Abstract
Background
Somatic mutations in the ubiquitin-specific peptidase 8 (USP8) gene are common in corticotropinomas of children with Cushing disease (CD). We report a unique patient with a germline USP8 mutation who presented with CD and a constellation of other findings that constitute an intriguing genetic syndrome.
Case Description
We describe a 16-year-old female with CD, developmental delay, dysmorphic features, ichthyosiform hyperkeratosis, chronic lung disease, chronic kidney disease, hyperglycemia, dilated cardiomyopathy with congestive heart failure, and previous history of hyperinsulinism and partial GH deficiency. She was diagnosed with CD at 14 years old and underwent transsphenoidal surgery. Despite initial improvement, she developed recurrent CD.
Methods
DNA was extracted from peripheral blood and tumor DNA; whole-exome and Sanger confirmatory sequencing were performed. Immunohistochemistry was performed on the resected adenoma.
Results
A de novo germline heterozygous USP8 mutation (c.2155T>C, p.S719P) in the critical 14-3-3 binding motif hot spot locus of the gene was identified in both the peripheral blood and tumor DNA. Histopathologic evaluation of the resected tumor confirmed an ACTH-secreting adenoma.
Conclusion
Somatic USP8 mutations are common in adenomas causing CD, but to date, no germline defects have been reported. We describe a patient with a de novo germline USP8 mutation with recurrent CD and multiple other medical problems. This unique patient informs us of the multitude of signaling events that may be controlled by USP8.
We report a patient with a de novo germline heterozygous USP8 gene mutation who presented with recurrent CD and other clinical features. Her presentation may characterize a new genetic syndrome.
Cushing disease (CD) describes the state of hypercortisolemia due to ACTH-producing pituitary adenomas, also known as corticotropinomas (1). These tumors are rare, especially in children; when they occur, they may be the result of a genetic defect at either the germline or somatic level (2, 3). Germline mutations leading to pediatric CD have been described in the MEN1, AIP, PRKAR1A, DICER1, CABLES1, and other genes (4–7). Nevertheless, the most frequent genetic defect in CD, both in pediatric and adult patients, are somatic mutations in the ubiquitin-specific peptidase 8 (USP8) gene, which are present in ∼20% to 60% of all corticotropinomas (8–13).
USP8 regulates epidermal growth factor receptor (EGFR) signaling by controlling its deubiquitination. Specifically, gain-of-function USP8 somatic mutations in corticotropinomas increase its deubiquitinating effect and thus the overall EGFR signaling activation, leading to enhanced POMC expression and ACTH secretion (8, 11). Notably, all USP8 somatic mutations associated with CD are located in the 14-3-3 binding motif (between amino acids 713 and 720) (8, 11).
In this report, we describe a unique patient that presented with developmental delay (DD), dysmorphic features, and other manifestations. She was also diagnosed with CD and multiple complications of hypercortisolemia, which only transiently improved after transsphenoidal surgery (TSS); she is currently under medical treatment. This patient has CD and a germline USP8 defect, and the constellation of her symptoms and signs likely represents a new genetic syndrome.
Subjects and Methods
Clinical protocol
The patient was referred to the National Institutes of Health (NIH) Clinical Research Center (CRC; protocol 97-CH-0076) at 16.5 years of age. All studies performed at CRC have been approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Previously obtained genetic testing was approved by the Rambam Health Care Campus Institutional Review Board. Informed consent was obtained from the patient’s parents.
DNA studies
Whole-exome sequencing (WES) was performed on peripheral blood-derived DNA (1 μg) from the patient, her parents, and her sister. Sequencing libraries were prepared using the Agilent Technologies SureSelect XT Human All Exon v6 kit and sequenced using an Illumina HiSeq sequencer (Illumina, San Diego, CA) to an average read depth of at least 120 times per individual. Raw sequence files were mapped to the reference genome (hg37), and variants were identified as previously described (14). An analysis of de novo variants was initially carried out, based on previous literature that de novo variants constitute the majority of events identified in clinical WES in patients in whom a genetic disorder is suspected and in the absence of family history suspicious for a recessive disorder, such as in our patient (15). All identified de novo variants are listed in an online repository (16). The WES data sets generated and analyzed during the current study are available from the Genetics Institute of Rambam Health Care Campus upon request to their Helsinki committee in accordance with their policies. To confirm and further investigate the results of WES, DNA from both peripheral blood and tumor samples was analyzed by Sanger sequencing for the identified USP8 defect using the PCR primers 5′-TCCTTCATCTGCACCTCCTT-3′ and 5′-TGGCTTCCTCTTCTCTTCCTC-3′, as previously described (10). Loss-of-heterozygosity studies for the USP8 locus were also performed as previously described (10). To assess the possibility of a mosaic mutation affecting certain tissues, including the pituitary gland, DNA was also extracted from multiple peripheral samples from the patient.
