Abstract
Objectives
This study aimed to evaluate the aetiologies of hyperprolactinaemia in the United Arab Emirates (UAE).
Methods
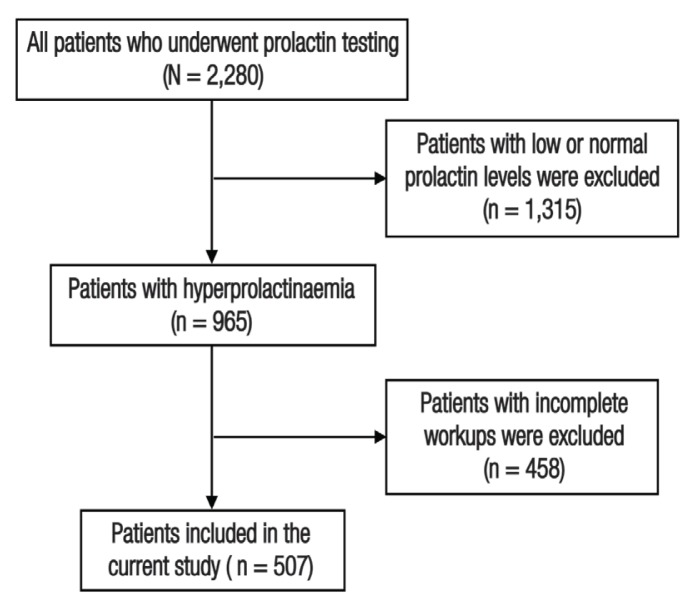
This retrospective study used laboratory databases to identify all patients who underwent evaluation for prolactin at Tawam Hospital, Al Ain, UAE, between 2009 and 2015. Of those 2,280 patients, all patients with low or normal prolactin (n = 1,315) were excluded. Subsequently, charts of the remaining patients (n = 965) with hyperprolactinaemia were reviewed and those with incomplete work-ups or insufficient documentation of the hyperprolactinaemia’s aetiology were excluded (n = 458).
Results
A total of 507 patients were included in the study. The average age at prolactin evaluation was 36 ± 13.2 years and the majority (67.1%) of patients were female. The most common reasons for requesting prolactin were menstrual disorders (29.5%), infertility (18%), evaluation of sellar masses (14.3%), ruling out seizures (13.4 %) and monitoring while on psychiatric medications (8.7%). The most common causes of hyperprolactinaemia were prolactinoma (17%), transient hyperprolactinaemia (14.6%), drug-induced side effects (14.4%), polycystic ovarian syndrome (11.8%) and seizure disorder (7.7%). In females, common aetiologies were prolactinomas, transient and idiopathic hyperprolactinaemia, while sellar masses, seizures, chronic kidney disease and acute illnesses were common aetiologies of hyperprolactinaemia in males. The prolactin level varied between the different aetiologies and a level of >250 ng/mL was suggestive of macro-prolactinoma.
Conclusion
A significant proportion of patients with hyperprolactinaemia have transient hyperprolactinaemia. Before further investigations are carried out, prolactin level assessment should be repeated, especially in patients with mild hyperprolactinaemia.
Keywords: Hyperprolactinemia, Prolactinoma, Etiology, Epidemiology, United Arab Emirates
Advances in Knowledge.
- A large proportion of patients with hyperprolactinaemia have a transient rise of prolactin that normalises spontaneously and requires no further evaluation.
- A prolactin level of >250 ng/mL is suggestive of the presence of macroprolactinoma.
Application to Patient Care
- To avoid unnecessary investigations, repeating prolactin level assessment is recommended in patients with mild hyperprolactinaemia.
- Knowledge of prolactin levels may predict the aetiology of hyperprolactinaemia and narrow the differential diagnosis.
