Abstract
Seed germination traits are key drivers of population dynamics, yet they are under-represented in community ecology studies, which have predominately focussed on adult plant and seed morphological traits. We studied the seed traits and germination strategy of eight woody plant species to investigate regeneration strategies in the arid zone of eastern Australia. To cope with stochastic and minimal rainfall, we predict that arid seeds will either have rapid germination across a wide range of temperatures, improved germination under cooler temperatures, or dormancy and/or longevity traits to delay or stagger germination across time. To understand how temperature affects germination responses, seeds of eight keystone arid species were germinated under laboratory conditions, and under three diurnal temperatures (30/20°C, 25/15°C and 17/7°C) for 30 days. We also tested for decline in seed viability across 24 months in a dry-aging treatment (~20°C). Six of the eight arid species studied had non-dormant, rapidly germinating seeds, and only two species had physiological dormancy traits. Seed longevity differed widely between species, from one recalcitrant species surviving only months in aging (P50 = <3 months) and one serotinous species surviving for many years (P50 = 84 months). Our results highlight the importance of understanding the reproductive strategies of plant species in arid environments. Rapid germination, the dominant seed trait of species included in this study, allows arid species to capitalise on sporadic rainfall. However, some species also exhibit dormancy and delayed germination; this an alternative strategy which spreads the risk of germination failure over time.
Introduction
Seed traits and germination strategies drive plant community dynamics and provide insight into species’ adaptations to environmental filters [1] and community composition [2]. Despite this, seed traits are under-represented in community ecology studies [3–5]. Knowledge of seed traits and germination strategies is necessary to describe plant niches, to anticipate population dynamics under changes in land use [6], and to assess plant responses to the environment [7]. By studying seed traits and germination responses we can obtain ecologically meaningful data about the functional properties of plant communities that improve predictions of plant assemblages under natural, and anthropogenic, environmental change [8].
Seed traits and germination strategies, which are often unrelated to other plant traits [9], can inform us about the reproductive strategies of species occurring in particular environments [10]. Seed mass is the most widely studied seed trait, and there are strong correlations among seed mass, germination rate, survival and establishment [11, 12]. Small seeds generally germinate faster than heavy seeds [13], however the chance of survival and establishment at the seedling or later plant stages is greater for heavier seeds [14, 15]. Furthermore, small-seeded species are generally able to produce more seeds [16]. Physiological responses of seeds to environmental cues enable germination to occur when conditions are most suitable for seedling establishment [17, 18]. Conversely, seedling mortality can increase if germination occurs in unfavourable environmental conditions; thus germination timing has strong fitness consequences [19–21].
The primary abiotic filters for recruitment of most arid plant species are water availability and the season it occurs in, and seeds of arid zone plants typically exhibit adaptive traits to tolerate or avoid drought conditions [22, 23]. Rainfall events that last several days are rare in arid zones, and smaller rainfall events are likely to result in the drying of upper soil layers before the germination process is complete [24], causing seedling mortality after germination. Conversely, a rainfall event during winter may present slower evaporation rates from the soil surface than during summer. Hence, for species that rely on avoidance strategies to survive drought conditions, the timing of recruitment must coincide with periods of reliable soil water availability [25]. Dormancy is an important drought avoidance mechanism for arid species as it restricts germination during unsuitable environmental conditions [26], whereas rapid germination is a drought tolerance strategy to facilitate the rapid exploitation of temporarily favourable conditions [27]. The evolution towards mature seeds with less nutritive tissue available to the embryo has enabled seeds to germinate faster [28, 29] and emerge when rainfall events are short.
The level of seed dormancy can vary among seeds within a population so that germination of individuals occurs over several seasons [30–32]. This reproductive strategy has been termed bet-hedging, as it limits synchronous germination events, spreading the risk of germination failure across seasons. This increases long-term fitness by preventing the mortality of the entire seed cohort during unfavourable conditions [33], provided seeds survive through multiple seasons of unfavourable conditions. Hence, dormancy and longevity are key traits of bet-hedging. However, in unpredictable environments, selection may favour plants that can reproduce rapidly and frequently, and hence bet-hedging may be less prevalent.
In arid ecosystems, Baskin and Baskin [34] estimate that 85% of plant species produce dormant seeds. The prevalence of dormancy generally increases with aridity [35], but the influence of rainfall predictability on seed traits is not as well studied. Modelling by Brown and Venable [36] predicts that the prevalence of dormancy traits should increase with decreasing rainfall predictability. Conversely, Harel et al. [37] found that dormancy decreased with rainfall predictability in desert annuals, hence further studies are required to ascertain the prevalence of seed dormancy in relation to aridity. Seeds of arid zone plants are often characterised by faster germination rates than those from regions of higher rainfall [38, 39], after dormancy is overcome. Species that germinate quickly are able to utilise the short pulses of water availability, reducing the likelihood of seed mortality [40], while the seedlings of slower-germinating species may be limited to using dwindling water availability at the end of longer rainfall pulses [41]. Further empirical evidence is required from a greater suite of species (particularly perennials), and from a greater range of environments, to test the effect of rainfall predictability on seed traits and to determine the prevalence of dormancy in the arid zone.
