Abstract
Castration-resistant prostate cancer (PCa) almost invariably occurs after androgen deprivation therapy for metastatic disease and is driven in part by androgen synthesis within the tumor. 3β-hydroxysteroid dehydrogenase isoenzyme-1 catalyzes the conversion of adrenal precursor steroids into potent androgens essential for PCa progression. A common 1245 A→C missense-encoding single nucleotide polymorphism in HSD3B1 (rs1047303), the gene that encodes this enzyme, leads to a more stable protein that is resistant to degradation and thus increased production of potent androgens from adrenal precursors, facilitating castration-resistant PCa development. Consistent with this mechanism, this adrenal-permissive HSD3B1(1245C) genotype is associated with inferior outcomes after androgen deprivation therapy for advanced PCa, and increased sensitivity to pharmacologic blockade of adrenal precursors in metastatic disease. Herein, we review current knowledge of the mechanisms conferred by HSD3B1 genotype to alter androgen physiology and accelerate development of castration-resistant disease and its associations with clinical PCa outcomes. In light of its effect on steroid physiology, we also discuss its potential associations with non-PCa phenotypes.
Prostate cancer (PCa) is the most common noncutaneous cancer in men and a leading cause of death, with an estimated 174,650 new cases and 31,620 deaths in the United States in 2019 (1). Localized disease is managed with radical prostatectomy, radiation, or active surveillance (2). Metastatic PCa has been initially treated by androgen deprivation therapy (ADT), which blocks the generation of gonadal androgens (i.e., testosterone) that fuel PCa growth by way of medical castration with gonadotropin-releasing hormone agonists (e.g., goserelin, leuprolide) or antagonists (degarelix), or surgical castration via bilateral orchiectomy (3, 4). Recent reports from the STAMPEDE and LATITUDE clinical trials demonstrate a survival benefit of upfront adrenal androgen ablation along with ADT (5–7). Although metastatic PCa initially responds to ADT, recurrence and progression to fatal castration-resistant PCa (CRPC) is usually inevitable (8). Intratumoral androgen synthesis from adrenal precursor steroids is a key mechanism behind tumor progression, as demonstrated by the survival benefit seen after treatment with abiraterone, which blocks the generation of adrenal androgens and inhibits androgen synthesis in the tumor, and enzalutamide, which competes with intratumoral androgens (i.e., testosterone and DHT) for the androgen receptor (AR) (9–13). Further evidence for this hypothesis comes from the observation that several enzymes that convert adrenal androgens to testosterone and/or DHT have increased expression in castration-resistant disease (14). One such enzyme is 3β-hydroxysteroid dehydrogenase isoenzyme-1 (3β-HSD1, encoded by HSD3B1), which catalyzes the rate-limiting step of potent androgen synthesis from adrenal precursor steroids in peripheral tissues (e.g., prostate, breast, skin, placenta) (15, 16). Here, we discuss the impact of an A→C missense-encoding single nucleotide polymorphism (SNP) in HSD3B1 nucleotide position 1245 (clustered refSNP ID 1047303) to augment potent androgen synthesis from adrenal precursors and thus enable faster CRPC development. We review the well-characterized associations between HSD3B1 genotype at nucleotide 1245 and PCa clinical outcomes and consider its potential implications on additional phenotypes. We and others have referred to the HSD3B1(1245C) allele as the variant allele because it is the minor allele. However, HSD3B1(1245C) is also the allele in the HSD3B1 reference sequence (17, 18). Given its effect on steroid physiology and consistent effects on clinical outcomes, HSD3B1(1245C) will be described as the adrenal-permissive allele that enables conversion from dehydroepiandrosterone (DHEA) to downstream steroids and HSD3B1(1245A) as the adrenal-restrictive allele that limits metabolism from DHEA to downstream steroids, which we believe is a more useful terminology.
3β-HSD1 in the androgen synthesis pathway
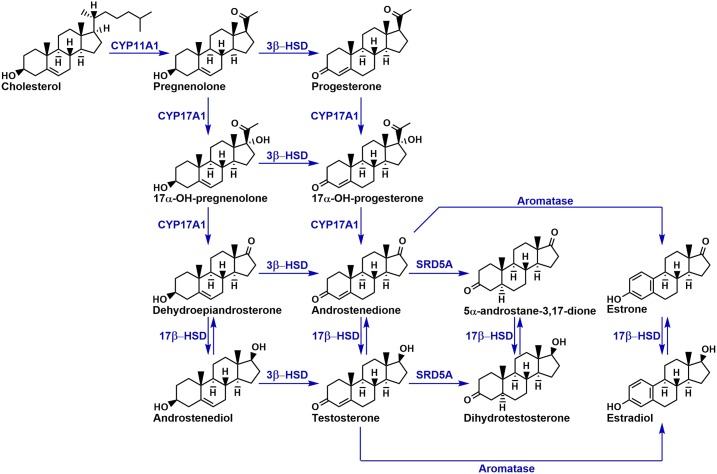
The androgen biosynthesis pathway is summarized in Figure 1. Androgen synthesis begins with the conversion of cholesterol to pregnenolone by cholesterol side-chain cleavage enzyme (CYP11A1) in the adrenal cortex and testes (19, 20). Of note, pregnenolone may be hydroxylated by 17α-hydroxylase/17,20-lyase (CYP17A1) to form 17-OH-pregnenolone, which is then converted into DHEA by the same enzyme. Notably, DHEA and its sulfated ester DHEAS are the most abundant steroid hormones in circulation (21). DHEA from the adrenal gland may be eventually converted to testosterone (T) and its 5α-reduced metabolite DHT, the most potent endogenous androgen, which can activate AR and thus stimulate PCa progression. Multiple routes to DHT synthesis exist, including the conventional adrenal pathway that goes through testosterone, the 5α-androstanedione pathway that uses adrenal precursors and circumvents testosterone, and the backdoor pathways (22, 23). Regardless of pathway, this process requires the activity of 3β-HSD, which oxidizes the 3β-hydroxyl group and isomerizes the double bond of Δ5-3β-OH-steroid-structures to create Δ4-3-keto-steroids (13). Thus, 3β-HSD has a crucial role in potent androgen production from adrenal precursors. This enzyme has two isoforms: type I (3β-HSD1, encoded by HSD3B1), which is present in peripheral tissues including the prostate, breast, skin, and placenta; and type II (3β-HSD2, encoded by HSD3B2), which is expressed in the adrenal gland and gonads. Both genes share a high degree of sequence similarity, but their tissue-specific and nonredundant expression is demonstrated in part by the observation that individuals with HSD3B2 mutations develop congenital adrenal hyperplasia despite possessing two normal copies of HSD3B1 (15).