Tumor immunohistochemistry
Formalin-fixed and paraffin-embedded tissue samples from the patient’s resected pituitary adenoma were analyzed with hematoxylin and eosin routine staining. Additional immunohistochemistry was performed using the following antibodies and conditions: antihuman Ki-67 (clone MIB-1) antibody (1:200 dilution; Agilent Technologies, Santa Clara, CA), anti-ACTH antibody (rabbit polyclonal predilute; Roche Diagnostics, Indianapolis, IN), and p53 (clone DO-7) antibody (1:1000 dilution; Agilent Technologies). Procedures were performed in Ventana Ultra Autostainers (Roche Diagnostics). All images were obtained using a Keyence BZ-X710 microscope with a ×20 magnification and processed with the BZ-X Analyzer software (Keyence, Itasca, IL).
Results
Patient description
The patient presented to the NIH at the age of 16.5 years old with a complex medical history, including recurrent CD, DD, dysmorphic features, ichthyosiform hyperkeratosis, chronic lung disease with hypoxia, stage 2 to 3 chronic kidney disease with proteinuria, hyperglycemia, dilated cardiomyopathy with congestive heart failure, and previous history of hyperinsulinism and partial GH deficiency.
She was born at 39 weeks of gestation, with a birth weight of 2310 g and head circumference of 31 cm (both less than the third percentile for gestational age). Pregnancy was complicated by oligohydramnios and intrauterine growth retardation. At 3 months of age, she presented with severe nonketotic hypoglycemia (glucose: 28 mg/dL) with elevated C-peptide level, detectable insulin, and negative workup for metabolic disease; she responded to diazoxide therapy. Due to borderline GH level obtained at the time of hypoglycemia (GH: 7.1 ng/mL), the patient underwent a GH stimulation test using glucagon at the age of 6 months old. Her peak GH level was 4.2 ng/mL, so she was started on GH treatment. At an early age, she was also noted to have considerable delay in attaining developmental milestones. Brain imaging at that time revealed delayed myelinization without anatomic defects.
The patient was initially diagnosed with CD around the age of 14 years old, when she presented with increased weight gain, increased appetite, and hirsutism. Review of her growth chart shows increasing weight Z score from −1.1 SD score (SDS) at the age of 11.5 years old to −0.42 SDS at 14.4 years and decreasing height Z score from −2.8 SDS at 11.5 years to −4.8 SDS at 14.4 years old (Fig. 1). Biochemical evaluation was consistent with CD, with elevated 24-hour urinary-free cortisol (94 μg/24 h), midnight serum cortisol (19.7 μg/dL), and ACTH (33.6 pg/mL) levels. Imaging studies demonstrated a pituitary adenoma (Fig. 3A). During the evaluation for CD, she was also diagnosed with hyperglycemia (persistent after discontinuation of diazoxide) requiring insulin therapy, restrictive lung disease with day- and nighttime desaturations requiring oxygen supplementation, chronic kidney disease, proteinuria of unknown cause (with inconclusive kidney biopsy), and dilated cardiomyopathy with congestive heart failure. She underwent TSS and histopathology confirmed an ACTH-secreting adenoma (see Immunohistochemistry section). She experienced transient improvement of her symptoms after TSS with decrease of her weight (4 kg). However, 7 months later, she was noted to have recurrent CD, and she was then referred to the NIH for further evaluation.
Figure 1.
The patient’s diagnosis of Cushing syndrome was made based on the characteristic for the disease height stagnation and concurrent weight gain. Despite a slight decrease of her weight after the TSS (marked with arrow), the patient presented with recurrent weight gain. (A discrepancy of height at the evaluation performed at the NIH, compared with that previously obtained at referring institution, is due to difficulties in assessing the height at the last visit because the patient was not standing independently.)
Figure 3.
(A) MRI T1 postcontrast coronal images of the pituitary at the initial diagnosis and (B) at time of recurrence showed an enlarged pituitary gland with hypoenhancing lesions (red arrows); (C) the brain MRI revealed diffuse white matter atrophy with ventriculomegaly.
