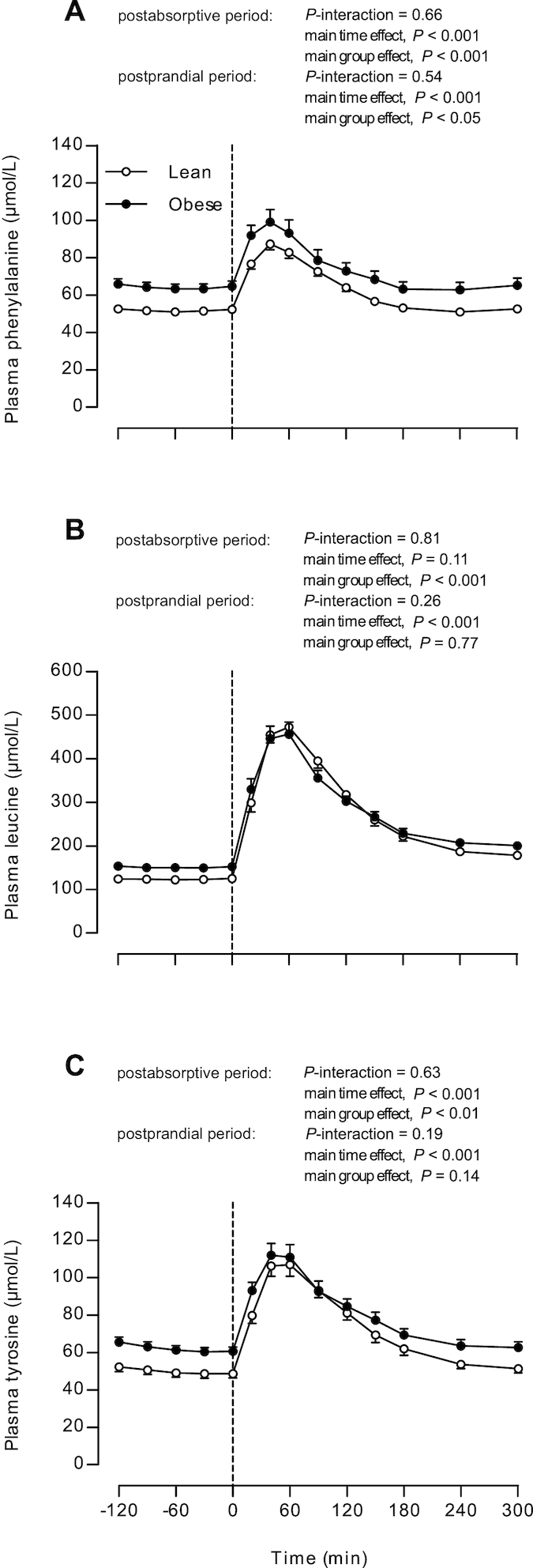
Plasma phenylalanine (A), leucine (B), and tyrosine (C) concentrations in lean and obese middle-aged men under postabsorptive conditions and after the ingestion of 25 g whey protein. Values are means ± SEMs, n = 12/group. The dotted line represents the ingestion of the protein beverage. Data were analyzed with repeated-measures (time × group) ANOVA.
Plasma amino acid enrichments
Plasma L-[ring-2H5]-phenylalanine (from the infused tracer) and L-[1-13C]-phenylalanine enrichments (from the ingested tracer) are presented in Figure 3. Postabsorptive plasma L-[ring-2H5]-phenylalanine enrichments were higher in lean subjects than in obese subjects (main group effect, P = 0.04). Plasma L-[ring-2H5]-phenylalanine enrichments decreased after protein ingestion in both groups (both P < 0.001). Despite a time × group interaction (P = 0.02), no differences were observed between individual time points in the postprandial period (all P > 0.05; Figure 3A). After protein ingestion, plasma L-[1-13C]-phenylalanine enrichments increased to a greater extent in lean than in obese men (P-interaction = 0.03), reaching higher peak values in lean (3.1 ± 0.1 MPE at t = 40) than in obese subjects (2.6 ± 0.1 MPE at t = 40 min; P < 0.001, Figure 3B). Higher postprandial plasma L-[1-13C]-phenylalanine enrichments in lean than in obese subjects were also observed for t = 40–120 min and for t = 240–300 min (post-hoc analyses for each time-point, P < 0.05).