Figure 1.
Overview of the androgen synthesis pathway. 3β-HSD serves to oxidize and isomerize Δ5-3β-OH steroids into Δ4-3-keto steroids and is essential for the generation of androgens from adrenal precursors.
Mechanism of the HSD3B1(1245C) adrenal-permissive genotype in augmented DHT synthesis and CRPC
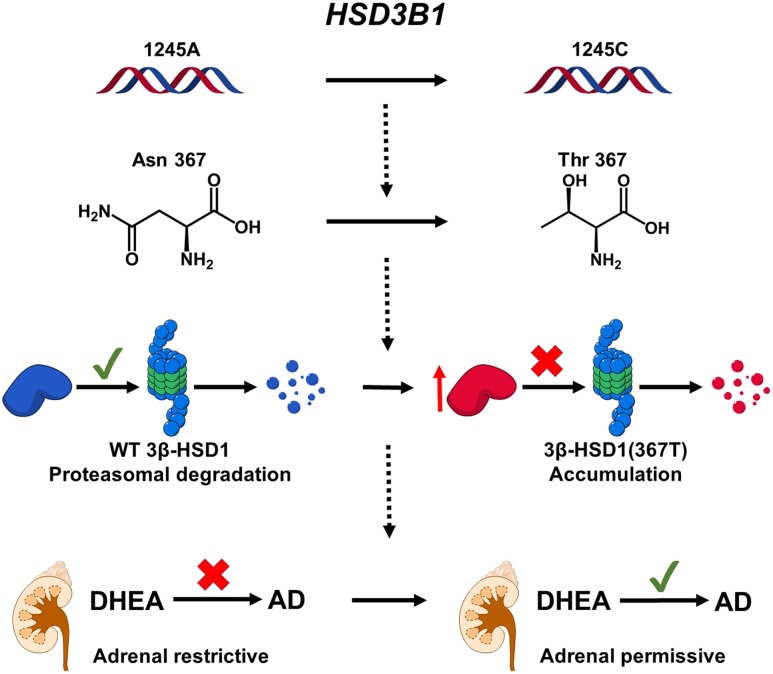
Our group mechanistically linked a germline single nucleotide polymorphism in HSD3B1 to CRPC (24). This A→C missense encoding change at HSD3B1 position 1245 encodes threonine in place of asparagine at amino acid 367. The resulting protein [3β-HSD1(367T)] was found to be resistant to AMFR-mediated ubiquitination and proteasome degradation, resulting in enzyme buildup. In agreement with 3β-HSD1’s function to convert DHEA to Δ4-androstenedione (AD) (Fig. 1), accumulation of 3β-HSD1(367T) from HSD3B1(1245C) resulted in increased production of AD and ultimately DHT from the adrenal precursor DHEA, resulting in an adrenal-permissive phenotype that allows more rapid development of CRPC. In contrast, the 3β-HSD1(367N) protein from HSD3B1(1245A) was readily degraded and thus incapable of robustly producing potent androgens from adrenal precursors, leading to an adrenal-restrictive phenotype (Fig. 2). The adrenal-permissive genotype occurs in CRPC as either a germline variant or a somatic mutation. The frequency of the germline 1245C allele varies widely with ancestry (34% European, 20% American, 16% South Asian, 9% African, and 8% East Asian) (25). Remarkably, the HSD3B1(1245C) allele was found to be selected for following androgen deprivation; 3/11 (27%) tumors from patients with CRPC with germline heterozygosity exhibited loss of heterozygosity with loss of HSD3B1(1245A) after ADT, and 3/25 (12%) of germline homozygous HSD3B1(1245A) CRPC tumors acquired the somatic mutation (24).
Figure 2.
