Abstract
Objectives
This study aimed to determine the effects of propolis on immune mediators and tissue histopathology in rats with L-arginine-induced acute pancreatitis (AP).
Methods
This study was conducted at Imam Abdulrahman Bin Faisal University, Dammam, Saudia Arabia between September and November 2017. A total of 24 male albino Wistar rats were divided into three equal groups. Group one was the negative control, group two was the positive control (L-arginine-induced AP) and group three received treatment (L-arginineinduced AP and propolis). The rats in group three were treated with 100 mg/kg propolis for seven days after AP induction. Pancreatic tissue was evaluated histologically and levels of interleukin (IL)-6, IL-22 and IL-1β and tumour necrosis factor-alpha (TNF-α) were measured.
Results
Propolis reduced the quanitity of proinflammatory molecules (TNF-α, IL-1β and IL-6) in group three compared to group two, significantly increased the overall anti-inflammatory effect of IL-22 (P <0.005) and reduced interstitial inflammation and neutrophil cell infiltration of the pancreatic tissues.
Conclusion
Propolis may exert a therapeutic effect in AP. Further studies are required to demonstrate the mechanisms of propolis in AP.
Keywords: Propolis, Arginine, Pancreatitis, Interleukins, Cytokinesis, Rats, Saudi Arabia
Advances in Knowledge.
- The present study identifies the anti-pancreatitis effect of propolis.
- The anti-pancreatitis effect takes potential inflammatory and proinflammatory pathways.
Application to patient care
- Propolis minimised inflammatory response and pancreatic tissue damage.
- Propolis should be considered an effective and promising natural compound for managing acute pancreatitis.
Pancreatitis is a major gastrointestinal problem worldwide.1 Despite the development of new therapeutic and diagnostic approaches, the clinical course of acute pancreatitis (AP) is associated with significant morbidity and a high mortality rate.2,3
Experimental studies focused on the molecular pathway, including proinflammatory cytokines, are shedding light on the pathophysiologic mechanisms of AP. Increased levels of proinflammatory cytokines—such as interleukin (IL)-1, IL-6 and tumour necrosis factor-alpha (TNF-α)—aggravate AP by increasing vascular permeability.4-6
Propolis is a natural resinous compound collected by bees from the gum of various plants and converted through salivary secretions to beeswax. Propolis has attracted global attention for its wide range of pharmacological and biological properties, making propolis a potentially promising therapeutic agent.7 The efficacy of propolis depends mainly on the presence of flavonoids, primarily caffeic acid phenethyl ester (CAPE), which provide an anti-inflammatory effect.8 Future studies should focus on standardising the therapeutic applications of propolis.9
Many studies have shown that the anti-inflammatory activity of propolis and/or its compounds inhibit the activation of cyclooxygenase (COX)-2 gene expression, suppress enzyme activities of COX-1 and COX-2 and inhibit the release of arachidonic acid from cell membranes. 10,11
Galangin, a propolis-associated flavonoid, has been shown to decrease prostaglandin E2 release, inhibit lipoxygenase, COX and the expression of the inducible isoform of COX-2.12 This study aimed to determine the effects of propolis on immune mediators and tissue histopathology of rats with L-arginine-induced AP.
Methods
This study was conducted at Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia from September to November 2017. A total of 24 male albino Wistar rats weighing 150–250 g were obtained from the university’s animal house for this study. All rats were maintained in a room at a constant temperature of 22 ± 1°C with 12 hour light/dark cycles and had free access to standard laboratory food pellets and water.