FIGURE 3.
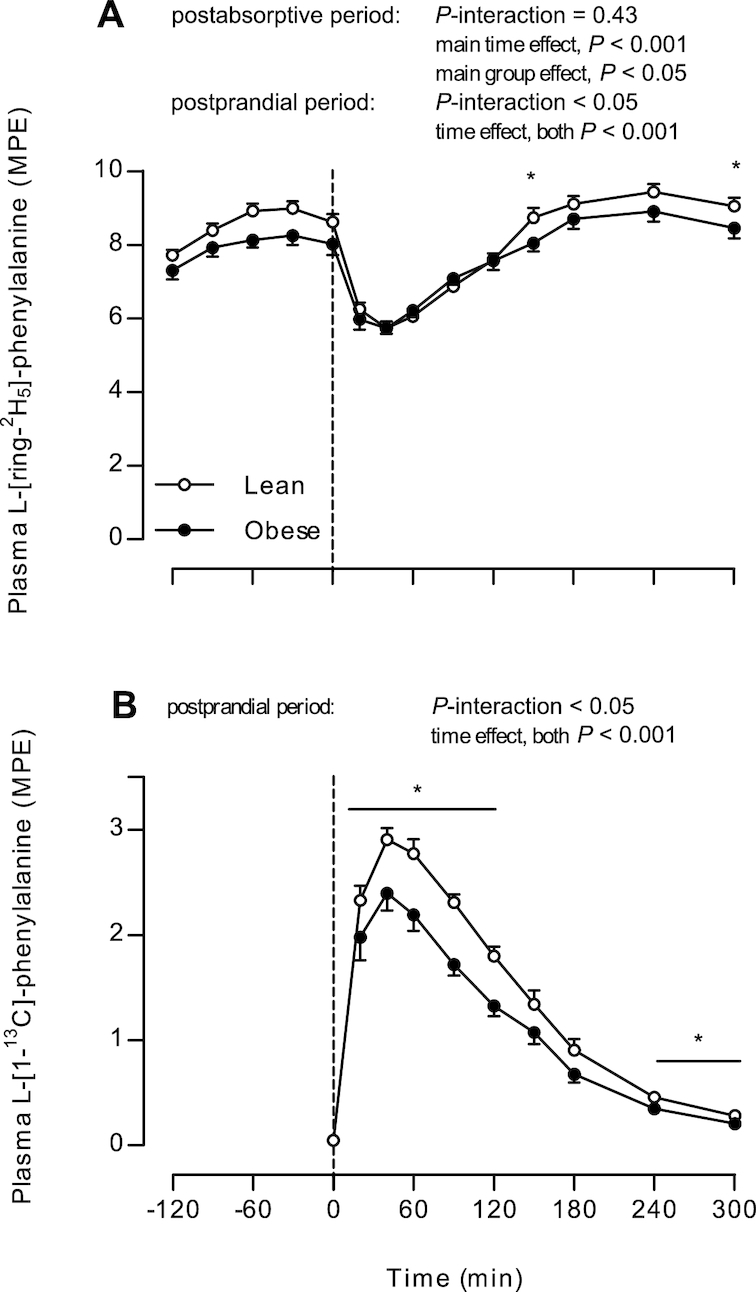
Plasma L-[ring-2H5]-phenylalanine (A) and L-[1-13C]-phenylalanine enrichments (B) in lean and obese middle-aged men under postabsorptive conditions and after the ingestion of 25 g whey protein. Values are means ± SEMs, n = 12/group. The dotted line represents the ingestion of the protein beverage. Data were analyzed with repeated-measures (time × group) ANOVA. Bonferroni post hoc test was used to locate differences over time and unpaired Student's t tests were used to locate differences between groups for each time point. *Different from obese, P < 0.05. MPE, mole percent excess.
