Abstract
STUDY QUESTION
What is the X chromosomal content of oocytes and granulosa cells of primordial/primary (small) follicles and stromal cells in ovaries of young patients with Turner’s syndrome (TS)?
SUMMARY ANSWER
Small ovarian follicles were detected in one-half of the patients studied, and X chromosome analysis revealed that most oocytes were normal, granulosa cells were largely monosomic, while stromal cells showed a high level of mosaicism.
WHAT IS KNOWN ALREADY
Most women with TS experience a premature reduction or complete loss of fertility due to an accelerated loss of gametes. To determine whether fertility preservation in this group of patients is feasible, there is a strong need for information on the X chromosomal content of ovarian follicular and stromal cells.
STUDY DESIGN, SIZE, DURATION
Small follicles (<50 μm) and stromal cells were isolated from ovarian tissue of young TS patients and analysed for their X chromosomal content. In addition to ovarian cells, several other cell types from the same patients were analysed.
PARTICIPANTS/MATERIALS, SETTING, METHODS
After unilateral ovariectomy, ovarian cortex tissue was obtained from 10 TS patients (aged 2–18 years) with numerical abnormalities of the X chromosome. Ovarian cortex fragments were prepared and cryopreserved. One fragment from each patient was thawed and enzymatically digested to obtain stromal cells and primordial/primary follicles. Stromal cells, granulosa cells and oocytes were analysed by FISH using an X chromosome-specific probe. Extra-ovarian cells (lymphocytes, buccal cells and urine cells) of the same patients were also analysed by FISH. Ovarian tissue used as control was obtained from individuals undergoing oophorectomy as part of their gender affirming surgery.
MAIN RESULTS AND THE ROLE OF CHANCE
Ovarian follicles were detected in 5 of the 10 patients studied. A method was developed to determine the X chromosomal content of meiosis I arrested oocytes from small follicles. This revealed that 42 of the 46 oocytes (91%) that were analysed had a normal X chromosomal content. Granulosa cells were largely 45,X but showed different levels of X chromosome mosaicism between patients and between follicles of the same patient. Despite the presence of a low percentage (10–45%) of 46,XX ovarian cortex stromal cells, normal macroscopic ovarian morphology was observed. The level of mosaicism in lymphocytes, buccal cells or urine-derived cells was not predictive for mosaicism in ovarian cells.
LIMITATIONS, REASONS FOR CAUTION
The results are based on a small number (n = 5) of TS patient samples but provide evidence that the majority of oocytes have a normal X chromosomal content and that follicles from the same patient can differ with respect to the level of mosaicism of their granulosa cells. The functional consequences of these observations require further investigation.
WIDER IMPLICATIONS OF THE FINDINGS
The results indicate that despite normal ovarian and follicular morphology, stromal cells and granulosa cells of small follicles in patients with TS may display a high level of mosaicism. Furthermore, the level of mosaicism in ovarian cells cannot be predicted from the analysis of extra-ovarian tissue. These findings should be considered by physicians when offering cryopreservation of ovarian tissue as an option for fertility preservation in young TS patients.
STUDY FUNDING/COMPETING INTEREST(S)
Unconditional funding was received from Merck B.V. The Netherlands (Number A16-1395) and the foundation ‘Radboud Oncologie Fonds’ (Number KUN 00007682). The authors have no conflicts of interest.
TRIAL REGISTRATION NUMBER
Keywords: Turner’s syndrome, fertility preservation, karyotyping, primordial/primary follicles, mosaicism
Introduction
Turner’s syndrome (TS) is a heterogeneous genetic disorder in women with one intact X chromosome and complete or partial absence of the second X chromosome that affects 1 in every 2500 female live births (Nielsen and Wohlert, 1991; Saenger, 1996). The presentation of TS is variable and includes typical phenotypic features, such as short stature, lymphedema, webbed neck, broad shield chest and congenital malformations of the heart and kidneys. The main concern for girls and women diagnosed with TS is the reduction or complete loss of fertility (Sylvén et al., 1993; Sutton et al., 2005). Impaired fertility in TS is thought to arise mainly from accelerated germ cell apoptosis and impaired folliculogenesis during fetal life, leading to premature follicular depletion and gonadal dysgenesis (Modi et al., 2003; Reynaud et al., 2004). The degree of oocyte loss, however, is variable, and the remaining function of the ovary after birth is thought to rely on the percentage of 46,XX cells in the ovaries (Grynberg et al., 2016). In women from the general population, the number of gametes declines from approximately 2 million at birth to about 400 000 at the start of puberty (Baker, 1963). In women with TS, the already-diminished oocyte pool at birth (Singh and Carr, 1966; Carr et al., 1968; Modi et al., 2003; Reynaud et al., 2004) combined with this subsequent post-natal loss of gametes leads to ovarian insufficiency and incomplete sexual development during childhood in more than 80% of cases (Pasquino et al., 1997). Several options exist to preserve fertility in women facing premature depletion of their gametes (Oktay et al., 2016). In adult women these include freezing of ovarian tissue, mature oocytes or embryos, while in prepubertal patients ovarian tissue cryopreservation (OTC) is currently the only available option. When offering OTC to girls with TS who cannot wait until sufficient maturity to undergo oocyte cryopreservation, it should be considered at the earliest age possible in order to store as many primordial follicles as possible before their premature disappearance (Borgstrom et al., 2009; Oktay et al., 2016). Although OTC is widely and successfully used for fertility preservation/restoration in other patients groups (Van der Ven et al., 2016; Jadoul et al., 2017; Jensen et al., 2017), this does not guarantee that it will also be successful in TS patients. OTC is already routinely offered to TS women in several countries (Hreinsson et al., 2002; Huang et al., 2008; Borgstrom et al., 2009; Balen et al., 2010; von Wolff et al., 2015), but there are no reports of children born from women with TS after auto-transplantation of their ovarian tissue (Schleedoorn et al., 2019). The efficacy of OTC in this particular group of patients therefore remains to be confirmed. The success of OTC not only depends on the developmental competence of follicles that persist in the ovaries, but also on the ability of ovarian tissue from TS patients to support normal ovarian function. The functional capacity of the tissue after auto-transplantation might depend on the percentage of normal 46,XX cells that are required for follicular growth and ovulation. Aneuploidy in oocytes of primordial/primary follicles is also very likely to have functional consequences. In addition, genetic abnormalities in the layer of granulosa cells present in small (primordial and primary) follicles might impair normal follicular development (Motta et al., 1997; Kidder and Mhawi, 2002), as these cells not only control the arrest of the oocyte during the prophase of meiosis I, but also play a crucial role in normal follicular maturation (McLaughlin and McIver, 2009). Very little is known about the karyotype of these follicular cells in TS women. This information, however, is essential for understanding the mechanisms of premature follicular depletion and gonadal dysgenesis in this specific group of patients and, hence, to evaluate if OTC is a realistic option to preserve their fertility.
