Abstract
Context
Anorexia nervosa (AN) is a psychiatric illness with considerable morbidity and no approved medical therapies. We have shown that relative androgen deficiency in AN is associated with greater depression and anxiety symptom severity.
Objective
To determine whether low-dose testosterone therapy is an effective endocrine-targeted therapy for AN.
Design
Double-blind, randomized, placebo-controlled trial.
Setting
Clinical research center.
Participants
Ninety women, 18 to 45 years, with AN and free testosterone levels below the median for healthy women.
Intervention
Transdermal testosterone, 300 μg daily, or placebo patch for 24 weeks.
Main Outcome Measures
Primary end point: body mass index (BMI). Secondary end points: depression symptom severity [Hamilton Depression Rating Scale (HAM-D)], anxiety symptom severity [Hamilton Anxiety Rating Scale (HAM-A)], and eating disorder psychopathology and behaviors.
Results
Mean BMI increased by 0.0 ± 1.0 kg/m2 in the testosterone group and 0.5 ± 1.1 kg/m2 in the placebo group (P = 0.03) over 24 weeks. At 4 weeks, there was a trend toward a greater decrease in HAM-D score (P = 0.09) in the testosterone vs placebo group. At 24 weeks, mean HAM-D and HAM-A scores decreased similarly in both groups [HAM-D: −2.9 ± 4.9 (testosterone) vs −3.0 ± 5.0 (placebo), P = 0.72; HAM-A: −4.5 ± 5.3 (testosterone) vs −4.3 ± 4.4 (placebo), P = 0.25]. There were no significant differences in eating disorder scores between groups. Testosterone therapy was safe and well tolerated with no increase in androgenic side effects compared with placebo.
Conclusion
Low-dose testosterone therapy for 24 weeks was associated with less weight gain—and did not lead to sustained improvements in depression, anxiety, or disordered eating symptoms—compared with placebo in women with AN.
Anorexia nervosa (AN), with an estimated prevalence of 0.5% to 2% among women (1), is a serious psychiatric condition with considerable premature mortality (2) and no approved medical therapies. Comorbid affective and anxiety disorders are very common (3–6), with lifetime rates of major depressive disorder (MDD) and anxiety disorders of 50% to 75% (7) and 55% to 71%, respectively (6, 8). Many studies have shown that depression and anxiety symptoms are associated with worse eating disorder outcomes (9–12), though antidepressant treatment has not proven effective in alleviating depression or anxiety associated with AN (13–15). Additionally, studies of psychotherapy, medications, and combination treatments have demonstrated little or no improvement in weight or core psychopathology in women with AN (16, 17). Relative androgen deficiency is common in women with AN (18–20) and is the result of decreased production of testosterone and testosterone precursors from the ovaries (21). We have shown that levels of testosterone in women with AN are inversely associated with depression and anxiety severity (22, 23). Therefore, relative androgen deficiency may be a promising treatment target in AN.
Testosterone administration has been shown to exert antidepressant effects in several populations, and studies using animal models have suggested that testosterone may also modulate food intake and body weight. Testosterone replacement improves mood in men with androgen deficiency secondary to hypopituitarism (24). Moreover, studies have suggested a possible antidepressant effect in hypogonadal men with refractory depression (25) and in eugonadal depressed men (26). Such data prompted investigators to ask whether low doses of testosterone administration (10 to 20 times lower than doses typically used in men) designed to raise testosterone levels within or near the normal female range might exert similar antidepressive effects in women. Several small, randomized, placebo-controlled studies have suggested this to be the case in women with relative androgen deficiency due to bilateral oophorectomy (27) and due to hypopituitarism (28). Moreover, our group demonstrated in a 3-week randomized, placebo-controlled pilot study that low-dose testosterone treatment improved depression symptom severity in depressed women with AN (22). Studies of testosterone administration in conjunction with estrogen replacement in postmenopausal women have demonstrated antianxiety effects (29, 30), though no studies have assessed the impact of low-dose testosterone therapy on anxiety in women with AN. Animal studies have implicated a role for testosterone in feeding behavior that may be independent of mood. For example, after orchiectomy, male rats display decreases in both food intake and weight gain, which can be reversed with testosterone replacement (31, 32). We hypothesized that physiologic testosterone therapy would improve weight, depression and anxiety symptoms, and eating disorder symptoms in women with AN and relative androgen deficiency.