Prolactin is an important hormone of the anterior pituitary gland with diverse effects on the body’s systems, especially in the reproductive system.1 Hyperprolactinaemia, defined as a prolactin level above the laboratory gender-specific normal range, is a common endocrine problem worldwide. 2–4 It is more common in females and its prevalence has increased over the last two decades.5 Hyperprolactinaemia can cause menstrual disorders, gynaecomastia, decreased libido, impotence and infertility.1
Hyperprolactinaemia can be caused by physiological changes, pathological conditions, medications, macroprolactin excess or can be idiopathic.6,7 The main physiological causes are pregnancy, lactation and stress, while common pathological causes include prolactinomas, other sellar masses, polycystic ovarian syndrome (PCOS), chronic kidney disease (CKD) and hypothyroidism.6,7 Antipsychotics, antidepressants and anti-emetics are among the most common medications that cause hyperprolactinaemia. 8 Macroprolactin is a large molecule of prolactin mostly attached to immunoglobulins and can result in hyperprolactinaemia due to reduced renal clearance.9
Knowledge of prevalence and causes of hyperprolactinaemia primarily stem from studies conducted outside the Middle East and North Africa (MENA) region.3–5,10 To the best of the authors’ knowledge, no data have been published on the aetiology of hyperprolactinaemia in the United Arab Emirates (UAE). Therefore, this study aimed to determine the causes of hyperprolactinaemia in a large referral centre in the UAE.
Methods
This retrospective study was conducted at Tawam Hospital, Al Ain, UAE, between 2009 and 2015. Tawam Hospital is the largest tertiary healthcare centre in Al Ain, a major city of the Abu Dhabi Emirate. Using an electronic laboratory database, a total of 2,280 patients with at least one laboratory record for serum prolactin were identified [Figure 1]. Of those identified, 1,315 patients with normal prolactin levels (male range: 2.6–13.1 ng/mL, female range: 3–27 ng/mL) were excluded. Additionally, 458 patients were excluded due to incomplete work-ups or insufficient documentation of their hyperprolactinaemia’s aetiology. The electronic medical records of the remaining patients were reviewed and demographic and clinical data were extracted.
Figure 1.
Flow chart showing this study’s inclusion process of hyperprolactinaemic patients who underwent evaluation for serum prolactin level at Tawam Hospital, Al Ain, United Arab Emirates, between 2009 and 2015.
Hyperprolactinaemia was defined as a serum prolactin >13.1 ng/mL in males and >27 ng/mL in females. Only one type of prolactin assay (Beckman Dxl 800, Beckman Coulter Inc, Atlanta, Georgia, USA) was used in this study; this assay has superior specificity for monomeric prolactin, therefore screening for macroprolactin excess was not routinely performed in the current cohort of patients. Prolactinomas were diagnosed based on elevated prolactin levels and evidence of pituitary adenomas from magnetic resonance imaging (MRI) scans.11 Prolactinomas were classified as macroprolactinomas (≥1 cm) or microprolactinomas (<1 cm) based on the size of the tumours. Prolactin levels <200 ng/mL (off-treatment) in patients with a sellar mass >1 cm were considered to be suggestive of the stalk effect.12 PCOS was accepted as the cause of hyperprolactinaemia if a diagnosis was documented by an experienced gynaecologist or endocrinologist. Hypothyroidism was considered responsible for hyperprolactinaemia in the absence of other causes of hyperprolactinaemia, a simultaneous elevation of both thyroid stimulating hormones (TSH) and prolactin levels and restoration of normal prolactin level after normalisation of serum TSH. Patients were designated as having drug-induced hyperprolactinaemia if they were receiving medications recognised to elevate prolactin such as anti-emetics, antipsychotics, tricyclic antidepressants, serotonin re-uptake inhibitors and dopamine antagonists. Idiopathic hyperprolactinaemia was diagnosed in patients with persistent hyperprolactinaemia and a negative pituitary MRI in the absence of other causes. Hyperprolactinaemia attributed to acute illness was diagnosed in patients with acute pain or infection, in those admitted to the intensive care unit and in the absence of any secondary cause of prolactin elevation. Transient hyperprolactinaemia was diagnosed in patients who had unexplained elevation of prolactin in a single reading that normalised spontaneously on repeat testing.
Data were extracted using Microsoft Excel 2011 (Microsoft Corp., Redmond, Washington, USA) and analysed in Stata SE, Version 15.1 (StataCorp LLC, College Station, Texas, USA). Qualitative variables were summarised using descriptive statistics. Results were presented as absolute and relative frequencies and quantitative variables as mean ± standard deviation or median with minimum and maximum as appropriate.
The study was approved by the Al Ain Medical District Human Research Ethics Committee (CRD 471/16, AAMDHREC Protocol No. 16/58).
