Abstract
Context
X-linked acrogigantism (X-LAG), a condition of infant-onset acrogigantism marked by elevated GH, IGF-1, and prolactin (PRL), is extremely rare. Thirty-three cases, including three kindreds, have been reported. These patients have pituitary adenomas that are thought to be mixed lactotrophs and somatotrophs.
Case Description
The patient’s mother, diagnosed with acrogigantism at 21 months, underwent pituitary tumor excision at 24 months. For more than 30 years, stable PRL, GH, and IGF-1 concentrations and serial imaging studies indicated no tumor recurrence. During preconception planning, X-LAG was diagnosed: single-nucleotide polymorphism microarray showed chromosome Xq26.3 microduplication. After conception, single-nucleotide polymorphism microarray on a chorionic villus sample showed the same microduplication in the fetus, confirming familial X-LAG. The infant grew rapidly with rising PRL, GH, and IGF-1 concentrations and an enlarging suprasellar pituitary mass, despite treatment with bromocriptine. At 15 months, he underwent tumor resection. The pituitary adenoma resembled the mother’s pituitary adenoma, with tumor cells arranged in trabeculae and glandular structures. In both cases, many tumor cells expressed PRL, GH, and pituitary-specific transcription factor-1. Furthermore, the tumor expressed other lineage-specific transcription factors, as well as SOX2 and octamer-binding transcription factor 4, demonstrating the multipotentiality of X-LAG tumors. Both showed an elevated Ki-67 proliferation index, 5.6% in the mother and 8.5% in the infant, the highest reported in X-LAG.
Conclusions
This is a prenatally diagnosed case of X-LAG. Clinical follow-up and biochemical evaluation have provided insight into the natural history of this disease. Expression of stem cell markers and several cell lineage-specific transcription factors suggests that these tumors are multipotential.
The clinical course and tumor pathology results of an infant with prenatally diagnosed X-LAG are described and compared with his affected mother. The significance of their findings is discussed.
Pituitary gigantism is extremely rare in infancy and childhood. In roughly one-half of childhood-onset cases, there is an identifiable genetic cause (1–3). The leading cause of nonsyndromic disease is a mutation of aryl hydrocarbon receptor interacting protein (AIP) (29% to 41%), followed by X-linked acrogigantism (X-LAG) resulting from G-protein coupled receptor 101 (GPR101) mutations (8% to 10%) (1–4). In familial isolated pituitary adenoma with two or more affected family members, the major known genetic cause is an AIP mutation (20%) followed by X-LAG (1–3, 5). In the 28 reported patients with X-LAG who have been analyzed by array comparative genomic hybridization, there are 24 nonrecurrent duplications with unique breakpoint junctions; these comprise 21 sporadic cases and 7 familial cases from 3 kindreds (4).
First described in 2014, X-LAG is pituitary gigantism occurring as early as the first few months of life (6). It is characterized by GH and IGF-1 excess leading to acromegaly, accelerated growth velocity, and increased height/weight deviation from the standard growth curve prior to epiphyseal closure (1, 2, 4, 6). In most cases, prolactin (PRL) concentrations are also markedly elevated (2, 7). X-LAG is caused by a microduplication of GPR101 on chromosome Xq26.3 (2, 3, 6, 7). GPR101 is thought to play a role in the development of the GHRH–GH axis and encodes a G-protein coupled receptor that strongly activates the cAMP pathway and stimulates proliferation of pituitary cells and secretion of PRL and GH (2, 5, 8). In contrast to other causes of gigantism for which male predominance is observed, X-LAG is female-predominant (78%), with median age of onset of 1.0 years (0.5 to 2.0), much younger than the median of 16.0 years (5.0 to 18.0) in other gigantism cases (1–4, 6, 8). Treatment is challenging, with a combination of therapeutic approaches necessary to achieve suppression of elevated hormone effects and control of tumor size (2, 3, 8, 9). Resection of the tumor reveals characteristic histologic features including sinusoidal and lobular architectures with scattered follicles and calcifications. The pituitary adenomas are thought to be mixed somatotroph-lactotroph adenomas with pituitary-specific transcription factor-1 (PIT1) expression (2, 6).
We now describe the clinical course for the son of a previously described case of X-LAG (10, 11). Additionally, we compare the mother and son’s pathology that provides further insight into the tumors of X-LAG. This report follows a patient with familial X-LAG prospectively starting at prenatal diagnosis and affords the unique opportunity to compare clinical courses and histopathology findings. These observations help elucidate the pathophysiology, tumorigenesis, and clinical presentation of pediatric pituitary adenomas.
Case Report and Methods
Clinical case
Clinical, laboratory, and radiological data were collected from the time of the infant’s prenatal diagnosis to his most recent clinic visit. Height and weight z scores were calculated using normative data from the World Health Organization (WHO) Child Growth Standards and the US Centers for Disease Control and Prevention Child Growth Standards. Appropriate consent as required by the Columbia University Irving Medical Center institutional review board was obtained from the parents of the patient for the genetic analyses, pathology studies, photograph use, and publication of his case report.