As a first step toward the characterization of ovarian tissue from TS patients, we determined the X chromosomal content of oocytes and granulosa cells of small follicles, and stromal cells of ovarian cortex tissue in a cohort of 10 young TS patients with numerical abnormalities of the X chromosome. To this end we developed protocols to isolate intact small follicles from ovarian cortex tissue and efficiently separate the follicular cells, followed by fluorescence in situ hybridization (FISH) analysis of the X chromosome. In addition, a procedure was developed for determining the number of X chromosomal sister DNA strands in the normally tetraploid oocytes of meiosis I arrested follicles. The karyotype in peripheral blood lymphocytes and cells of buccal smears and urine was determined to assess their predictive value for the karyotype of ovarian cells.
Materials and Methods
Patients
The current investigation is part of a nationwide trial ‘Preservation of Ovarian Cortex Tissue in Girls With Turner Syndrome’ (ClinicalTrials.gov Identifier: NCT03381300). Patients were recruited nationwide and referred to our tertiary fertility clinic between January 2018 and December 2018. Patients included in the current study are women with TS diagnosed with numerical X chromosome aberrations (45,X or 47,XXX), aged 2–18 years. Human ovarian tissue that was used as control was obtained from female-to-male transgender individuals undergoing oophorectomy as part of their gender affirming surgery.
Ethics
The study was approved by the Dutch Central Committee on Research Involving Human Subjects (CCMO NL57738.000.16). Written informed consent was obtained from all patients and/or their parents.
Collection of ovarian cortical tissue and estimation of the number of follicles per ovary
After unilateral ovariectomy, the ovary was collected in cold L15 medium (Lonza, Switzerland), immediately transferred to the laboratory and placed on a pre-cooled surface at 4°C. The medulla was removed from the cortex, after which cortex fragments of ~5 × 8 mm were prepared. Fragments were cryopreserved according to clinical standards (Peek et al., 2015). For each patient a single representative ovarian cortex fragment was available for research purposes. The fragment was thawed and cut in half. One-half was used for the isolation of small follicles and stromal cells (see below), the other half for histological analysis. The number of follicles was determined by complete serial sectioning (4 μm sections) of the tissue followed by haematoxylin–eosin (HE) staining of sections at 24 μm intervals. By examining stained sections at 24 μm intervals none of the primordial/primary follicles (follicle diameter ~45 μm) could have been missed. Since the follicular density in the ovaries of TS patients is low, counting the same follicle twice could be easily avoided and staining of all sections was therefore not necessary. In addition, in view of the heterogeneous distribution of follicles in the ovary, follicle density is expressed as the number of follicles per mm3 of cortex tissue.
Dissociation of ovarian cortex tissue
Individual small follicles and the stromal cells were isolated as described previously, with several modifications (Dolmans et al., 2006; Vanacker et al., 2011). To this end the tissue was cut into small pieces of ~1 × 1 × 1 mm and enzymatically digested in 4 ml of pre-warmed (37°C) L15 medium containing 0.1 mg/ml Liberase DH, 10 μg/ml DNase I (both from Roche diagnostics, Mannheim, Germany) and 1 mg/ml collagenase I from Clostridium histolyticum (Sigma life sciences, Israel) for 75 min at 37°C. The digestion mix was pipetted up and down every 15 min. The enzymatic reaction was stopped by the addition of 4 ml of cold L15 supplemented with 10% of fetal bovine serum (FBS; Life technology, Paisley, UK). The dissociated tissue was washed once with 8 ml of cold L15 medium by centrifugation at 500g and resuspended in 500 μl L15 medium. Next the cell suspension was transferred to a Petri dish and examined under a stereomicroscope. Small follicles (<50 μm) were manually picked up using a 75 μm plastic pipette (Research instruments, Falmouth, UK) and transferred to a droplet of L15 medium supplemented with 10% FBS at 4°C to prevent aggregation of follicles. To improve follicular cell spreading prior to FISH analysis, the follicles were treated with a solution of 0.06% trypsin, 1 mg/ml EDTA and 1 mg/ml glucose for 20 min at 37°C. Ovarian stromal cells were obtained from the cortex cell suspension, taking special care to avoid picking up any remaining follicles.
FISH analysis of lymphocytes, buccal cells, urine cells and ovarian cells from TS patients
FISH analysis of extra-ovarian cells was performed following standard protocols (Freriks et al., 2013). Ovarian follicles or stromal cells were transferred to 100 μl droplets of 0.04 mM KCl on a slide and incubated for 20 min at 37°C. Next, slides were allowed to dry and then pre-fixed in 300 μl of 0.05 mM KCl/7.5% acetic acid/22.5% methanol for 2 min at room temperature. Final fixation was performed by covering the slide with methanol/acetic acid (3:1) for 2 min at room temperature. FISH was performed according the manufacturer’s instructions with chromosome X and chromosome 18-specific centromeric probes (CEP X (DXZ1) and CEP 18 (D18Z1); Vysis, Abbott, IL, USA). Fluorescent images were captured, and the signal(s) for the X chromosome was evaluated in somatic cells only when two signals of chromosome 18 were visible. In most oocytes only one signal could be detected for each chromosome. Slides were counterstained with 4′,6-Diamidino-2-Phenylindole (DAPI). The presence of different cell lines in the patients was determined by routine cytogenetic analysis of peripheral blood lymphocytes (30 cells), followed by FISH analysis of buccal cells (100 cells) and urine-derived cells (100 cells), following clinical standards.