Results
A total of 507 patients with hyperprolactinaemia were included in this study. The mean age of patients at the time of prolactin evaluation was 36 ± 13.2 years. Most patients were female (67.1%) and UAE nationals (76%). The reason for ordering prolactin testing was available for 461 patients (90.9%). The test was most commonly used to evaluate menstrual disorders (29.5%), infertility (18%) and sellar masses (14.3%) and to rule out seizures (13.4%). In females, prolactin was requested most commonly to investigate menstrual disorders, infertility and hirsutism compared to excluding true seizures and evaluating sellar masses and infertility in males [Table 1].
Table 1.
Common reasons for requesting serum prolactin level testing in patients with hyperprolactinaemia (N = 461)
| Reason* | n (%)† | ||
|---|---|---|---|
| Female (n = 316) | Male (n = 145) | ||
| Menstrual disorders | 136 (43) | - | 136 (29.5) |
| Infertility | 57 (18) | 26 (17.9) | 83 (18) |
| Work-up/follow-up on sellar masses | 33 (10.4) | 33 (22.8) | 66 (14.3) |
| To exclude seizures | 18 (5.7) | 44 (30.3) | 62 (13.4) |
| Screening while on psychiatric drugs | 23 (7.3) | 17 (11.7) | 40 (8.7) |
| Hirsutism | 31 (9.8) | - | 31 (6.7) |
| Galactorrhoea | 24 (7.6) | 3 (2.1) | 27 (5.9) |
| Other‡ | 30 (9.5) | 14 (9.7) | 44 (9.7) |
| Erectile dysfunction | - | 13 (9) | 13 (2.8) |
Reasons for prolactin testing were unknown in 46 patients (25 females and 21 males).
Percentages do not add up to 100 as patients may have had multiple reasons.
Other reasons included headaches (n = 9), hypopituitarism (n = 7), lactation difficulties (n = 7), breast pain (n = 6), gynaecomastia (n = 5), delayed puberty (n = 3), testicular pain (n = 2), recurrent miscarriages (n = 2), decreased libido (n = 2) and acne (n = 1).
The most common causes were prolactinomas (17%), transient hyperprolactinaemia (14.6%) and druginduced hyperprolactinaemia (14.4%). Of the 86 patients with prolactinomas, 27 patients had macroprolactinomas. Of the 73 patients with transient hyperprolactinaemia, 58 patients were females and 27 underwent prolactin measurement for evaluating infertility. Of the 73 patients who were diagnosed with drug-induced hyperprolactinaemia, 22 were on antipsychotics, 15 were on antimotility drugs, three were taking anti-depressants and one was on anti-epileptic monotherapy. Fewer patients (n = 32) were using multiple medications. The most frequently used monotherapy drugs were risperidone (41.5%), metoclopramide (19.5%) and domperidone (17.1%). In total, 60 patients (11.8%) were diagnosed with PCOS and seizures were present in 7.7% of cases. A small proportion of patients (6.7%) had been diagnosed with sellar masses other than prolactinomas. Microprolactinomas and transient hyperprolactinaemia were more frequently diagnosed in females compared with other sellar masses, seizures, CKD and acute illnesses, all of which were found to be related to hyperprolactinaemia in males [Table 2].
Table 2.
The aetiologies of hyperprolactinaemia in a sample of hyperprolactinaemic patients (N = 507)
| Cause of hyperprolactinaemia | n (%)* | ||
|---|---|---|---|
| Female (n = 340) | Male (n = 167) | ||
| Prolactinoma | 63 (18.5) | 23 (13.8) | 86 (17) |
| Microprolactinoma | 48 (14.1) | 11 (6.6) | 59 (11.6) |
| Macroprolactinoma | 15 (4.4) | 12 (7.2) | 27 (5.3) |
| Transient | 58 (17.1) | 16 (9.6) | 74 (14.6) |
| Drug-induced | 48 (14.1) | 25 (15) | 73 (14.4) |
| PCOS | 60 (17.6) | - | 60 (11.8) |
| Seizure | 10 (2.9) | 29 (17.4) | 39 (7.7) |
| Sellar masses excluding prolactinomas | 12 (3.5) | 22 (13.2) | 34 (6.7) |
| Acute illness | 11 (3.2) | 23 (13.8) | 34 (6.7) |
| Idiopathic | 25 (7.4) | 5 (3) | 30 (5.9) |
| CKD | 9 (2.6) | 19 (11.4) | 28 (5.5) |
| Pregnancy | 20 (5.9) | - | 20 (3.9) |
| Other† | 13 (3.8) | 3 (1.8) | 16 (3.2) |
| Empty sella syndrome | 8 (2.4) | 1 (0.6) | 9 (1.8) |
PCOS = polycystic ovarian syndrome; CKD = chronic kidney disease.