Nucleic acid preparation, sequencing, and analysis
Genomic DNA was purified from the blood of the infant and his mother and father using the QIAsymphony DNA mini kit (QIAGEN Inc., Germantown, MD). In addition, DNA was purified from formalin-fixed, paraffin-embedded tissue from the infant’s pituitary adenoma using the QIAGEN QIAamp Tissue Kit and from the infant’s buccal swab with the QIAGEN DNA Micro Kit. RNA purification from the infant’s pituitary adenoma was performed with the QIAGEN RNeasy FFPE Kit. DNA whole exome libraries were prepared with the SureSelectXT All Exon V5+UTRs capture kit (Agilent Technologies, Santa Clara, CA). For genomic DNA, the Agilent SureSelect Target Enrichment System was used to target the exome, and sequencing was performed on the Hiseq2500 (Illumina Inc., San Diego, CA). RNA was sequenced on the HiSeq2500 using the Illumina TruSeq Stranded Total RNA LT Sample Prep Kit (12, 13).
DNA sequence was analyzed for variants using NextGENe software (SoftGenetics, State College, PA) and filtered with the Columbia Laboratory of Personalized Genomic Medicine proprietary analytical pipeline (13). The sequences are referenced to human genome build GRCh37/UCSC hg19. Whole exome samples had an average coverage of at least 150-fold, and 10× coverage of >98% of the region of interest. Copy number variation was analyzed with EXCAVATOR (14). RNA-sequencing analysis, including relative expression analysis, was performed as previously described (13). The tumor transcriptome had at least 50 million independent mappable reads.
Immunohistochemistry
Histological analysis of tumor specimens was performed with hematoxylin and eosin staining on 4-μm sections of formalin-fixed, paraffin-embedded tissue. Immunoperoxidase staining was done on paraffin-embedded sections using antibodies to Ki-67, CAM5.2, SOX2 (sex determining region Y-box 2), octamer-binding transcription factor 4 (OCT4) (Ventana, Tucson, AZ); chromogranin, PRL, TSH, GH, ACTH, LH, FSH (Agilent Technologies); steroidogenic factor-1 (SF1) (Invitrogen, Carlsbad, CA); PIT1 (Santa Cruz Biotechnology, Dallas TX); and T box factor pituitary cell-restricted (TPIT)/T-box transcription factor 19 (Atlas Antibodies, Bromma, Sweden). Immunohistochemical staining for all antibodies was performed on a Ventana BenchMark XTautomated stainer.
Relevant maternal-fetal history
The patient’s mother was diagnosed with acrogigantism at 21 months based on elevated concentrations of PRL [370 ng/mL; normal for age, 3.5 to 16 ng/mL (16,087 pmol/L; normal for age, 152 to 696 pmol/L)], GH [135 ng/mL; normal for age, ≤5 ng/mL (135 μg/L; normal for age, ≤5 μg/L)], and IGF-1 [1540 ng/mL; normal for age, 27 to 157 ng/mL (202 nmol/L; normal for age, 4 to 21 nmol/L)], having been noted to be large for age as early as 4 months old. By 21 months of age, she had grown to height 97.6 cm (+4.4 SD), weight 20.6 kg (+6.2 SD), and head circumference 55 cm (+5.5 SD). At 24 months of age, she underwent right frontotemporal craniotomy and microsurgical gross total resection, with the expected subsequent development of hypopituitarism and diabetes insipidus, as well as a seizure disorder (10). She required treatment with hydrocortisone, levothyroxine, desmopressin, and antiepileptic medication. GH therapy was given from age 9 years until early adulthood, when she elected to discontinue treatment, and puberty was initiated with estrogen therapy at 13 years old. To date, for more than 30 years, serial laboratory evaluations demonstrated stable concentrations of PRL [19 to 43 ng/mL (826 to 1870 pmol/L)], GH [0.38 to 1 ng/mL (0.38 to 1 μg/L)], and IGF-1 [101 to 166 ng/mL (13 to 22 nmol/L)], and serial MRI studies showed no evidence of tumor recurrence. As described by Gordon et al. (11), the mother underwent genetic testing: chromosome microarray showed a de novo 516-kb microduplication on chromosome Xq26.3 that included GPR101, establishing the diagnosis of X-LAG. She conceived at 32 years of age with intrauterine insemination. A single-nucleotide polymorphism microarray was performed on a chorionic villus sample and showed the same chromosome Xq26.3 microduplication in the male fetus that was present in the mother (nucleotides 135,590,323 through 136,122,117, including GPR101) (11).
Serial prenatal ultrasounds demonstrated disproportionate fetal growth, with abdominal circumference and biparietal diameter greater than 97th percentile and greater than 90th percentile respectively by 34 weeks’ gestation, whereas the femur length was consistently less than the 5th percentile throughout the pregnancy. Estimated fetal weight ranged from 50th to 80th percentiles, and head circumference ranged from 75th to 85th percentiles.
Infant clinical course
The infant was born full-term via cesarean section, with a birthweight of 4.04 kg (+1.26 SD), length 52 cm (+1.04 SD), and head circumference 37 cm (+1.94 SD) (WHO Child Growth Standards) (Fig. 1A). Examination was notable for mild dysmorphic facial features (frontal bossing, posteriorly rotated low-set ears with underdeveloped superior helix and tragus, mild hypertelorism, depressed and broad nasal bridge), shortened neck, posterior white hair lock, small bell-shaped thoracic cavity with wide-spaced nipples, rhizomelic limbs, and mild fifth finger clinodactyly with tapering fingers. Testes were descended with a normal volume of 2.5 mL bilaterally. He was admitted to the neonatal intensive care unit for respiratory support, and required continuous positive airway pressure until day of life 20 because of lung hypoplasia related to the small thoracic cavity. A skeletal survey at 2 days of life showed irregular mineralization of the vertebral bodies; trident acetabula; shortened long bones with irregular, dysplastic metaphyses; and short, dysplastic ribs with handlebar-appearing clavicles. His clinical findings indicated a skeletal dysplasia primarily affecting the thoracic cavity, as well as rhizomelic shortening of the upper and lower limbs. He initially exhibited poor feeding and required nasogastric feeds; a gastrostomy tube was placed at 4 months of life and subsequently removed at 11 months with resolution of weight issues and improved oral intake.
