ABSTRACT
Human papillomavirus (HPV) is recognized as a necessary, but insufficient cause of cervical cancer. Recent in vitro and in vivo evidence from our laboratory demonstrate that hyperactivation of yes-associated protein 1 (YAP1) is sufficient to induce cervical carcinogenesis. We found that synergism between hyperactivated YAP1 and high-risk HPV is a key driver of cervical cancer initiation and progression.
keywords: The Hippo Pathway, YAP1, HPV, cervical cancer, innate immunity
Cervical cancer is the most frequently diagnosed gynecological cancer and is the fourth leading cause of cancer death in women worldwide. According to an estimation from the International Agency for Research on Cancer (IARC), around 527,000 women were diagnosed with cervical cancer, and more than 310,000 women died of this disease in 2018.1 Since more than 95% of the cervical cancer patients test positive for human papillomavirus (HPV), HPV has been thought to be a necessary causative agent of cervical cancer.2 With the improvement of experimental tools in the past two decades, accumulating evidence demonstrates that HPV infection alone is not sufficient to cause cervical cancer. It is believed that unknown genetic and epigenetic alterations are required for HPV induction of cervical carcinogenesis. Currently, HPV is identified as a necessary, but insufficient cause of cervical cancer development.3 The exact molecular mechanisms underlying cervical cancer development are unclear.
Our previous in vitro research showed that yes-associated protein 1 (YAP1), a major effector of the Hippo tumor suppressive signaling pathway, forms a feedforward loop with the EGFR signaling pathway to drive the progression of cervical cancer.4 Moreover, we found that HPV16 E6 oncoprotein may stabilize YAP1 to promote its tumorigenic action in the cervical cancer cells.4 Because these data were derived from cultured cervical epithelial cell lines or cancer cell lines, it is not clear whether the disrupted Hippo/YAP signaling pathway plays a role in the development of cervical cancer in vivo (under pathological conditions).
Recently, we developed several unique transgenic mouse models to examine the potential role of the Hippo/YAP1 signaling pathway in cervical carcinogenesis in vivo. Consistent with a previous report,5 we found that the expression of HPV E6/E7 proteins alone in the cervical epithelium of Krt14-E6/E7 mice did not induce invasive cancer. However, hyperactivation of YAP1 in the cervical epithelium of Krt14-E6/E7 mice induced invasive cancer, suggesting that YAP1 interacts with HPV to promote cervical cancer development.5 Surprisingly, we also found that hyperactivation of YAP1 alone in the cervical epithelial cells was able to induce invasive cervical cancer.5 This finding is against the current dogma that HPV is required for cervical cancer development. Our mechanistic studies show that HPV synergizes with the YAP1 oncogene to promote the initiation and progression of cervical cancer. Specifically, YAP1 increases the expression of the putative HPV receptor molecules and suppressed innate immunity in host cells to facilitate the establishment of HPV infection. HPV, in turn, stabilizes the YAP1 protein and induces YAP1 activation in cervical epithelial cells to promote YAP1-induced cervical carcinogenesis (Figure 1).
Figure 1.
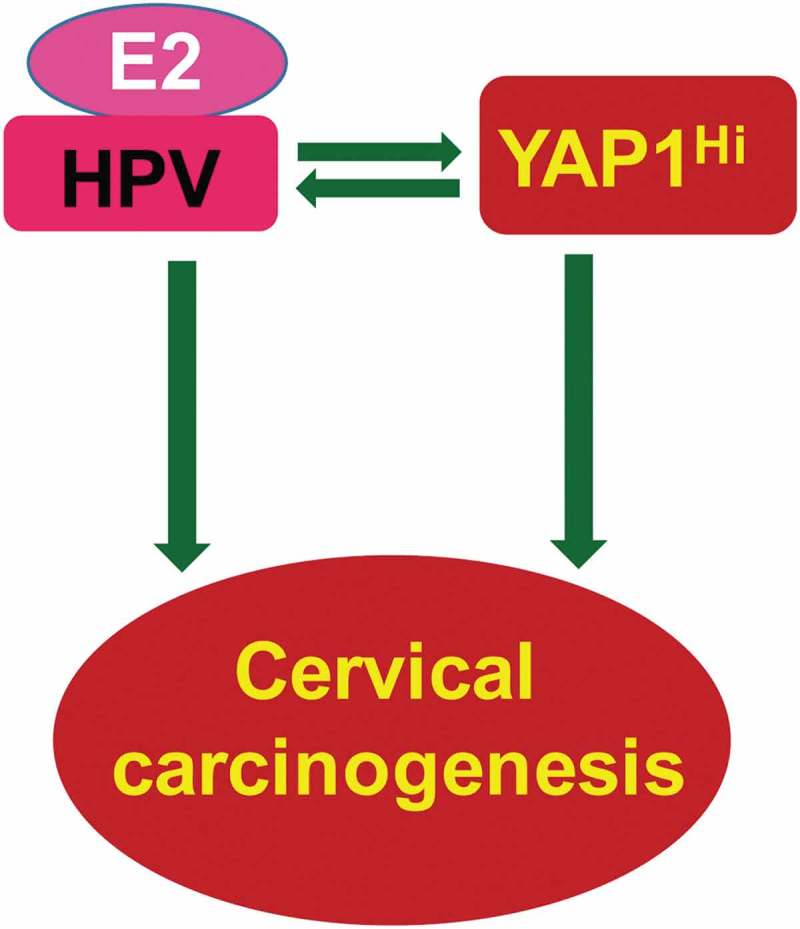
Synergism between YAP1 and HPV oncoproteins plays a central role in cervical carcinogenesis. Research results generated from our in vitro and in vivo experimental models and data derived from multi-dimensional cancer genetic/genomic analyses of TCGA database suggest that the disruption of the Hippo signaling pathway and the subsequent hyperactivation of YAP1 is sufficient to induce invasive cervical cancer, implying that HPV is not necessary for cervical cancer development. However, HPV synergizes with YAP1 to drive the initiation and progression of cervical carcinogenesis. YAP1, via upregulating the putative HPV receptor molecules and suppressing host cell innate immunity, facilitates HPV infection (and potentially the establishment of persistent HPV infection). HPV oncoproteins, in turn, suppress the Hippo pathway and stabilize YAP1 protein to promote the oncogenic action of YAP1. Although HPV alone is insufficient to induce cervical cancer, it is worth noting that a combination of HPV and high-level of 17β-estradiol is able to induce the development of cervical cancer.6,7