Mechanism of HSD3B1 1245 A→C in the adrenal-permissive phenotype. A→C at HSD3B1 position 1245 results in Asn→Thr at amino acid 367. This change renders the enzyme resistant to ubiquitination and proteasome degradation, leading to protein buildup and increased flux from DHEA to AD. This leads to increased DHT synthesis and ultimately hastens progression to CRPC.
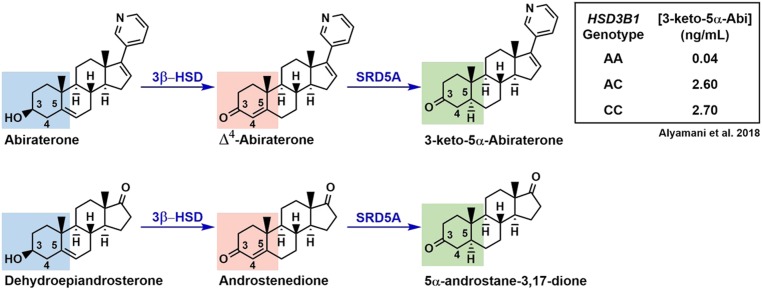
The adrenal-permissive HSD3B1(1245C) genotype’s effect on the metabolism of drugs that chemically mimic adrenal precursors further supports its effect on steroid physiology. As with other 3β-HSD substrates (pregnenolone, 17α-OH-pregnenolone, DHEA, and androstenediol), the CYP17A1 inhibitor abiraterone contains a Δ5-3β-OH structure. 3β-HSD1 converts abiraterone into Δ4-abiraterone (D4A), which is an AR antagonist in addition to a CYP17A1 inhibitor. D4A is subsequently metabolized into 3-keto-5α-abiraterone (5α-abi), which is actually an AR agonist, by steroid 5α-reductase (SRD5A) (26–28). This metabolism is analogous to the conversion of adrenal precursors into Δ4-3-keto steroids by 3β-HSD and these Δ4-3-keto steroids into 5α-reduced compounds by SRD5A (Fig. 3). Thus, individuals with the adrenal-permissive genotype, which increases flux of the Δ5-3β-OH–containing DHEA to the Δ4-3-keto–containing AD and subsequently the 5α-reduced 5α-androstanedione, would be expected to have elevated levels of 5α-abi following abiraterone treatment compared with those with the adrenal-restrictive genotype. Indeed, we found a progressive increase in 5α-abi levels with each copy of HSD3B1(1245C) (0.04 ng/mL 0 copies vs 2.60 ng/mL 1 copy vs 2.70 ng/mL 2 copies, P = 0.002) in CRPC patients receiving abiraterone after adjusting for pharmacokinetics (29).
Figure 3.
Effect of the adrenal-permissive genotype on abiraterone metabolism. Akin to its function to convert the Δ5-3β-OH structures of substrates such as DHEA into Δ4-3-keto steroids such as AD, 3β-HSD1 metabolizes abiraterone into D4A, which is then converted to 5α-abi by SRD5A. In agreement with the adrenal-permissive genotype’s effect to increase DHEA flux to 5α-reduced androgens, patients undergoing abiraterone treatment of CRPC who harbor HSD3B1(1245C) have in increased 5α-abi levels compared with those with the adrenal-restrictive one (29). The Δ5-3β-OH structure is shown in blue; Δ4-3-keto in red; and 5α-reduced in green.
Association of the HSD3B1 adrenal-permissive genotype with clinical PCa outcomes
Given the data mechanistically linking HSD3B1(1245C) to CRPC, it was hypothesized that men with PCa carrying the adrenal-permissive allele would show greater resistance to ADT because it enables tumor generation of testosterone and DHT from extragonadal precursor steroids (i.e., DHEA) compared with men who possess the adrenal-restrictive one. We tested the hypothesis that possession of the adrenal-permissive allele would be associated with quicker progression to CRPC and inferior metastasis-free survival (MFS) in three independent cohorts of total 443 patients (primary and validation cohorts with men treated with ADT for biochemical recurrence after radical prostatectomy, and a metastatic validation cohort) (30). In the primary cohort, possession of each copy of the adrenal-permissive allele was associated with stepwise inferior progression-free survival (PFS) from ADT (6.6 years 1245AA vs 4.1 years 1245AC vs 2.5 years 1245CC, P = 0.011). This trend was also found in the prostatectomy validation cohort (3.3 years 1245AA vs 2.8 years 1245AC vs 0.9 years 1245CC, P = 0.0022) and the metastatic group (1.8 years 1245AA vs 1.4 years 1245AC vs 0.8 years 1245CC). In the primary cohort, the adrenal-permissive genotype was also associated with distant MFS, with shorter MFS seen with each copy of the adrenal-permissive allele (9.1 years 1245AA vs 6.8 years 1245AC vs 3.6 years 1245CC, P = 0.014). Insufficient follow-up time in the prostatectomy validation cohort and the presence of metastasis already in the metastatic cohort prevented this analysis in these groups. An association between HSD3B1 genotype and overall survival (OS) was also observed in the primary and metastatic cohort.
