Abstract
The emergence and spread of Lyme disease and other infections associated with black-legged ticks is causing a public health crisis. No human vaccines are currently available, and both diagnosis and treatment are sometimes ineffectual, leading to advocacy for self-directed preventative measures. These recommendations are widely communicated to the public, but there is limited evidence for their efficacy. We undertook a systematic review and mixed-effects meta-regression analysis of factors purported to increase or decrease risk of black-legged tick bites and tick-borne disease. Published articles used in the study spanned the years 1984–2018. Variables associated with increased probability of tick-borne disease, with odds ratios significantly greater than 1, included deer abundance, high density of nymph-stage black-legged ticks, landscapes with interspersed herbaceous and forested habitat, low human population density, gardens, cat ownership, and race. Contrary to recommendations, use of landscape-related tick control measures, such as clearing brush, trimming branches, and having a dry barrier between lawn and woods, tended to increase risk. Pet ownership increased bite risk. Bite risk was highest for children aged 5 years or less, with a secondary peak in persons aged 50–70 years. Although some widely disseminated recommendations are supported by the research analyzed, others require further evaluation. Additional research is also needed to understand the mechanisms underlying significant relationships.
Keywords: black-legged tick, Ixodes scapularis, Lyme disease, meta-analysis, tick bites
The United States has an estimated 300,000 cases of Lyme disease annually (1). Throughout eastern North America, the causative agent of Lyme disease, the bacterium Borrelia burgdorferi, is transmitted by the black-legged tick, Ixodes scapularis; on the West Coast, the western black-legged tick, Ixodes pacificus, is the vector for B. burgdorferi. I. pacificus and I. scapularis both also transmit Anaplasma phagocytophilum, the bacterium that causes anaplasmosis. I. scapularis transmits Babesia microti, the protozoan that causes babesiosis. Nymphal I. scapularis exhibit peak questing (host-seeking) behavior in late spring and early summer; most human Lyme disease cases are thought to result from bites by nymphs (2).
Black-legged ticks present a public health crisis in North America. No human vaccines are currently available, and both diagnosis and treatment are sometimes ineffective, leading to advocacy for self-directed preventative measures to reduce encounters with ticks. For example, the Centers for Disease Control and Prevention recommends treating clothes with permethrin; using insect repellent; avoiding “wooded and brushy areas with high grass and leaf litter”; walking in the center of trails; checking clothing, pets, and people (including oneself) for ticks; and showering after being outdoors (3). It has also issued a series of recommendations for yard management (4). While these recommendations are widely communicated to the public, there is limited evidence for their efficacy.
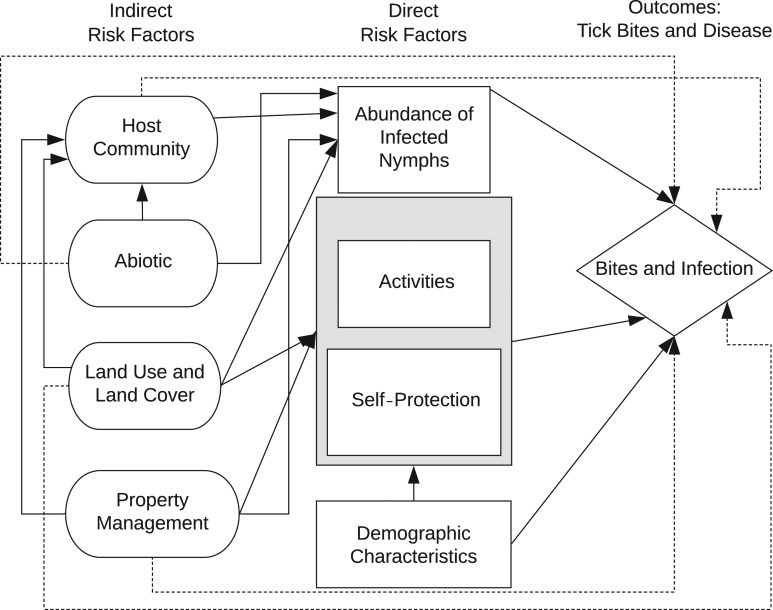
Overall risk of exposure to tick-borne disease is determined by entomological risk (defined as the abundance and/or infection prevalence of questing ticks in a location) and human behavior (Figure 1). These 2 categories of direct risk are influenced by indirect risk factors, including the community of vertebrate hosts for ticks, land use and land cover, property management, and abiotic variables (aspects of the physical environment, such as weather). Conclusions regarding the influences of these risk factors on public health outcomes have been mixed, contributing to uncertainty about prevention (5). The literature on these risk factors has not previously been characterized through quantitative meta-analysis.
Figure 1.
Conceptual diagram of tick-borne disease risk. Arrows indicate established or hypothesized causal relationships. Direct risk factors include entomological risk, human behavior (activities, self-protection), and sociodemographic factors, which also may influence human behavior. Entomological risk is the density of black-legged ticks (Ixodes scapularis or Ixodes pacificus) infected with pathogens. The interaction between human behavior and entomological risk influences the probability of tick bites and tick-borne disease. Indirect risk factors may affect entomological risk and human behavior and therefore tick-bite and disease outcomes.