Percentages do not add up to 100 as reported causes are not mutually exclusive.
Other reasons included breastfeeding (n = 8), localised breast irritation/infection/surgery (n = 6), hypothyroidism (n = 4), hypoplastic pituitary (n = 1) and syncope (n = 1).
The highest median serum concentration of prolactin was 191 ng/mL (range: 14.6–2000 ng/mL) and was noted in patients with prolactinoma. For the other aetiologies, there was an overlap in the median prolactin level which was not predictive of aetiology. Patients with transient hyperprolactinaemia had a prolactin level ranging from 13.4–77 ng/mL. Similarly, mild-to-moderate hyperprolactinaemia was seen in PCOS cases with only one patient having a prolactin level >85 ng/mL. Prolactin levels in patients with drug-induced hyperprolactinaemia ranged from 19.6–240 ng/mL. Patients with other sellar masses had prolactin levels which ranged from 13–196 ng/mL; of which, 32 patients (94.1%) had levels <100 ng/mL and only two (5.9%) had levels >150 ng/mL. Overall, prolactin levels >250 ng/mL were only found in 14 patients (2.8%). In two patients (14.2%), hyperprolactinaemia was due to pregnancy while in the remaining cases it was due to prolactinoma. Serum prolactin levels higher than 500 ng/mL were only seen in macroprolactinoma patients [Table 3].
Table 3.
Frequency and serum prolactin levels of endocrine and non-endocrine causes of hyperprolactinaemia (N = 507)
| Cause of hyperprolactinaemia | n (%) | Median (minimum–maximum) in ng/mL |
|---|---|---|
| Endocrine | ||
| Prolactinoma | 86 (17) | 191 (14.6–2000) |
| Sellar masses except prolactinoma | 34 (6.7) | 41.3 (13–196) |
| PCOS | 60 (11.8) | 42.2 (27–92.2) |
| Empty sella syndrome | 9 (1.8) | 48.4 (29.4–78) |
| Hypothyroidism | 4 (0.8) | 28.3 (13.4–38) |
| Non-endocrine | ||
| Transient | 74 (14.6) | 32.5 (13.4–77) |
| Drug-induced | 73 (14.4) | 68.4 (19.6–240) |
| Seizures | 39 (7.7) | 37.9 (13.6–221.2) |
| Acute illness | 34 (6.7) | 32.4 (14–70.2) |
| Idiopathic | 30 (5.9) | 47.7 (13.6–115) |
| CKD | 28 (5.5) | 50.1 (13.9–151.7) |
| Pregnancy | 20 (3.9) | 87.4 (24–490) |
| Other* | 16 (3.2) | 48.2 (18–167.8) |
PCOS = polycystic ovarian syndrome; CKD = chronic kidney disease
Other reasons included breastfeeding (n = 8), localised breast irritation/ infection/surgery (n = 6), hypoplastic pituitary (n = 1) and syncope (n = 1).
Discussion
This is the first study to comprehensively describe the aetiologies of hyperprolactinaemia in a large cohort of patients presenting to a tertiary referral center in UAE. Results showed that a significant proportion of patients were diagnosed with transient hyperprolactinaemia.
Hyperprolactinaemia negatively affects gonadotropin releasing hormones and results in menstrual disorders and symptoms of hypogonadism.1 Hence, most patients in the current study were found to have undergone prolactin evaluation because of menstrual disorders, infertility or erectile dysfunction. A large number of patients underwent prolactin evaluation to rule out a seizure disorder because prolactin has been shown to be elevated in patients with seizures.13 In fact, levels two times above the baseline obtained 10–20 minutes after seizure activity have been reported as suggestive of seizure diagnosis with 46.1–60% sensitivity and 96% specificity.14 However, patients with syncope may also experience mild prolactin elevation.14 Furthermore, a recent study of patients admitted to an epilepsy monitoring unit to distinguish psychogenic from epileptic seizures questioned the role of prolactin, with 29% and 16% false positive and false negative rates, respectively.15 Therefore, physicians ordering prolactin level for assessing seizures or pseudo-seizures should be cognisant of the above limitations. Another reason for ordering prolactin testing was to evaluate sellar masses, which are relatively common with a prevalence of 0.1%.16 The distinction between prolactinomas and other sellar masses is an essential diagnostic step, as medical therapy is the treatment of choice for the former while surgery may be needed for the latter. Some of the current patients (7.8%) underwent prolactin testing to screen for hyperprolactinaemia while on psychotropic medications. This practice is in-line with the current guidelines advocating for hyperprolactinaemia screening in such patients because of the high prevalence (44%) of hyperprolactinaemia in this setting.17,18
In the current study, prolactinoma was the most frequent cause of hyperprolactinaemia (17%). Previous studies have similarly shown that prolactinoma is the most common cause of hyperprolactinaemia but with much higher rates (25.6–56.6%).4,5 The lower rate in the current study could be attributed to the patients’ heterogeneity and the inclusion of prolactin testing for both inpatients and outpatients.