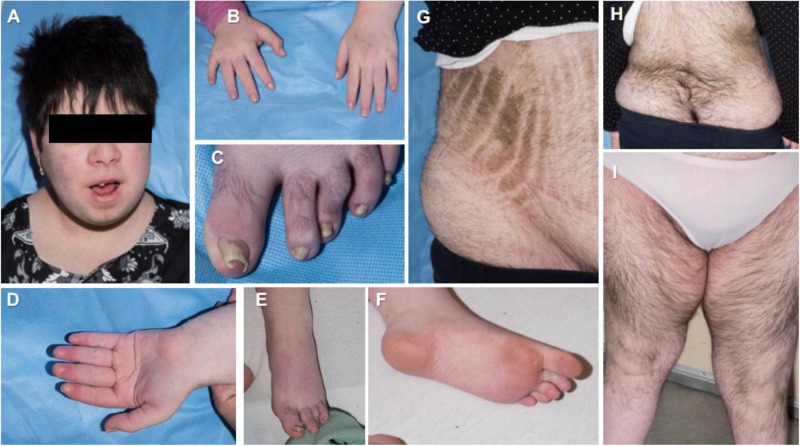
During her admission at NIH, she was noted to have several dysmorphic facial features, including downward palpebral fissures, mild esotropia, low-set and long ears, and small mouth and chin. Skeletal defects included high arch palate, genu valgum, pes planus, and scoliosis. She also had various integumentary manifestations including hypertrichosis, ichthyosiform hyperkeratosis over the abdomen and flank, dysplastic nails with onychomycosis, as well as findings consistent with CD (striae, hirsutism, and acne) (Fig. 2).
Figure 2.
Clinical features of the reported patient: (A) facial plethora and acne, typical of Cushing syndrome; dysmorphism with evident low-set and long ears; and a small mouth and chin; (B–F) multiple defects of the extremities, including dysplastic nails with onychomycosis and palmoplantar keratosis; (G) ichyosiform keratodermal plaques on the abdominal skin, complicated by Cushing syndrome–related striae; and (H and J) considerable hypertrichosis throughout the patient’s trunk and extremities.
Laboratory evaluation revealed loss of the circadian rhythm of cortisol secretion with elevated midnight cortisol (23.6 μg/dL) and ACTH levels (57.6 pg/mL). The patient responded to ovine CRH stimulation test with increase of ACTH by 64%, and she had sufficient suppression of cortisol after a high-dose dexamethasone suppression test (dose of 120 μg/kg) from 20.3 μg/dL to 1.8 μg/dL (decreased by 91%), both suggesting a pituitary source of hypercortisolemia. MRI of brain and pituitary showed a 9-mm hypoenhancing pituitary lesion; the brain MRI showed diffuse cerebral volume loss associated with ventriculomegaly (Fig. 3B and 3C).
Her cardiac evaluation revealed dilatation of the left ventricle with decreased ejection fraction at 38% and global ventricular hypokinesis. Cardiac catheterization confirmed the ventricular dilatation and revealed a patent foramen ovale. She was also found to have increased mean pulmonary arterial pressures. A CT of her chest demonstrated diffuse atelectasis, potentially partly due to chronic aspiration, contributing to her chronic hypoxia and oxygen requirements. Given her evaluation at the time, it was decided that the patient was not a surgical candidate until stabilization of her underlying cardiac and respiratory comorbidities; she was started on ketoconazole for control of hypercortisolemia.