The density of infected ticks, a primary entomological risk factor, represents the probability that a person will be exposed to a tick-borne infection given entry into tick habitat. The infection prevalence of a tick population represents the probability of exposure given that a tick feeds on a person long enough for transmission to occur. Entomological risk is often correlated with disease incidence (6–8), yet the correlation is, in some cases, weak (6, 9–11), with human behavior hypothesized to contribute to this inconsistency (6, 10, 11). Exposure to ticks outside the yard, for example, was one possible explanation for why a randomized controlled trial of yard treatment with acaricides resulted in a reduction in entomological risk without a reduction in tick bites or disease (10).
Human behavioral factors include activities that bring people into contact with ticks and self-protective actions. Social and demographic patterns of tick-borne disease, including peak incidence rates in boys aged 5–9 years and adults aged 60–64 years, have been attributed to behavior (1). Activities associated with increased disease include yard work (12), outdoor work (13), and outdoor recreation, including camping (14) and trail use (15). The relationships between self-protection and disease have varied across studies, with reduced disease being associated with bathing (16), repellents (17), and tick checks (16, 18) in some studies but not in others (12, 14).
Entomological risk and human behavior are influenced by indirect factors, including the community of hosts for ticks (which are required for the presence of ticks and the pathogens they transmit), land use and land cover (which can affect host communities and human behavior), property management (which can affect habitat quality for ticks and hosts), and abiotic variables (such as temperature and humidity, which can affect tick abundance and human behavior) (Figure 1). Abundance of small mammals, which are key hosts for immature ticks and reservoirs for tick-borne pathogens, predicts entomological risk (19) and disease incidence (20). An increase in numbers of coyotes and a decline in numbers of red foxes was found to be correlated with Lyme disease incidence, suggesting that suppression of foxes by coyotes has led to population growth of small mammals (21). In one study, removing deer from a small island substantially reduced tick abundance (22), but otherwise results of studies on deer removal for reduction of entomological risk have been inconsistent (23). In a recent synthesis, Kugeler et al. (24) concluded there is not strong evidence for deer culling reducing disease incidence. Abiotic ecosystem factors are also correlated with disease and entomological risk; for example, hot, dry summers are associated with reduced tick questing and disease (25).
Land use and land cover can influence entomological risk; for example, risk increases with increasing forest fragmentation (26). This pattern is hypothesized to occur because small patches of forest support high density of white-footed mice (Peromyscus leucopus), the most competent reservoir host for B. burgdorferi, and low diversity of other, incompetent reservoir hosts. In a study in Old Lyme, Connecticut, landscapes with higher forest fragmentation had higher entomological risk, yet Lyme disease incidence was negatively correlated with forest fragmentation (9). Human behavior may explain this pattern, if people tend not to use highly fragmented areas with high entomological risk (5, 23).
To reduce ticks and prevent tick bites, public health officials have recommended property management measures, such as removal of leaf litter, clearing of brush, and placing a barrier between lawn and woods (4). The presence of wood piles (18), stone walls (18), and leaf litter (14) in yards is associated with increased disease.
Results across studies are mixed regarding the influences of risk factors on disease and tick bites. This inconsistency has led to uncertainty among the public (27) and public health officials (28) about where to focus prevention efforts and to the advocacy of poorly supported recommendations. To better inform prevention decisions, we conducted a meta-analysis of risk factors associated with black-legged ticks. The meta-analysis examined direct and indirect risk factor categories, as well as the roles of specific variables within each risk factor category.
METHODS
Search strategy and selection criteria
We performed a meta-analysis following PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] guidelines (29). The search strategy we used (see Web Appendix 1, available at https://academic.oup.com/aje) included the risk factor categories (Figure 1) and variables in studies included in a meta-analysis on spatial components of tick-borne disease risk (Ilya R. Fischhoff et al., Cary Institute of Ecosystem Studies, unpublished manuscript, 2018). We restricted the search to all pathogens transmitted by I. scapularis or I. pacificus. The search included US states and Canadian provinces with recent Lyme disease incidence of at least 1 per 100,000 people (30, 31), as well as all remaining states in the US Northeast, Midwest, and West Coast and Canadian British Columbia.
We performed a search of Web of Science (Clarivate Analytics, Philadelphia, Pennsylvania) on February 20, 2018. Two people screened each article’s title and abstract. If at least 1 screener determined an abstract to be possibly relevant, we assessed the article in full. If the article reported on relevant risk factors in relation to disease or tick-bite data, we included it in the meta-analysis. We excluded reviews, articles that were primarily on other species of ticks, articles on studies that lacked controls, and articles that dissociated risk factor and disease or tick-bite data.
For studies on disease, we excluded data on the risk factors age and sex, as these factors have been analyzed in recent national-scale studies (1, 32). However, we included studies that addressed the relationship of these factors to tick bites. We included, within the category of tick bites, both tick encounters and tick bites (based on self-reports and serological evidence of tick bites). Biologically, it is appropriate to link tick bites and tick encounters in assessing transmission risk for tick-borne diseases. Any tick bite must have been preceded by a tick encounter. A tick encounter that does not result in a tick bite implies that the person detected the tick in time to prevent the bite. We excluded composite variables that included multiple categories (e.g., “NDVI [normalized difference vegetation index] and precipitation” (33), comprising land cover and the abiotic environment). Data were excluded if information on the same variable, place, and time was presented in a different paper by the same authors, indicating redundancy. See Web Figure 1 for the screening and review steps used and the numbers of articles remaining at each step. Where data on effect sizes were incomplete, we requested missing information from the authors. If data were presented in graphs alone, we digitized the data using the Engauge Digitizer, version 10.4 (Mark Mitchell, Torrance, California).