Methods
Study participants
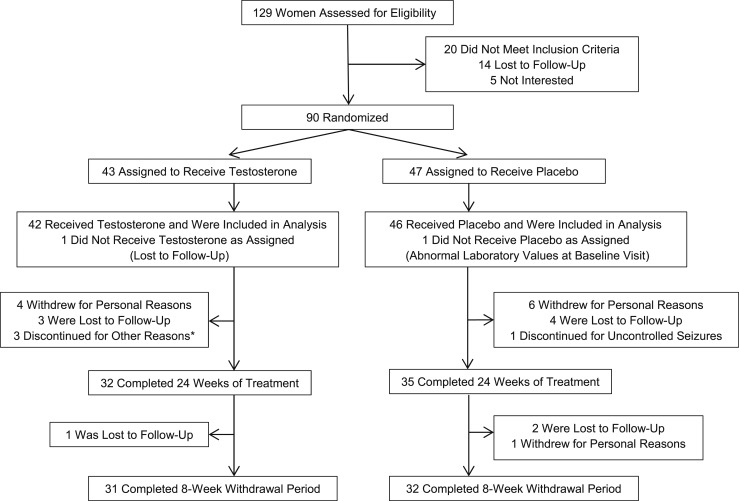
Ninety women with AN, aged 18 to 45 years, were enrolled in the study, and 88 were included in the analysis (Fig. 1). Subjects fulfilled criteria for AN (33) as assessed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (34). Women with low-weight AN and atypical AN (individuals who meet psychological criteria for AN but are not low weight) were included. Subjects were required to have a serum free testosterone level less than the median of the reference range for premenopausal women, and all AN subjects who were screened met this inclusion criterion. Subjects were excluded if they had an unstable medical illness or psychiatric illness, including severe current depressive symptoms (defined as a Hamilton Depression Rating Scale score >20, excluding two eating/weight loss items related to the symptoms of AN) or serious suicide risk. Subjects were also excluded if they had substance use disorder active within the 6 months prior to study enrollment, bipolar I disorder, or a psychotic disorder. All subjects were required to be followed by a treatment team and were permitted to start/change psychotropic drug regimens as clinically indicated, though all had been on stable psychotropic drug regimens for at least 6 weeks prior to enrollment. No subjects had received androgens or androgen precursors within the 3 months before study enrollment. All subjects were required to use a medically accepted means of contraception for the duration of the study; subjects taking estrogen had been on stable doses for at least 1 month prior to study enrollment.
Figure 1.
Flow of study participation. *Reasons for discontinuation: increased irritability, relapse of alcohol use disorder, and pregnancy.
Protocol
The study was approved by the Massachusetts General Hospital Institutional Review Board, and all subjects gave informed written consent before study participation. Study subjects were recruited through collaborating physicians and through advertisements. At the screening visit, a medical history and physical examination were performed. Nutritional evaluation, including weight in a gown and height, were measured, and percent ideal body weight and body mass index (BMI) were calculated. Blood was drawn for total and free testosterone levels, TSH, free T4 level, complete blood count, alanine aminotransferase (ALT), and creatinine, and a urine toxicology screen was performed. Eligible subjects returned for a baseline visit during which nutritional evaluation was repeated, a urine pregnancy test was performed, and blood was drawn for total and free testosterone, dehydroepiandrosterone sulfate (DHEAS), ALT, and lipid profile. Serum was frozen at −80°C for batched measurement of estradiol (for subjects not taking exogenous estrogen) and IGF-1 after study completion. Psychiatric disorders, including MDD and generalized anxiety disorder (GAD), were diagnosed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (35). The Hamilton Depression Rating Scale (HAM-D) (36) and Hamilton Anxiety Rating Scale (HAM-A) (37) were administered to all subjects to assess depression and anxiety symptom severity. Eating disorder psychopathology was measured by the Eating Disorder Examination-Questionnaire (EDE-Q) (38) and the Eating Disorder Inventory-2 (EDI-2) (39).
After completion of all baseline testing, participants were randomly assigned to receive transdermal testosterone (300 μg; Intrinsa; Procter & Gamble Pharmaceuticals, Cincinnati, OH) or placebo applied twice weekly for 24 weeks. Randomization assignment was blinded to investigators and subjects. A health care professional not involved with the study monitored free testosterone levels and implemented dose reductions from 300 μg to 150 μg in subjects with serum free testosterone levels above the upper limit of normal for women of reproductive age. To maintain blinding of subjects and investigators, a study subject receiving placebo was sham dose-reduced concurrently with each subject receiving testosterone. After completion of 24 weeks of treatment, subjects were tapered from one 300-μg patch applied twice weekly to one 300-μg patch applied weekly for 2 weeks before discontinuation. Subjects who had been dose-reduced to 150 μg were tapered from one 150-μg patch applied twice weekly to one 150-μg patch applied weekly for 2 weeks before discontinuation. Following discontinuation, subjects were monitored for an additional 8-week withdrawal period.
Subjects returned for study visits at 4, 12, 18, 24, and 32 weeks after the baseline visit. The primary outcome (BMI) and secondary outcomes (HAM-D, HAM-A, EDE-Q, and EDI-2 scores) were assessed at all visits. Safety evaluation was also performed at all visits. This included assessment of suicidality using the suicide module of the Eating Disorders Longitudinal Interval Follow-up Evaluation (40), assessment of aggression by the Buss-Perry Aggression Questionnaire (BPAQ) (41), Lorenzo hirsutism evaluation (42), urine pregnancy test, and blood tests for free testosterone, ALT, and lipid profile. At the 24-week visit, blood was drawn for total testosterone and DHEAS, and serum was frozen at −80°C for batched measurement of estradiol (for subjects not taking exogenous estrogen) and IGF-1 after study completion.