DNA studies
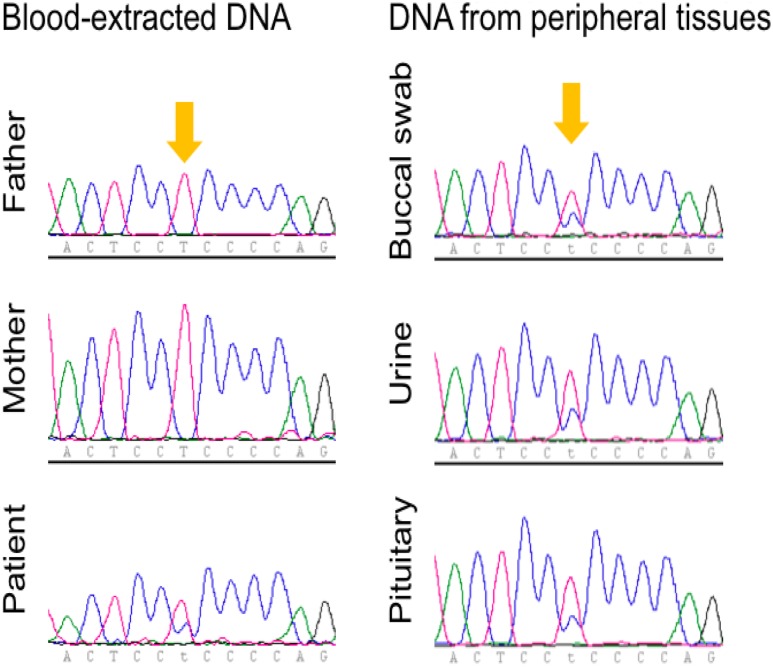
WES analysis of the patient’s peripheral blood-derived DNA identified the c.2155T>C, p.S719P variant in the USP8 gene. This variant was heterozygous based on identical number of reads sequenced for the wild-type (n = 31) and mutant (n = 31) alleles. A table with all de novo variants identified is included in an online repository (16). Sanger sequencing of peripheral and tumor DNA confirmed the presence of the USP8 defect in both. There was no loss of heterozygosity in the tumor, and the p.S719P variant was identified in normal pituitary tissue sampled from multiple sites, ruling out the possibility of a mosaic genetic defect (Fig. 4). DNA samples from both developmentally appropriate parents and her sister were negative for any USP8 gene defect (Fig. 4). Thus, WES and Sanger sequencing together confirm that the USP8 defect identified in the patient occurred de novo.
Figure 4.
Sanger sequencing of the USP8 hot spot in blood-extracted DNA from the patient and her parents (left) and in DNA extracted from various tissues of the patient (right). The locus of the USP8 variant c.2155T>C, p.S719P is indicated with yellow arrows.
Immunohistochemistry
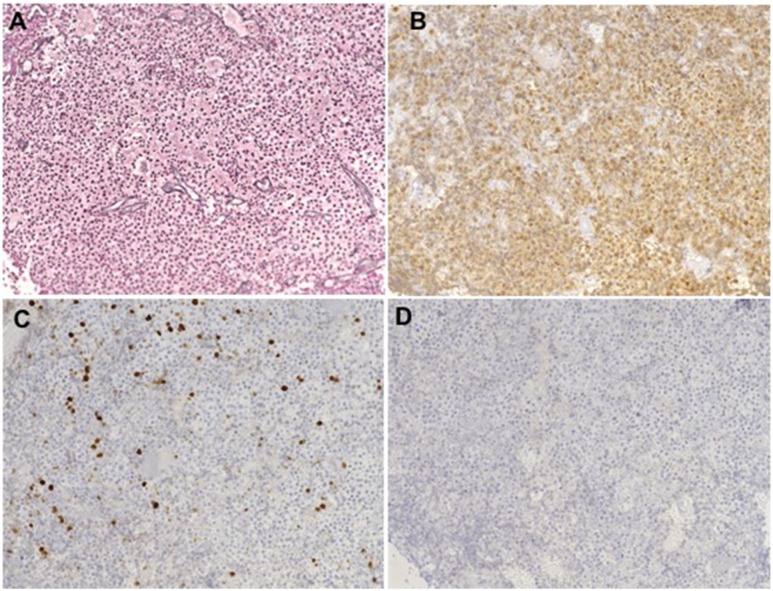
Immunohistochemical evaluation of the resected tumor is shown in Fig. 5. There was diffuse disruption of the reticulin network, consistent with pituitary adenoma, and strong ACTH staining confirming the presence of corticotroph cells in the tumor sample. The proliferation marker Ki-67 was present in 8% of the cells, suggesting a highly proliferating tumor. There was no TP53 immunoreactivity in the analyzed tumor sample.
Figure 5.
Immunohistochemistry analyses (×20 magnification) of the resected pituitary tumor revealed disruption of the (A) reticulin network and (B) strong ACTH staining. (C) The Ki-67 marker was present in 8% of the cells, and (D) TP53 was negative.
Discussion
In this report, we describe a unique patient with severe CD, DD, dysmorphic features, and other medical problems. The patient was found to have a de novo germline heterozygous USP8 mutation, which had been previously described in the somatic level (11). The constellation of findings in this patient represents a clinical genetic syndrome, presumably due to the identified USP8 defect. As expected, from the known involvement of USP8 in sporadic corticotropinomas causing CD, the patient also developed a severe form of CD, which was not surgically cured.