Statistical analysis
Each data point comprised the measure associated with 1 variable in 1 study. Study results were reported in multiple ways, including incidence or number of cases and population, numbers of cases and controls or computed odds ratios, and numbers of persons associated with a variable versus continuous variables measured for individuals. Where numbers of cases were presented without population size, the latter was obtained from government sources (34, 35) for computation of incidence. We converted a range of data types into log odds ratios and standard errors around log odds ratios. The procedures involved in processing different types of data are described in Web Appendix 2. If data on effect sizes were present but not variances, then variance was imputed using multiple imputation with the R package “mi” (R Foundation for Statistical Computing, Vienna, Austria). If authors reported on risk associated with multiple levels of a variable—for example, across locations or years—we computed a pooled estimate. If authors of a tick-bite study reported on multiple age classes, we found the odds ratio relating the lowest-risk age class to each higher-risk age class and then computed a pooled odds ratio. All analyses were conducted in R, version 3.4.4 (36).
Variables with similar meanings were reclassified under unified variable names (Web Table 1). For example, types of repellent were reclassified as “repellent”; descriptors of forests were reclassified as “forest.” At this stage, certain variables (e.g., “male vs. female”) were changed to their opposites (“female vs. male”), with their log odds ratio values multiplied by −1. The odds ratio associated with each variable was estimated, using an intercept-only model for each variable, with the function “lm” in base R. Factors that were protective were converted to their opposites; for example, if self-protective action “X” had a negative log odds ratio (corresponding to an odds ratio less than 1), the risk-increasing factor “not X” was used in the analysis, after multiplying the log odds ratio values for the variable by −1. We made this conversion to compare the relative magnitudes of effect sizes.
We performed meta-analyses to estimate associations between odds ratios and risk factor categories and specific variables. Risk factors may have different influences on the 2 outcomes we analyzed: tick bites and disease. Using Akaike Information Criterion values, we fitted 2 alternative mixed-effects models to the data predicting the log odds ratios—one model that included risk factor category and a second model that included both category and outcome (disease or bite). In both models, study was a random effect. The model including outcome was a better fit (difference in Akaike Information Criterion for small sample sizes = 6.75; weight = 0.97). Therefore, separate analyses were conducted with tick bite and disease data sets. For each data set, to examine the influence of category (Figure 2), we fitted a linear mixed model, with a fixed effect of category and a random effect of study. We used the R package “nlme” (function “lme”). The model had an intercept of 0, to produce estimates of the risk associated with each category. We analyzed log odds ratios, but we present results after transforming them to odds ratios, applying correction factors to obtain unbiased back-transformation (37). We did not incorporate study quality into the meta-analysis, given the lack of objective means of assessing quality.
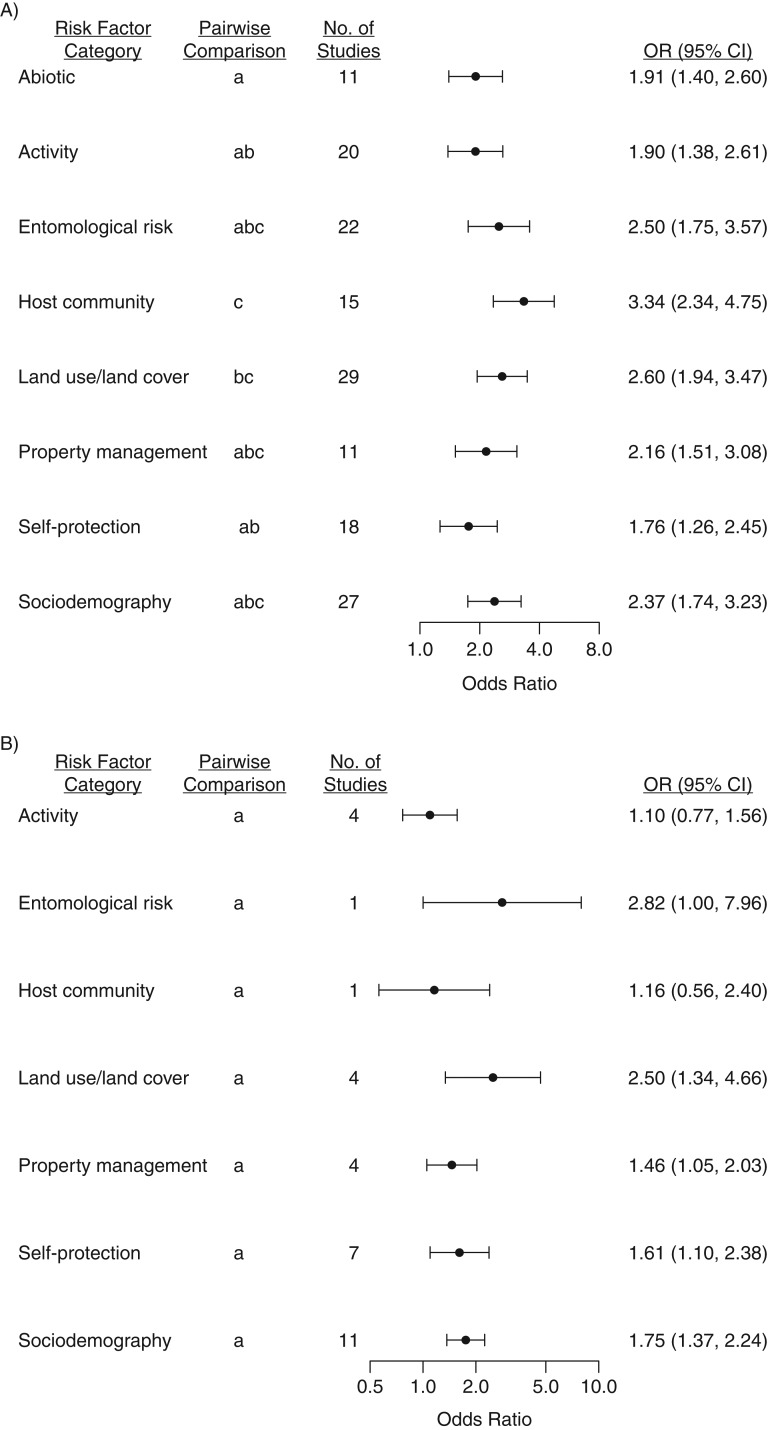
Figure 2.