Laboratory methods
Total testosterone was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Mayo Clinic Laboratories, Rochester, MN), with a normal range of total testosterone for women of reproductive age of 8 to 60 ng/dL. Free testosterone concentration was calculated as the product of percent free testosterone, measured by equilibrium dialysis (Mayo Medical Laboratories, Rochester, MN), and total testosterone concentration, with a normal range of free testosterone for women of reproductive age of 0.3 to 1.9 ng/dL. Estradiol was measured by LC-MS/MS (Brigham Research Assay Core, Boston, MA), with a lower limit of detection of 1 pg/mL and intra-assay coefficient of variation <5%. IGF-1 was measured by the Immulite 2000 immunoassay analyzer (Siemens Healthcare, Malvern, PA). DHEAS was measured by LC-MS/MS (Endocrine Sciences, Calabasas Hills, CA). TSH, free T4, complete blood count, ALT, creatinine, and lipid profile were measured using standardized clinical methods.
Statistical analysis
JMP Statistical Discoveries (version 12.0; SAS Institute, Cary, NC) was used for statistical analyses of baseline clinical variables, within-group changes from baseline to 24 weeks, and side effects. SAS software (SAS Institute) was used for all other statistical analyses. Baseline mean clinical characteristics were compared by ANOVA after log transformation of variables. Within-group analyses were performed using paired t tests after log transformation of variables. The analysis of variables at 4 weeks was performed using the model in Diggle et al. (43), which is equivalent to an analysis of covariance with the baseline as a covariate. The model used at 24 weeks was a random slopes model with a fixed time and time-treatment interaction and a random intercept and time term. The term of primary interest was the time-treatment interaction that measures the difference in the mean slope between the treatment groups. Significance levels were not corrected for multiple comparisons. With 90 subjects, the chance of detecting a difference in the rate of BMI increase of 0.13 kg/m2/mo at a two-sided significance level of 0.05 was >85%. The estimates in the tables and text are average treatment differences calculated directly. Data from the 8-week withdrawal period is not reported given lack of significant treatment effects from baseline to 24 weeks. Fisher exact testing was used to compare rates of side effects between groups. Statistical significance was defined as a two-tailed P value ≤0.05. Data are reported as mean ± SD.
Results
Participant characteristics
Of 129 women who were assessed for eligibility, 90 were eligible and agreed to be randomized to receive testosterone or placebo (Fig. 1). Of the 43 subjects randomized to receive testosterone, 1 was excluded from analysis because she was lost to follow-up immediately after randomization and did not receive testosterone. Of the 47 subjects randomized to receive placebo, 1 was excluded from analysis because of laboratory abnormalities prior to initiation of study drug that made her ineligible to participate.
Clinical characteristics of the study subjects are shown in Table 1. There were no significant differences between the two groups in the variables tested, including mean pretreatment HAM-D score, HAM-A score, EDE-Q global score, and EDI-2 subscales (Table 2). Forty-two percent of subjects met criteria for atypical AN with BMI >18.5 kg/m2 (P = 1.00 for testosterone vs placebo), and 67% of subjects with atypical AN had a past history of low weight (BMI <18.5 kg/m2) (P = 0.29 for testosterone vs placebo). The percentage of subjects taking combined hormonal contraceptives or progestin-only contraceptives was similar between groups.
Table 1.
Baseline Characteristics
| Testosterone (n = 42) | Placebo (n = 46) | P Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 28 ± 7 | 27 ± 7 | 0.29 |
| Weight, kg | 49 ± 5 | 50 ± 6 | 0.43 |
| BMI, kg/m2 | 18.3 ± 1.8 | 18.3 ± 1.5 | 0.89 |
| Duration of AN, y | 12 ± 8 | 11 ± 7 | 0.93 |
| AN subtype: restricting/binge-purge, % | 67/33 | 72/28 | 0.65 |
| MDD, % | 64 | 59 | 0.66 |
| GAD, % | 74 | 67 | 0.64 |
| Psychiatric medication use, % | 71 | 76 | 0.64 |
| Current amenorrhea, % | 19 | 24 | 0.61 |
| Estrogen statusa: replete/deplete, % | 76/24 | 70/30 | 0.47 |
| Combination oral contraceptive pill use, % | 17 | 28 | 0.21 |
| Hormone measurements | |||
| Total testosterone, ng/dL | 26 ± 15 | 24 ± 14 | 0.42 |
| Free testosterone, ng/dL | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.85 |
Data are mean ± SD unless otherwise indicated.
Estrogen replete is defined as having menses or taking exogenous estrogen within the last 3 mo, whereas estrogen deplete is defined as having no menses or exogenous estrogen within the last 3 mo.
Table 2.