The p.S719P USP8 variant identified in our patient lies at the 14-3-3 binding motif of the gene, and its pathogenic effects have been tested in vitro (11). Specifically, although p.S719P mutant protein is expressed at similar levels as the wild-type protein, it fails to bind 14-3-3 proteins; these proteins normally lead to USP8 phosphorylation and inactivation. Thus, it presents as a gain-of-function mutation with increased deubiquitination and decreased degradation of its substrate (EGFR). These effects are similar to those of other frequent USP8 pathogenic mutations identified in corticotropinomas (11).
The prognosis of patients with somatic USP8 mutations differs in various studies. Our group and others have shown a potentially increased risk for recurrence in patients with somatic USP8 mutations; this has not been consistently reported in other studies (10, 13, 17). However, given the heterozygous germline carrier status of our patient, she apparently bears a more ominous prognosis because her genetic defect affects all the cells of the pituitary gland, and thus, presumably all corticotroph cells present with inappropriate ACTH secretion. The appearance of distinct adenomas may follow the natural history of the disease, similar to other conditions, in which a germline defect originally leads to hyperplasia of the gland and later to distinct adenomas (18). This assumption is very important for therapeutic decisions, because excision of the identified defect may not lead to sustainable cure of the patient. Further studies are required to provide evidence on the functional status of the normal corticotroph cells to make proper medical decisions.
The uncommon features presenting in our patient may either represent direct effects of the USP8 mutation on EGFR or other pathways or derive from long-standing hypercortisolemia. It seems plausible to presume that the dermatologic findings may be the result of aberrant EGFR action on the skin epidermis, where EGFR is known to stimulate the epidermal growth and differentiation of keratinocytes (19). Recent data also report a potential role for USP8 in insulin secretion, which may explain the presence of infantile hyperinsulinemia and later diabetes (although hypercortisolemia constitutes an obvious risk factor for the latter) (20). Further studies are required to provide insight on the specific effects of USP8 in various organ systems, such as cardiovascular, pulmonary, renal, etc.
In conclusion, germline USP8 defects constitute a cause of CD in pediatric patients. Our patient also presented with additional clinical features that together may characterize an intriguing genetic syndrome.
Acknowledgments
We thank Drs. Elena Belyavskaya and Charalampos Lyssikatos for coordinating successfully all of the complexities of the care of this (and other) patients. Additionally, we thank our patient and the family for participation in our studies, as well as the nursing and technical staff of the 1NW ward of the NIH CRC, without whom this study could never have been completed.
Financial Support: This study was funded in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Intramural Research Program and in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK112846 (to D.A.B.).
Disclosure Summary: C.A.S. holds patents on technologies involving PRKAR1A and related genes, and his laboratory has already received research funding from Pfizer Inc. unrelated to this subject investigation. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CD
Cushing disease
- CRC
Clinical Research Center
- DD
developmental delay
- EGFR
epidermal growth factor receptor
- NIH
National Institutes of Health
- SDS
SD score
- TSS
transsphenoidal surgery
- USP8
ubiquitin-specific peptidase 8
- WES
whole-exome sequencing
References and Notes
- 1. Lodish MB, Keil MF, Stratakis CA. Cushing’s syndrome in pediatrics: an update. Endocrinol Metab Clin North Am. 2018;47(2):451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117–123. [DOI] [PubMed] [Google Scholar]