Pooled odds ratio (OR) estimates for categories of risk of tick-borne disease (1984–2017) (A) and tick bites (1991–2018) (B) in a meta-analysis, North America, 1984–2018. To enable comparison of the magnitudes of effect sizes (some of which increase risk, while others decrease risk), we converted any specific variable that was protective (odds ratio < 1) to the opposite variable (one that increased risk). Data have been plotted on a log scale. Within each outcome (disease or bite), categories sharing a letter did not differ significantly (P > 0.05, unadjusted) in pairwise comparison of least-squares mean values. Bars, 95% confidence intervals (CIs).
To investigate specific variables within categories, we conducted separate analyses within each category. Sample sizes were inadequate to obtain stable model results in a meta-analysis of the influence of variable, within each category. Therefore, we carried out univariate analyses with respect to each variable. In the case of land use and land cover, complementary sets of analyses were conducted, reflecting 2 components of the landscape: First, variables were classified according to land cover and land use type (e.g., forest, population density), and second, variables were classified according to type of landscape metric (herbaceous forest edge, forest fragmentation, land cover, land use). Univariate analyses were conducted with the function “lm” in base R. Weights were set to equal the inverse of the log standard error for each data point.
For age as a risk factor for tick bites, we conducted 2 analyses. First, we determined the mean odds ratio for less risky age classes versus the most risky age classes. This enabled us to compare effect sizes for age with those for other risk factors. Second, tick-bite incidence patterns were investigated qualitatively, by calculating, across studies for each age class, the mean and standard error in incidence values. Where the oldest age class had no end, we assumed an upper age limit of 100 years. For each age interval, we assumed that all data referred to the mean age to the nearest 5-year mark.
We evaluated heterogeneity in effect sizes, which can originate from publication bias or variation in methods or populations, by inspecting a funnel plot (log odds ratio vs. log standard error) and by conducting a regression test for funnel plot asymmetry (38). The regression test was carried out using the function “regtest” (package “metafor”) based on an intercept-only linear mixed model (function “rma.uni”). These analyses used the original log odds ratio values, prior to protective variables being converted to risky ones.
RESULTS
From 1,976 citations returned by the literature search, 89 studies met the criteria for inclusion; we also reviewed and included 5 additional studies that came to our attention independently of the search, bringing the total to 94 studies spanning the years 1984–2018 (Web Table 2). For 14 papers, we contacted authors to request missing information. In 4 of those cases, authors responded with data; in 2 cases, authors responded to indicate that data were unavailable; and in 8 cases, we received no response. The 94 studies provided 326 estimates of disease risk (in 83 studies) and 57 estimates of bite risk (in 16 studies) (39).
For the disease data, analysis of variance identified a significant association between category and odds ratio (F8,236 = 10.15, P < 0.0001). Each category had a log odds ratio different from 0 (corresponding to an odds ratio different from 1), indicating significant risk (Table 1). Host community had the highest odds ratio, similar to the values for land use and land cover, entomological risk, sociodemography, and property management, based on pairwise comparisons of least-squares means (function “emmeans” in the package “emmeans”) (Figure 2).
Table 1.
Estimated Odds Ratios and P Values for Associations of Various Categories of Risk Factors With Tick-Borne Disease in a Meta-Analysis, North America, 1984–2017a
| Risk Factor Category | t Test | P Value | Odds Ratio | 95% Confidence Interval | Pairwise Comparisonb | No. of Observations | No. of Studies |
|---|---|---|---|---|---|---|---|
| Abiotic | 4.14 | <0.0001 | 1.93 | 1.42, 2.64 | a | 18 | 11 |
| Activity | 4.04 | 0.0001 | 1.93 | 1.40, 2.65 | a,b | 58 | 20 |
| Entomological risk | 5.09 | <0.0001 | 2.53 | 1.79, 3.61 | a,b,c | 32 | 22 |
| Host community | 6.73 | <0.0001 | 3.38 | 2.37, 4.81 | c | 20 | 15 |
| Land use/land cover | 6.51 | <0.0001 | 2.63 | 1.97, 3.52 | b,c | 67 | 29 |
| Property management | 4.29 | <0.0001 | 2.19 | 1.53, 3.13 | a,b,c | 32 | 11 |
| Self-protection | 3.41 | 0.0008 | 1.78 | 1.28, 2.49 | a,b | 53 | 18 |
| Sociodemography | 5.59 | <0.0001 | 2.42 | 1.78, 3.30 | a,b,c | 46 | 27 |
a 236 degrees of freedom.
b Categories with different letters had significant pairwise differences (P < 0.05, unadjusted) between least-squares mean values.