Results
Determining the X chromosomal content of peripheral blood lymphocytes and cells from buccal smears and urine in patients with TS
From the study population five patients with a non-structural, mosaic karyotype 45,X/46,XX (/47,XXX) and five patients with a 45,X monosomy were selected based on order of inclusion in the study (Table I). In the mosaic patients four out of five were of the 45,X/46,XX karyotype (Patients B–E), while in Patient A three cell lines (45,X/46,XX/47,XXX) were found. The ratio between the different cell lines varied considerably between tissues of the same patient (Fig. 1). For example, in Patient A the majority of lymphocytes had a 47,XXX karyotype while in urine comparable numbers of 45,X and 47,XXX cells were observed. In Patient E the ratio between 45,X and 46,XX cells in lymphocytes and buccal cells was approximately 1, while in urine only 45,X cells were found without any detectable mosaicism.
Table I.
Characteristics of the patients with Turner’s syndrome.
| Patient | Age (years) | Cell Lines (lymphocytes and buccal cells) | Number of follicles per mm3 tissue |
|---|---|---|---|
| A | 8 | 45,X/46,XX/47,XXX | 11 |
| B | 5 | 45,X/46,XX | 64 |
| C | 15 | 45,X/46,XX | 45 |
| D | 16 | 45,X/46,XX | 6 |
| E | 15 | 45,X/46,XX | 3 |
| F | 14 | 45,X | 0 |
| G | 9 | 45,X | 0 |
| H | 13 | 45,X | 0 |
| I | 3 | 45,X | 0 |
| J | 17 | 45,X | 0 |
In this study 10 patients with Turner’s syndrome and numerical abnormalities of the X chromosomes were included.
Figure 1.
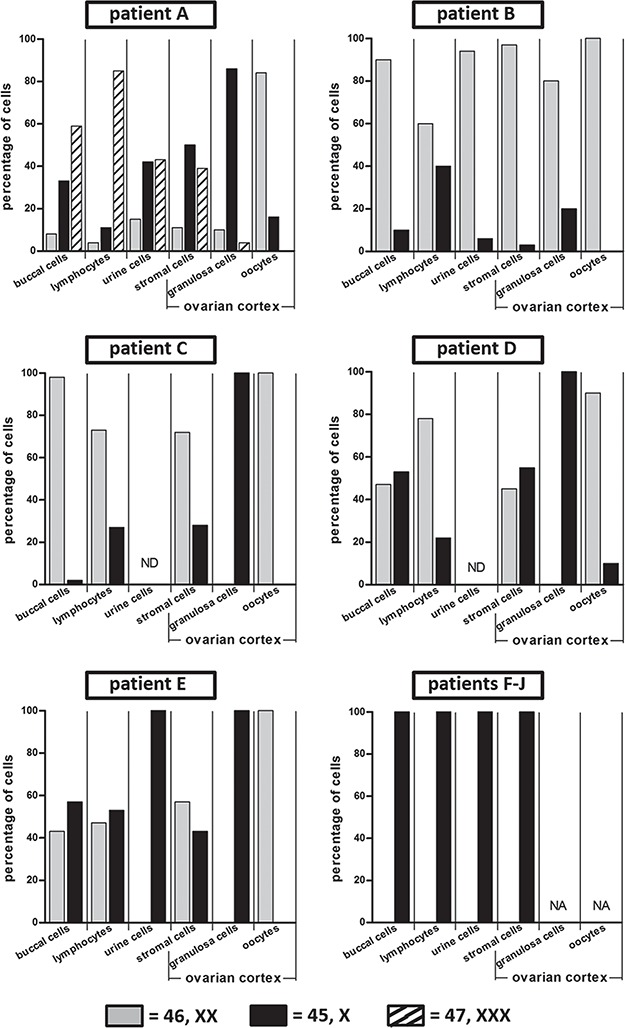
Mosaicism in extra-ovarian tissues and ovarian stromal cells, granulosa cells and oocytes from patients with Turner syndrome. In patients with Turner’s syndrome (TS), lymphocytes (n = 30), cells from buccal smears (n = 100) and urine (n = 100) were analysed by FISH with an X chromosomal peri-centromeric probe. The percentage of cells with a particular karyotype is indicated. In Patient A three different cell lines (45X/46,XX/47,XXX) were detected, Patients B–E were mosaic for 45,X and 46,XX cell lines, while in Patients F–J only 45,X cells were found. In addition, ovarian cortex components including stromal cells (n = 100), granulosa cells (n ≥ 100) and oocytes were karyotyped. The number of oocytes was 19, 12, 2, 9 and 4 for Patients A, B, C, D and E, respectively. In Patients F–J no follicles were detected. ND = not done; NA = not applicable.
Macroscopic morphology of ovaries
Intact ovaries were obtained by laparoscopic unilateral ovariectomy and photographed before preparation of cortex fragments. As illustrated in Fig. 2, the monosomy patients showed gonadal dysgenesis with bilateral streak ovaries. The ovaries of the five mosaic patients were of normal macroscopic morphology.
Figure 2.
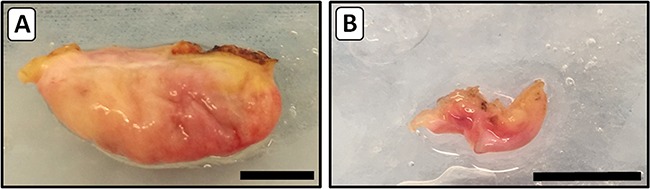
Macroscopic images of ovaries from TS patients. After unilateral ovariectomy intact ovaries were transported to the laboratory and photographed. A representative example of an ovary from a mosaic 45,X/46,XX girl (Patient D) with normal morphology and volume is shown in panel A. The small fibrous streak ovary shown in photo B is from a girl with 45,X monosomy (Patient I). The brown discoloration of the tissue is due to electrocauterization during surgery. Bars represent 1 cm.