Psychometric and Behavioral Measures
| Testosterone | Placebo | P Valuea | |||
|---|---|---|---|---|---|
| Pretreatment | 24 wk | Pretreatment | 24 wk | ||
| Depression and anxiety symptom severity | |||||
| HAM-D scoreb | 15.2 ± 3.6 | 13.1 ± 5.5 | 15.5 ± 4.9 | 12.0 ± 6.0 | 0.72 |
| HAM-A scorec | 14.8 ± 5.5 | 10.8 ± 4.9 | 15.1 ± 5.3 | 10.8 ± 5.0 | 0.25 |
| Eating disorder symptoms/behaviors | |||||
| Vomiting over last 4 wk, number of episodes | 6 ± 18 | 7 ± 21 | 3 ± 7 | 3 ± 7 | 0.83 |
| Overexercise over last 4 wk, number of episodes | 6 ± 9 | 3 ± 6 | 5 ± 8 | 5 ± 8 | 0.05 |
| EDE-Q global score | 3.1 ± 1.3 | 2.9 ± 1.5 | 3.3 ± 1.3 | 2.8 ± 1.2 | 0.93 |
| EQE-Q subscales | |||||
| Dietary restraint | 3.0 ± 1.7 | 2.7 ± 2.0 | 3.0 ± 1.5 | 2.7 ± 1.4 | 0.63 |
| Eating concern | 2.5 ± 1.5 | 2.3 ± 1.5 | 2.7 ± 1.4 | 2.3 ± 1.4 | 0.57 |
| Weight concern | 3.2 ± 1.5 | 3.0 ± 1.6 | 3.6 ± 1.5 | 3.0 ± 1.5 | 0.90 |
| Shape concern | 3.8 ± 1.4 | 3.5 ± 1.7 | 3.9 ± 1.5 | 3.3 ± 1.4 | 0.93 |
| EDI-2 subscales | |||||
| Drive for thinness | 9.8 ± 6.3 | 9.3 ± 6.8 | 11.1 ± 6.8 | 9.6 ± 6.7 | 0.89 |
| Bulimia | 2.8 ± 4.6 | 1.8 ± 2.7 | 4 0.0 ± 5.8 | 2.6 ± 4.8 | 0.58 |
| Body dissatisfaction | 12.5 ± 7.1 | 10.6 ± 7.8 | 10.9 ± 6.5 | 10.0 ± 7.0 | 0.32 |
| Ineffectiveness | 7.8 ± 6.4 | 8.2 ± 7.0 | 9.1 ± 6.1 | 7.2 ± 5.7 | 0.52 |
| Perfectionism | 9.1 ± 4.8 | 7.1 ± 3.9 | 9.1 ± 5.2 | 8.4 ± 5.3 | 0.54 |
| Interpersonal distrust | 4.8 ± 3.7 | 3.8 ± 3.7 | 5.4 ± 4.1 | 4.4 ± 3.9 | 0.70 |
| Interoceptive awareness | 7.8 ± 5.9 | 6.0 ± 5.6 | 9.8 ± 6.9 | 7.9 ± 6.3 | 0.99 |
| Maturity fears | 5.4 ± 5.8 | 5.0 ± 5.6 | 5.6 ± 4.3 | 2.7 ± 3.2 | 0.22 |
| Asceticism | 6.7 ± 4.7 | 5.7 ± 4.7 | 8.5 ± 6.0 | 8.0 ± 6.4 | 0.84 |
| Impulse regulation | 4.3 ± 4.8 | 3.1 ± 4.8 | 5.7 ± 5.5 | 4.3 ± 5.2 | 0.75 |
| Social insecurity | 6.5 ± 4.3 | 6.4 ± 4.6 | 7.6 ± 4.3 | 6.1 ± 4.0 | 0.11 |
| BPAQd | |||||
| Physical aggression | 18.9 ± 8.7 | 17.8 ± 6.4 | 17.4 ± 4.9 | 17.3 ± 4.3 | 0.21 |
| Verbal aggression | 13.3 ± 5.4 | 12.5 ± 5.1 | 15.3 ± 5.1 | 14.2 ± 4.9 | 0.45 |
| Anger | 18.5 ± 7.3 | 17.3 ± 4.7 | 19.8 ± 7.3 | 19.2 ± 6.2 | 0.04 |
| Hostility | 24.1 ± 11.2 | 23.5 ± 10.6 | 24.2 ± 11.4 | 21.5 ± 9.7 | 0.40 |
For all scales/questionnaires, higher scores indicate worse symptom severity. Data are mean ± SD.
P value for comparison of change in psychometric or behavioral measure over 24 wk in subjects randomized to receive testosterone vs placebo.
HAM-D: 0–7, not depressed; 8–13, mild depression; 14–18, moderate depression; 19–22, severe depression; ≥23, very severe depression.
HAM-A: 0–17, mild anxiety; 18–24, moderate anxiety; ≥25, severe anxiety.
Performed as part of safety evaluation.