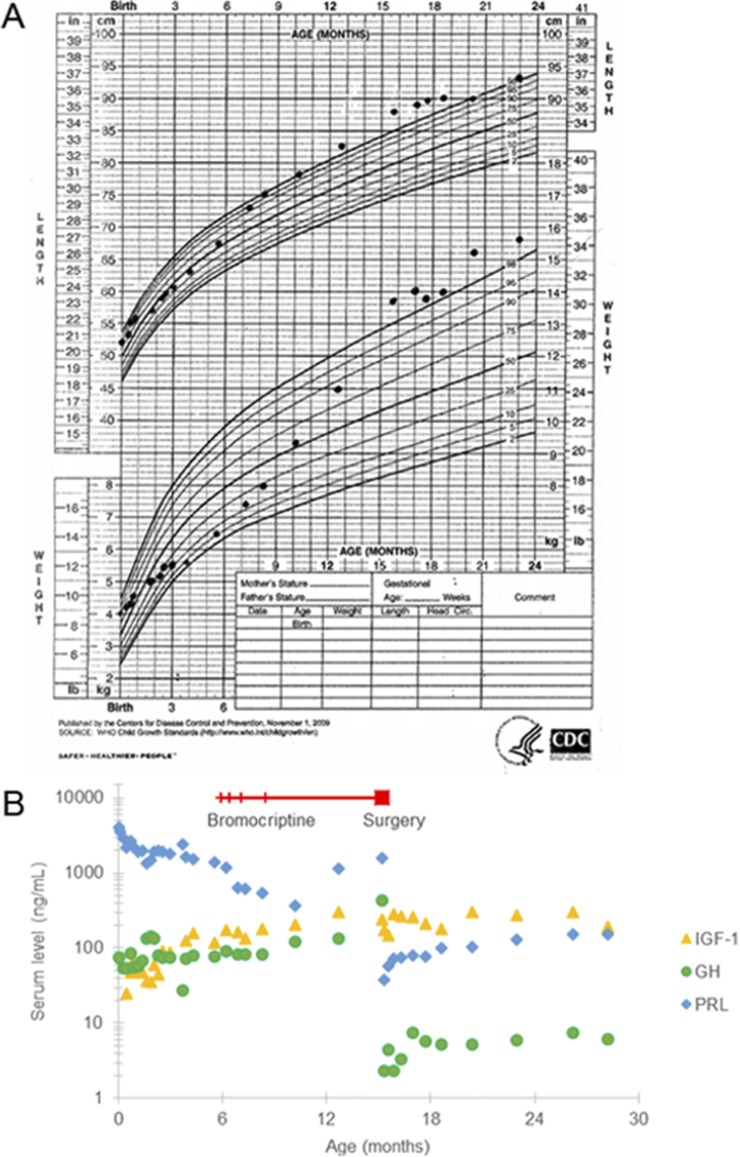
Figure 1.
Growth parameters and biochemical markers with medical and surgical intervention from 0 to 24 mo of age. (A) The infant’s height and weight, plotted on the WHO Growth Curve, increase over time despite medical and surgical intervention. (B) Serum concentrations of PRL [normal for age, 3.5 to 16 ng/mL (152 to 696 pmol/L)], GH [normal for age, ≤5 ng/mL (≤5 µg/L)], and IGF-1 [normal for age, 27 to 157 ng/mL (4 to 21 nmol/L)] are shown. The superimposed timeline indicates treatment with bromocriptine starting at 6 mo of age; hatch marks show increases in dosing and end at 15 mo of age at the time of surgical intervention.
Concentrations of hormones and growth factor were closely monitored with the known diagnosis of X-LAG. In the first 6 months, PRL ranged from 1360 to 4102 ng/mL (59,130 to 178,347 pmol/L), normal for age 3.5 to 16 ng/mL (152 to 696 pmol/L); GH ranged from 27 to 142 ng/mL (27 to 142 µg/L), normal for age, ≤5 ng/mL (≤5 μg/L); and IGF-1 ranged from <25 to 159 ng/mL (<3 to 21 nmol/L), normal for age, 27 to 157 ng/mL (4 to 21 nmol/L) (Fig. 1B). Thyroid tests were within normal limits on the second day of life. Serial cortisol measurements were all within normal limits, with robust random cortisol of 25 µg/dL (690 nmol/L) at 7 weeks of life. MRI at 3 weeks of life showed a heterogeneously enhancing mass arising from the sella with suprasellar extension measuring 8.1 × 7.5 × 10.7 mm (anteroposterior × transverse × craniocaudal) (Fig. 2A and 2B). Ophthalmology examination showed normal visual acuity for age and no evidence of optic nerve atrophy or swelling; serial reevaluations demonstrated no changes in vision. Repeat MRI at 3.5 months of age showed interval increase in size of the tumor to 9.0 × 10.0 × 12.0 mm; no impingement on the optic chiasm was observed (Fig. 2C and 2D).