Designing a protocol for the separation of ovarian cortex cell components prior to FISH analysis
To determine the X chromosomal content of ovarian stromal cells, follicular granulosa cells and oocytes by FISH, we first designed a protocol to efficiently separate these ovarian cell components (Fig. 3). To this end ovarian cortex fragments were prepared after unilateral ovariectomy. One part of the ovarian cortex fragment that was available for research purposes was used for histological analysis to determine the number of follicles (Fig. 3C and F). The remaining part was enzymatically digested to yield a suspension consisting mainly of single stromal cells and, if present, individual follicles (Fig. 3D and G). When no follicles were observed histologically, part of the stromal cells was used directly after the enzymatic digestion for FISH with centromere probes specific for the X chromosome and, as a control, chromosome 18. (Fig. 3E). When small follicles (<50 μm) were present in the cell suspension these were picked up manually and analysed separately by FISH (Fig. 3K). After follicle isolation, part of the stromal cells from the cell suspension (Fig. 3h) was used for FISH analysis on separate slides (Fig.3I). Interpretation of the FISH signals of follicles that were isolated directly after the enzymatic digestion of the cortex tissue proved to be problematic, as nuclei of the granulosa cells of individual follicles were generally not sufficiently spaced to unambiguously determine the number of hybridization spots per nucleus. In addition, the oocyte-specific FISH signals were frequently obscured by granulosa cell DNA (Fig. 4A and B). To obtain more dispersed granulosa cell nuclei for FISH analysis we introduced an additional enzymatic treatment of isolated follicles with trypsin. This treatment visibly affected the follicles, showing partial rounding of granulosa cells on the surface of the follicle, but did not lead to detachment of cells from the follicle (Fig. 3J). FISH analysis and DAPI counterstaining of these follicles clearly showed more dispersed granulosa cell nuclei and, more importantly, oocyte nuclei that were not covered by granulosa cell nuclei (Figs 3K, 4C and D).
Figure 3.
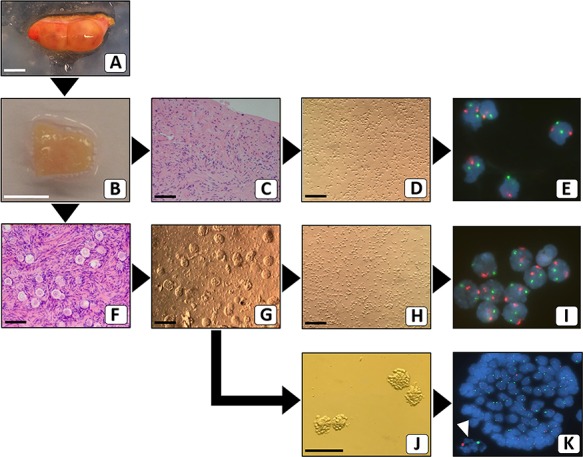
Flow scheme for separating ovarian cortex cellular constituents prior to FISH analysis. After the surgical removal of the intact ovary (A), cortical fragments were prepared. Part of one representative cortex fragment (B) was analysed by standard haematoxylin–eosin staining for the presence of follicles. When no follicles were present (C), the remaining part of the fragment was used to make a suspension of stromal cells (D), for interphase FISH with chromosome X (green) and chromosome 18 (red)-specific probes (E). When follicles were present (F), the remaining part of the cortex fragment was used to make a cell suspension (G) from which small follicles were manually picked up. These isolated follicles were subjected to further digestion (J) and subsequently analysed by FISH. The white arrowhead points to the signals from the oocyte (K). Part of the remaining cell suspension (H) was used for FISH analysis of stromal cells (I). Bars represent 1 cm (A and B) or 100 μm (C, D, F–H and J). Original magnification of FISH signals was ×630 (E, I and K).
Figure 4.
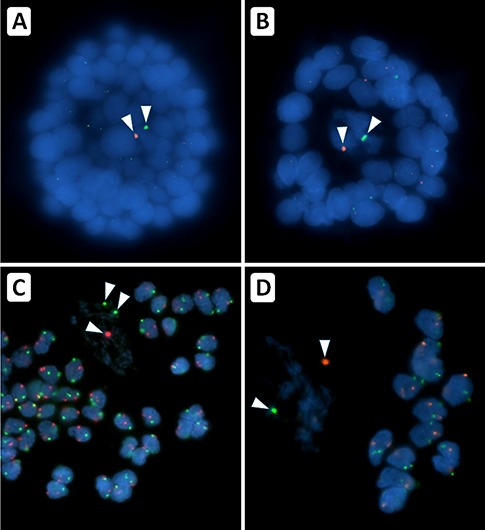
After treatment of isolated follicles with trypsin the follicular cells become more available for FISH. Cells of individual small follicles isolated from a suspension of ovarian cortex remain clumped during preparation for FISH, obscuring signals of individual granulosa cells and the oocyte (panels A and B). Treatment with trypsin of isolated follicles prior to FISH resulted in less cell clumping and allowed karyotyping of granulosa cells and oocyte of the same follicle (panels C and D). Note that the DAPI counterstain in the trypsin treated follicles reveals that the DNA of the oocyte is much more diffuse and can be easily distinguished from DNA of the granulosa cells. FISH signals for the X chromosome (green) and chromosome 18 (red) of the oocytes are indicated by arrowheads. Original magnification was ×630.
Determining the karyotype of ovarian stromal cells
In the five patients (F–J) with 45,X monosomy in lymphocytes, buccal cells and urine cells, no follicles were found in the HE stained sections of ovarian cortex tissue, nor in the cell suspensions used for FISH (Table I, Fig. 3C and D). Karyotyping of the ovarian stromal cells of these patients showed exclusively 45,X cells (Fig. 1). In the mosaic TS patients the karyotype of the stromal cells showed a ratio of cell lines (45,X; 46,XX and 47,XXX) that was similar to that of cells from urine (Patients A and B), lymphocytes (Patients C and E) or buccal cells (Patients B, D and E).
Karyotyping granulosa cells from small ovarian follicles
The number of X chromosomes was determined in granulosa cells from small follicles isolated from ovarian tissue from the five mosaic TS patients (A–E). Although over 98% of the follicles in these tissues were either in the primordial or primary stages of development (Fig. 5A), a small number of secondary follicles was observed as well (Fig. 5B). After enzymatic digestion of the tissue and subsequent purification of the small follicles, the X chromosomal content of their granulosa cells was determined. Remarkably, in three out of five patients with a mosaic pattern all granulosa cells were 45,X (Patients C–E). In the other two mosaic patients the granulosa cells displayed a mosaic karyotype with the majority being 45,X in Patient A, and 46,XX in Patient B (Fig. 1). In Patients B, D and E, efficient spreading of follicular cells allowed us to determine the number of X chromosomes in at least 22 granulosa cells from individual small follicles (Fig. 6). The ratio of the 45,X and 46,XX granulosa cells varied considerably between follicles from the same ovary. In Patient B a follicle was observed with exclusively 46,XX granulosa cells, while six other follicles of this patient showed a percentage of 45,X granulosa cells varying from 13% to 59%. The granulosa cells of 10 individual follicles of Patients D and 5 individual follicles of Patient E were all 45,X (Figs 1 and 6). Although a large number (45–72%) of the stromal cells of Patients C–E was 46,XX, we did not observe any cells with a 46,XX karyotype amongst the granulosa cells of these patients, strongly suggesting that stromal cells do not co-purify with the follicles. The co-purification of theca cells is also very unlikely since small follicles do not yet contain this cell type (Young and McNeilly, 2010).
