ABSTRACT
MLN4924, a small molecular inhibitor of NEDD8 (neuronal precursor cell-expressed developmentally downregulated protein 8) activating enzyme (NAE), blocks cullin neddylation to inactivate cullin-RING ligase. We found that MLN4924 has additional activities: it triggers EGFR dimerization and activation of RAS/MAPK and PI3K/AKT1 signals to stimulate tumor sphere formation and inhibit ciliogenesis; and it triggers PKM2 tetramerization to promote glycolysis.
KEYWORDS: Neddylation, EGFR, AKT1, tumor-sphere, ciliogenesis, PKM2, glycolysis, dimerization, tetramerization
Neddylation, a reversible post-translational modification, conjugates a conserved ubiquitin-like molecule NEDD8 (neuronal precursor cell-expressed developmentally downregulated protein 8) to a lysine residue of targeted protein.1 Analogous to ubiquitylation, neddylation requires a three-step enzymatic cascade reaction, catalyzed by E1 NEDD8-activating enzyme (NAE), one of two E2 NEDD8-conjugating enzymes (UBE2M/UBC12 or UBE2F), and one of several substrate-specific NEDD8 E3 ligases.1,2 Unlike ubiquitylation that mainly regulates protein homeostasis through ubiquitin-proteasome system (UPS) for targeted degradation, neddylation modulates function, activity, conformation, and localization of its substrates. Among the growing list of neddyaltion substrates, the cullin subunits of Cullin-RING ligase (CRL) are the best characterized ones, which ubiquitylate about 20% of cellular proteins for degradation through UPS.3 Besides cullins, several biological significant proteins such as p53, VHL (von-Hippel-Lindau), mouse double minitue 2 (Mdm2), and epidermal growth factor receptor (EGFR) are also the reported substrates of neddylation.2 Thus, protein neddylation plays a pivotal role in many important aspects of biological processes including transcription, proteolysis, inflammatory responses, differentiation, signaling transduction, and tumorigenesis.1,2
Accumulating lines of evidence have shown that neddylation pathway is over-activated during tumorigenesis due to overexpression of NEDD8 and several neddylation enzymes.2,4 Thus, targeting protein neddylation system, especially through pharmacological approach, would be an appealing strategy for cancer therapy. MLN4924, also known as Pevonedistat, is a specific small molecule inhibitor of NAE.3 It binds to the NAE active site and forms a stable MLN4924-NEDD8 adduct in analog to adenylate-NEDD8, but inhibits further enzymatic process and terminates the cascade at this proximal step, thus blocking the entire neddylation modification.5 Preclinical studies have clearly demonstrated that MLN4924 suppresses the growth of various cancer cell lines by inducing growth arrest, apoptosis, senescence and autophagy, mainly through inactivation of CRLs to cause accumulation of tumor suppressor substrates.2,4 Due to its potent anticancer activity and well-tolerated toxicity in mice, MLN4924 has been advanced into several phase II clinical trials alone or in combination with chemotherapeutic drugs against a variety of human cancers.2,4
Although MLN4924 is a well-characterized selective inhibitor of NAE to block the entire process of neddylation,3 several lines of evidence from our laboratory surprisingly showed that MLN4924 has other cellular activities other than neddylation inhibition. Here, we summarize the MLN4924 activities beyond neddylation inhibition as follows:
1. Stimulation of tumor sphere formation and wound healing
We recently found that MLN4924 at nanomolar concentrations stimulates in vitro tumor sphere formation and in vivo tumorigenesis of both cancer cells and embryonic stem cells.6 Mechanistic studies revealed MLN4924, on one hand, inactivates SCFFBWX7 E3 to cause accumulation of c-Myc, and one the other hand, triggers EGFR dimerization to activate EGFR and its downstream signals, including RAS/RAF/MEK/ERK and PI3K/AKT1/mTOR pathways. The studies with various signal inhibitors revealed that tumor-sphere stimulating activity of MLN4924 is mainly mediated by the RAS/MAPK pathway. In addition, MLN4924 promotes EGF-induced wound healing in the mouse skin. Therefore, low dose of MLN4924 regulates stem cell proliferation and differentiation, which differs from its high-dose anticancer property. MLN4924 may, therefore, have new application for stem cell therapy and tissue regeneration.6
2. Inhibition of ciliogenesis
Our most recent study showed that MLN4924 blocks ciliogenesis by inhibiting cilia initiation and promoting cilia disassembly.7 Mechanistic study revealed that this activity is not mediated by its CRL inhibitory effect, since there is no accumulation of several cilia-associated proteins, known to be CRL substrates. Rather, it is mediated by MLN4924-mediated AKT1 activation via inducing EGFR dimerization,6,7 given both inhibitors of AKT1 and EGFR completely abrogate MLN4924 inhibitory effect on cilia formation.7 More specifically, both siRNA-based genetic and small molecular inhibitor-based pharmacological approaches demonstrated that MLN4924 induced AKT1 phosphorylation at the Ser473 plays a major role in suppression of cilia formation, but has no effect on cilia growth/length. Thus, MLN4924 may have a novel application for the treatment of human cancers which rely on cilia for growth or drug resistance.8
3. Promotion of glycolysis
In our newly published study, we found, through untargeted metabolomics strategy and a series of glycolytic detection assays, that MLN4924 can also promote glycolysis.9 Mechanistic study revealed that MLN4924 markedly increases PK (pyruvate kinase) activity in a manner solely dependent on PKM2 (M2 isoform of pyruvate kinase). To dissect how MLN4924 activates PKM2, we first excluded the possibility that PKM2 is a neddylation substrate. Interestingly, we found that MLN4924 has a similar chemical structure with a known PKM2 activator SAICAR (succinyl-5-aminoimidazole-4-carboxamide-1-ribose-5ʹ-phosphate), which triggers PKM2 tetramerization.10 Indeed, MLN4924 effectively triggers PKM2 tetramerization with a potency better than SAICAR.9 Biologically, MLN4924-induced PKM2 activation confers a better survival for breast cancer cells, and the combination of MLN4924 and PKM2 inhibitor shikonin significantly suppresses cancer cell growth both in vitro cell culture setting and in vivo xenograft model.9 Thus, activation of PKM2 to promote glycolysis and cell survival could be a side-effect of MLN4924 for cancer therapy, which should be cautiously noted.