Data on the prevalence of transient hyperprolactinaemia is limited. In the current study, transient hyperprolactinaemia was the second most common aetiology of hyperprolactinaemia and was diagnosed mostly in women being evaluated for infertility. One study documented a transient rise of prolactin in females during their mid-cycle; the rate rises to 94% in those with infertility, ranging between 25–75 ng/mL and usually lasting 1–3 days.19 Additionally, prolactin follows a circadian rhythm with the highest reading occurring during sleep and immediately after awakening.12 It also increases after sexual intercourse, following a protein-rich meal and during stress.12 Whyte et al. reported using antecubital fossa cannula to avoid stress, normalising prolactin levels in 17% of referred patients with hyperprolactinaemia with repeat testing only; this increased by 10% after a 120-minute rest period.20 Moreover, prolactin levels ≥94 ng/mL had 97% specificity in identifying true hyperprolactinaemia.20 In keeping with this finding, the current patients had prolactin levels ranging between 13.4–77 ng/mL. As knowledge of stress at the time of blood extraction or other physiological processes described earlier may not be readily available to the physician, assessment of serum prolactin levels should be repeated in patients with mild hyperprolactinaemia or in the absence of typical symptoms. This recommendation conflicts with the Endocrine Society Guidelines, which suggest that a single prolactin evaluation is enough to diagnose hyperprolactinaemia. However, this recommendation supports other experts’ recommendations.7,20,21 Such an approach is expected to reduce both patient anxiety and unnecessary pituitary imaging costs.
Drugs such as antipsychotics, antidepressants and anti-emetics may alter the neuroendocrine control mechanisms regulating prolactin secretion and cause modest prolactin elevation.8 Medications inducing hyperprolactinaemia constituted 14% of all cases in the current study, where most patients were on antipsychotics or anti-emetics. Vilar et al. reported a similar rate (12%) in Brazil; however, the rate was much higher (45.9%) in Scotland with rising prevalence during the last years of the study.4,5 Recently, Alosaimi et al. reported a high prevalence of hyperprolactinaemia in psychiatric patients as well as guidelines that recommend screening for hyperprolactinaemia in such patients.18 The current institution is not a referral centre for psychiatric patients and has no inpatient psychiatric services, which may contribute to this difference. Any treatment modifications in psychiatric patients such as switching to agents with no or only a minor effect on prolactin levels (e.g. bupropion, mirtazapine and aripiprazole) or even initiating dopamine agonist therapy should be done cautiously and in consultation with the treating psychiatrist to avoid exacerbating underlying psychiatric conditions.8,21
PCOS is a common disorder in young adults and contributes to 13–16% of all cases of hyperprolactinaemia. 7 The rate in the current study is slightly lower (11.8%) as all cases of hyperprolactinemia were analysed without limiting the sample to outpatient cases as has been done in other studies. On the other hand, the rate in the current study might be over-estimated since Tawam Hospital is the largest and only governmental fertility centre in Al Ain. Moreover, no data are available on the number of PCOS patients who had pituitary MRIs; therefore, microprolactinomas may have been missed. However, the prolactin levels in those patients were moderate, ranging between 28–92 ng/mL and only one patient (1.7 %) had a prolactin level >85 ng/mL. A recent study suggested that in patients with PCOS, a prolactin >85 ng/mL might indicate the presence of adenomas with 77% sensitivity and 100% specificity.22
Sellar masses, excluding prolactinomas, could cause mild hyperprolactinaemia by inhibiting dopamine transport on the hypothalamus-pituitary axis. In this study, 6.7% of all cases of hyperprolactinaemia had other sellar masses with a median prolactin level of 33 ng/mL; the measured levels rarely rose above 100 ng/mL. Vilar et al. showed that a non-functioning adenoma contributed to 6.6% of all hyperprolactinaemia cases with 82% of patients having prolactin levels <100 ng/mL.4
Idiopathic hyperprolactinaemia has been reported as the cause of hyperprolactinaemia in 3.6–27.8% of all cases. This wide range reflects the differences found in the studied population and may be due to possible underestimation of macroprolactin or an incomplete work-up of patients.3–5 In the current study, 3.2% of patients were diagnosed with idiopathic hyperprolactinaemia; none had adenoma on a pituitary MRI scan.