Figure 5.
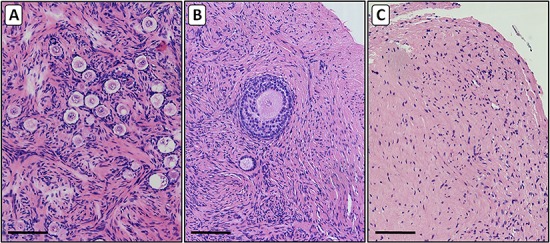
Histological sections of ovarian cortex from patients with TS. Haematoxylin–eosin stained 4-μm sections were prepared from cortical tissue from ovaries of mosaic (panels A and B) and 45,X monosomy TS patients (panel C). In addition to the variable number of small follicles in the tissue of the mosaic patients (panel A), a low number (<2%) of secondary follicles were observed (panel B). The ovarian tissue of the monosomic 45,X patients contained no follicles and showed a fibrous texture with relatively low number of cells (panel C). Bars represent 100 μm.
Figure 6.
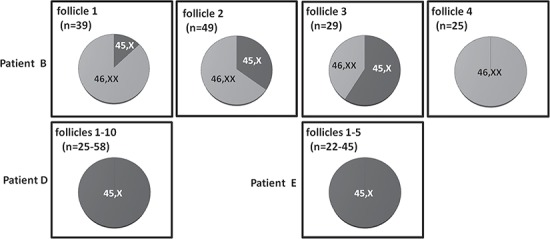
FISH analysis of granulosa cells from individual follicles. The ratio between 46,XX and 45,X granulosa cells from the same small follicle varied considerably. FISH analysis of six individual small follicles from Patient B revealed that the percentage of 45,X granulosa cells varied from 0% to 59% (top row; four follicles are shown). In Patients D (337 granulosa cells from 10 follicles) and E (152 granulosa cells from 5 follicles) all granulosa cells were 45,X. For Patients B and D at least 25 granulosa cells per follicle were analysed and for Patient E at least 22 granulosa cells per follicle.
Karyotyping the oocytes from small ovarian follicles
The FISH signals for the X chromosome and the control chromosome 18 were considerably more intense in the nuclei of oocytes than in the granulosa cells. Oocytes in primordial/primary follicles are arrested in the prophase of meiosis I and contain four copies of each chromosome held closely together by a synaptonemal complex (Burgoyne et al., 2009). This close proximity results in just a single but strong hybridization signal for the four clustered copies of each chromosome (Fig. 4 A, B and D). In contrast to somatic cells it is therefore not possible to simply count the hybridization spots to determine the number of X chromosomes of a particular oocyte. Should an oocyte lack half of its X chromosomes it is likely that the corresponding FISH signal becomes less intense. To validate this assumption, we analysed the FISH results from a number of oocyte nuclei in which the hybridization signal for either the X chromosome or chromosome 18, or both, was split into two discrete spots (Fig. 7A–C). By determining the surface of the hybridization spots as a measure for intensity, we found that the ratio of FISH signals between a single spot of a split signal and the control (non-split) signal in the same oocyte was approximately 0.6 (mean, 0.6; range 0.4–0.9; Fig. 7D). Next we analysed the ratio of FISH signals for the X chromosome and chromosome 18 in the oocytes of small follicles from TS patients and two non-Turner 46,XX control patients (Fig. 8). The mean of the ratio of the X chromosome/chromosome 18 FISH signals of individual oocytes was between 1.0 and 1.1, which was similar to the oocytes of the control patients. This indicates that most oocytes from the patients with a mosaic pattern had the normal number of four X chromosomal sister DNA strands. However, in Patients A and D the FISH signal ratio was found to be approximately 0.5 in 3 out of 19 oocytes and 1 out of 9 oocytes, respectively. This implies that in these oocytes probably only half of the normal X chromosomal DNA content was present.
Figure 7.
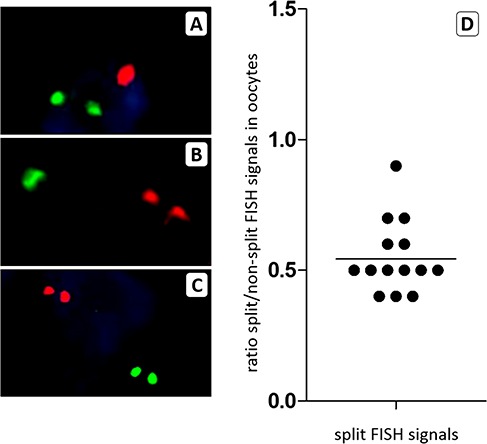
The surface of FISH signals can be used to karyotype oocytes from small follicles. To assess whether the surface of FISH signals can be used to determine the X chromosomal content of oocytes in the prophase of meiosis I, we measured the surface of split signals for the X chromosome (panel A), for chromosome 18 (panel B) or for both chromosomes (panel C) and compared these to the signal surface of the other chromosome. The surface ratio is shown in panel D and indicates that the surface of a split FISH signal is approximately 0.5 compared to the non-split signal of the other chromosome in the same oocyte. A 50% reduction in the number of X chromosomal sister chromatids will therefore lead to a reduction in the surface ratio between FISH signals for the X chromosome and chromosome 18.
Figure 8.
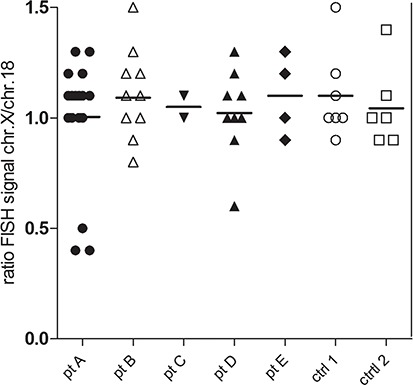
Most oocytes of small follicles from mosaic TS patients are 46,XX. The ratio between FISH signals from the X chromosome and chromosome 18 was measured for 46 oocytes from 5 mosaic TS patients (pt) and 13 oocytes from 2 controls (ctrl). The ratio in the controls varied between 0.9 and 1.5. In TS Patients B, C and E, a similar distribution was seen, indicating that these oocytes had a normal X chromosomal content. In Patients A and D the majority of the oocytes displayed a normal ratio, while in three oocytes from Patient A and one oocyte from Patient D, the ratio varied between 0.4 and 0.6, which is indicative of a reduction in the number of the X chromatids by half.