The finding of inferior post-ADT PFS associated with HSD3B1(1245C) was independently validated in a study of 102 men with newly diagnosed metastatic PCa by Agarwal et al. (31). Men harboring the homozygous adrenal-permissive allele had worse PFS after ADT compared with those homozygous for the adrenal-restrictive one after adjusting for Gleason score and baseline prostate-specific antigen (PSA) [11 vs 21 months, respectively; hazard ratio (HR), 2.16; P = 0.046]. However, PFS did not differ significantly between men homozygous and heterozygous for the adrenal-permissive allele (19 vs 21 months, respectively; HR, 1.04; P = 0.86).
We also showed that the adrenal-permissive genotype is associated with inferior clinical outcomes after androgen deprivation for BCR following radiation therapy in a cohort of 213 men (32). Time to composite progression did not differ significantly across HSD3B1 allele (2.3, 2.3, and 1.4 years for those carrying 0, 1, and 2 1245C allele copies, respectively; P = 0.68). OS likewise did not vary significantly with HSD3B1 genotype (7.7, 6.9, and 7.2 years for those carrying 0, 1, and 2 1245C allele copies, respectively; P = 0.31). However, a significant decrease in MFS with each adrenal-permissive allele inherited was observed (7.4, 5.8, and 4.4 years for those carrying 0, 1, and 2 1245C allele copies, respectively; P = 0.03). Multivariable analysis controlling for Gleason score, baseline PSA, age, and prior ADT found a significantly increased HR of metastasis in the homozygous adrenal-permissive allele compared with the homozygous adrenal-restrictive one (adjusted HR, 2.01, P = 0.045), but no significant differences in time to progression or OS across adrenal-permissive genotype status. However, the effect of the adrenal-permissive genotype on progression and OS may be masked by treatment with AR antagonists, which may reduce the effect of DHT from adrenal precursors, in 56% of patients in this cohort. Further, that 49% of men in this cohort were treated with ADT at the time of radiation therapy may dilute any HSD3B1-dependent signal.
Shiota et al. (33) again validated the association between the adrenal-permissive genotype and worse post-ADT clinical outcomes in 104 Japanese men treated with AD for metastatic disease. Men possessing one or two copies of the adrenal-permissive allele had a higher HR of progression compared with homozygous wild-type men (HR, 2.16; P = 0.02), but not OS (HR, 1.3; P = 0.50). The low frequency of the adrenal-permissive allele in this Japanese cohort (6.7% 1245AC and 1.9% 1245CC) may explain why no differences in OS across adrenal-permissive genotype status were detected. Similarly, in a cohort of 44 Spanish men with PCa treated with ADT, Gil et al. (34) found the adrenal-permissive genotype to be associated with inferior PFS compared with the adrenal-restrictive one (24 vs 57 months, respectively, P = 0.038). Further, a post hoc analysis of 197 patients in a randomized phase 3 clinical trial (35) shows that inheritance of one or two copies of the adrenal-permissive allele is associated with more rapid development of CRPC and shorter OS in men with low-volume metastatic PCa treated with ADT ± docetaxel (36). Taken together, these validated findings in independent cohorts of multiple ancestries indicate that the adrenal-permissive genotype is a predictive biomarker of poor response to ADT in those with low volume metastatic disease or BCR after prostatectomy or RT.
The finding that CRPC appears to rely on extragonadal steroids for androgen synthesis in the adrenal permissive genotype gives rise to the hypothesis that men with CRPC harboring it would exhibit greater sensitivity to and superior clinical outcomes with adrenal precursor blockade. We examined this hypothesis in 90 men with metastatic CRPC treated with ketoconazole, which reduces these adrenal precursor steroids by inhibiting CYP17A1 (Fig. 1) (37). A stepwise increase in length of ketoconazole therapy was found with each copy of the adrenal-permissive 1245C allele (5.0 months, 0 copies; 7.5 months, 1 copy; and 12.3 months, 2 copies; P = 0.01). Time to progression likewise progressively improved with inheritance of the adrenal-permissive allele (5.4 months, 0 copies; 9.7 months, 1 copy; and 15.2 months, 2 copies; P = 0.03). These results suggest that HSD3B1 genotype may serve as a biomarker of susceptibility to adrenal precursor inhibition.
Abiraterone inhibits CYP17A1 and thus synthesis of adrenal androgen precursors, but also has increased conversion in the adrenal-permissive genotype to the downstream metabolite 5α-abi that can stimulate AR and promote tumor growth (27, 29). Thus, the adrenal-permissive genotype would be expected to either improve or worsen clinical outcomes of abiraterone treatment of CRPC depending on the relative contributions of the adrenal-permissive allele on adrenal androgens compared with abiraterone metabolism. Hahn et al. (38) investigated the effect of the adrenal-permissive genotype on PFS in 76 metastatic CRPC patients treated with upfront abiraterone. No statistically significant difference in PFS was found comparing men possessing one or two copies of the adrenal-permissive allele to men homozygous for the adrenal-restrictive allele (HR, 0.87; P = 0.64, and HR, 1.68; P = 0.28, respectively). On the other hand, after adjusting for baseline PSA, metastasis stage, and prior drug treatment, Shiota et al. (33) found that the heterozygous genotype is associated with a lower HR of treatment failure (HR, 0.35; P = 0.01) and all-cause mortality (HR, 0.40; P = 0.04) compared with the homozygous adrenal-restrictive genotype in 99 Japanese metastatic CRPC patients (33). This finding implies that abiraterone’s effect to ablate adrenal precursors and thus reduce DHT appears to outweigh AR agonism by increased 5α-abi in men with the adrenal-permissive genotype, similar to the findings observed in nonsteroidal CYP17A1 inhibition by Almassi et al. (37). However, abiraterone metabolite levels were not measured in either study.