The rats were equally divided into three groups. Group one was the untreated negative control group, group two consisted of the positive controls and group three was the experimental treatment group. Group two and three were injected with L-arginine to induce AP. Two intraperitoneal (IP) injections of L-arginine (Sigma-Aldrich Chemical, Merck KGaA, Darmstadt, Germany) at a dose of 250 mg/100 g of body weight (BW) prepared in isotonic saline (20% 0.15 M sodium chloride) were administered at a one-hour interval to induce AP.13 The rats in group three were treated orally with Brazilian green propolis alcohol extract (Uniflora Apicultores Associados Ltda, Olímpia, Brazil) 100 mg/kg of BW after two hours of L-arginine injection and daily for seven days.14 This dose has previously been shown to be anti-inflammatory.15 AP was diagnosed clinically as rats became sluggish and lethargic. The condition was most severe 72 hours after the L-arginine-injections and was confirmed by histopathological examination.16
The treatment regimens were stopped after seven days and 12 hours before the rats were anaesthetised with an IP injection of ketamine (50 mg/kg of BW; Alfasan International BV, Woerden, the Netherlands). Rats were euthanised and blood was collected from the abdominal aorta by means of a vacutainer.17 Antiinflammatory cytokines (IL-1β, IL-6, TNF-α and IL-22) were assessed by enzyme-linked immunosorbent assay (ELISA). The IL-22 ELISA Kit (R&D Systems, Minneapolis, Minnesota, USA) and all other cytokine assays (Bio-Rad Laboratories Inc., Hercules, California, USA) were quantified in accordance with the manufacturers’ guidelines and instructions.18,19
Pancreatic tissues were fixed in a 10% formaldehyde solution for 48 hours, embedded in paraffin wax and sectioned. The sections were stained with haematoxylin and eosin and evaluated under a light microscope to detect inflammatory manifestations, including oedema, leukocyte infiltration, parenchymal necrosis and haemorrhage in the pancreatic tissue. The general morphology and histological features were evaluated with a BX51 photomicroscope (Olympus Corporation, Tokyo, Japan).20
Histopathologic scoring for AP included assessment of inflammatory cell infiltration, oedema and acinar degenerative changes. The inflammatory cells were counted in five high power field (HPF) × 400 images and the mean number of cells was calculated and rated. Fewer than 50 inflammatory cells/HPF was rated 1+ (mild), 50–100 inflammatory cells/HPF was rated 2+ (moderate) and >100 inflammatory cells/HPF was rated 3+ (severe).
Statistical analysis was performed using Statistical Package of Social Science (SPSS), Version 21 (IBM Corp., Armonk, New York, USA). Data are presented as means ± standard error of the mean. One-way analysis of variance followed by Tukey’s multiple comparison post-hoc test was used to compare the means. Statistical significance was set at P <0.05.
All experiments were performed in accordance with the recommendations of the national guidelines for the care and handling of laboratory animals. The experimental protocol was approved by the Local Animal Ethics Committee (IRB 2015-01-185).
Results
Serum proinflammatory cytokines (IL-1β, IL-6 and TNF-α) and anti-inflammatory IL-22 in the three studied groups are shown in Table 1. Injecting two doses of L-arginine induced an increase in proinflammatory measurements and a decrease in the measured anti-inflammatory cytokine concentration in group two. Moreover, pathohistological features of AP were present.
Table 1.
Serum analysis of proinflammatory (interleukin [IL]-1β, IL-6 and tumour necrosis factor-alpha) and anti- inflammatory (IL-22) cytokine in three rat groups
| Group | Mean ± SEM | ||
|---|---|---|---|
| I (negative control) | II (positive control) | III (propolis-treated group) | |
| IL-1β in pg/mL | 36.39 ± 3.07 | 116.13 ± 4.28* | 41.43 ± 4.01 |
| IL-6 in pg/mL | 39.02 ± 3.13 | 85.44 ± 3.39*‡ | 45.21 ± 2.05 |
| TNF-α in pg/mL | 59.74 ± 1.40 | 96.91 ± 2.51*‡ | 48.83 ± 1.51 |
| IL-22 in pg/mL | 0.19 ± 0.020 | 0.15 ± 0.023 | 0.30 ± 0.056*† |
SEM = standard error of mean; IL = interleukin; TNF-α = tumour necrosis factor-alpha.
significantly different from group one (P <0.005);
significantly different from group two (P <0.001);
significantly different from group three (P <0.001).