Discussion
Fertility preservation by cryopreservation of ovarian cortex tissue is being performed on an experimental basis in young women with TS in several countries (Hreinsson et al., 2002; Huang et al., 2008; Borgstrom et al., 2009; Balen et al., 2010; von Wolff et al., 2015). However, very little is known about the genetics of ovarian cells in this group of patients, and this may well determine the final outcome of this fertility preservation procedure. In this study, we have therefore developed methods to analyse the X chromosomal content of both oocytes and granulosa cells from small (primordial and primary) follicles, and stromal cells from ovaries of young TS patients. Determining the number of chromosomes in diploid somatic cells is relatively straightforward. FISH with a centromere-specific probe will give two signals in each cell representing a pair of homologous chromosomes. However, analysis of oocytes from small follicles is more challenging as these oocytes are arrested in the prophase of meiosis I, and therefore tetraploid. At this stage of meiosis, each pair of homologous maternal and paternal chromosomes is held together by a synaptonemal complex (Burgoyne et al., 2009). The very close proximity of the homologous chromosomes in these complexes prevents the identification of individual chromosomes by FISH analysis. This unique organization of genetic material in the oocytes, as present in small follicles, explains why we observed only a single strong signal after hybridization with chromosome-specific probes, which was more intense compared to the signals in the nuclei of granulosa cells or stromal cells. Determining the karyotype of these oocytes is therefore not possible by simply counting the number of signals for each chromosome. Instead, the intensity of the FISH signal should be used for analysis, assuming that a reduction in number of a certain chromosome in the tetraploid oocyte will lead to a less intense FISH signal. To evaluate whether the number of sister DNA strands of a certain chromosome is indeed reflected by the intensity of the FISH signals in oocytes, we analysed images of oocytes of both TS patients and controls, in which the FISH signal for the X chromosome, chromosome 18 or both chromosomes was separated into two discrete spots. In these oocytes the synaptonemal complex holding the four chromatids together was probably disrupted resulting in not one (as in most oocytes) but two signals, each containing half of the genetic material. By using the surface area of the FISH spots as a measure for spot intensity, we found that intensity for each of these spots was indeed reduced by ~50% and concluded that the surface area of FISH signals can be used to determine the number of X chromosomal sister DNA strands in oocytes. There are several other techniques described for quantitative analysis of fluorescent intensity in microscopy images, such as epifluorescence imaging of FITC signals relative to their associated DAPI-stained signals (Gartler et al., 2006), or by determining the ratio between two different FISH signals in the same cell (Aida et al., 2007). However, most of the techniques suffer from lack of precision in the results and complexity of existing software and are therefore not straightforward for a researcher to use (Fontenete et al., 2016). Furthermore, these techniques are optimized for cells during interphase or metaphase and have never been used to analyse the FISH signals of the tightly clustered chromosomes of a tetraploid human oocyte.
Karyotyping oocytes of TS patients from follicles at more advanced stages of maturation has been presented in a case report (Balen et al., 2010). However, the oocytes analysed in that study already had demonstrated their capacity for development and might be part of a subset of gametes capable of progressing through meiosis and may therefore not be representative for the resting pool of small follicles in the ovary. The results of our oocyte FISH analysis seem reassuring; of the 46 oocytes from small follicles isolated from TS patients, 42 oocytes appeared to have a normal number of X chromosomes. Four oocytes with an ~50% reduction of the FISH signal for the X chromosome were observed in two patients, suggesting that these oocytes were 45,X. In 45,X oocytes the X chromosome lacks a homologue and can therefore not achieve homologous synapsis during diplonema of prophase I. The finding of 45,X oocytes in humans is remarkable since this asynapsis is known to lead to oocyte elimination through meiotic silencing of unsynapsed chromatin (Burgoyne et al., 2009). Our findings may be explained by a process observed in mice, in which the 45,X oocytes evade elimination by non-homologous self-synapsis of the X chromosome (Turner et al., 2005). The complete absence of follicles in ovarian tissue of the 45,X patients in our study argues against rescue of 45,X oocytes through self-synapsis. However, in contrast to the mosaic TS patients, the ovaries of the 45,X patients had a fibrous streak-morphology with a large amount of extracellular matrix that may not allow 45,X oocytes to develop or persist. Low numbers of follicles have been reported in ovaries of TS patients with 45,X monosomy, but karyotyping of the oocytes was not performed and cryptic mosaicism in these patients cannot be fully excluded (Borgstrom et al., 2009). Although most oocytes from TS patients we analysed had a normal X chromosomal content, this is not a guarantee that these follicles are truly functional. The human primordial/primary follicle is a multicellular structure in which the oocyte is surrounded by a single layer of granulosa cells. The oocyte depends on this layer of granulosa cells for its long-term arrest in meiosis I. During follicular growth there is extensive bidirectional signaling between the oocyte and its surrounding granulosa cells to ensure normal follicular development (McLaughlin and McIver, 2009). Our observation that all granulosa cells were 45,X in three of the five mosaic TS patients may have functional consequences for follicular development. In addition to follicles containing only 45,X granulosa cells, we observed heterogeneity in the ratio of 45,X/46,XX granulosa cells between different follicles from the same patient. This low-level or absence of mosaicism in granulosa cells that populate the small follicles suggests that these cells originate from a relatively small number of ovarian surface epithelium derived progenitor cells (Mork et al., 2012). During follicle maturation the single layer of granulosa cells surrounding the oocyte in small follicles undergoes at least 10 mitotic divisions to produce the more than 2000 cells of the mature antral follicle (Hirshfield, 1991). As 45,X cells show an increase in apoptosis and have a prolonged cell cycle, the timing of expansion of the granulosa cell layer during maturation may be distorted (Nielsen and Krag-Olsen, 1980; Barrenäs et al., 2000; Gupta et al., 2003). The apparent histologically normal follicles populated with a high percentage of, or exclusively, 45,X granulosa cells could therefore be functionally impaired. Although the functional consequences of our observations require additional research, it could be possible that the mere presence of small follicles in the ovarian cortex tissue of TS patients does not guarantee normal ovarian function.