Hormone levels
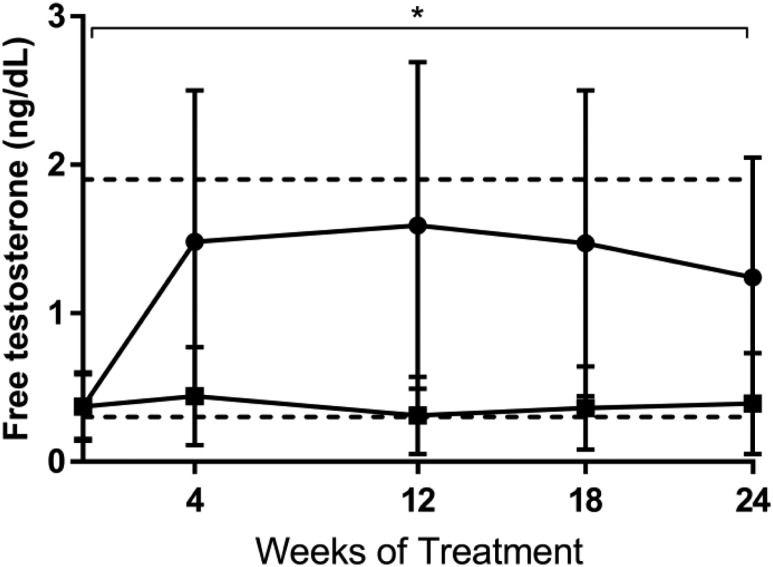
By study design, mean serum free testosterone at baseline was below the median for healthy women of reproductive age (Table 1, Fig. 2). In the testosterone group, serum free testosterone increased by 1.1 ± 0.9 ng/dL from 0 to 4 weeks and remained significantly higher than serum free testosterone in the placebo group during the 24 weeks of testosterone administration (P < 0.001; Fig. 2). Estradiol and IGF-1 levels did not change with testosterone administration vs placebo. Mean DHEAS level decreased by 4.0 μg/dL in the testosterone group and by 18.4 μg/dL in the placebo group (P = 0.05).
Figure 2.
Serum free testosterone in subjects receiving testosterone (black circles) or placebo (black squares). Horizontal dotted lines delineate the normal range of serum free testosterone for women of reproductive age. *P < 0.001 for the difference between groups over 24 wk. Error bars indicate SD.
Weight and mood symptoms
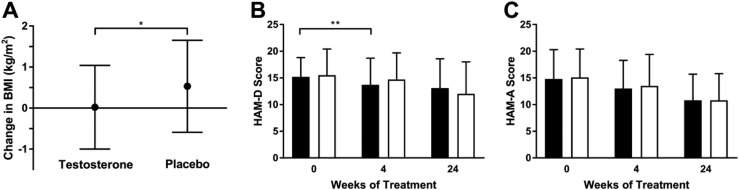
Mean BMI increased by 0.0 ± 1.0 kg/m2 in the testosterone group compared with 0.5 ± 1.1 kg/m2 in the placebo group over 24 weeks (P = 0.03; Fig. 3A). At 4 weeks, there was a trend toward a greater decrease in mean depression severity score by HAM-D score in the testosterone vs placebo group (−1.6 ± 2.8 vs −0.7 ± 3.0, P = 0.09; Fig. 3B) but no difference between groups in mean HAM-A score [−1.8 ± 3.1 (testosterone) vs −1.6 ± 3.1 (placebo), P = 0.59; Fig. 3C]. At 24 weeks, HAM-D and HAM-A scores had decreased similarly from baseline in testosterone and placebo groups [HAM-D: −2.9 ± 4.9 (testosterone) vs −3.0 ± 5.0 (placebo), P = 0.72, Table 2, Fig. 3B; HAM-A: −4.5 ± 5.3 (testosterone) vs −4.3 ± 4.4 (placebo), P = 0.25, Table 2, Fig. 3C]; these findings remained nonsignificant when including only subjects with MDD in the HAM-D analysis (P = 0.71) and only subjects with GAD in the HAM-A analysis (P = 0.78). Six women in the testosterone group and four women in the placebo group experienced a reduction in HAM-D score by >50% (P = 0.51). No subject experienced remission of depression (HAM-D score ≤7). Groups did not differ in the percentage of subjects who started or discontinued psychiatric medication [44% (testosterone) vs 43% (placebo); P = 1.00] or changed psychiatric medication dose [25% (testosterone) vs 34% (placebo); P = 0.44] during the study. One subject in the testosterone group was hospitalized for worsening depression and passive suicidality, and one subject in the placebo group was hospitalized after a suicide attempt (after completion of study drug taper).
Figure 3.
(A) Mean BMI increased by 0.0 ± 1.0 kg/m2 in the testosterone group and by 0.5 ± 1.1 kg/m2 in the placebo group over 24 wk (P = 0.03). (B) At 4 wk, there was a trend toward a greater decrease in mean depression severity score by HAM-D in the testosterone (black bars) vs placebo group (white bars) (P = 0.09). There was no difference in change in HAM-D scores between groups at 24 wk. (C) There was no difference in change in mean anxiety severity score by HAM-A between the testosterone (black bars) or placebo group (white bars) at 4 wk or 24 wk. Error bars indicate SD. *P < 0.05; **P < 0.1.
Within-group analyses demonstrated that over 24 weeks, BMI did not change within the testosterone group (P = 0.93) and increased within the placebo group (P = 0.01). Depression severity score by HAM-D and anxiety severity score by HAM-A decreased in both groups from baseline to 24 weeks (P < 0.001).
Eating disorder symptoms
Among all subjects, self-reported frequency of overexercise over the preceding 4 weeks decreased by a mean of 3.0 ± 7.7 episodes in the testosterone group vs 1.2 ± 5.7 episodes in the placebo group (P = 0.05; Table 2) at 24 weeks. There was no difference in mean self-reported frequency of vomiting between groups (P = 0.83; Table 2). There were no significant differences in the mean change in EDE-Q global score, EDE-Q subscales (dietary restraint, eating concern, shape concern, or weight concern), or EDI-2 scores between the testosterone and placebo groups over 24 weeks (Table 2). One subject in the testosterone group, and none in the placebo group, was hospitalized for weight loss.