Whole-body protein kinetics
Whole-body phenylalanine kinetics are presented in Figure 4. Exogenous phenylalanine rates of appearance (Figure 4A) (i.e., the rate at which dietary protein–derived phenylalanine enters the circulation) increased to a higher extent in lean than in obese subjects (P-interaction < 0.001), with higher values observed at t = 40–300 min in lean than in obese subjects (post-hoc analyses for each time point, P < 0.05). The fraction of dietary protein–derived phenylalanine that appeared in the plasma over the 5-h postprandial period did not differ between groups: 53 ± 1% and 53 ± 2% in lean and obese subjects (P = 0.82), respectively. Endogenous phenylalanine rates of appearance (Figure 4B) (i.e., the rate at which phenylalanine derived from whole-body protein breakdown enters the circulation) decreased after protein ingestion in both groups (P < 0.001), with absolute rates being higher in lean than in obese subjects (main group effect, P = 0.02). In addition, total phenylalanine rates of appearance and disappearance (Figure 4C, D, respectively) increased in both groups after protein ingestion, with rates being higher in lean than in obese subjects (P-interaction < 0.001, post hoc analyses for each time point t = 40–300 min, P < 0.001).
FIGURE 4.
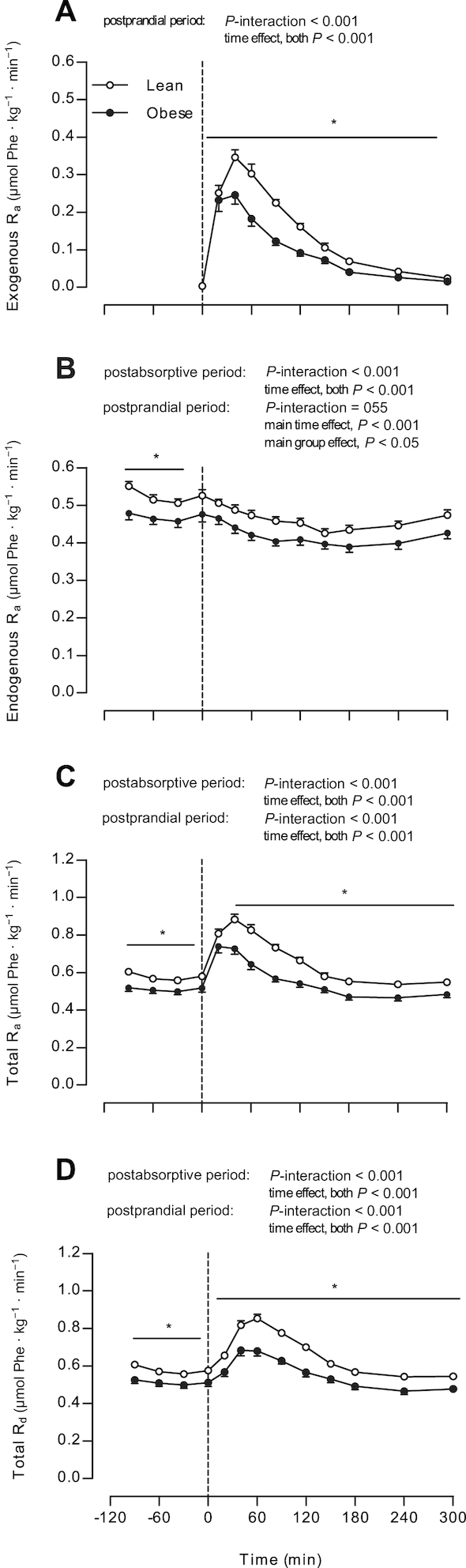
Exogenous Ra (A), endogenous Ra (B), total Ra (C), and total Rd (D) in lean and obese middle-aged men under postabsorptive conditions and after the ingestion of 25 g whey protein. Values are means ± SEMs, n = 12/group. The dotted line represents the ingestion of the protein beverage. Data were analyzed with repeated-measures (time × group) ANOVA. Bonferroni post hoc test was used to locate differences over time and unpaired Student's t tests were used to locate differences between groups for each time point. *Different from obese, P < 0.05. Ra, rate of appearance; Rd, rate of disappearance.