No obvious correlation was found between the number of follicles in the ovary and the patient’s karyotype of either the somatic cells or the oocytes. However, estimating the follicular density in a single piece of cortex should be interpreted with care in view of their uneven distribution throughout the cortex (Schmidt et al., 2003; Lambalk et al., 2004).
In addition to the karyotype of the oocyte and the granulosa cells, the karyotype of the stromal cellular compartment may be of importance for the formation and persistence of a functional ovary as well. Stromal cells not only support the growing follicle physically and metabolically, but also provide the theca cells during follicular development (Erickson et al., 1985; Orisaka et al., 2006; Honda et al., 2007). In the fibrous streak ovaries of the 45,X patients we found no evidence of mosaicism, which may well have contributed to their abnormal morphology. However, a certain degree of aneuploidy in the ovarian stromal cells does not seem to drastically influence the morphology of the ovary. Macroscopically, ovarian morphology was normal in the 45,X/46,XX mosaic patients with 45–97% 46,XX stromal cells. Remarkably, normal ovarian macroscopic morphology was also found in a patient presenting a mosaic with only 10% 46,XX stromal cells. These findings are in line with previous published results. Ovarian stromal cells from a 45,X/46,XX patient, from which normal 46,XX oocytes were retrieved after FSH stimulation, also showed 60% of 45,X cells (Balen et al., 2010). This indicates that follicular development is possible even as the majority of the ovarian stromal cells are aneuploid.
In this study the analysis of extra-ovarian cells (lymphocytes, cells from buccal smears and urine) in TS patients with mosaicism does not seem to have any predictive value for the X chromosomal content of the ovarian cells (oocytes, granulosa and stromal cells). This substantiates the difference in levels of mosaicism, not only between patients but also between tissues, follicles and cells of the same type of a particular patient. Although the number of patients we investigated is relatively low, it is likely that the analysis of additional patients will only further support the notion that no two TS patients are the same with regard to the variation in mosaicism. In none of the extra-ovarian tissues of the 45,X patients did we find evidence of mosaicism. This suggests that analysis of not only lymphocytes but also buccal and urine cells, all revealing a 45,X pattern, might be indicative of presence of a non-functional ovary. Over the years the presence of oocytes and even pregnancies in apparently 45,X women have been reported (Cools et al., 2004; Mortensen et al., 2010). However, the karyotyping methods in these cases were not clearly defined, and one can assume that these apparently 45,X women were in fact individuals with cryptic mosaicism (Magee et al., 1998; Hook and Warburton, 2014).
In this paper we have confined our study to patients with numerical abnormalities of the X chromosome. Structural abnormalities (e.g. isochromosome X, ring chromosome X, X deletions or translocations) are also frequently found in TS. Karyotyping cells of TS patients with structural abnormality of the second X chromosome is not possible with the probes we used, as these are directed at repetitive peri-centromeric sequences and will not reveal chromosomal aberrations that leave these sequences intact. Structural aberrations of the X chromosome can be detected with probes against single copy sequences in somatic cells. However, due to the unique organization of the genetic material in meiosis I arrested oocytes, karyotyping with single copy probes is likely to be more difficult due to their less intense hybridization signal compared to the centromere-specific probes.
In conclusion, we believe this to be the first report that presents a detailed analysis of the X chromosomal content of oocytes, granulosa cells and ovarian stromal cells from ovaries of young patients with TS. We show that the majority of oocytes from small follicles of mosaic TS patients are normal, but all or part of the granulosa cells of individual follicles may be aneuploid. Follicles derived from the same ovary can differ regarding the level of mosaicism of their granulosa cells. High-level mosaicism was found in ovarian stromal cells without deviant macroscopic morphology of the ovary. The level of mosaicism observed in lymphocytes, buccal cells or urine-derived cells did not correlate with that of ovarian cells. These new findings suggest that despite the presence of morphologically normal ovaries and follicles in mosaic TS patients, the aberrant karyotype of their granulosa cells and ovarian stromal compartment may limit their capacity to support fertility. As a consequence, caution should be taken when counseling TS patients and their parents about fertility preservation options, to avoid unrealistic expectations regarding the success rate of this treatment. Clearly more research is required to reveal the functional consequences of high-level monosomy in ovarian tissue cells. With these data a more informed decision can be made on whether OTC should be offered to girls and women with TS, and whether the procedure is able to restore fertility.
Acknowledgements
We would like to acknowledge Marjo van Brakel, Sandra Kemp and Anne Janssen for expert technical assistance.
Authors’ roles
The project and experiments were designed by R.P., M.S., D.S., J.V., D.B., F.G. and K.F. Experiments were performed by R.P., M.S. and G.Z. R.P. drafted the original manuscript. All authors critically reviewed the manuscript and approved the final version.
Funding
Merck B.V. (The Netherlands; A16-1395); Radboud Oncologie Fonds (KUN 00007682).
Conflict of interest
The authors report no financial or other conflict of interest relevant to the subject of this article.