Adverse events
Testosterone was generally well tolerated with no differences between groups in frequency of self-reported androgenic side effects (Table 3). Patch site irritation occurred in nearly one-third of subjects, with a similar frequency in the testosterone and placebo groups (Table 3). There were no significant changes in mean total cholesterol or lipoprotein levels in subjects receiving testosterone vs placebo. The mean BPAQ anger score decreased more in the testosterone group than placebo group at 24 weeks (P = 0.04; Table 2); otherwise, no significant differences in BPAQ scores between groups were seen. One subject in the testosterone group dropped out of the study because of increased irritability. Four subjects in the testosterone group and one subject in the placebo group were hospitalized during the study; reasons for hospitalization are described in Table 3.
Table 3.
Subject-Reported Adverse Events
| Testosterone | Placebo | P Value | |
|---|---|---|---|
| Patch site irritation | 12 (29) | 15 (33) | 0.82 |
| Increased acne | 14 (33) | 18 (39) | 0.66 |
| Increased oily skin | 3 (7) | 2 (4) | 0.67 |
| Increased facial hair growth | 7 (17) | 7 (15) | 1.00 |
| Increased depilatory rate | 2 (5) | 4 (9) | 0.68 |
| Increased irritability | 4 (10) | 3 (7) | 0.70 |
| Increased scalp hair loss | 2 (5) | 1 (2) | 0.60 |
| Hospitalization related to AN | 1a (2) | 0 (0) | 0.48 |
| Hospitalization unrelated to AN | 3b (7) | 1c (2) | 0.34 |
Data are n (%) unless otherwise indicated.
Reason for hospitalization was weight loss.
Reasons for hospitalizations were gastrointestinal symptoms (prior to initiation of study drug), worsening depression and passive suicidality, and pancreatitis.
Reason for hospitalization was suicide attempt (after completion of study drug taper).
Discussion
Effective therapies for women with AN targeting disordered eating psychopathology and/or comorbid affective symptoms are lacking. AN in women is characterized by relative testosterone deficiency, and we have reported an inverse association between androgen levels and depression and anxiety symptom severity in three separate cohorts of women with AN (20, 22, 23). Moreover, we have demonstrated a significant reduction in depression symptom severity with low-dose testosterone compared with placebo over a 3-week period (22). Given the known association between depression and anxiety symptoms and poor eating disorder outcomes (9–12), we hypothesized that a treatment such as testosterone targeting affective symptoms may improve eating disorder symptoms and lead to weight gain. This study examines whether long-term physiologic testosterone therapy improves weight, affective symptoms, and eating disorder psychopathology. Contrary to our hypothesis, we found that testosterone administration resulted in less weight gain, only modest and nonsustained improvements in depression, and no improvements in anxiety or disordered eating symptoms compared with placebo.
Although depression and anxiety symptoms improved in both women receiving testosterone and in women receiving placebo, we found that those who received testosterone demonstrated a marginally greater decrease in depression severity by HAM-D at 4 weeks. However, this effect was not sustained at 24 weeks. Our data are consistent with a randomized, placebo-controlled trial by our group evaluating 3 weeks of low-dose testosterone therapy in women with AN, which demonstrated a greater improvement in mood as measured by the Beck Depression Inventory (BDI) in the subgroup of depressed women who received testosterone vs placebo (22). Regarding sustained treatment effects, two longer-term studies of transdermal testosterone in women with severe androgen deficiency secondary to hypopituitarism (28) and in women status post–bilateral oophorectomy (27) demonstrated antidepressant effects of 300 μg testosterone. In our study of androgen-deficient women with hypopituitarism, depression severity scores by Beck Depression Inventory (BDI) were significantly lower after 12 months of testosterone treatment compared with placebo (28). In the study by Shifren et al. (27) of women who had undergone bilateral oophorectomy, 12 weeks of 300 μg of testosterone and conjugated equine estrogen treatment resulted in significant improvements in the composite score of the Psychological General Well-Being Index as well as the positive well-being and depressed mood subscales.
The reasons for the lack of sustained psychiatric effects of testosterone therapy in women with AN are unclear, but there are several factors in this study that may have contributed. Usual medical and psychiatric care was continued by subjects’ treatment teams for the duration of the study, which resulted in initiation or discontinuation of psychiatric medication in nearly half of all subjects and in psychiatric medication dose changes in nearly one-third of subjects. Although the frequency of these medication changes was not different between the testosterone and placebo groups, the sheer number may have attenuated any effects of testosterone that could have been seen if psychiatric medications were held constant. Additionally, given that prior studies of similar duration have shown treatment effects of testosterone in women with severe androgen deficiency (27, 28), we could speculate that normalization of free testosterone from low-normal values with physiologic testosterone administration is not sufficient or that a severe deficiency in testosterone is requisite for a therapeutic effect. It is also possible that we might have seen greater improvements in mood if we had only studied women who met criteria for MDD or who had more severe depression symptoms. Studies of men with depression, including treatment-refractory depression, have shown antidepressant benefits of testosterone (25, 26), and, in our study in which testosterone was administered to women with AN for 3 weeks, improvement in depression symptom severity was seen only in the subgroup of women who were depressed at baseline (22). Finally, we note that the lack of difference between the effect of testosterone and placebo likely reflects the lack of a strong therapeutic effect. Therefore, our data do not support testosterone therapy for treatment of depression or depressive symptoms in women with AN.