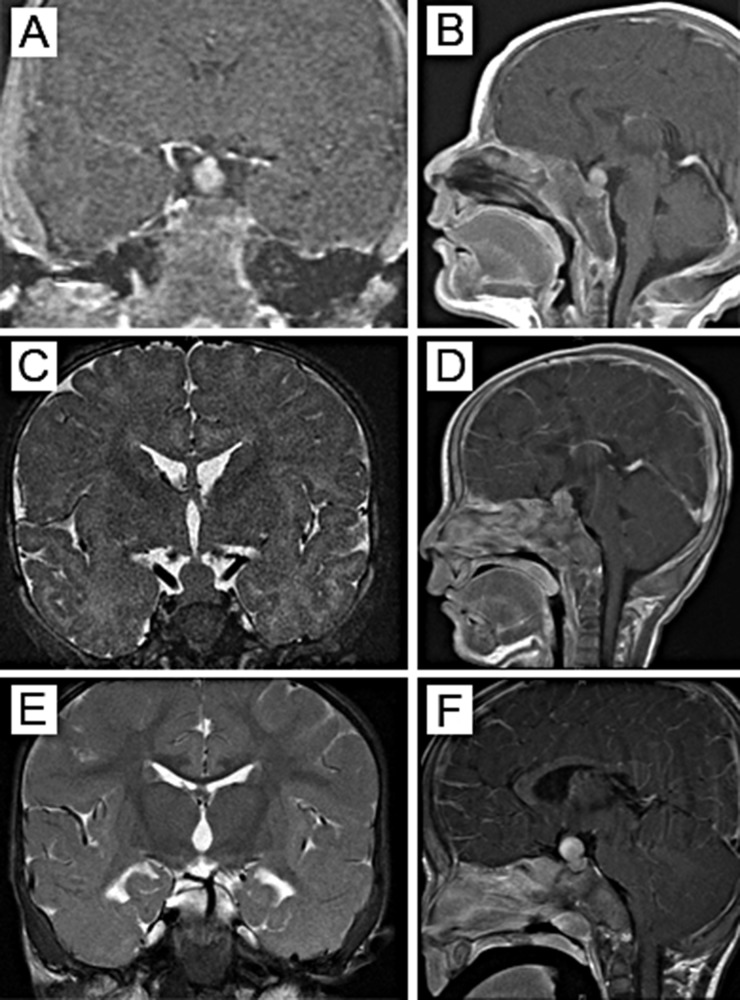
Figure 2.
MRI of the pituitary tumor in (A, C, E) coronal and (B, D, F) sagittal views. (A, B) At 3 wk of age, the pituitary mass measured 8.1 × 7.5 × 10.7 mm in the sella with suprasellar extension. (C, D) At 3.5 mo of age, the mass increased to 9.0 × 10.0 × 12.0 mm, without signs of impingement on the optic chiasm. (E, F) By 10 mo of age, the tumor measured 12.5 × 12.5 × 15.1 mm, abutting onto the optic chiasm.
At 6 months of age, the infant was started on oral bromocriptine therapy at 0.625 mg daily; however, he continued to grow (Fig. 1A). Hormone and growth factor concentrations continued to rise; by 15 months of age, PRL was 1585 ng/mL (68,913 pmol/L), GH was 436 ng/mL (436 µg/L), and IGF-1 was 243 ng/mL (32 nmol/L) (Fig. 1B). After 4 months of pharmacological treatment, the pituitary mass seen on MRI had grown to 12.5 × 12.5 × 15.1 mm with abutment onto the optic chiasm (Fig. 2E and 2F), and by 13 months of age (after 7 months of treatment), the tumor had grown to 12.0 × 14.0 × 18.0 mm with mild elevation of the optic chiasm and posterior displacement of the pituitary stalk.
Because of continued evidence of tumor growth and elevation in hormone and growth factor concentrations at 15 months of age, he underwent right frontotemporal craniotomy and microsurgical gross total resection. The tumor was found to displace the optic chiasm with extension to the stalk; the stalk was partially preserved with thin residual posterior pituitary. Postoperatively, the infant developed the expected hypopituitarism, with a brief period of diabetes insipidus followed by syndrome of inappropriate antidiuretic hormone. He had a seizure in the setting of a sodium concentration of 129 mEq/L (129 mmol/L). The sodium concentrations normalized with oral sodium chloride supplementation, and he demonstrated intact thirst with good oral intake to match urine output, with no evidence of diabetes insipidus. He was discharged home on antiepileptic medication, hydrocortisone, levothyroxine, and sodium chloride supplementation.
After surgical intervention, all hormone and growth factor concentrations sharply decreased initially, with PRL of 37 ng/mL (1609 pmol/L), GH of 2.3 ng/mL (2.3 µg/L), and IGF-1 of 146 ng/mL (19 nmol/L). Starting at 21 months of age (6 months postoperatively), the IGF-1 level rose, and PRL and GH concentrations also slowly increased. By 28 months of age, PRL was 151 ng/mL (6578 pmol/L), GH was 6.1 ng/mL (6.1 µg/L), and IGF-1 was 189 ng/mL (25 nmol/L) (Fig. 1B). He measured in the 99th percentile for height (+2.26 SD) and the 99th percentile for weight (+2.52 SD) (US Centers for Disease Control and Prevention Child Growth Standards) (Fig. 1A). MRI at 28 months of age showed an area of hypointensity measuring 7.0 × 5.0 × 6.0 mm in the right side of the sella suggestive of possible residual disease. Compared with the previous imaging, there was greater than 95% resection of the overall mass. As of age 28 months, he has disproportionately large hands and feet, which likely represent acromegaly, but his facial features resemble those of his unaffected father (Fig. 3, photo at 24 months of age).