The mean serum concentration of IL-1β was significantly higher in group two than group one (P <0.005). Group two also had significantly higher TNF-α and IL-6 than groups one and three (P <0.005 and P <0.001 each). The mean serum level of IL-22 for the propolis-treated group increased significantly compared to groups one and two (P <0.005 and P <0.001, respectively) [Table 1]. Group two was rated as 3+ and group three was rated at 2+.
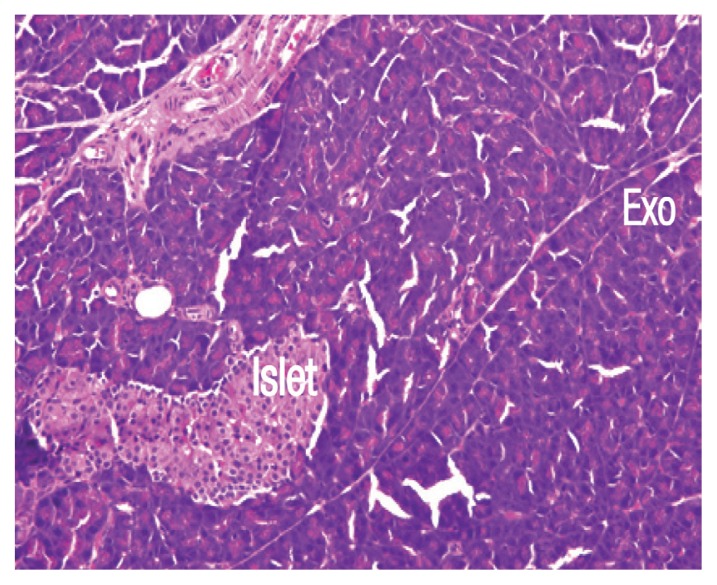
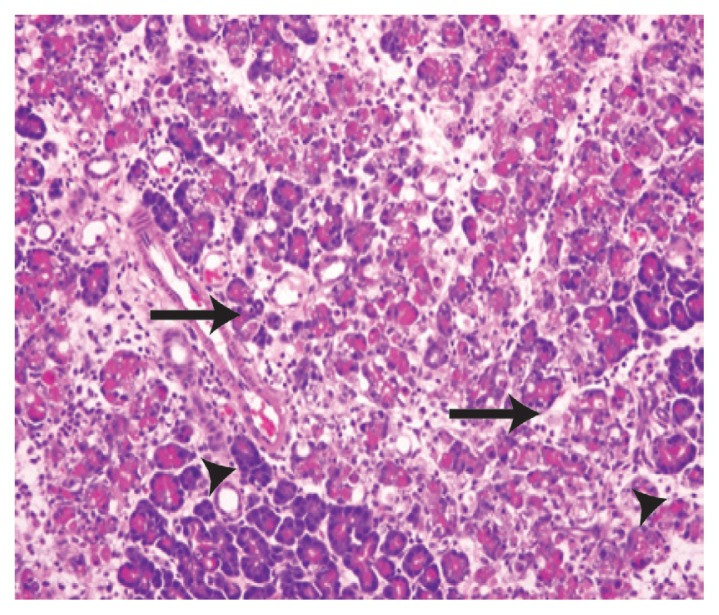
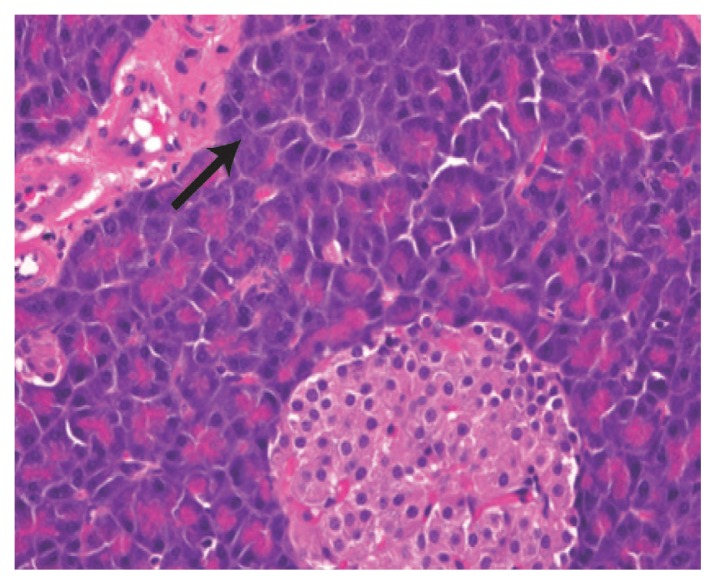
While regular pancreas morphology was observed in the tissues of rats in group one [Figure 1], severe degrees of inflammatory cell infiltration, pancreatic tissue necrosis, haemorrhage and parenchymal degenerative changes were observed in the tissues of rats in group two compared with the other groups (P <0.05) [Figure 2]. However, treatment with propolis in group three significantly decreased the degree of cellular infiltration, interstitial oedema, acinar cell necrosis and parenchymal degenerative changes compared to group two (P <0.05) [Figure 3].