Aside from a modest decrease in the self-reported frequency of overexercise, we found that testosterone administration did not result in improvements in eating disorder psychopathology or behaviors. Similarly, mean BMI was unchanged in women receiving testosterone, whereas mean BMI increased minimally in women receiving placebo. The lack of sustained psychiatric effects makes these results less surprising if one hypothesizes that testosterone therapy may mediate weight and eating disorder symptoms through improvements in depression and anxiety. However, some studies have suggested a direct role of testosterone in eating disorder–specific changes in brain metabolism. For example, our group identified distinct loci of regional brain hypometabolism on fluorodeoxyglucose-positron emission tomography in women with AN compared with healthy controls and demonstrated increased metabolism in several of these areas, including to near normal in one area, after 3 weeks of low-dose testosterone administration (44). The increases in cerebral metabolism were independent of changes in mood; effects on eating disorder symptoms and weight were not reported.
Testosterone was well tolerated in this study and was not complicated by an increase in androgenic side effects compared with placebo. The most common side effect, patch site irritation, was equally prevalent among women receiving testosterone and women receiving placebo and therefore was likely related to ingredients other than the testosterone itself. One subject receiving testosterone dropped out of the study due to increased emotional irritability, although the overall self-reported frequency of irritability was <10% and equally distributed between the testosterone and placebo groups. Testosterone did not lead to increases in physical aggression, verbal aggression, or hostility and resulted in lower scores on the anger subscale of BPAQ compared with placebo.
In summary, our data demonstrate that low-dose testosterone therapy for 24 weeks in women with AN and relative androgen deficiency resulted in less weight gain and did not lead to sustained improvements in depression, anxiety, or disordered eating symptoms relative to placebo and therefore cannot be recommended to treat eating disorder, depressive, or anxiety symptoms in women with AN.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants R01MH083657, T32DK007028, K24HL092902, and K23MH092560. This work was also conducted with support from the Harvard Catalyst/The Harvard Clinical and Translational Science Center (Grants 1UL1TR001102 and 8UL1TR000170 from the National Center for Advancing Translational Science and 1UL1RR025758 from the National Center for Research Resources).
Clinical Trial Information: ClinicalTrials.gov no. NCT01121211 (registered 12 May 2010).
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- AN
anorexia nervosa
- BMI
body mass index
- BPAQ
Buss-Perry Aggression Questionnaire
- DHEAS
dehydroepiandrosterone sulfate
- EDE-Q
Eating Disorder Examination-Questionnaire
- EDI-2
Eating Disorder Inventory-2
- GAD
generalized anxiety disorder
- HAM-A
Hamilton Anxiety Rating Scale
- HAM-D
Hamilton Depression Rating Scale
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MDD
major depressive disorder
Additional Information
Disclosure Summary: Procter & Gamble provided the study medication at no cost. E.A.L. has served as a consultant for and has a financial interest in OXT Therapeutics, Inc. K.K.M. is the recipient of an investigator-initiated research grant from Amgen and receives study drug at no cost and support for assays from Marinus Pharmaceuticals. The remaining authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164(8):1259–1265. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. [DOI] [PubMed] [Google Scholar]
- 3. Herzog DB, Keller MB, Sacks NR, Yeh CJ, Lavori PW. Psychiatric comorbidity in treatment-seeking anorexics and bulimics. J Am Acad Child Adolesc Psychiatry. 1992;31(5):810–818. [DOI] [PubMed] [Google Scholar]
- 4. Kaye WH, Bulik CM, Plotnicov K, Thornton L, Devlin B, Fichter MM, Treasure J, Kaplan A, Woodside DB, Johnson CL, Halmi K, Brandt HA, Crawford S, Mitchell JE, Strober M, Berrettini W, Jones I. The genetics of anorexia nervosa collaborative study: methods and sample description. Int J Eat Disord. 2008;41(4):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halmi KA, Eckert E, Marchi P, Sampugnaro V, Apple R, Cohen J. Comorbidity of psychiatric diagnoses in anorexia nervosa. Arch Gen Psychiatry. 1991;48(8):712–718. [DOI] [PubMed] [Google Scholar]
- 6. Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215–2221. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association Work Group on Eating Disorders. Practice guideline for the treatment of patients with eating disorders (revision). Am J Psychiatry. 2000;157(1, Suppl):1–39. [PubMed] [Google Scholar]
- 8. Godart NT, Flament MF, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: a review. Int J Eat Disord. 2002;32(3):253–270. [DOI] [PubMed] [Google Scholar]
- 9. Bizeul C, Brun JM, Rigaud D. Depression influences the EDI scores in anorexia nervosa patients. Eur Psychiatry. 2003;18(3):119–123. [DOI] [PubMed] [Google Scholar]