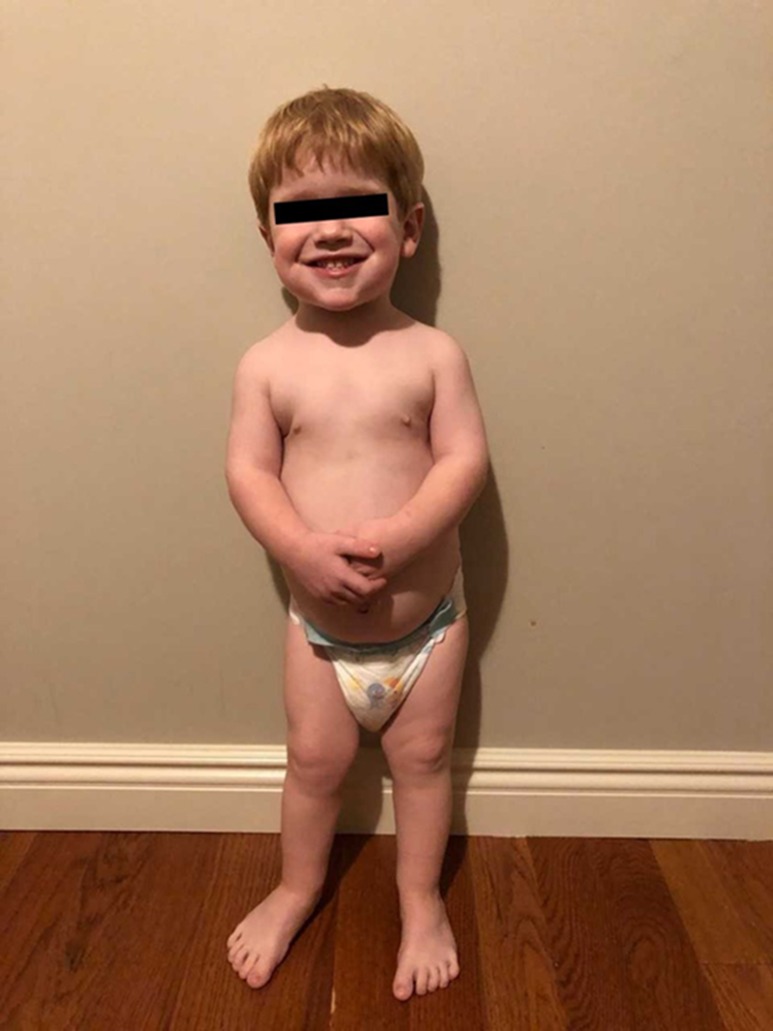
Figure 3.
Photo of infant at 24 mo. He has rhizomelic limbs with a narrow, bell-shaped chest. The jaw is not acromegalic and his facial features resemble those of his unaffected father. His hands and feet are large for age; here, he wears size 4T clothing and 8W toddler shoe, correlating to a foot length of 15 to 16 cm.
Characterization of infant’s exome and transcriptome
Whole exome sequencing (WES) did not report any pathogenic somatic or germline variants in the infant relevant to the patient’s presentation of X-LAG with pituitary adenoma, including in the AIP gene. Transcriptome sequencing did not identify any abnormal fusion transcripts. Taken together, these data support that the infant’s acrogigantism is due solely to chromosome Xq26.3 microduplication. Of note, RNA-sequencing data also showed that the most abundantly expressed transcripts in the pituitary adenoma were PRL, GH1, and GNAS.
Histological analysis of the pituitary adenomas
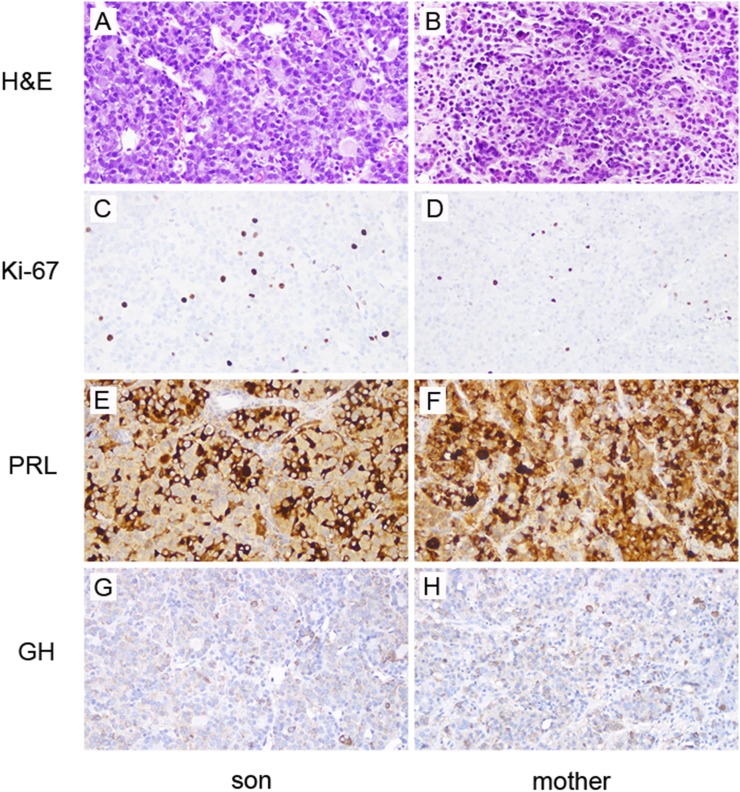
Hematoxylin and eosin–stained sections of the infant’s pituitary adenoma and his mother’s pituitary adenoma show similar neoplasms arranged in trabeculae and glandular structures with colloid-like material (Fig. 4A and 4B). Necrosis and hemorrhage were not identified. The adenomas are composed predominantly of chromophobes, with relatively fewer, scattered acidophils. The neoplastic cells are pleomorphic, with round to oval nuclei and occasional prominent nucleoli. Several mitotic figures are seen (for example, Fig. 4B, arrow). The Ki-67 proliferation index is increased in both adenomas, with 8.5% of cells staining positive in the infant and 5.6% in the mother (Fig. 4C and 4D). PRL is the most commonly and abundantly expressed hormone in both adenomas. In many tumor cells, strong diffuse PRL expression is seen, consistent with the presence of many densely granulated lactotrophs. A smaller subset of cells has exclusive paranuclear localization of PRL, consistent with relatively fewer sparsely granulated lactotrophs (Fig. 4E and 4F). A subset of tumor cells expresses GH in both adenomas (Fig. 4G and 4H). A CAM5.2 immunostain demonstrates fibrous bodies in some tumor cells, indicative of sparsely granulated somatotrophs, though most tumor cells exhibit a cytoplasmic pattern of expression, indicating that there are more densely granulated somatotrophs in these tumors (data not shown). Rare, scattered cells in both resection specimens express FSH, LH, and ACTH, and no cells express TSH (data not shown).