In summary, our studies unexpectedly reveal that MLN4924 has additional biochemical activities other than NAE inhibition (Figure 1). These activities may provide new application of MLN4924 for stem cell therapy and tissue regeneration, for the treatment of abnormal ciliogenesis, and for rationale drug combination in cancer therapy.
Figure 1.
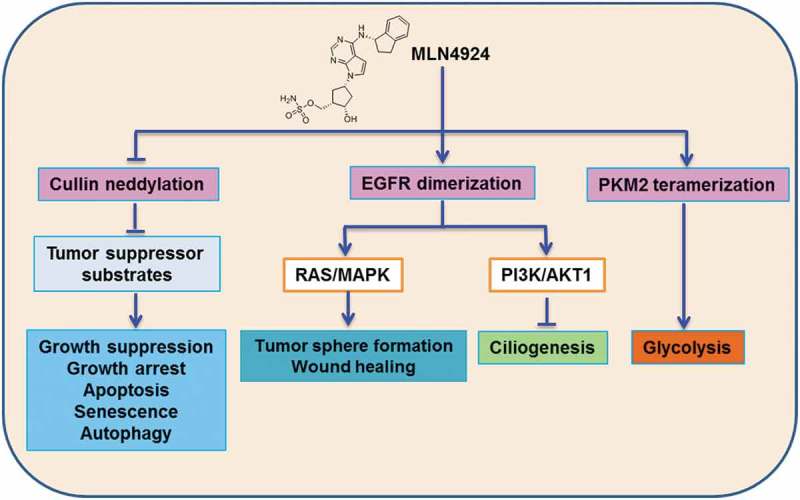
Neddylation dependent and independent activities of MLN4924. (1) Inactivating CRL (Cullin-RING ligase) E3: MLN4924 inactivates CRLs by blocking cullin neddylation to cause the accumulation of a number of tumor suppressor proteins, leading to growth suppression via inducing growth arrest, apoptosis, senescence, or autophagy in a variety of cancer cell lines. (2) Inducing EGFR (epidermal growth factor receptor) dimerization: MLN4924 activates EGFR by triggering its dimerization to activates its downstream RAS/MAPK pathway to promotes tumor sphere formation and wound healing, and PI3K/AKT1 pathway to inhibits ciliogenesis, respectively. (3) Inducing PKM2 (M2 isoform of pyruvate kinase) tetramerization: MLN4924 activates PKM2 via promoting its tetramerization to increase glycolysis.
Funding Statement
This work was supported by the Natural Science Foundation of Zhejiang Province [LY17C070001]; National Natural Science Foundation of China [81572718]; Chinese NSFC [31701167]; National Key R&D Program of China [2016YFA0501800].
Acknowledgments
This work is supported by the National Key R&D Program of China (2016YFA0501800) (YS), the Chinese NSFC grant 31701167 (QZ) and 81572718 (YS), and Natural Science Foundation of Zhejiang Province grant LY17C070001 (QZ).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Enchev RI, Schulman BA, Peter M.. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol. 2015;16:30–44. doi: 10.1038/nrm3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou L, Zhang W, Sun Y, Jia L. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. 2018;44:92–102. doi: 10.1016/j.cellsig.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature [pii]10.1038/nature07884 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Jiang Y, Luo Q, Li L, Jia L. Neddylation: a novel modulator of the tumor microenvironment. Mol Cancer. 2019;18:77. doi: 10.1186/s12943-019-0979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt AL, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell [pii]10.1016/j.molcel.2009.12.024 2010;37:102–111. doi: 10.1016/j.molcel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Tan M, Nyati MK, Zhao Y, Wang G, Sun Y. Blockage of neddylation modification stimulates tumor sphere formation in vitro and stem cell differentiation and wound healing in vivo. Proc Natl Acad Sci U S A. 2016;113:E2935–44. doi: 10.1073/pnas.1522367113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao H, Tang Z, Li H, Sun B, Tan M, Fan S, Zhu Y, Sun Y. Neddylation inhibitor MLN4924 suppresses cilia formation by modulating AKT1. Protein Cell. 2019. doi: 10.1007/s13238-019-0614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenks AD, Vyse S, Wong JP, Kostaras E, Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M, Michaelis M, et al. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep. 2018;23:3042–3055. doi: 10.1016/j.celrep.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Li H, Li Y, Tan M, Fan S, Cao C, Meng F, Zhu L, Zhao L, Guan M-X, et al. Inhibiting neddylation modification alters mitochondrial morphology and reprograms energy metabolism in cancer cells. JCI Insight. 2019 Jan 22. pii: 121582. doi: 10.1172/jci.insight.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller KE, Tan IS, Lee YS. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science. 2012;338:1069–1072. doi: 10.1126/science.1224409. [DOI] [PMC free article] [PubMed] [Google Scholar]