- 3. Hannah-Shmouni F, Stratakis CA. An update on the genetics of benign pituitary adenomas in children and adolescents. Curr Opin Endocr Metab Res. 2018;1:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernández-Ramírez LC, Tatsi C, Lodish MB, Faucz FR, Pankratz N, Chittiboina P, Lane J, Kay DM, Valdés N, Dimopoulos A, Mills JL, Stratakis CA. Corticotropinoma as a component of Carney complex. J Endocr Soc. 2017;1(7):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D, Hamel N, Choi JH, Park SH, Deal CL, Kelsey MM, Dishop MK, Esbenshade A, Kuttesch JF, Jacques TS, Perry A, Leichter H, Maeder P, Brundler MA, Warner J, Neal J, Zacharin M, Korbonits M, Cole T, Traunecker H, McLean TW, Rotondo F, Lepage P, Albrecht S, Horvath E, Kovacs K, Priest JR, Foulkes WD. Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014;128(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernández-Ramírez LC, Gam R, Valdés N, Lodish MB, Pankratz N, Balsalobre A, Gauthier Y, Faucz FR, Trivellin G, Chittiboina P, Lane J, Kay DM, Dimopoulos A, Gaillard S, Neou M, Bertherat J, Assié G, Villa C, Mills JL, Drouin J, Stratakis CA. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr Relat Cancer. 2017;24(8):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 9. Perez-Rivas LG, Theodoropoulou M, Ferraù F, Nusser C, Kawaguchi K, Stratakis CA, Faucz FR, Wildemberg LE, Assié G, Beschorner R, Dimopoulou C, Buchfelder M, Popovic V, Berr CM, Tóth M, Ardisasmita AI, Honegger J, Bertherat J, Gadelha MR, Beuschlein F, Stalla G, Komada M, Korbonits M, Reincke M. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100(7):E997–E1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faucz FR, Tirosh A, Tatsi C, Berthon A, Hernández-Ramírez LC, Settas N, Angelousi A, Correa R, Papadakis GZ, Chittiboina P, Quezado M, Pankratz N, Lane J, Dimopoulos A, Mills JL, Lodish M, Stratakis CA. Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y, Li YM, Hu RG, Zhang ZY, Ye HY, Shen M, Shou XF, Li ZQ, Peng H, Wang QZ, Zhou DZ, Qin XL, Ji J, Zheng J, Chen H, Wang Y, Geng DY, Tang WJ, Fu CW, Shi ZF, Zhang YC, Ye Z, He WQ, Zhang QL, Tang QS, Xie R, Shen JW, Wen ZJ, Zhou J, Wang T, Huang S, Qiu HJ, Qiao ND, Zhang Y, Pan L, Bao WM, Liu YC, Huang CX, Shi YY, Zhao Y. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballmann C, Thiel A, Korah HE, Reis AC, Saeger W, Stepanow S, Köhrer K, Reifenberger G, Knobbe-Thomsen CB, Knappe UJ, Scholl UI. USP8 mutations in pituitary Cushing adenomas-targeted analysis by next-generation sequencing. J Endocr Soc. 2018;2(3):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Losa M, Mortini P, Pagnano A, Detomas M, Cassarino MF, Pecori Giraldi F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine. 2019;63(2):240–246. [DOI] [PubMed] [Google Scholar]
- 14. Chen A, Tiosano D, Guran T, Baris HN, Bayram Y, Mory A, Shapiro-Kulnane L, Hodges CA, Akdemir ZC, Turan S, Jhangiani SN, van den Akker F, Hoppel CL, Salz HK, Lupski JR, Buchner DA. Mutations in the mitochondrial ribosomal protein MRPS22 lead to primary ovarian insufficiency. Hum Mol Genet. 2018;27(11):1913–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, Veeraraghavan N, Hawes A, Chiang T, Leduc M, Beuten J, Zhang J, He W, Scull J, Willis A, Landsverk M, Craigen WJ, Bekheirnia MR, Stray-Pedersen A, Liu P, Wen S, Alcaraz W, Cui H, Walkiewicz M, Reid J, Bainbridge M, Patel A, Boerwinkle E, Beaudet AL, Lupski JR, Plon SE, Gibbs RA, Eng CM. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312(18):1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen M, Persky R, Stegemann R, Hernández-Ramírez LC, Zeltser D, Lodish MB, Chen A, Keil MF, Tatsi C, Faucz FR, Buchner DA, Stratakis CA, Tiosano D. Data from: Germline USP8 mutation associated with pediatric Cushing disease and other clinical features: a new syndrome. Figshare 2019. Deposited 2 May 2019. https://figshare.com/s/c5e68fe3b20f50905a0a. [DOI] [PMC free article] [PubMed]
- 17. Albani A, Pérez-Rivas LG, Dimopoulou C, Zopp S, Colón-Bolea P, Roeber S, Honegger J, Flitsch J, Rachinger W, Buchfelder M, Stalla GK, Herms J, Reincke M, Theodoropoulou M. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2018;89(4):454–458. [DOI] [PubMed] [Google Scholar]
- 18. Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney Complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006;9(3):203–209. [DOI] [PubMed] [Google Scholar]
- 19. Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10(7):505–510. [PubMed] [Google Scholar]
- 20. Pearson G, Chai B, Vozheiko T, Liu X, Kandarpa M, Piper RC, Soleimanpour SA. Clec16a, Nrdp1, and USP8 form a ubiquitin-dependent tripartite complex that regulates β-cell mitophagy. Diabetes. 2018;67(2):265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]