Figure 4.
Histological features of (A, C, E, G) infant’s and (B, D, F, H) mother’s pituitary adenomas in the left and right columns, respectively. (A, B) Hematoxylin and eosin–stained sections show neoplastic cells arranged in cords and acinar structures filled with eosinophilic, colloid-like substance. (C, D) The Ki-67 antibody labels many cells in both adenomas, with 8.5% of cells staining positive in the infant and 5.6% in the mother. Many of the neoplastic cells are strongly positive for (E, F) PRL with (G, H) fewer GH positive cells. Magnification, ×20.
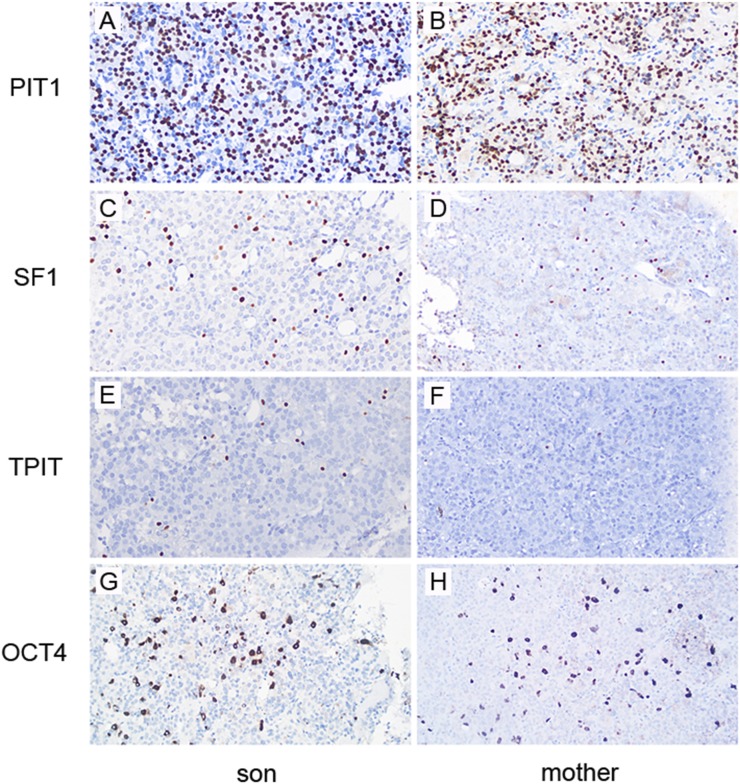
To examine the cell lineages present in these tumors, expression of lineage-specific transcription factors was evaluated. Both adenomas are positive for PIT1 (lactotroph and somatotroph lineages), but are not diffusely positive (Fig. 5A and 5B). There are a fairly large number of SF1 positive (gonadotroph lineage) tumor cells (Fig. 5C and 5D) and relatively rare TPIT positive (corticotroph lineage) tumor cells (Fig. 5E and 5F). OCT4, a stem cell/progenitor cell marker expressed in pituitary (15), is abundant in both tumors (Fig. 5G and 5H). SOX2, another stem cell/progenitor cell marker, is also highly expressed (data not shown). Taken together, these histological features are compatible with a mixed lactotroph and somatotroph adenoma for both the infant and his mother, with more than one cell lineage present in each tumor. The abundant expression of OCT4 and SOX2 provide evidence that many tumor cells are in an early stage of differentiation in these adenomas.
Figure 5.
The pituitary adenomas contain multiple cell lineages and express stem cell markers. A majority of the cells are positive for PIT1, a transcription factor expressed in lactotrophs and somatotrophs, in the (A) infant’s adenoma and the (B) mother’s adenoma. Relatively fewer cells are positive for SF1, a transcription factor expressed in (C, D) gonadotrophs, and rare cells express TPIT, a transcription factor expressed in (E, F) corticotrophs. (G, H) An OCT4 immunostain is positive in the cytoplasm and/or nuclei of large subset of cells. Magnification, ×20.
Discussion
This case reports the postnatal clinical course of a prenatally diagnosed case of X-LAG, offering the unique prospective follow-up of an infant in comparison with his affected mother who has been previously reported (10, 11). For more than 30 years, the mother has had a good clinical outcome. She is on hormone replacement therapies for the expected hypopituitarism that followed the pituitary tumor resection in early childhood, has had no signs of tumor recurrence on serial laboratory measurements or imaging, and has no stigmata of acromegaly, likely in part because of the early timing of her surgical intervention.