Role of the adrenal-permissive HSD3B1(1245C) allele in non-PCa phenotypes
The mechanistic data demonstrating enhanced DHT synthesis in the adrenal-permissive genotype along with its reproducible association with inferior PCa outcomes in the absence of gonadal T raise the question of whether associations between HSD3B1(1245C) and additional phenotypes attributable to increased flux from adrenal precursors exist. Studies have identified associations between the adrenal-permissive genotype and mammographic density and alopecia. An association between and hypertension has been explored, but the mechanism and results are unclear.
Mammary gland tissue is sensitive to hormonal changes. Androgens inhibit mammary epithelial cell proliferation (39), whereas estrogens promote it (40). Mammographic density (MD), which refers to the percentage of mammogram with radiodense breast tissue and is an established predictor of breast cancer, is accordingly influenced by hormones (41). Because the adrenal-permissive genotype results in enhanced androgen synthesis from adrenal precursors, it is possible that possession of this genotype would lead to decreased mammary cell proliferation and thus be associated with decreased MD. However, because androgens may also be converted into mammary-stimulating estrogens via aromatase (Fig. 1), it may also be hypothesized that HSD3B1(1245C) would be associated with increased MD. Three studies have generally found a negative association between the adrenal-permissive genotype and MD.
Haiman et al. (42) examined the relationship between polymorphisms in steroid biosynthesis genes and MD in 396 African-American and Caucasian women. An association between the adrenal-permissive genotype and MD was found, but only after race-specific analysis. African-American women who were heterozygous or homozygous for the HSD3B1(1245C) allele had increased MD compared with those homozygous for the HSD3B1(1245A) allele (difference of +5.9% or +5.1%, respectively; P = 0.02), whereas Caucasian women harboring this allele had decreased MD compared with wild-type (difference of −5.5% for 1245 AC and −19.7% for 1245 CC, P = 0.04). However, the validity of the analysis among African-American women may be limited by the small sample size (n = 4 for 1245 CC). No significant differences were observed when the analysis was limited to premenopausal women or stratified by estrogen plus progestin hormone replacement therapy (HRT) status.
The finding of decreased MD in individuals harboring the adrenal-permissive genotype has been replicated in additional studies. In a twins and sister study of 457 predominantly Caucasian women, Stone et al. (43) found an average stepwise decrease in MD of 3.47% with each additional copy of HSD3B1(1245C) when the entire cohort was analyzed together cross-sectionally (P = 0.035). A stronger association but with lower statistical significance was found when only premenopausal women (n = 150) were included (β = −4.88, P = 0.07). The association remained when the analysis was limited to only pairs of related sisters. Biong et al. (44) validated this negative association in a study of 454 postmenopausal Norwegian women. A correlation was found between the adrenal-permissive genotype and MD in individuals undergoing HRT (P = 0.0343 in a discovery cohort and P = 0.0877 in a validation cohort). A relationship between HSD3B1(1245C) and testosterone levels in individuals not treated with HRT was also reported (P = 0.054 discovery and P = 0.040 validation), which is in accordance with its ability to increase androgen synthesis.
In summary, interpretation of the data on MD is challenged by the fact that some studies accounted for menopausal status, whereas others did not. One might anticipate that, just as the effect of adrenal-permissive and adrenal-restrictive HSD3B1 genotypes in prostate cancer is dependent on the presence or absence of gonadal testosterone (i.e., castration), any effect that enables adrenal conversion to sex steroids in breast tissue should similarly be colored by the presence or absence of ovarian estrogens.
Androgenic alopecia, also known as male/female pattern hair loss, describes hair loss in the front and top of scalp. This condition is caused in part by the action of DHT at the dermal papillae (45). The adrenal-permissive genotype, which results in increased DHT synthesis from adrenal precursor steroids, may thus be expected to be positively associated with androgenic alopecia. Indeed, Tu et al. (46) described an association between the adrenal-permissive genotype and female pattern hair loss among women with polycystic ovarian syndrome (PCOS). HSD3B1 genotype was determined in 472 Taiwanese women diagnosed with PCOS, and the associations with several PCOS phenotypes were evaluated. Women with female pattern hair loss were more likely to possess the HSD3B1 1245 AC or CC genotypes compared with those without hair loss (18.8% vs 10.7%, respectively; P = 0.068). After stratifying by weight status, overweight women carrying the adrenal-permissive allele had a statistically significantly higher risk of having female pattern hair loss (odds ratio, 3.53; P = 0.009), whereas those with normal weight carrying the allele did not did not (OR, 1.087; P = 0.88). The association between HSD3B1 genotype status and female pattern hair loss remained after adjusting for age, body mass index, hypertension (HTN), and certain laboratory values (OR, 2.312; P = 0.021).