For bite data, there was a significant association between category and risk (F7,35 = 4.89, P = 0.0006). The categories of land use and land cover, sociodemography, self-protection, and property management had odds ratios different from 1, while odds ratios for entomological risk, activity, and host community did not differ significantly from 1 (Figure 2, Table 2). No bite studies considered abiotic factors.
Table 2.
Estimated Odds Ratios and P Values for Associations of Various Categories of Risk Factors With Tick Bites in a Meta-Analysis, North America, 1991–2018a,b
| Risk Factor Category | t Test | P Value | Odds Ratio | 95% Confidence Interval | No. of Observations | No. of Studies |
|---|---|---|---|---|---|---|
| Activity | 0.50 | 0.6219 | 1.10 | 0.77, 1.56 | 6 | 4 |
| Entomological risk | 1.96 | 0.0577 | 2.82 | 1.00, 7.96 | 1 | 1 |
| Host community | 0.40 | 0.6918 | 1.16 | 0.56, 2.40 | 1 | 1 |
| Land use/land cover | 2.88 | 0.0068 | 2.50 | 1.34, 4.66 | 6 | 4 |
| Property management | 2.26 | 0.0303 | 1.46 | 1.05, 2.03 | 13 | 4 |
| Self-protection | 2.43 | 0.0203 | 1.61 | 1.10, 2.38 | 14 | 7 |
| Sociodemography | 4.43 | 0.0001 | 1.75 | 1.37, 2.24 | 16 | 11 |
a 35 degrees of freedom.
b No categories had significant pairwise differences (P > 0.05, unadjusted) between least-squares mean values.
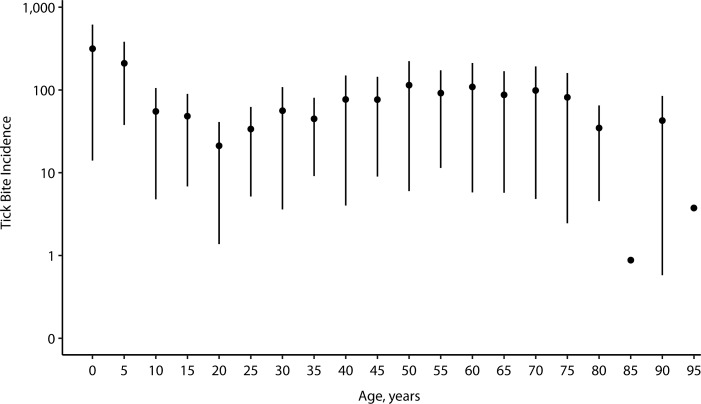
Among host community variables, an abundance of deer increased disease risk. Among entomological risk measures in relation to disease, density of nymphs had odds ratios significantly higher than 1 (Web Figure 2, Web Table 3). Disease risk was greater for people with a garden in their yards, in areas with lower human population density, and in areas with greater herbaceous forest edge. Among measures of land use and land cover, there were significant associations of land cover and land use with disease risk, with trends for greater herbaceous edge and forest fragmentation increasing risk of disease. Owning a cat and being a white person increased risk. No significant associations with risk were detected for specific variables in the human behavior categories. For tick bites, owning pets or domestic animals was associated with risk (Web Figure 3). Tick-bite incidence had a bimodal distribution with respect to age, reaching the highest values in children aged approximately 5 years or less, with a smaller peak among adults around ages 50–70 years (Figure 3) (40–43).
Figure 3.
Mean incidence of tick bites per 100,000 people in a meta-analysis, by age, North America, 1996–2016. Bars, standard errors.
There was a significant relationship between log odds ratios and log standard errors for disease (Z = 5.77, P < 0.0001) (Web Figure 4) but not for bites (Z = 0.015, P = 0.99) (Web Figure 5). This identifies potential publication bias, or heterogeneity due to other causes, in the disease data set (44).
DISCUSSION
Each category of risk had an odds ratio significantly greater than 1 and thus merits attention for disease prevention. Odds ratios for disease were greater for the direct factors of entomological risk and sociodemography and for certain indirect factors (host community, property management, land use and land cover) than for abiotic variables or human behavior. For tick bites, there were significant associations with risk for the categories of land use, land cover, sociodemography, property management, and self-protection. There was limited statistical power to test the tick-bite categories of human activity, host community, entomological risk, and n = 0 abiotic variables. To identify effective interventions against tick bites, we need more data on tick encounters in relation to risk factors.
Among factors contributing to disease risk, the highest odds ratio was associated with host community variables. The results are compatible with managing deer abundance to reduce risk. Among deer-targeted control measures, there is greater support for hunts than for the use of 4-poster tick control devices (45), although we cannot draw strong conclusions with each factor represented by 1 study. The results for deer differ from Kugeler et al.’s conclusion, reached in a recent review (24), that culling deer is unlikely to reduce the incidence of Lyme disease. A possible explanation is that the present meta-analysis included several citations, providing data on disease in relation to deer abundance, that were not used in the previous review, which addressed culling (24). In the current meta-analysis, we did not have data with which to address sources of variation that the previous review identified as important, such as incidence trends independent of culls, or differences in deer influences for island versus mainland areas. In one study (21), an increase in the number of coyotes tended to increase risk, as did a reduction in the number of foxes; this identifies the benefit of additional studies on predators’ roles in risk. Compared with deer, fewer studies examined small mammals. Studies that address multiple species within host communities offer potential to resolve questions about the relative influences of different species.