Prolactin levels were shown to be predictive of macroprolactinoma in the current study as patients with macroprolactinomas had the highest prolactin levels. Except during pregnancy (n = 2), all other patients with prolactin levels >250 ng/mL were diagnosed with macroprolactinomas. Similarly, Vilar et al. found that only 4.2% of non-prolactinoma patients had prolactin levels >250 ng/mL and readings of >500 ng/mL were seen exclusively in patients with prolactinoma.4 Prolactin levels varied and were of no predictive value for other aetiologies.
The current study had several strengths. It is the largest study assessing hyperprolactinaemia in the MENA region. Furthermore, real-world data have been reported on all inpatients and outpatients with hyperprolactinaemia, unlike many previous reports primarily focusing on patients encountered in outpatient clinics. Also, a single prolactin assay was used in this study. However, the study also has some limitations. As this was a single centre study, the results may not be generalisable to other clinical settings. Additionally, data were collected retrospectively. In total, 20% of the patients with hyperprolactinaemia were excluded because of an incomplete work-up. Also, macroprolactinaemia may have been underdiagnosed in the study as macroprolactin is not screened routinely. Excessive evaluation of patients for hyperprolactinaemia could have been conducted by non-endocrinologists with limited knowledge of macroprolactin interference.
Conclusion
Prolactin testing is most commonly requested for evaluation of menstrual disorders, infertility and sellar masses. As a sizable proportion of the current patients had transient hyperprolactinaemia, repeated prolactin measurement is recommended before further investigations are considered. Prolactinomas and medications were the commonest causes of non-transient hyperprolactinaemia in the current study. A prolactin level >250 ng/mL was highly suggestive of macroprolactinoma.
ACKNOWLEDGEMENTS
The authors would like to thank Mrs Amani Alshamisi for extracting the laboratory data and Mr Anvar Kandanath for his help with data management.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
No funding was received for this study.
References
- 1.Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: Pathological implications. Nat Rev Endocrinol. 2015;11:265–75. doi: 10.1038/nrendo.2015.36. [DOI] [PubMed] [Google Scholar]
- 2.Hussein SH, Wahedi TS, Johani NA, Hakami YA, Alzahrani K, AlMalki MH. Clinical and epidemiological characteristics of pituitary tumours in a single centre in Saudi Arabia. Hormones (Athens) 2018;17:261–7. doi: 10.1007/s42000-018-0030-8. [DOI] [PubMed] [Google Scholar]
- 3.Zargar AH, Laway BA, Masoodi SR, Bhat MH, Wani AI, Bashir MI, et al. Clinical and etiological profile of hyperprolactinemia--data from a tertiary care centre. J Assoc Physicians India. 2005;53:288–90. [PubMed] [Google Scholar]
- 4.Vilar L, Freitas MC, Naves LA, Casulari LA, Azevedo M, Montenegro R, Jr, et al. Diagnosis and management of hyperprolactinemia: Results of a Brazilian multicenter study with 1234 patients. J Endocrinol Invest. 2008;31:436–44. doi: 10.1007/BF03346388. [DOI] [PubMed] [Google Scholar]
- 5.Soto-Pedre E, Newey PJ, Bevan JS, Greig N, Leese GP. The epidemiology of hyperprolactinaemia over 20 years in the Tayside region of Scotland: The Prolactin Epidemiology, Audit and Research Study (PROLEARS) Clin Endocrinol (Oxf) 2017;86:60–7. doi: 10.1111/cen.13156. [DOI] [PubMed] [Google Scholar]