Whole-body protein turnover rates are presented in Figure 5. Postabsorptive whole-body protein breakdown, synthesis, and oxidation rates were lower in obese subjects than in lean controls (all, P < 0.05). Postabsorptive whole-body protein net balance did not differ between groups (0.1 ± 0.2 and 0.3 ± 0.1 µmol phenylalanine · h−1 · kg−1 in obese and lean men, respectively; P = 0.36). During the 5-h postprandial period, whole-body protein breakdown rates decreased to a similar extent (∼12%) in both groups (P-interaction = 0.15), whereas protein synthesis (∼9% compared with 4%) and oxidation rates (∼27% compared with 13%) increased to a greater extent in the lean than in the obese, respectively (P-interaction < 0.05). Postprandial whole-body protein breakdown, synthesis, and oxidation rates were lower in obese subjects than in lean subjects (all, P < 0.05). Whole-body protein net balance increased from the postabsorptive to the postprandial state in both the lean (∼24-fold; 7.1 ± 0.2 µmol phenylalanine · h−1 · kg−1) and in the obese men (∼41-fold; 4.6 ± 0.4 µmol phenylalanine · h−1 · kg−1, P-interaction < 0.001).
FIGURE 5.
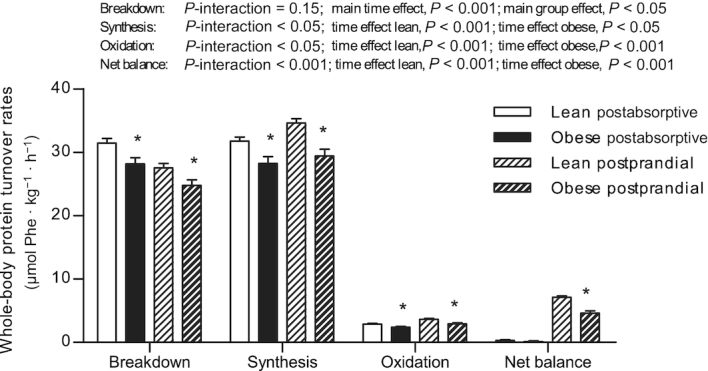
Rates of whole-body protein breakdown, synthesis, oxidation, and net protein balance in lean and obese middle-aged men under postabsorptive conditions and after the ingestion of 25 g whey protein. Values are means ± SEMs, n = 12/group. Data were analyzed with unpaired Student's t test (between groups) in the postabsorptive period. Repeated-measures (time × group) ANOVAs were used to analyze changes from the postabsorptive to the postprandial period. In case of significant time × group interaction, time effects within groups were analyzed using repeated-measures ANOVA and between-group effects using unpaired Student's t tests. *Different from lean, P < 0.05.
Muscle protein synthesis
Myofibrillar protein synthesis rates, expressed as FSR, are presented in Figure 6. Postabsorptive myofibrillar protein synthesis rates did not differ between lean and obese subjects (0.030 ± 0.002 compared with 0.028 ± 0.003%/h, respectively; P = 0.60). After the ingestion of 25 g whey protein, myofibrillar FSR increased to 0.031 ± 0.002%/h compared with 0.033 ± 0.005%/h during the “early” 0- to 2-h period, and 0.036 ± 0.002%/h compared with 0.036 ± 0.003%/h during the “late” 2- to 5-h period in lean and obese subjects (P = 0.05), respectively, with no differences between groups (P-interaction = 0.84). Myofibrillar protein synthesis rates assessed over the entire 5-h postprandial period (0.034 ± 0.002 and 0.035 ± 0.003%/h in lean and obese subjects, respectively) were significantly higher than postabsorptive myofibrillar FSR (P = 0.03) but no differences were observed between groups (P-interaction = 0.58).
FIGURE 6.