Recent analysis of the mother’s pituitary tumor tissue is notable for an unusually high Ki-67 proliferation index of 5.6%, which would be expected to correlate with a more aggressive tumor with high likelihood of recurrence (16). The quantitative approach used counts the number of Ki-67 positive cells divided by the total number of cells in the highest labeled field of 1000 total cells, and underestimates the fraction of tumor that is actively dividing. Such high Ki-67 levels are rarely seen in pituitary adenomas from patients with X-LAG. Of the 33 cases reported in the literature, 8 had Ki-67 reported. Of those, four were less than 1%, and the rest ranged from 2% to 5% (4). In comparison, the infant’s Ki-67 index is even higher at 8.5%: the highest reported for X-LAG to date. Although the mother’s tumor’s high Ki-67 index does not seem to correlate with her clinical outcome of no signs of tumor recurrence, the infant has been refractory to pharmacological treatment before surgery, and now 13 months postoperatively, there are increasing concentrations of hormones and biochemical markers (PRL, IGF-1, GH) as well as an increasing height z score. Of note, the infant’s superimposed skeletal dysplasia, characterized by small chest cavity and rhizomelic limbs, is likely reducing the degree of overgrowth noted in gigantism resulting from X-LAG. The child’s much more severe phenotype compared with the mother may be related to the male child having the microduplication in all cells, whereas the mother has random X inactivation of one-half of the cells with the microduplication.
The histochemical staining of the two pituitary adenoma tumors are both positive for PIT1, a finding previously reported for other X-LAG tumors (2), and indicate that the majority of the tumor cells are somatotrophs and lactotrophs. Surprisingly, both tumors are not diffusely positive for PIT1, indicating that there is more than one cell lineage present. This suggestion of multipotentiality is further supported by the findings of a subset of SF1-positive tumor cells and relatively rare TPIT-positive tumor cells, demonstrating the existence of gonadotrophs and corticotrophs, respectively. The abundant OCT4-positive and SOX2-positive cells suggest that the cell of origin is upstream of specific hormone-restricted pathways that lead to somatotrophs, lactotrophs, gonadotrophs, and corticotrophs. Double stains of the tumor specimens will help determine if some tumor cells coexpress lineage-specific transcription factors and/or multiple hormones. Alternatively, the gonadotrophs and corticotrophs may be entrapped anterior pituitary gland cells; in this case, the double stains may reveal only one type of hormone from these cells without positive staining for SOX2, OCT4, or other stem cell markers. Future studies to examine the stem cell/progenitor populations (using stem cell markers including SOX2 and OCT4) of larger cohorts of pediatric pituitary adenomas, as well as prospective long-term follow-up of clinical outcomes in infants and comparison with adults, will enable us to better understand how GPR101 ultimately leads to neoplasia and the clinical presentation and outcomes seen in patients with X-LAG.
The patient has an associated skeletal dysplasia that manifests as a small thoracic cage and rhizomelia, similar to but different from Jeune asphyxiating thoracic dystrophy (MIM #208500). At this time, there is no identified genetic cause for the skeletal dysplasia; genetic testing for Jeune syndrome and other targeted skeletal dysplasia panels done in infancy were negative, as were WES studies of both peripheral leukocyte-derived DNA and the pituitary tumor. Furthermore, the affected mother with the same microduplication has proportionate body measurements with no signs of skeletal dysplasia, nor to date has the patient’s skeletal phenotype been reported in other patients with X-LAG (4, 7). He likely has an undescribed skeletal dysplasia, and the negative WES in such a case is not unusual if the gene is not known.
Treatment of X-LAG can be difficult; somatostatin analogs and dopamine agonists (such as bromocriptine used for the infant initially) are often given as first-line therapy or in combination with surgery, but are usually ineffective. Pegvisomant can be used to control concentrations of IGF-1 and growth velocity, but there are few pediatric studies. Surgical resection may achieve disease control in some cases, but postoperative hypopituitarism and diabetes insipidus are common (2, 7, 8). The mother only required one surgery with stable postsurgical clinical outcomes for more than 30 years, whereas the infant may require further intervention. Depending on tumor regrowth, this will likely require additional surgical resection, and the use of medical agents discussed previously will also be considered. With the aggressive nature of the tumor suggested from the pathology, close monitoring and early treatment of the infant initiated with the prenatal diagnosis may have minimized more severe outcomes that may have resulted without early intervention, and also provide opportunity for prospective longitudinal clinical follow-up.
Conclusions
In this report, we have presented familial X-linked acrogigantism cases, in a mother and her son, resulting from pituitary adenomas that presented during infancy. For more than 30 years, stable PRL, GH, and IGF-1 concentrations and serial imaging studies indicated no recurrence following tumor resection in the mother. The son, who was prenatally diagnosed with X-LAG, also has an associated skeletal dysplasia of unclear etiology. His postsurgical clinical course has been relatively stable, although recent biochemical markers are gradually increasing, and recent imaging indicates possible residual tumor. Analysis of both pituitary adenomas reveals highly proliferative tumors that express multiple lineage-specific transcription factors, including PIT1, and stem cell markers such as SOX2 and OCT4. Early surgical intervention may prevent the substantial stigmata of acromegaly.
Acknowledgments
Molecular studies (whole exome and transcriptome) were performed in the Columbia Laboratory of Personalized Genomic Medicine.
Financial Support: This work was supported in part by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 5T32DK065522-14 (to B.K.W.-O. and S.E.O.) and the JPB Foundation (to W.K.C.).