References
- Aida J, Izumiyama-Shimomura N, Nakamura K, Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS, Arai T et al. . Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol 2007;38:1192–1200. [DOI] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 1963;158:417–433. [DOI] [PubMed] [Google Scholar]
- Balen AH, Harris SE, Chambers EL, Picton HM. Conservation of fertility and oocyte genetics in a young woman with mosaic Turner syndrome. BJOG 2010;117:238–242. [DOI] [PubMed] [Google Scholar]
- Barrenäs M, Landin-Wilhelmsen K, Hanson C. Ear and hearing in relation to genotype and growth in Turner syndrome. Hear Res 2000;144:21–28. [DOI] [PubMed] [Google Scholar]
- Borgstrom B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, Fridstrom M, Hovatta O. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab 2009;94:74–80. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 2009;10:207–216. [DOI] [PubMed] [Google Scholar]
- Carr DH, Haggar RA, Hart AG. Germ cells in the ovaries of XO female infants. Am J Clin Pathol 1968;49:521–526. [DOI] [PubMed] [Google Scholar]
- Cools M, Rooman RP, Wauters J, Jacqemyn Y, Du Caju MV. A nonmosaic 45,X karyotype in a mother with Turner's syndrome and in her daughter. Fertil Steril 2004;82:923–925. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, Donnez J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod 2006;21:413–420. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev 1985;6:371–399. [DOI] [PubMed] [Google Scholar]
- Fontenete S, Carvalho D, Lourenco A, Guimaraes N, Madureira P, Figueiredo C, Azevedo NF. FISHji: new ImageJ macros for the quantification of fluorescence in epifluorescence images. Bioch Eng J 2016;112:61–69. [Google Scholar]
- Freriks K, Timmers HJ, Netea-Maier RT, Beerendonk CC, Otten BJ, van Alfen-van der Velden JA, Traas MA, Mieloo H, van de Zande GW, Hoefsloot LH et al. . Buccal cell FISH and blood PCR-Y detect high rates of X chromosomal mosaicism and Y chromosomal derivatives in patients with Turner syndrome. Eur J Med Genet 2013;56:497–501. [DOI] [PubMed] [Google Scholar]
- Gartler SM, Varadarajan KR, Luo P, Norwood TH, Canfield TK, Hansen RS. Abnormal X: autosome ratio, but normal X chromosome inactivation in human triploid cultures. BMC Genet 2006;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg M, Bidet M, Benard J, Poulain M, Sonigo C, Cédrin-Durnerin I, Polak M. Fertility preservation in Turner syndrome. Fertil Steril 2016;105:13–19. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chiplunkar S, Gupta A, Gollapudi S. Increased spontaneous, tumor necrosis factor receptor- and CD95 (Fas)-mediated apoptosis in cord blood T-cell subsets from Turner's syndrome. Genes Immun 2003;4:239–243. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991;124:43–101. [DOI] [PubMed] [Google Scholar]
- Honda A, Hirose M, Hara K, Matoba S, Inoue K, Miki H, Hiura H, Kanatsu-Shinohara M, Kanai Y, Kono T. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. PNAS 2007;104:12389–12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB, Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet 2014;133:417–424. [DOI] [PubMed] [Google Scholar]
- Hreinsson JG, Otala M, Fridstrom M, Borgstrom B, Rasmussen C, Lundqvist M et al. . Follicles are found in the ovaries of adolescent girls with Turner’s syndrome. J Clin Endocrinol Metab 2002;87:3618–3623. [DOI] [PubMed] [Google Scholar]
- Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, Chian RC. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: case report. Hum Reprod 2008;23:336–339. [DOI] [PubMed] [Google Scholar]
- Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, Dolmans MM. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod 2017;32:1046–1054. [DOI] [PubMed] [Google Scholar]
- Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P. Andersen CY.86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet 2017;34:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions in ovarian folliculogenesis. Reproduction 2002;123:613–620. [DOI] [PubMed] [Google Scholar]
- Lambalk CB, de Koning CH, Flett A, Van Kasteren Y, Gosden R, Homburg R. Assessment of ovarian reserve. Ovarian biopsy is not a valid method for the prediction of ovarian reserve. Hum Reprod 2004;19:1055–1059. [DOI] [PubMed] [Google Scholar]
- Magee AC, Nevin NC, Armstrong MJ, McGibbon D, Nevin J. Ullrich-Turner syndrome: seven pregnancies in an apparent 45,X woman. Am J Med Genet 1998;75:1–3. [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction 2009;137:1–11. [DOI] [PubMed] [Google Scholar]
- Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 2003;9:219–225. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 2012;86:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen KH, Rohde MD, Uldbjerg N, Gravholt CH. Repeated spontaneous pregnancies in 45,X Turner syndrome. Obstet Gynecol 2010;115:446–449. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S, Nottola SA. The ultrastructure of human reproduction. I: the natural history of female germ cell origin, migration and differentiation inside the developing ovary. Hum Reprod Update 1997;3:281–295. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Krag-Olsen B. Cell selection in vivo. Follow-up of nine unselected mixoploid children. Hum Genet 1980;55:357–361. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet 1991;87:81–83. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G, Berkowitz K, Bronson R, Kashani B, McGovern P, Pal L, Quinn G, Rubin K. Fertility preservation in women with Turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol 2016;29:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, Yoshida Y, Kotsuji F. 2006 Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod 2006;75:734–740. [DOI] [PubMed] [Google Scholar]
- Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner's syndrome. Italian study group for Turner's syndrome. J Clin Endocrinol Metab 1997;82:1810–1813. [DOI] [PubMed] [Google Scholar]
- Peek R, Bastings L, Westphal JR, Massuger LF, Braat DD, Beerendonk CC. A preliminary study on a new model system to evaluate tumour-detection and tumour-purging protocols in ovarian cortex tissue intended for fertility preservation. Hum Reprod 2015;30:870–876. [DOI] [PubMed] [Google Scholar]
- Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil Steril 2004;81:1112–1119. [DOI] [PubMed] [Google Scholar]
- Saenger P. Turner's syndrome. N Engl J Med 1996;335:1749–1754. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Byskov AG, Nyboe Andersen A, Müller J, Yding AC. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod 2003;18:1158–1164. [DOI] [PubMed] [Google Scholar]
- Schleedoorn MJ, van der Velden VA, Braat D, Peek R, Fleischer K. To freeze or not to freeze? An update on fertility preservation in females with Turner syndrome. Pediatr Endocrinol Rev 2019;16:369–382. [DOI] [PubMed] [Google Scholar]
- Singh RP, Carr DH. The anatomy and histology of XO human embryos and fetuses. Anat Rec 1966;155:369–383. [DOI] [PubMed] [Google Scholar]
- Sutton EJ, McInerney-Leo A, Bondy CA, Gollust SE, King D, Biesecker B. Turner syndrome: four challenges across the lifespan. Am J Med Genet A 2005;139:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvén L, Magnusson C, Hagenfeldt K, von Schoultz B. Life with Turner's syndrome--a psychosocial report from 22 middle-aged women. Acta Endocrinol 1993;129:188–194. [DOI] [PubMed] [Google Scholar]
- Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, Burgoyne PS. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 2005;37:41–47. [DOI] [PubMed] [Google Scholar]
- Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, Amorim CA. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril 2011;96:379–383. [DOI] [PubMed] [Google Scholar]
- Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krüssel J, Frambach T, Kupka M, Hohl MK, Winkler-Crepaz K et al. . Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod 2016;31:2031–2041. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Dittrich R, Liebenthron J, Nawroth F, Schüring AN, Bruckner T, Germeyer A. Fertility-preservation counselling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online 2015;31:605–612. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction 2010;140:489–504. [DOI] [PubMed] [Google Scholar]