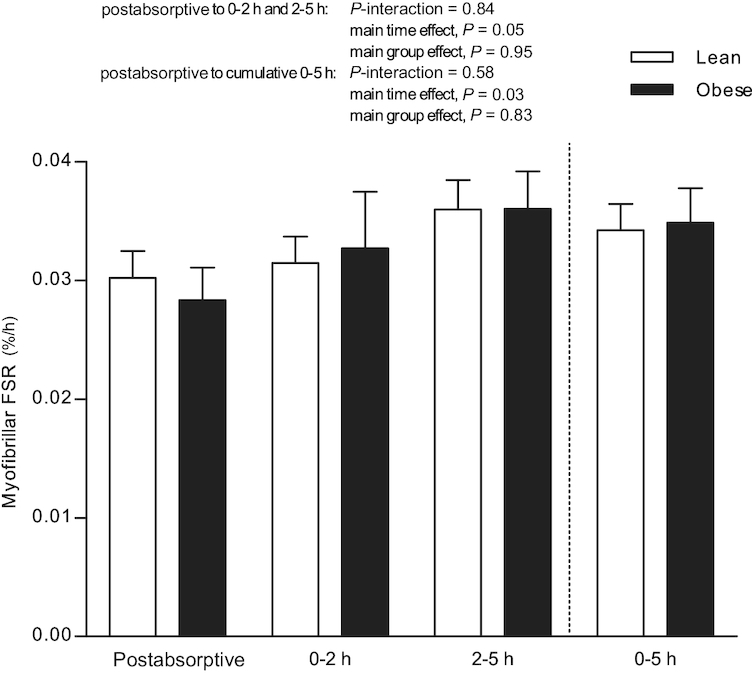
Myofibrillar protein FSRs during the postabsorptive, early (0–2 h), late (2–5 h), and cumulative (0–5 h) postprandial period, using intravenous L-[ring-2H5]-phenylalanine infusions in lean and obese middle-aged men. Values are means ± SEMs, n = 12/group. Data were analyzed with unpaired Student's t test (between groups) in the postabsorptive period. Repeated-measures (time × group) ANOVAs were used to analyze changes from the postabsorptive to the postprandial period. FSR, fractional synthetic rate.
Discussion
The present study assessed basal, postabsorptive and postprandial muscle protein synthesis rates after the ingestion of 25 g whey protein in obese men and age-matched lean controls. Both postabsorptive myofibrillar protein synthesis rates as well as postprandial protein handling, including protein digestion and amino acid absorption, and the postprandial muscle protein synthetic response to protein ingestion were not impaired in obese men when compared with age-matched lean controls.
Obesity has been suggested to be accompanied by impairments in protein metabolism in skeletal muscle tissue. Basal, postabsorptive muscle protein synthesis rates have been reported to be either lower (16, 23) or similar (18, 20, 22, 24, 25) in obese when compared with lean individuals. In the present study, we selected 12 obese middle-aged men and 12 age-matched lean controls. The obese men were morbidly obese [class II obesity based upon the WHO classification (43)] with a BMI > 35 and a body fat percentage of ∼32, with most fat deposited in the visceral area (Tables 1 and 2). In contrast, the lean men had a healthy BMI (<25) with a body fat percentage of ∼21 (P < 0.001). In line with this, the obese men presented a reduced insulin sensitivity [impaired fasting glucose (≥5.6 mmol/L)], higher postabsorptive plasma IL-6 and TG concentrations (Table 1), and a tendency for increased postabsorptive NEFA concentrations (Figure 1) when compared with lean controls. Despite clear phenotypic and metabolic distinctions between the groups, postabsorptive muscle protein synthesis rates (P = 0.60) did not differ between lean and obese men (Figure 6). Our data confirm previous observations (18, 20, 22, 24, 25), but certainly not all (16, 23), showing that there are no impairments in basal, postabsorptive muscle protein synthesis rates in obese men.
Over the last decade, it has become evident that skeletal muscle maintenance is largely dependent on the muscle protein synthetic response to food intake and physical activity (8). The muscle protein synthetic response to protein ingestion is regulated by protein digestion and subsequent amino acid absorption, resulting in a rapid postprandial rise in plasma amino acid concentrations (1–3, 8). In the present study, we combined contemporary stable isotope methodology with the use of intrinsically L-[1-13C]-phenylalanine labeled whey protein (36, 37) to allow us to compare postprandial protein digestion and amino acid absorption kinetics in vivo between lean and obese middle-aged men. After the ingestion of 25 g intrinsically labeled whey protein, plasma amino acid concentrations increased to the same extent in lean and obese men (Figure 2). Plasma L-[1-13C]-phenylalanine enrichments increased rapidly in both groups, although values increased to a greater extent in the lean than in the obese men (Figure 3B). The provided 25-g protein dose was similar for the lean and obese men in absolute terms, but smaller for the obese when expressed per kilogram of bodyweight. The lower plasma L-[1-13C]-phenylalanine enrichments observed in the obese men can, therefore, be attributed to the greater distribution volume in the obese than in the lean men. In total, 53 ± 1% and 53 ± 2% of the dietary protein–derived phenylalanine appeared in the circulation over the 5-h postprandial period in the lean and obese men, respectively. In previous work, we observed similar postprandial amino acid availability after the ingestion of a comparable dose of whey protein in (lean) young and older adults (2, 42, 44). Despite the greater visceral organ mass in the obese individuals (Table 2) which could have increased the first-pass splanchnic extraction of the dietary protein–derived amino acids, we did not detect any impairments in protein digestion and amino acid absorption kinetics in the obese men when compared with the lean men. Consequently, the present study is the first, to our knowledge, to show that postprandial protein handling, including protein digestion and subsequent protein-derived amino acid appearance in the circulation, is not impaired in obese but otherwise healthy men.