Figure 1.
Haematoxylin and eosin stain of pancreatic tissue from group one at x200 maginification showing normal, unremarkable pancreatic tissue in both exocrine (Exo) and endocrine parts (Islet).
Figure 2.
Haematoxylin and eosin stain of pancreatic tissue from group two at x200 maginification showing significant severe interstitial inflammation, oedema with mixed mononuclear inflammatory cells (arrows) and neutrophils. The pancreatic acini show moderate parenchymal degenerative changes (arrowheads).
Figure 3.
Haematoxylin and eosin stain of pancreatic tissue from group three at x400 maginification showing moderate interstitial inflammation and neutrophil cell infiltration of the pancreatic tissue (arrow).
Discussion
Previous studies have used experimental AP induced by L-arginine to study the effect of various therapeutic agents and the pathophysiologic mechanisms of the disease.21 The present study aimed to determine the effects of propolis on immune mediators and tissue histopathology in rats with AP. The effect of propolis was evidenced by a decrease in proinflammatory cytokines (TNF-α, IL-1β and IL-6) and a significant increase in the level of anti-inflammatory IL-22 to levels close to that of the negative controls.
Several studies have focused on the pathogenesis of AP.2,6 Overproduction of inflammatory mediators, including TNF-α, IL-1β, IL-6 and IL-8, was found to play an important role in the pathogenesis of AP.22 In the present study, propolis significantly reduced the total pathologic score and the extent of oedema, most likely due to the anti-inflammatory action of propolis and/or its active compounds.23 These results agree with those of previous studies from different AP models.24
Interestingly, an important and novel result of this study was the finding that propolis significantly increased levels of IL-22, which had not been tested in previous studies. The anti-inflammatory activities of propolis are still not fully understood. Pooran et al. suggested that propolis has an anti-inflammatory effect as it inhibits the release of histamine, prostaglandins and leukotrienes.22 Another study reported that the anti-inflammatory properties of propolis are due to CAPE.25 CAPE exerts its anti-inflammatory actions by suppressing the inflammatory enzyme activities of COX-1 and COX-2 and inhibiting the release of arachidonic acid from cell membranes.26
In the current study, there was non-significant minimal change in IL-22 levels between groups one and two. The increase of IL-22 in only group three could be via the signal transducer and activator of transcription (STAT) 3 signaling pathway in which exogenous recombinant IL-22 protected mice from L-arginine-induced AP.27 The favourable effect of IL-22 is thought to depend on the extent of AP inflammation. In mild cases of induced AP, administration of exogenous IL-22 successfully aborted disease development.28 Furthermore, over-expressed animal models of IL-22 were resistant to AP development.29 Xue et al. was reported that the administration of the anti-IL-22 anti-body, to block the receptors, endogenously aggravated pancreatic injury which supports the essential role of IL-22.30 Collectively, these findings strongly suggest that IL-22 plays a vital role in AP prevention. Furthermore, IL-22 may mediate a protective effect against L-arginine-induced AP via activation of the STAT3 signaling pathway, which can suppress apoptosis by inducing downstream genes, including Bcl-xL and Bcl-2.31