3β-HSD catalyzes the synthesis of precursors that may be converted into the mineralocorticoid aldosterone, which is secreted by the adrenal cortex and leads to water retention and thus increased blood pressure. HSD3B2, not HSD3B1, is expressed specifically in the adrenal cortex; thus, polymorphisms in HSD3B2, but not HSD3B1, may be expected to be associated with HTN. However, this notion has been challenged by the observation that HSD3B1 expression is found in some aldosterone-secreting tumors (47). This suggests that 3β-HSD1 may be present in the adrenal cortex and thus that the adrenal-permissive genotype may increase aldosterone synthesis and lead to HTN. Studies investigating the association between HSD3B1(1245C) and HTN have conflicting results. Shimodaira et al. (48) found that the diplotype formed by the adrenal-permissive genotype and HSD3B1 SNP rs3088283 was associated with HTN and elevated aldosterone levels in a study of 275 Japanese patients, and Tripodi et al. (49) found a substantial association between the adrenal-permissive genotype and systolic blood pressure in a cohort of 729 individuals with untreated HTN. On the other hand, Verwoert et al. (50) found no association between the adrenal-permissive genotype and HTN (β = 0.73, P = 0.24) in a genetic association meta-analysis of total 11,192 Dutch individuals. Further, the authors demonstrated minimal HSD3B1 expression in aldosterone-secreting adenomas compared with HSD3B2.
Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate gland and the most common cause of lower urinary tract symptoms. Prostate enlargement is thought to be due to increased DHT synthesis (51). Therefore, the adrenal-permissive genotype, which augments DHT production, may be hypothesized to be associated with BPH. Roberts et al. (52) investigated the association between SNPs in androgen biosynthesis and BPH in 510 Caucasian men. Surprisingly, the adrenal-permissive allele was associated with decreased risk of prostate enlargement vs the adrenal-restrictive one (HR, 0.79; P = 0.04), although this effect did not remain after adjusting for DHT inactivation polymorphism genes.
Can HSD3B1 genotype status explain the discrepancies in DHEA supplementation outcomes?
DHEA and DHEAS together have the highest circulating levels of any steroid hormone and decline progressively with age (21). These observations, in conjunction with evidence that low levels are associated with aging in animal models, have prompted investigation into whether supplementation can affect clinical outcomes in individuals with low DHEA levels (53). Initial observational studies identified an association between low DHEA levels and multiple diseases, including cardiovascular and ischemic heart disease (54) and premenopausal breast cancer (55). Randomized trials of DHEA supplementation, however, are inconsistent or demonstrate only a modest benefit on clinical outcomes (53). Arlt et al. (56) randomized 24 women with adrenal insufficiency to receive either 4 months of 50 mg DHEA or placebo. DHEA supplementation lead to statically significant improvement of sexual thoughts, interest, and satisfaction compared with control. Beaulieu et al. (57) randomized 280 healthy individuals to 50 mg DHEA or placebo and found some benefits only among women: increase in bone turnover and libido among older women and improved skin status. Nair et al. (58) discovered no effect of DHEA supplementation on body composition or quality of life in a trial of 57 elderly women and 87 elderly men with low DHEAS randomized to receive 50 mg DHEA (women)/75 mg DHEA or 5 mg T (men) or placebo. In contrast, Villareal and Holloszy found DHEA supplementation to decrease subcutaneous and visceral fat and increase insulin sensitivity in a randomized trial of 58 elderly men and women assigned to either 50 mg DHEA or placebo (59).
The mechanism behind DHEA’s potential benefits has not been fully elucidated, but may involve an agonistic or antagonistic effect of DHEA itself or its metabolites on multiple receptors, including those for PPARα, estrogen, neurotransmitters, and TRPV-1 (53). That the adrenal-permissive genotype increases flux from DHEA raises suspicion that it could alter levels of the hormone or its metabolites and thus affect outcomes of DHEA supplementation studies. Importantly, the population frequency of the adrenal-permissive genotype differs by ethnicity, with Europeans having the highest frequency (∼34%) and East Asians and Africans the lowest (∼8% or less) (25). The adrenal-permissive genotype’s effect on clinical outcomes may also differ by ethnicity. For instance, Haiman et al. (42) found opposite effects of the HSD3B1(1245C) on MD on African-American and Caucasian women, although the sample size of African-American women was small. Oncologic outcomes associated with the adrenal-permissive genotype also vary with ethnicity. Overall, these observations suggest that stratification by ancestry and HSD3B1 genotype status may explain the discrepancy between DHEA supplementation studies and should be taken into account in future DHEA trials.
Conclusion
HSD3B1(1245 A→C) is a well-studied missense polymorphism that encodes a more stable enzyme resistant to ubiquitin-mediated degradation, leading to an adrenal-permissive phenotype in which adrenal precursor steroids are converted into androgens that drive CRPC. This adrenal-permissive genotype has been associated with both inferior outcomes following ADT in cohorts of multiple ethnicities and superior outcomes after adrenal androgen ablation. Because 3β-HSD1 plays a critical role in converting adrenal precursors to sex steroids, which confer multiple effects, it appears likely that HSD3B1(1245C) alters additional phenotypes. Three studies have linked the adrenal-permissive genotype with MD, although the mechanism has not yet been elucidated. In line with its effect on increased androgen synthesis, one study found an association with alopecia in overweight women with PCOS. It has been hypothesized that the adrenal-permissive genotype could contribute to HTN via increased aldosterone production, but results are inconsistent and in disagreement with the fact that HSD3B2, not HSD3B1, is predominantly expressed in the adrenal cortex. The population frequency of the adrenal-permissive allele differs by ancestry, with the highest frequency occurring in Europeans and the lowest in Africans and Asians. This remarkable difference may explain the inconclusive results of DHEA supplementation studies, and suggests that future DHEA investigations should incorporate ancestry and HSD3B1 genotype status. In-depth explorations of additional phenotypes associated with HSD3B1(1245C) are warranted.