The postprandial rise in circulating (essential) amino acids, and leucine in particular, stimulates muscle protein synthesis with a rapid rise in myofibrillar protein synthesis rates for ≤5 h after protein ingestion. The muscle protein synthetic response to protein ingestion has been shown to be blunted in the older population, now commonly referred to as anabolic resistance (5, 7). Previous work has suggested that anabolic resistance to protein ingestion may also develop as a consequence of obesity, due to overfeeding and/or lipid oversupply (12, 24, 25). In support, lipid infusion has previously been shown to induce a state of anabolic resistance blunting the amino acid–induced increase in muscle protein synthesis rates (12, 17). Consequently, we hypothesized that obese individuals would show a blunted muscle protein synthetic response to protein ingestion when compared with age-matched lean controls. Upon the ingestion of 25 g whey protein, whole-body protein synthesis rates increased and whole-body protein breakdown rates decreased, causing net protein balance to become positive in both the lean and obese men (Figure 5). However, because whole-body protein turnover rates do not necessarily reflect skeletal muscle tissue, we obtained skeletal muscle biopsies to assess postprandial muscle protein synthesis rates. Myofibrillar protein synthesis rates increased by ∼26% during the 5-h postprandial period, with no differences between the lean and obese men (Figure 6; P = 0.58). In line with this, we observed no differences in myofibrillar protein synthesis rates assessed during the early (0–2 h) or late (2–5 h) postprandial period (both P > 0.05). Obviously, we were unable to detect any differences in the muscle protein synthetic response to protein ingestion between lean and obese men, despite the considerably higher circulating plasma NEFA, TG, and IL-6 concentrations and insulin resistance in the obese men. Consequently, we need to reject our hypothesis and conclude that the muscle protein synthetic response to protein ingestion is not impaired in obese, but otherwise healthy, middle-aged men.
Our data seem to agree with some (16, 22), but certainly not all (12, 16, 24, 25), studies that have addressed the proposed impairments in postprandial muscle protein synthesis rates in the obese state. Murton et al. (24) observed a blunted increase in muscle protein synthesis rates under hyperinsulinemic-hyperaminoacidemic clamp conditions in older, obese compared with age-matched lean men. However, these experimental clamped conditions differ from a normal postprandial response upon the ingestion of a single meal-like amount of protein. More recently, Beals et al. (25) reported anabolic resistance to the ingestion of pork meat (36 g protein) in young overweight and obese men as opposed to lean controls. In contrast, Smeuninx et al. (22) observed no differences in postprandial muscle protein synthesis rates after the ingestion of 15 g protein between older obese men and women compared with lean. There is no clear explanation for the apparent discrepancy in the proposed impact of obesity on postprandial muscle protein synthesis in the literature. As discussed previously, anabolic resistance can be the consequence of a more sedentary lifestyle and is not related to age, whole-body insulin resistance, and/or body fat content per se (45). Whereas physical activity increases the anabolic response to protein ingestion (46, 47), physical inactivity or disuse rapidly lowers the anabolic response to protein ingestion and, as such, induces anabolic resistance (6, 48, 49). Therefore, habitual physical activity level may be the key parameter explaining the presence or absence of differences in basal and/or postprandial muscle protein synthesis rates between lean and obese individuals. In agreement, Smeuninx et al. (22) showed that the muscle protein synthetic response to protein ingestion correlated well with daily step count. In the present study, we aimed to include lean and obese men with a (normal) physical activity pattern as opposed to a more sedentary lifestyle. Although daily step count was lower in the obese than in the lean men (Table 3, P = 0.03), overall step count was relatively high in both groups (>7000 steps/d) when compared with previous reports on (morbidly) obese adults (22, 50). In the recent work by Beals et al. (25), surprisingly large differences in postprandial muscle protein synthesis rates were observed between young lean and obese men. Although physical activity levels were not assessed in that study, it is likely that the obese men had adopted a severe sedentary lifestyle. That level of obesity (i.e., a BMI > 35 with an unhealthy metabolic phenotype) at such a young age (24–27 y) can only be explained by the combined impact of excess energy intake and a severe sedentary lifestyle. Whereas anabolic resistance can be induced by skeletal muscle disuse and exogenous lipid administration, we conclude that obesity per se does not impair postprandial muscle protein synthesis rates after the ingestion of a meal-like amount of dietary protein.