While some studies have discussed several promising mechanisms, the proposed therapeutic effect of propolis needs further investigation to better characterise the mechanism by which it exerts the observed therapeutic effect.26,32 The push-and-pull between the anti-inflammatory and proinflammatory cytokines are believed to determine the outcome of AP.33,34 Immune-modifying therapeutic approaches have been used for many inflammatory conditions with the aim of promoting the release of the anti-inflammatory cytokines and/or hindering the release of the inflammatory cytokines. Recently, Lattanzi et al. provided evidence for the potential contributions of the inflammatory reaction and/or inflammatory-induced oxidative stress in the aetiopathogenesis of several complicated disorders such as acute stroke and degenerative and secondary dementia.35 The anti-inflammatory properties of propolis could suggest novel pathophysiology-oriented treatment options in the management of various medical conditions where inflammation plays a pivotal role in the development and progression of tissue damage.36
Conclusion
Propolis attenuated the severity of inflammation of the pancreatic tissues of rats with L-arginine-induced AP. Therefore, propolis could be investigated as a potential treatment for many inflammatory conditions in humans.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
Financial support was received from King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia (M S 302-35).
References
- 1.Goulden MR. The pain of chronic pancreatitis: a persistent clinical challenge. British journal of pain. 2013;7:8–22. doi: 10.1177/2049463713479230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bülbüller N, Doğru O, Umaç H, Gürsu F, Akpolat N. [The effects of melatonin and pentoxiphylline on L-arginine induced acute pancreatitis]. Ulus Travma Acil Cerrahi Derg. 2005;11:108–14. [PubMed] [Google Scholar]
- 3.Banks PA, Freeman ML. Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–62. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–42. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- 6.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–14. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 7.Greenaway W, Scaysbrook T, Whatley FR. The composition and plant origins of propolis: A report of work at Oxford. Bee World. 1990;71:107–18. doi: 10.1080/0005772X.1990.11099047. [DOI] [Google Scholar]
- 8.Okutan H, Ozcelik N, Yilmaz HR, Uz E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin Biochem. 2005;38:191–6. doi: 10.1016/j.clinbiochem.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hariri MT, Eldin TAG, Al-Harb MM. Protective effect and potential mechanisms of propolis on streptozotocin-induced diabetic rats. J Taibah Univ Med Sci. 2016;11:7–12. doi: 10.1016/j.jtumed.2015.11.002. [DOI] [Google Scholar]
- 10.Rossi A, Ligresti A, Longo R, Russo A, Borrelli F, Sautebin L. The inhibitory effect of propolis and caffeic acid phenethyl ester on cyclooxygenase activity in J774 macrophages. Phytomedicine. 2002;9(6):530–5. doi: 10.1078/09447110260573164. [DOI] [PubMed] [Google Scholar]
- 11.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase- 2 in human oral epithelial cells and in a rat model of inflammation. Cancer research. 1999;59:2347–52. [PubMed] [Google Scholar]
- 12.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–52. [PubMed] [Google Scholar]
- 13.Dawra R, Saluja AK. L-arginine-induced experimental acute pancreatitis. Pancreapedia: Exocrine Pancreas Knowl Base. 2012 doi: 10.3998/panc.2012.6. [DOI] [Google Scholar]
- 14.Kaur J, Sidhu S, Chopra K, Khan MU. Calendula officinalis ameliorates l-arginine induced acute necrotizing pancreatitis in rats. Pharm Biol. 2016;54:2951–9. doi: 10.1080/13880209.2016.1195848. [DOI] [PubMed] [Google Scholar]
- 15.Park EH, Kahng JH. Suppressive effects of propolis in rat adjuvant arthritis. Arch Pharm Res. 1999;22:554–8. doi: 10.1007/BF02975325. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Rana SV, Rana S, Bhasin DK, Nada R, Malhotra S. Severe chronic pancreatitis due to recurrent acute injury: Noninvasive chronic pancreatitis model of rat. JOP J Pancreas (Online) 2017;18:107–20. [Google Scholar]