- 10. Pollice C, Kaye WH, Greeno CG, Weltzin TE. Relationship of depression, anxiety, and obsessionality to state of illness in anorexia nervosa. Int J Eat Disord. 1997;21(4):367–376. [DOI] [PubMed] [Google Scholar]
- 11. Löwe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31(5):881–890. [DOI] [PubMed] [Google Scholar]
- 12. Fichter MM, Quadflieg N, Hedlund S. Twelve-year course and outcome predictors of anorexia nervosa. Int J Eat Disord. 2006;39(2):87–100. [DOI] [PubMed] [Google Scholar]
- 13. Attia E, Haiman C, Walsh BT, Flater SR. Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry. 1998;155(4):548–551. [DOI] [PubMed] [Google Scholar]
- 14. Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, Pike KM, Devlin MJ, Woodside B, Roberto CA, Rockert W. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA. 2006;295(22):2605–2612. [DOI] [PubMed] [Google Scholar]
- 15. Claudino AM, Hay P, Lima MS, Bacaltchuk J, Schmidt U, Treasure J. Antidepressants for anorexia nervosa. Cochrane Database Syst Rev. 2006;(1):CD004365. [DOI] [PubMed] [Google Scholar]
- 16. Bulik CM, Berkman ND, Brownley KA, Sedway JA, Lohr KN. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):310–320. [DOI] [PubMed] [Google Scholar]
- 17. Attia E, Mayer L, Killory E. Medication response in the treatment of patients with anorexia nervosa. J Psychiatr Pract. 2001;7(3):157–162. [DOI] [PubMed] [Google Scholar]
- 18. Monteleone P, Luisi M, Colurcio B, Casarosa E, Monteleone P, Ioime R, Genazzani AR, Maj M. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med. 2001;63(1):62–68. [DOI] [PubMed] [Google Scholar]
- 19. Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92(4):1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dichtel LE, Lawson EA, Schorr M, Meenaghan E, Paskal ML, Eddy KT, Pinna G, Nelson M, Rasmusson AM, Klibanski A, Miller KK. Neuroactive steroids and affective symptoms in women across the weight spectrum. Neuropsychopharmacology. 2018;43(6):1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawson EA, Misra M, Meenaghan E, Rosenblum L, Donoho DA, Herzog D, Klibanski A, Miller KK. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(4):1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab. 2005;90(3):1428–1433. [DOI] [PubMed] [Google Scholar]
- 23. Miller KK, Wexler TL, Zha AM, Lawson EA, Meenaghan EM, Misra M, Binstock AB, Herzog DB, Klibanski A. Androgen deficiency: association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry. 2007;68(6):959–965. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89(5):2085–2098. [DOI] [PubMed] [Google Scholar]
- 25. Pope HG Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(1):105–111. [DOI] [PubMed] [Google Scholar]
- 26. Perry PJ, Yates WR, Williams RD, Andersen AE, MacIndoe JH, Lund BC, Holman TL. Testosterone therapy in late-life major depression in males. J Clin Psychiatry. 2002;63(12):1096–1101. [DOI] [PubMed] [Google Scholar]
- 27. Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343(10):682–688. [DOI] [PubMed] [Google Scholar]
- 28. Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2006;91(5):1683–1690. [DOI] [PubMed] [Google Scholar]
- 29. Montgomery JC, Appleby L, Brincat M, Versi E, Tapp A, Fenwick PB, Studd JW. Effect of oestrogen and testosterone implants on psychological disorders in the climacteric. Lancet. 1987;1(8528):297–299. [DOI] [PubMed] [Google Scholar]
- 30. Sherwin BB. Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J Affect Disord. 1988;14(2):177–187. [DOI] [PubMed] [Google Scholar]
- 31. Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol. 1999;276(5):R1366–R1373. [DOI] [PubMed] [Google Scholar]
- 32. Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90(1):18–25. [DOI] [PubMed] [Google Scholar]
- 33. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 34. First MB, Williams JW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) Arlington: American Psychiatric Association; 2015. [Google Scholar]
- 35. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research; 2002. [Google Scholar]
- 36. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. [DOI] [PubMed] [Google Scholar]
- 38. Fairburn CG, Beglin S. Appendix B: Eating disorder examination questionnaire. In: Fairburn CG, ed. Cognitive Behavior Therapy and Eating Disorders. New York, NY: Guildford Press; 2008:309–314. [Google Scholar]
- 39. Fairburn CG, Cooper Z. Eating Disorder Inventory-2. Professional Manual Odessa: Psychological Assessment Research, Inc; 1991. [Google Scholar]
- 40. Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. [DOI] [PubMed] [Google Scholar]
- 41. Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63(3):452–459. [DOI] [PubMed] [Google Scholar]
- 42. Moncada E. Familial study of hirsutism. J Clin Endocrinol Metab. 1970;31(5):556–564. [DOI] [PubMed] [Google Scholar]
- 43. Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data New York: Oxford University Press; 1994. [Google Scholar]
- 44. Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, Klibanski A. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res. 2004;132(3):197–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.