Results in the “land use and land cover” category are consistent with greater risk in rural and suburban settings. The results point to the value of land-use planning, specifically of reducing herbaceous forest edge arising from forest fragmentation. Such edges have intermediate entomological risk, between the high levels of forest and the low levels of herbaceous habitat (46). Elevated risk associated with the prevalence of herbaceous forest edge may result in part from people frequenting these edges, indicating the need for better understanding about where people encounter ticks. Herbaceous forest edge is also favorable deer habitat (47). With respect to disease and bites, there was a trend toward greater yard size contributing to risk, which is consistent with yards being important sites for exposure (48, 49).
Actions that reduce numbers of ticks in the environment, especially nymphal ticks, reduce risk. Density of nymphs increased disease risk more than other variables, although with a small sample size (n = 2 studies). As the prevalence of nymphal infection increased, there was a modest trend toward reduction in risk (n = 2), suggesting that this may be a less useful measure than others. Diverse measures of entomological risk have been used. If investigators were to present data on multiple measures, this would aid in resolving which measure best predicts human outcomes.
Social and demographic factors, including race and cat ownership, increased risk. Increased risk for white people may be due to correlation between race and exposure associated with where people live, work, and recreate. The tick-bite patterns with respect to age mirror those published for disease (1), lending support for use of passive tick-bite surveillance as a predictor of disease risk. The sociodemographic patterns identify the advantages of tailoring public health messages to populations at elevated risk. Further study could help elucidate whether the risk associated with cats is specific to outdoor cats (and, if so, whether it is due to cats’ transporting ticks or affecting host communities) or rather is correlated with another factor.
Despite the focus of public health messaging on peridomestic risk, property management is among the least studied categories of risk, based on the number of published studies (48). Use of landscaping tick control measures appeared to increase risk (confidence intervals excluding 1), which contrasts with recommendations made by public health specialists. The yard work entailed in implementing these measures may expose people to ticks, with this increase in risk outweighing any potential (but poorly documented) benefits for tick control. Acaricide use had a pattern of reducing risk, while the presence of a stone wall or wood pile tended to increase risk; these trends are consistent with recommendations from public health agencies (4, 50). Combining several types of data—data on human behavior, garden presence, and entomological risk—would allow evaluation of how gardens influence risk. Such integrated analyses could be enabled by the publication of anonymized raw data.
Within the activity category, travel to high-risk areas had the highest odds ratios, indicating the benefits of educating travelers. All activities, except for camping, had confidence intervals excluding 1 for disease, indicating a diverse set of exposures. Occupational exposure appears to have a relatively small influence (n = 11 studies), suggesting that domestic and recreational exposure may have a greater influence. The relative magnitudes of risk from recreational exposures versus occupational exposures, and of exposures incurred around the home versus elsewhere, could be better understood by further studies that simultaneously examined both dimensions.
Hot, dry conditions exhibited a trend toward increasing disease risk. These conditions, however, reduced numbers of questing ticks (25). This counterintuitive result prompts the hypothesis that, in spite of hot, dry weather’s reducing entomological risk, these impacts may be counterbalanced by people spending more time in tick habitat in such weather.
The lack of several self-protection behaviors (bathing, wearing protective clothing, tick avoidance, repellent use, tick checks) each had odds ratios with confidence intervals that excluded 1 (Web Table 3), indicating that each step may reduce disease risk, possibly with the greatest benefits being derived from bathing or showering after potential exposure. Wearing protective clothing, repellent use, and avoidance of ticks similarly had trends toward protective influences on bites. While there was a pattern of bathing being associated with increased risk of tick bites, this may reflect increased detection of ticks or, alternatively, people at higher risk being more likely to adopt this behavior. We detected no influence of awareness on risk, indicating the need for education directed toward action rather than basic knowledge.
The current meta-analysis results differ from those of past studies that have identified strong associations with entomological risk of small mammal abundance (19), host diversity (51), and (in some studies (45, 52) but not others (19)) deer. With few exceptions, studies used in the meta-analysis were not placebo-controlled (10, 53–55) or double-blinded (10, 53). In most studies, therefore, the probability of reports of bites or disease may have been influenced by perceived risk—for example, related to frequency of deer sightings or participant behavior. This potential source of bias would probably be stronger in studies where tick bites or cases were the response variable, as compared with studies of entomological risk.
Significant relationships between effect sizes and standard errors in the disease data set but not in the tick-bite data sets indicate potential publication bias or, alternatively, heterogeneity in methods or populations (44). We included all diseases associated with I. scapularis and I. pacificus; variation across pathogens is one potential source of heterogeneity, although not one we would expect to bias results with respect to particular risk factors. Similarly, site-specific variables may influence variation across studies in influences of deer or other variables. As the literature expands, it may become possible to control for additional sources of variation in meta-analyses. Compared with the number of studies eligible for meta-analysis, there were approximately twice as many studies that could not be used because they presented either disease or bite data or risk factor data, but not both. This highlights the challenge, and value for guiding prevention decisions, of carrying out studies that test the links between risk factors and human outcomes.