- 6.Serri O, Chik CL, Ur E, Ezzat S. Diagnosis and management of hyperprolactinemia. CMAJ. 2003;169:575–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Vilar L, Fleseriu M, Bronstein MD. Challenges and pitfalls in the diagnosis of hyperprolactinemia. Arq Bras Endocrinol Metabol. 2014;58:9–22. doi: 10.1590/0004-2730000003002. [DOI] [PubMed] [Google Scholar]
- 8.Vilar L, Abucham J, Albuquerque JL, Araujo LA, Azevedo MF, Boguszewski CL, et al. Controversial issues in the management of hyperprolactinemia and prolactinomas - An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab. 2018;62:236–63. doi: 10.20945/2359-3997000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimatsu A, Hattori N. Macroprolactinemia: Diagnostic, clinical, and pathogenic significance. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/167132. 167132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saejong R, Dangrat C, Techatrisak K, Angsuwatthana S, Rattanachaiyanont M, Tanmahasamut P. Hyperprolactinemia: A 12-year retrospective study at gynecologic endocrinology unit, Siriraj Hospital. J Med Assoc Thai. 2013;96:1247–56. [PubMed] [Google Scholar]
- 11.Byrne B, O’Shea P, Barrett P, Tormey W. The Beckman DxI 800 prolactin assay demonstrates superior specificity for monomeric prolactin. Clin Chem Lab Med. 2010;48:205–8. doi: 10.1515/CCLM.2010.038. [DOI] [PubMed] [Google Scholar]
- 12.López MAC, Rodríguez JLR, García MR. Physiological and pathological hyperprolactinemia: Can we minimize errors in the clinical practice? In: Nagy GM, Toth BE, editors. Prolactin. Rijeka, Croatia: InTech; 2013. pp. 213–30. [DOI] [Google Scholar]
- 13.Pritchard PB, 3rd, Wannamaker BB, Sagel J, Daniel CM. Serum prolactin and cortisol levels in evaluation of pseudoepileptic seizures. Ann Neurol. 1985;18:87–9. doi: 10.1002/ana.410180115. [DOI] [PubMed] [Google Scholar]
- 14.Chen DK, So YT, Fisher RS Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:668–75. doi: 10.1212/01.wnl.0000178391.96957.d0. [DOI] [PubMed] [Google Scholar]
- 15.Abubakr A, Wambacq I. Diagnostic value of serum prolactin levels in PNES in the epilepsy monitoring unit. Neurol Clin Pract. 2016;6:116–19. doi: 10.1212/CPJ.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Dahmani K, Mohammad S, Imran F, Theriault C, Doucette S, Zwicker D, et al. Sellar masses: An epidemiological study. Can J Neurol Sci. 2016;43:291–7. doi: 10.1017/cjn.2015.301. [DOI] [PubMed] [Google Scholar]
- 17.Grigg J, Worsley R, Thew C, Gurvich C, Thomas N, Kulkarni J. Antipsychotic-induced hyperprolactinemia: Synthesis of worldwide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology (Berl) 2017;234:3279–97. doi: 10.1007/s00213-017-4730-6. [DOI] [PubMed] [Google Scholar]
- 18.Alosaimi FD, Fallata EO, Abalhassan M, Alhabbad A, Alzain N, Alhaddad B, et al. Prevalence and risk factors of hyperprolactinemia among patients with various psychiatric diagnoses and medications. Int J Psychiatry Clin Pract. 2018;22:274–81. doi: 10.1080/13651501.2018.1425459. [DOI] [PubMed] [Google Scholar]
- 19.Ben-David M, Schenker JG. Transient hyperprolactinemia: A correctable cause of idiopathic female infertility. J Clin Endocrinol Metab. 1983;57:442–4. doi: 10.1210/jcem-57-2-442. [DOI] [PubMed] [Google Scholar]
- 20.Whyte MB, Pramodh S, Srikugan L, Gilbert JA, Miell JP, Sherwood RA, et al. Importance of cannulated prolactin test in the definition of hyperprolactinaemia. Pituitary. 2015;18:319–25. doi: 10.1007/s11102-014-0576-7. [DOI] [PubMed] [Google Scholar]
- 21.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 22.Kyritsi EM, Dimitriadis GK, Angelousi A, Mehta H, Shad A, Mytilinaiou M, et al. The value of prolactin in predicting prolactinoma in hyperprolactinaemic polycystic ovarian syndrome. Eur J Clin Invest. 2018;48:e12961. doi: 10.1111/eci.12961. [DOI] [PubMed] [Google Scholar]