In conclusion, postabsorptive muscle protein synthesis rates and postprandial protein handling, including protein digestion and amino acid absorption kinetics and the postprandial muscle protein synthetic response to protein ingestion, are not impaired in the obese state.
Acknowledgments
We greatly appreciate the assistance of Henrike Hamer, Stefan Gorissen, Joey Smeets, and Michelle Weijzen in the execution of the experiments. Technical expertise from Jos Stegen and Wendy Sluijsmans during the sample analyses was greatly appreciated. The authors’ responsibilities were as follows—JWvD, LBV, and LJCvL: designed the research; IWKK and JWvD: conducted the experimental trials with medical assistance from IFK and FMHvD; JPBG: performed the stable isotope analyses; IWKK, JWvD, and AMHH: performed the (statistical) data analysis and wrote the manuscript together with LJCvL; IWKK, JWvD, and LJCvL: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by Top Institute Food and Nutrition (TiFN), a public–private partnership on precompetitive research in food and nutrition. The researchers are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The industrial partners have contributed to the project through regular discussion.
Author disclosures: JWvD, AMHH, IFK, JPBG, and FMHvD, no conflicts of interest. LJCvL has received research grants, consulting fees, speaking honoraria, or a combination of these, from FrieslandCampina and Nutricia Research. IWKK and LBV have received speaking honoraria from Nutricia Research.
Abbreviations used: CSA, cross-sectional area; En%, energy percentage; FFM, fat-free mass; FSR, fractional synthesis rate; HU, Hounsfield Units; MPE, mole percent excess; NEFA, nonesterified fatty acid; OGTT, oral-glucose-tolerance test; Ra, rate of appearance; TG, triacylglycerol; VAT, visceral adipose tissue.
References
- 1. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73. [DOI] [PubMed] [Google Scholar]
- 2. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–62. [PMC free article] [PubMed] [Google Scholar]
- 4. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 5. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10:e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wall BT, Dirks ML, Snijders T, van Dijk JW, Fritsch M, Verdijk LB, van Loon LJ. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am J Physiol Endocrinol Metab. 2016;310:E137–47. [DOI] [PubMed] [Google Scholar]
- 7. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41:169–73. [DOI] [PubMed] [Google Scholar]
- 8. Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106:2040–8. [DOI] [PubMed] [Google Scholar]
- 9. Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52:1889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss?. Diabetes Metab. 2005;31(S1):5S20–5S26. [DOI] [PubMed] [Google Scholar]
- 12. Stephens FB, Chee C, Wall BT, Murton AJ, Shannon CE, van Loon LJ, Tsintzas K. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes. 2015;64:1615–20. [DOI] [PubMed] [Google Scholar]
- 13. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85. [DOI] [PubMed] [Google Scholar]
- 15. Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355–65. [DOI] [PubMed] [Google Scholar]
- 16. Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94:3044–50. [DOI] [PubMed] [Google Scholar]
- 17. Liebau F, Jensen MD, Nair KS, Rooyackers O. Upper-body obese women are resistant to postprandial stimulation of protein synthesis. Clin Nutr. 2014;33:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villareal DT, Smith GI, Shah K, Mittendorfer B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity (Silver Spring). 2012;20:1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring). 2011;19:312–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chevalier S, Burgos SA, Morais JA, Gougeon R, Bassil M, Lamarche M, Marliss EB. Protein and glucose metabolic responses to hyperinsulinemia, hyperglycemia, and hyperaminoacidemia in obese men. Obesity (Silver Spring). 2015;23:351–8. [DOI] [PubMed] [Google Scholar]