Glossary
Abbreviations:
- AIP
aryl hydrocarbon receptor interacting protein
- GPR101
GTP-binding protein coupled receptor 101
- OCT4
octamer-binding transcription factor 4
- PIT1
pituitary-specific transcription factor-1
- PRL
prolactin
- SF1
steroidogenic factor-1
- TPIT
T box factor pituitary cell-restricted
- WES
whole exome sequencing
- WHO
World Health Organization
- X-LAG
X-linked acrogigantism
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017;20(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iacovazzo D, Caswell R, Bunce B, Jose S, Yuan B, Hernández-Ramírez LC, Kapur S, Caimari F, Evanson J, Ferraù F, Dang MN, Gabrovska P, Larkin SJ, Ansorge O, Rodd C, Vance ML, Ramírez-Renteria C, Mercado M, Goldstone AP, Buchfelder M, Burren CP, Gurlek A, Dutta P, Choong CS, Cheetham T, Trivellin G, Stratakis CA, Lopes MB, Grossman AB, Trouillas J, Lupski JR, Ellard S, Sampson JR, Roncaroli F, Korbonits M. Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol Commun. 2016;4(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22(5):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trivellin G, Hernández-Ramírez LC, Swan J, Stratakis CA. An orphan G-protein-coupled receptor causes human gigantism and/or acromegaly: Molecular biology and clinical correlations. Best Pract Res Clin Endocrinol Metab. 2018;32(2):125–140. [DOI] [PubMed] [Google Scholar]
- 5. Daly AF, Yuan B, Fina F, Caberg JH, Trivellin G, Rostomyan L, de Herder WW, Naves LA, Metzger D, Cuny T, Rabl W, Shah N, Jaffrain-Rea ML, Zatelli MC, Faucz FR, Castermans E, Nanni-Metellus I, Lodish M, Muhammad A, Palmeira L, Potorac I, Mantovani G, Neggers SJ, Klein M, Barlier A, Liu P, Ouafik L, Bours V, Lupski JR, Stratakis CA, Beckers A. Somatic mosaicism underlies X-linked acrogigantism syndrome in sporadic male subjects. Endocr Relat Cancer. 2016;23(4):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, Sierra ML, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenický P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, Yuan B, Choong CS, Caberg JH, Verrua E, Naves LA, Cheetham TD, Young J, Lysy PA, Petrossians P, Cotterill A, Shah NS, Metzger D, Castermans E, Ambrosio MR, Villa C, Strebkova N, Mazerkina N, Gaillard S, Barra GB, Casulari LA, Neggers SJ, Salvatori R, Jaffrain-Rea ML, Zacharin M, Santamaria BL, Zacharieva S, Lim EM, Mantovani G, Zatelli MC, Collins MT, Bonneville JF, Quezado M, Chittiboina P, Oldfield EH, Bours V, Liu P, W de Herder W, Pellegata N, Lupski JR, Daly AF, Stratakis CA. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22(3):353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodd C, Millette M, Iacovazzo D, Stiles CE, Barry S, Evanson J, Albrecht S, Caswell R, Bunce B, Jose S, Trouillas J, Roncaroli F, Sampson J, Ellard S, Korbonits M. Somatic GPR101 duplication causing X-linked acrogigantism (XLAG)—diagnosis and management. J Clin Endocrinol Metab. 2016;101(5):1927–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naves LA, Daly AF, Dias LA, Yuan B, Zakir JC, Barra GB, Palmeira L, Villa C, Trivellin G, Júnior AJ, Neto FF, Liu P, Pellegata NS, Stratakis CA, Lupski JR, Beckers A. Aggressive tumor growth and clinical evolution in a patient with X-linked acro-gigantism syndrome. Endocrine. 2016;51(2):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blumberg DL, Sklar CA, David R, Rothenberg S, Bell J. Acromegaly in an infant. Pediatrics. 1989;83(6):998–1002. [PubMed] [Google Scholar]
- 11. Gordon RJ, Bell J, Chung WK, David R, Oberfield SE, Wardlaw SL. Childhood acromegaly due to X-linked acrogigantism: long term follow-up. Pituitary. 2016;19(6):560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dela Cruz FS, Diolaiti D, Turk AT, Rainey AR, Ambesi-Impiombato A, Andrews SJ, Mansukhani MM, Nagy PL, Alvarez MJ, Califano A, Forouhar F, Modzelewski B, Mitchell CM, Yamashiro DJ, Marks LJ, Glade Bender JL, Kung AL. A case study of an integrative genomic and experimental therapeutic approach for rare tumors: identification of vulnerabilities in a pediatric poorly differentiated carcinoma. Genome Med. 2016;8(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oberg JA, Glade Bender JL, Sulis ML, Pendrick D, Sireci AN, Hsiao SJ, Turk AT, Dela Cruz FS, Hibshoosh H, Remotti H, Zylber RJ, Pang J, Diolaiti D, Koval C, Andrews SJ, Garvin JH, Yamashiro DJ, Chung WK, Emerson SG, Nagy PL, Mansukhani MM, Kung AL. Implementation of next generation sequencing into pediatric hematology-oncology practice: moving beyond actionable alterations. Genome Med. 2016;8(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RRID:SCR_007358, https://scicrunch.org/resolver/SCR_012766.
- 15. Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4(3):e4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schreiber S, Saeger W, Lüdecke DK. Proliferation markers in different types of clinically non-secreting pituitary adenomas. Pituitary. 1999;1(3-4):213–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.