- 17.Al-Hariri M, Eldin TG, Abu-Hozaifa B, Elnour A. Glycemic control and anti-osteopathic effect of propolis in diabetic rats. Diabetes Metab Syndr Obes. 2011;4:377–84. doi: 10.2147/DMSO.S24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frossard JL, Hadengue A, Pastor CM. New serum markers for the detection of severe acute pancreatitis in humans. Am J Respir Crit Care Med. 2001;164:162–70. doi: 10.1164/ajrccm.164.1.2008026. [DOI] [PubMed] [Google Scholar]
- 19.Shukla R, Santoro J, Bender FC, Laterza OF. Quantitative determination of human interleukin 22 (IL-22) in serum using Singulex-Erenna® technology. J Immunol Methods. 2013;390:30–4. doi: 10.1016/j.jim.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Bulut NE, Özkan E, Ekinci O, Dulundu E, Topaloğlu Ü, Şehirli AÖ, et al. Beneficial effects of alpha lipoic acid on cerulein-induced experimental acute pancreatitis in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:383–9. doi: 10.5505/tjtes.2011.99835. [DOI] [PubMed] [Google Scholar]
- 21.Rakonczay Z, Jr, Hegyi P, Dósa S, Iványi B, Jármay K, Biczó G, et al. A new severe cute necrotizing pancreatitis model induced by L-ornithine in rats. Crit Care Med. 2008;36:2117–27. doi: 10.1097/CCM.0b013e31817d7f5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263–6. doi: 10.1097/00004836-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73:S53–63. doi: 10.1016/S0367-326X(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 24.Büyükberber M, Savaş M, Bağci C, Koruk M, Gülşen MT, Tutar E, et al. The beneficial effect of propolis on cerulein-induced experimental acute pancreatitis in rats. Turk J Gastroenterol. 2009;20:122–8. [PubMed] [Google Scholar]
- 25.Hu F, Hepburn HR, Li Y, Chen M, Radloff SE, Daya S. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J Ethnopharmacol. 2005;100:276–83. doi: 10.1016/j.jep.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, Longo R, Russo A, Borrelli F, Sautebin L. The role of the phenethyl ester of caffeic acid (CAPE) in the inhibition of rat lung cyclooxygenase activity by propolis. Fitoterapia. 2002;73:S30–7. doi: 10.1016/S0367-326X(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 27.Qiao YY, Liu XQ, Xu CQ, Zhang Z, Xu HW. Interleukin-22 ameliorates acute severe pancreatitis-associated lung injury in mice. World J Gastroenterol. 2016;22:5023–32. doi: 10.3748/wjg.v22.i21.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8:249–57. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huan C, Kim D, Ou P, Alfonso A, Stanek A. Mechanisms of interleukin-22’s beneficial effects in acute pancreatitis. World J Gastrointest Pathophysiol. 2016;7:108–16. doi: 10.4291/wjgp.v7.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–80. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu XQ, Qiao YY, Xu CQ, Zhu ST, Xu HW. Protective effects of interleukin-22 on severe acute pancreatitis-associated kidney injury in mice. Austin Intern Med. 2017;2:1016. [Google Scholar]
- 32.Sud’ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21–4. doi: 10.1016/0014-5793(93)80184-V. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73:S53–63. doi: 10.1016/S0367-326X(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 34.Singh VK, Bollen TL, Wu BU, Repas K, Maurer R, Yu S, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098–103. doi: 10.1016/j.cgh.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophilto-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8:57489–94. doi: 10.18632/oncotarget.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lattanzi S, Carbonari L, Pagliariccio G, Bartolini M, Cagnetti C, Viticchi G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307–15. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]