Acknowledgments
Financial Support: This work is supported by grants from the National Cancer Institute (R01CA168899, R01CA172382, R01CA190289) and the Prostate Cancer Foundation (to N.S.).
Glossary
Abbreviations:
- 3β-HSD1
3β-hydroxysteroid dehydrogenase isoenzyme-1
- 5α-abi
3-keto-5α-abiraterone
- AD
androstenedione
- ADT
androgen deprivation therapy
- AR
androgen receptor
- BPH
benign prostatic hyperplasia
- CRPC
castration-resistant prostate cancer
- D4A, Δ
4-abiraterone
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- HR
hazard ratio
- HRT
hormone replacement therapy
- HTN
hypertension
- MD
mammographic density
- MFS
metastasis-free survival
- OS
overall survival
- PCa
prostate cancer
- PCOS
polycystic ovarian syndrome
- PFS
progression-free survival
- PSA
prostate-specific antigen
- SNP
single nucleotide polymorphism
- SRD5A
steroid 5α-reductase
- T
testosterone
Additional Information
Disclosure Summary: N.S. has been a paid consultant for Janssen and Pfizer. The remaining author has nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, Drake CG, de Bono JS. Prostate cancer. Lancet. 2016;387(10013):70–82. [DOI] [PubMed] [Google Scholar]
- 3. Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010;17(4):R305–R315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. [DOI] [PubMed] [Google Scholar]
- 7. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. [DOI] [PubMed] [Google Scholar]
- 8. Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67(3):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI; COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 12. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B; PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7(9):a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. [DOI] [PubMed] [Google Scholar]
- 15. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26(4):525–582. [DOI] [PubMed] [Google Scholar]
- 16. Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology. 2010;151(8):3514–3520. [DOI] [PubMed] [Google Scholar]
- 17. National Center for Biotechnology Information. Homo sapiens hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (HSD3B1), RefSeqGene on chromosome 1. https://www.ncbi.nlm.nih.gov/nuccore/1039673932. Accessed 5 July 2019.
- 18. National Center for Biotechnology Information. NM_000862.3(HSD3B1):c.1100C= (p.Thr367=). https://www.ncbi.nlm.nih.gov/clinvar/variation/620577/#summary-evidence. Accessed 5 July 2019.
- 19. Auchus RJ. The physiology and biochemistry of adrenarche. Endocr Dev. 2011;20:20–27. [DOI] [PubMed] [Google Scholar]
- 20. Sharifi N. Minireview: androgen metabolism in castration-resistant prostate cancer. Mol Endocrinol. 2013;27(5):708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arlt W. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab. 2004;18(3):363–380. [DOI] [PubMed] [Google Scholar]
- 22. Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108(33):13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharifi N. Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology. 2013;154(11):4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, Mirzaei H, Auchus RJ, Sharifi N. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. IGSR: The International Genome Sample Resource. Genome browsers. http://www.internationalgenome.org/1000-genomes-browsers/. Accessed 5 July 2019.
- 26. Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ, Sharifi N. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523(7560):347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Alyamani M, Li J, Rogacki K, Abazeed M, Upadhyay SK, Balk SP, Taplin ME, Auchus RJ, Sharifi N. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alyamani M, Li Z, Berk M, Li J, Tang J, Upadhyay S, Auchus RJ, Sharifi N. Steroidogenic metabolism of galeterone reveals a diversity of biochemical activities. Cell Chem Biol. 2017;24:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alyamani M, Emamekhoo H, Park S, Taylor J, Almassi N, Upadhyay S, Tyler A, Berk MP, Hu B, Hwang TH, Figg WD, Peer CJ, Chien C, Koshkin VS, Mendiratta P, Grivas P, Rini B, Garcia J, Auchus RJ, Sharifi N. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Invest. 2018;128(8):3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hearn JWD, AbuAli G, Reichard CA, Reddy CA, Magi-Galluzzi C, Chang KH, Carlson R, Rangel L, Reagan K, Davis BJ, Karnes RJ, Kohli M, Tindall D, Klein EA, Sharifi N. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17(10):1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3(6):856–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hearn JWD, Xie W, Nakabayashi M, Almassi N, Reichard CA, Pomerantz M, Kantoff PW, Sharifi N. Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 2018;4(4):558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shiota M, Narita S, Akamatsu S, Fujimoto N, Sumiyoshi T, Fujiwara M, Uchiumi T, Habuchi T, Ogawa O, Eto M. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw Open. 2019;2(2):e190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gil SG, Rodríguez RR, Bello AP, Nazco-Casariego GJ, Marrero RG, Jurado JC, Batista López JN, García JG, Nicolás FG. Relationship between mutations in the HSD3B1 gene and response time to androgen deprivation therapy in the treatment of prostate cancer. In Proceedings of the 4th European Conference of Oncology Pharmacy; 25–27 October 2018; Nantes, France. Abstract 107.