- 21. Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, White PJ, Bain JR, Muehlbauer MJ, Ilkayeva OR et al.. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58:2324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smeuninx B, McKendry J, Wilson D, Martin U, Breen L. Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol Metab. 2017;102:3535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patterson BW, Horowitz JF, Wu G, Watford M, Coppack SW, Klein S. Regional muscle and adipose tissue amino acid metabolism in lean and obese women. Am J Physiol Endocrinol Metab. 2002;282:E931–6. [DOI] [PubMed] [Google Scholar]
- 24. Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ, Greenhaff PL. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes. 2015;64:3160–71. [DOI] [PubMed] [Google Scholar]
- 25. Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, Ulanov AV, Li Z, Paluska SA, De Lisio M et al.. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am J Clin Nutr. 2016;104:1014–22. [DOI] [PubMed] [Google Scholar]
- 26. Beals JW, Mackenzie RWA, van Vliet S, Skinner SK, Pagni BA, Niemiro GM, Ulanov AV, Li Z, Dilger AC, Paluska SA et al.. Protein-rich food ingestion stimulates mitochondrial protein synthesis in sedentary young adults of different BMIs. J Clin Endocrinol Metab. 2017;102:3415–24. [DOI] [PubMed] [Google Scholar]
- 27. Horstman AMH, Kouw IWK, van Dijk JW, Hamer HM, Groen BBL, van Kranenburg J, Gorissen SHM, van Loon LJC. The muscle protein synthetic response to whey protein ingestion is greater in middle-aged women compared with men. J Clin Endocrinol Metab. 2019;104:994–1004. [DOI] [PubMed] [Google Scholar]
- 28. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 29. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–10. [DOI] [PubMed] [Google Scholar]
- 32. Strandberg S, Wretling ML, Wredmark T, Shalabi A. Reliability of computed tomography measurements in assessment of thigh muscle cross-sectional area and attenuation. BMC Med Imaging. 2010;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–40. [DOI] [PubMed] [Google Scholar]
- 34. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. [PubMed] [Google Scholar]
- 35. Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJ. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci. 2011;94:4366–73. [DOI] [PubMed] [Google Scholar]
- 36. van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92:4812–22. [DOI] [PubMed] [Google Scholar]
- 37. Burd NA, Cermak NM, Kouw IW, Gorissen SH, Gijsen AP, van Loon LJ. The use of doubly labeled milk protein to measure postprandial muscle protein synthesis rates in vivo in humans. J Appl Physiol (1985). 2014;117:1363–70. [DOI] [PubMed] [Google Scholar]
- 38. Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290:E1245–52. [DOI] [PubMed] [Google Scholar]
- 39. Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996;271:E1083–91. [DOI] [PubMed] [Google Scholar]
- 40. Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed Hoboken, NJ: John Wiley and Sons, Inc; 2005. [Google Scholar]
- 41. Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O, Beaufrere B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. [DOI] [PubMed] [Google Scholar]
- 43. WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii., 1–253. [PubMed] [Google Scholar]
- 44. Gorissen SH, Horstman AM, Franssen R, Kouw IW, Wall BT, Burd NA, de Groot LC, van Loon LJ. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: a randomized trial. Am J Clin Nutr. 2017;105:332–42. [DOI] [PubMed] [Google Scholar]
- 45. Burd NA, Wall BT, van Loon LJ. The curious case of anabolic resistance: old wives’ tales or new fables?. J Appl Physiol. 2012;112:1233–5. [DOI] [PubMed] [Google Scholar]
- 46. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–31. [DOI] [PubMed] [Google Scholar]
- 47. Holwerda AM, Kouw IW, Trommelen J, Halson SL, Wodzig WK, Verdijk LB, van Loon LJ. Physical activity performed in the evening increases the overnight muscle protein synthetic response to presleep protein ingestion in older men. J Nutr. 2016;146:1307–14. [DOI] [PubMed] [Google Scholar]
- 48. Biolo G, Pisot R, Mazzucco S, Di Girolamo FG, Situlin R, Lazzer S, Grassi B, Reggiani C, Passaro A, Rittweger J et al.. Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: relationships with changes in muscle mass. Clin Nutr. 2017;36:1420–6. [DOI] [PubMed] [Google Scholar]
- 49. Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98:2604–12. [DOI] [PubMed] [Google Scholar]
- 50. Tudor-Locke C, Brashear MM, Johnson WD, Katzmarzyk PT. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Act. 2013;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]