Behavioral factors, while representing significant disease risk, had smaller influences on risk than other factors. While behavior is under the control of individuals, other categories of risk, including land use/land cover and property management, are affected by decisions of communities, businesses, and governments. The patterns found in this meta-analysis indicate the need to investigate the efficacy of interventions, such as wildlife management or land-use planning, at these larger scales of decision-making.
In conclusion, while we found support for some widely disseminated recommendations, others require further evaluation. It appears that landscape-related tick control methods, such as clearing brush, trimming branches, and having a dry barrier at the lawn-woods edge, increase rather than decrease risk. The results of this meta-analysis provide additional clarity on the contradictory findings obtained from individual studies—for example, regarding pet ownership, the importance of wildlife species, and self-protective behaviors.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Cary Institute of Ecosystem Studies, Millbrook, New York (Ilya R. Fischhoff, Richard S. Ostfeld); and Biology Program, Bard College, Annandale-on-Hudson, New York (Felicia Keesing).
This work was supported by the Steven and Alexandra Cohen Foundation.
We thank the authors of several studies (Drs. Curtis Fritz, Neeta Connally, Ellen Cromley, and William Hallman) for providing supplementary data. We also thank Ashley Pfister, Stacy Mowry, Deanna DePietro, Carly Barbera, Megan Schierer, and Monica Marrone for screening abstracts.
This article is a contribution to the program of the Cary Institute of Ecosystem Studies (Millbrook, New York).
Conflict of interest: none declared.
REFERENCES
- 1. Nelson CA, Saha S, Kugeler KJ, et al. . Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis. 2015;21(9):1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore SM, Eisen RJ, Monaghan A, et al. . Meteorological influences on the seasonality of Lyme disease in the United States. Am J Trop Med Hyg. 2014;90(3):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention Preventing tick bites. 2019. https://www.cdc.gov/ticks/avoid/on_people.html Accessed February 17, 2019.
- 4. Centers for Disease Control and Prevention Preventing ticks in the yard. 2018. https://www.cdc.gov/ticks/avoid/in_the_yard.html Accessed August 27, 2018.
- 5. Eisen RJ, Piesman J, Zielinski-Gutierrez E, et al. . What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J Med Entomol. 2012;49(1):11–22. [DOI] [PubMed] [Google Scholar]
- 6. Pepin KM, Eisen RJ, Mead PS, et al. . Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the eastern United States. Am J Trop Med Hyg. 2012;86(6):1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stafford KC 3rd, Cartter ML, Magnarelli LA, et al. . Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36(5):1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mather TN, Nicholson MC, Donnelly EF, et al. . Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144(11):1066–1069. [DOI] [PubMed] [Google Scholar]
- 9. Brownstein JS, Skelly DK, Holford TR, et al. . Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 2005;146(3):469–475. [DOI] [PubMed] [Google Scholar]
- 10. Hinckley AF, Meek JI, Ray JA, et al. . Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J Infect Dis. 2016;214(2):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connally NP, Ginsberg HS, Mather TN. Assessing peridomestic entomological factors as predictors for Lyme disease. J Vector Ecol. 2006;31(2):364–370. [DOI] [PubMed] [Google Scholar]
- 12. Orloski KA, Campbell GL, Genese CA, et al. . Emergence of Lyme disease in Hunterdon County, New Jersey, 1993: a case-control study of risk factors and evaluation of reporting patterns. Am J Epidemiol. 1998;147(4):391–397. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz BS, Goldstein MD. Lyme disease in outdoor workers: risk factors, preventive measures, and tick removal methods. Am J Epidemiol. 1990;131(5):877–885. [DOI] [PubMed] [Google Scholar]
- 14. Klein JD, Eppes SC, Hunt P. Environmental and life-style risk factors for Lyme disease in children. Clin Pediatr (Phila). 1996;35(7):359–363. [DOI] [PubMed] [Google Scholar]
- 15. Ley C, Olshen EM, Reingold AL. Case-control study of risk factors for incident Lyme disease in California. Am J Epidemiol. 1995;142(9 suppl):S39–S47. [DOI] [PubMed] [Google Scholar]
- 16. Connally NP, Durante AJ, Yousey-Hindes KM, et al. . Peridomestic Lyme disease prevention: results of a population-based case-control study. Am J Prev Med. 2009;37(3):201–206. [DOI] [PubMed] [Google Scholar]
- 17. Vazquez M, Muehlenbein C, Cartterj M, et al. . Effectiveness of personal protective measures to prevent Lyme disease. Emerg Infect Dis. 2008;14(2):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith G, Wileyto EP, Hopkins RB, et al. . Risk factors for Lyme disease in Chester County, Pennsylvania. Public Health Rep. 2001;116(suppl 1):146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ostfeld RS, Canham CD, Oggenfuss K, et al. . Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006;4(6):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schauber EM, Ostfeld RS, Evans AS. What is the best predictor of annual Lyme disease incidence: weather, mice, or acorns? Ecol Appl. 2005;15(2):575–586. [Google Scholar]
- 21. Levi T, Kilpatrick AM, Mangel M, et al. . Deer, predators, and the emergence of Lyme disease. Proc Natl Acad Sci U S A. 2012;109(27):10942–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rand PW, Lubelczyk C, Holman MS, et al. . Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme disease. J Med Entomol. 2004;41(4):779–784. [DOI] [PubMed] [Google Scholar]
- 23. Ostfeld RS. Lyme Disease: The Ecology of a Complex System. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 24. Kugeler KJ, Jordan RA, Schulze TL, et al. . Will culling white-tailed deer prevent Lyme disease? Zoonoses Public Health. 2016;63(5):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burtis JC, Sullivan P, Levi T, et al. . The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasit Vectors. 2016;9:Article 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conserv Biol. 2003;17(1):267–272. [Google Scholar]
- 27. Butler AD, Sedghi T, Petrini JR, et al. . Tick-borne disease preventive practices and perceptions in an endemic area. Ticks Tick Borne Dis. 2016;7(2):331–337. [DOI] [PubMed] [Google Scholar]
- 28. Poland GA. Prevention of Lyme disease: a review of the evidence. Mayo Clin Proc. 2001;76(7):713–724. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Shamseer L, Clarke M, et al. . Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention Lyme disease data tables: historical data. Reported cases of Lyme disease by state or locality, 2006–2016. 2017. https://www.cdc.gov/lyme/stats/tables.html Accessed August 27, 2018.