- 35. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hearn JW, Sweeney CJ, Almassi N, Reichard CA, Reddy C, Hobbs B, Jarrard DF, Chen Y-H, Dreicer R, Garcia J, Carducci MA, DiPaola RS, Sharifi N. HSD3B1 and overall survival (OS) in men with low-volume (LV) metastatic prostate cancer (PCa) treated with androgen deprivation therapy (ADT) or chemohormonal therapy in the CHAARTED Randomized trial. In Proceedings of the ASCO Annual Meeting 2019; 31 May–4 June 2019; Chicago, IL. Abstract 5020.
- 37. Almassi N, Reichard C, Li J, Russell C, Perry J, Ryan CJ, Friedlander T, Sharifi N. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 2018;4(4):554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hahn AW, Gill DM, Nussenzveig RH, Poole A, Farnham J, Cannon-Albright L, Agarwal N. Germline variant in HSD3B1 (1245 A > C) and response to abiraterone acetate plus prednisone in men with new-onset metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16(4):288–292. [DOI] [PubMed] [Google Scholar]
- 39. Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11(5):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. [DOI] [PubMed] [Google Scholar]
- 41. Huo CW, Chew GL, Britt KL, Ingman WV, Henderson MA, Hopper JL, Thompson EW. Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat. 2014;144(3):479–502. [DOI] [PubMed] [Google Scholar]
- 42. Haiman CA, Bernstein L, Berg D, Ingles SA, Salane M, Ursin G. Genetic determinants of mammographic density. Breast Cancer Res. 2002;4(3):R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stone J, Gurrin LC, Byrnes GB, Schroen CJ, Treloar SA, Padilla EJ, Dite GS, Southey MC, Hayes VM, Hopper JL. Mammographic density and candidate gene variants: a twins and sisters study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1479–1484. [DOI] [PubMed] [Google Scholar]
- 44. Biong M, Suderman M, Haakensen VD, Kulle B, Berg PR, Gram IT, Dumeaux V, Ursin G, Helland Å, Hallett MH, Børresen-Dale AL, Kristensen VN. Candidate SNP analyses integrated with mRNA expression and hormone levels reveal influence on mammographic density and breast cancer risk. bioRxiv. 2018;259002 https://www.biorxiv.org/content/10.1101/259002v1.article-info. Accessed 5 July 2019. [Google Scholar]
- 45. Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, Sansone A, Lombardo F. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. [DOI] [PubMed] [Google Scholar]
- 46. Tu YA, Lin SJ, Chen PL, Chou CH, Huang CC, Ho HN, Chen MJ. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome [published online ahead of print 2 May 2019]. J Formos Med Assoc. doi: 10.1016/j.jfma.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 47. Wu VC, Wu CK, Chang YC, Young GH, Chen SC, Yang WS, Chen CY, Wang WJ, Lin CY, Lin YH, Lin SL, Chueh SC, Wu KD; TAIPAI study group. Association of the variations in the HSD3β gene with primary aldosteronism. J Hypertens. 2013;31(7):1396–1405, discussion 1405. [DOI] [PubMed] [Google Scholar]
- 48. Shimodaira M, Nakayama T, Sato N, Aoi N, Sato M, Izumi Y, Soma M, Matsumoto K. Association of HSD3B1 and HSD3B2 gene polymorphisms with essential hypertension, aldosterone level, and left ventricular structure. Eur J Endocrinol. 2010;163(4):671–680. [DOI] [PubMed] [Google Scholar]
- 49. Tripodi G, Citterio L, Kouznetsova T, Lanzani C, Florio M, Modica R, Messaggio E, Hamlyn JM, Zagato L, Bianchi G, Staessen JA, Manunta P. Steroid biosynthesis and renal excretion in human essential hypertension: association with blood pressure and endogenous ouabain. Am J Hypertens. 2009;22(4):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verwoert GC, Hofland J, Amin N, Mattace-Raso FU, Sijbrands EJ, Hofman A, van den Meiracker AH, Uitterlinden AG, van Duijn CM, de Jong FH, Danser AH. Expression and gene variation studies deny association of human HSD3B1 gene with aldosterone production or blood pressure. Am J Hypertens. 2015;28(1):113–120. [DOI] [PubMed] [Google Scholar]
- 51. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7(Suppl 9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts RO, Bergstralh EJ, Farmer SA, Jacobson DJ, Hebbring SJ, Cunningham JM, Thibodeau SN, Lieber MM, Jacobsen SJ. Polymorphisms in genes involved in sex hormone metabolism may increase risk of benign prostatic hyperplasia. Prostate. 2006;66(4):392–404. [DOI] [PubMed] [Google Scholar]
- 53. Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014;74(11):1195–1207. [DOI] [PubMed] [Google Scholar]
- 54. Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315(24):1519–1524. [DOI] [PubMed] [Google Scholar]
- 55. Helzlsouer KJ, Gordon GB, Alberg AJ, Bush TL, Comstock GW. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992;52(1):1–4. [PubMed] [Google Scholar]
- 56. Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013–1020. [DOI] [PubMed] [Google Scholar]
- 57. Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharrière O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97(8):4279–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ III, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. [DOI] [PubMed] [Google Scholar]
- 59. Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292(18):2243–2248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.