The development of cervical squamous cell carcinoma in Krt14-Yap1S127A mice, regardless of HPV infection, clearly supports the idea that disrupted Hippo pathway signaling and subsequent hyperactivation of Yap1 is a key mechanism of cervical carcinogenesis. A major concern for our study is that the cervical carcinogenesis in mice may be different from that in humans. Fortunately, evidence from human cervical cancer patients supports a role for the Hippo/YAP1 pathway in human cervical cancer development. First, cross-cancer genetic analysis (based on TCGA datasets) demonstrated that the most frequent alteration of the Hippo pathway occurs in cervical carcinoma. The upstream tumor suppressors of the Hippo pathway, such as MST1, LATS1/2, FAT1/2/3/4, were frequently deleted and/or mutated, while the downstream oncogenic genes, such as YAP1, WWTR1, and TEAD1/2/3/4, were frequently upregulated.5 Second, our IHC studies showed that increased expression of YAP1, the major effector of the Hippo pathway, is positively correlated with the progression of cervical intraepithelial neoplasia (CIN) and the development of invasive cancer.5 Third, a recent genome-wide screening by TCGA also showed that the copy number of genes in chromosome 11q22 was frequently amplified in cervical squamous cell carcinoma.8 Interestingly, YAP1 and its downstream target genes such as BACR2 and BACR3 are located in this area. Moreover, the TCGA study found that YAP1 was significantly amplified in cervical cancer samples of the epithelial–mesenchymal transition (EMT) cluster. The EMT cluster was classified based on DNA methylation status and featured with the worst survival outcomes among all cervical cancer subtypes.8 In fact, YAP1 protein was the most differentially expressed protein that distinguishes the EMT cluster from all others.8
Research findings in this study have significant impacts on basic research and clinical practice of cervical cancer. First, results from this study challenge the dogma that HPV is a necessary agent for the development of cervical cancer and shed new light on the etiology of cervical cancer. Results from this study indicate that hyperactivated YAP1 alone is sufficient to induce the development of cervical cancer in vivo and identify the Hippo/YAP signaling pathway as a critical regulator of cervical carcinogenesis. Importantly, evidence from this study suggests that HPV is not a necessary agent for cervical cancer development. Instead, HPV may serve as a causative risk factor and a potent promoter of cervical carcinogenesis. HPV synergizes with hyperactivated YAP1 to drive the initiation and progression of cervical cancer. Second, research from the current study showed that YAP1 expression/activation is significantly increased with the progression of CINs and the development of invasive cancer, suggesting that nuclear YAP1 (active form) could be used as a marker to screening early lesion of cervical cancer. The dual-marker screening strategy (nuclear YAP1 and HPV infection) may greatly help the early detection of precancerous lesions in the cervix of the high-risk patients, and enable clinicians to better assess risk, manage patients, and improve outcomes. Third, our studies suggest that targeting the Hippo/YAP1 pathway represents a new opportunity for better prevention and improved treatment of cervical cancer. Finally, animal models developed in this study will provide powerful in vivo tools for studying the relationships among viral oncoproteins, host cell transformation, and cervical cancer progression. Importantly, the results of this study do not weaken the importance of HPV in cervical cancer initiation and progression. On the contrary, our findings indicate that HPV is a critical promoter of cervical cancer development, further emphasizing the importance of HPV vaccination.
Funding Statement
This work was supported by the National Cancer Institute (US) [1R01CA197976, 1R01CA201500]
Acknowledgments
This work was supported by the National Cancer Institute/National Institute of Health (1R01CA197976, 1R01CA201500); the Colleen’s Dream Foundation (no number), the Olson Center for Women’s Health (no number), and the Vincent Center for Reproductive Biology (no number).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanya D, Pd G. HPV and cervical cancer: updates on an established relationship. Postgrad Med. 2008;120:7–13. doi: 10.3810/pgm.2008.11.1928. [DOI] [PubMed] [Google Scholar]
- 4.He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7:1426–1449. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He C, Lv X, Huang C, Angeletti PC, Hua G, Dong J, Zhou J, Wang Z, Ma B, Chen X, et al. A Human Papillomavirus-Independent Cervical Cancer Animal Model Reveals Unconventional Mechanisms of Cervical Carcinogenesis. Cell Rep. 2019;26:2636–2650.e5. doi: 10.1016/j.celrep.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007(67):1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Research Network Integrated genomic and molecular characterization of cervical cancer. Nature. 2017; 543: 378–384. 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]