- 31. Government of Canada National Lyme disease surveillance in Canada 2013: Web report. 2015. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/national-lyme-disease-surveillance-canada-2013-web-report.html Accessed August 27, 2018.
- 32. Schwartz AS, Hinckley AF, Mead PS, et al. . Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ. 2017;66(22):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen JM, Civitello DJ, Brace AJ, et al. . Spatial scale modulates the strength of ecological processes driving disease distributions. Proc Natl Acad Sci U S A. 2016;113(24):E3359–E3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Institut de la Statistique Quebec Population and age and sex structure. 2015. http://www.stat.gouv.qc.ca/statistiques/population-demographie/structure/index_an.html Accessed November 2, 2018.
- 35. National Cancer Institute Download U.S. population data—1969–2016. 2017. https://seer.cancer.gov/popdata/download.html Accessed October 1, 2018.
- 36. R Core Team R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 37. Baskerville GL. Use of logarithmic regression in the estimation of plant biomass. Can J For Res. 1972;2(1):49–53. [Google Scholar]
- 38. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. [DOI] [PubMed] [Google Scholar]
- 39. Fischhoff IR, Ostfeld R, Keesing F Dataset: risk factors for bites and disease associated with blacklegged ticks: systematic review and meta-analysis. 2019. https://figshare.com/articles/Dataset_Risk_factors_for_bites_and_disease_associated_with_blacklegged_ticks_systematic_review_and_meta-analysis/7324226 Accessed May 16, 2019.
- 40. Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143(2):187–192. [DOI] [PubMed] [Google Scholar]
- 41. Xu G, Mather TN, Hollingsworth CS, et al. . Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis. 2016;16(8):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rand PW, Lacombe EH, Dearborn R, et al. . Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol. 2007;44(6):1118–1129. [DOI] [PubMed] [Google Scholar]
- 43. Gasmi S, Ogden NH, Lindsey LR, et al. . Surveillance for Lyme disease in Canada: 2009–2015. Can Commun Dis Rep. 2017;43(10):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koricheva J, Gurevitch J, Mengersen K. Handbook of Meta-Analysis in Ecology and Evolution. Princeton, NJ: Princeton University Press; 2013. [Google Scholar]
- 45. Horobik V, Keesing F, Ostfeld RS. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest-field edges. EcoHealth. 2006;3(4):262–268. [Google Scholar]
- 46. Alverson WS, Waller DM, Solheim SL. Forests too deer: edge effects in northern Wisconsin. Conserv Biol. 1988;2(4):348–358. [Google Scholar]
- 47. Stafford KC III, Williams SC, Molaei G. Integrated pest management in controlling ticks and tick-associated diseases. J Integr Pest Manag. 2017;8(1):Article 28. [Google Scholar]
- 48. Falco RC, Fish D. A survey of tick bites acquired in a Lyme disease endemic area in southern New York State. Ann N Y Acad Sci. 1988;539(1):456–457. [Google Scholar]
- 49. Stafford KC., III Tick Management Handbook: An Integrated Guide for Homeowners, Pest Control Operators, and Public Health Officials for the Prevention of Tick-Associated Disease. New Haven, CT:Connecticut Agricultural Experiment Station; 2004. [Google Scholar]
- 50. LoGiudice K, Ostfeld RS, Schmidt KA, et al. . The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100(2):567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daniels TJ, Fish D, Schwartz I. Reduced abundance of Ixodes scapularis (Acari, Ixodidae) and Lyme disease risk by deer exclusion. J Med Entomol. 1993;30(6):1043–1049. [DOI] [PubMed] [Google Scholar]
- 52. Hoen AG, Rollend LG, Papero MA, et al. . Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 2009;9(4):431–438. [DOI] [PubMed] [Google Scholar]
- 53. Daltroy LH, Phillips C, Lew R. A controlled trial of a novel primary prevention program for Lyme disease and other tick-borne illnesses. Health Educ Behav. 2007;34(3):531–542. [DOI] [PubMed] [Google Scholar]
- 54. Vaughn MF, Meshnick SR. Pilot study assessing the effectiveness of long-lasting permethrin-impregnated clothing for the prevention of tick bites. Vector Borne Zoonotic Dis. 2011;11(7):869–875. [DOI] [PubMed] [Google Scholar]
- 55. Malouin R, Winch P, Leontsini E, et al. . Longitudinal evaluation of an educational intervention for preventing tick bites in an area with endemic Lyme disease in Baltimore County, Maryland. Am J Epidemiol. 2003;157(11):1039–1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.