This study investigates the seed traits and germination strategy of eight Australian, arid zone species. The species selected are considered as keystone plants, as they are the dominant species and the only prevalent woody species in the ecosystem. We focused on traits that may be critical to species’ persistence in arid ecosystems, and that are essential to understand if we are to successfully restore these species using seed. We hypothesise that seeds will be categorised by one of three strategies beneficial to recruitment in arid zones. To cope with stochastic and low rainfall, we predict that species adapted to the arid zone produce seeds that will either 1) have low seed mass but with rapid germination, across a wide range of temperatures, to ensure seedling establishment before soil moisture evaporates, 2) show improved germination under cool, winter temperatures where soil evaporation rates are lowest, or 3) have dormancy and/or longevity traits to delay or stagger germination, therefore spreading the risk of germination failure across time. Specifically, we measure seed dormancy and embryo traits, germination responses under different temperature regimes, and seed longevity under ambient aging conditions. We also test if seed mass, and other traits, are related to germination strategy and whether seed traits can be used as a proxy for germination strategy. We highlight important germination strategies of plants from an arid zone with stochastic rainfall, and discuss the evolution of seed traits that favour seed survival and germination when rainfall is infrequent and unpredictable.
Materials and methods
Study species and seed collection
We chose the following Australian arid zone species for this study: trees Casuarina pauper F.Muell. ex L.A.S.Johnson (Casuarinaceae), Myoporum platycarpum ssp. platycarpum R.Br., Geijera parviflora Lindl. (Scrophulariaceae), Alectryon oleifolius ssp. canescens S.T.Reynolds (Sapindaceae) and Hakea tephrosperma R.Br. (Proteaceae), and understory shrubs from the Chenopodiaceae family, Atriplex rhagodioides F.Muell., Maireana sedifolia (F.Muell.) Paul G.Wilson and Maireana pyramidata (Benth.) Paul G.Wilson. All species in this study are targeted for restoration after mineral sand mining. Vegetation communities prior to clearing include three major plant community types; shrublands dominated by M. sedifolia, woodlands dominated by C. pauper and M. platycarpum and woodlands with shrubland understory, dominated by C. pauper and understory shrubs M. sedifolia and M. pyramidata [42]. Less dominant tree species occur as small, scattered patches across the landscape and include H. tephrosperma, A. oleifolius and G. parviflora.
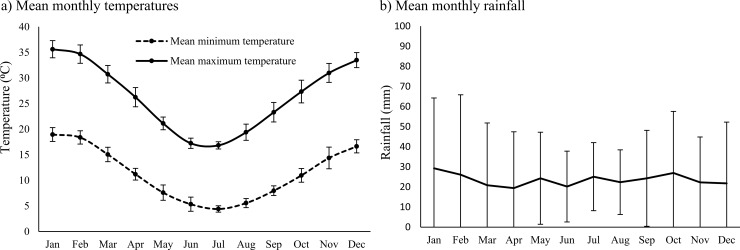
The climate of the study area is arid (250 mm mean annual rainfall) although average annual rainfall can often fall below 200 mm for consecutive years. Temperatures range from 2°C to 47°C, with cooler mean daily temperatures from May to August (Fig 1A; [43]). Across 60 years of climate data, average monthly rainfall was 24 mm and, unlike most arid zones across the globe, there is no distinct wet season (Fig 1B; [43]).
Fig 1.
(a) Mean monthly minimum and maximum temperatures from 2003–2016, and (b) mean monthly rainfall, from 1956–2016, at the study site. Unlike most other arid zones, there is no predictable wet season.
Mature seeds of each species were collected from remnant populations adjacent to a mine site (33°22’05”S, 142°13’36”E) at which restoration of these species is planned, however, for species with infrequent seeding events (H. tephrosperma, A. oliefolius and A. rhagodioides), seeds were collected within a 200-km radius of the mine site. Seeds were either collected by hand, donated from Ecotypic Pty Ltd and Tronox Mining, or purchased from Ogyris Pty Ltd. Where possible, seeds were collected within the six months prior to testing. However, due to lack of seeding events, it was necessary to use seeds stored for over one year in some species (seed ages shown in Table 1). Seeds were manually cleaned and stored in paper bags, under cool dark conditions. Bracts and seed covering structures were removed prior to seed weight measurements and germination experiments.
Table 1. Measured seed traits of species in this study.
| Species | Lifeform | Age (months) |
Dry mass (g) | SF (%) | V (%) | ET | E:S | I72 (%) | DT |
|---|---|---|---|---|---|---|---|---|---|
| Atriplex rhagodioides | Shrub | 12 | 0.06 (0.001) | 94 (0.6) | 67 (1.1) | Peripheral | >1 | 21 (1.4) | PD |
| Maireana. sedifolia | Shrub | 2 | 0.13 (0.001) | 97 (0.3) | 93 (0.5) | Peripheral | >1 | 30 (2.5) | ND |
| Maireana pyramidata | Shrub | 8 | 0.24 (0.005) | 89 (0.9) | 62 (0.9) | Peripheral | >1 | 16 (0.4) | ND |
| Casuarina pauper | Tree | 5 | 0.32 (0.007) | 72 (0.9) | 35 (0.6) | Investing | ~1 | 10 (0.6) | ND |
| Hakea tephrosperma | Tree | 16 | 3.31 (0.058) | 96 (0.8) | 96 (0.6) | Investing | ~1 | 91 (1.8) | ND |
| Alectryon oleifolius | Tree | 15 | 2.75 (0.111) | 68 (0.8) | 48 (1.2) | Bent | ~1 | 87 (3.4) | ND |
| Geijera parviflora | Tree | 2 | 2.95 (0.136) | 94 (0.5) | 88 (1.1) | Spatulate | 0.89 | 29 (0.7) | PD |
| Myoporum platycarpum | Tree | 0.5 | 0.26 (0.005) | 20 (0.7) | 13 (0.6) | Straight | ~1 | N/A | ND |
Age = age of seed used in tests; Dry mass = mean weight of 100 seeds; SF = initial seed fill; V = seed viability; ET = embryo type; E:S = embryo to seed ratio (seeds without endosperm indicated as ~1 and seeds with embryo longer than seed indicated as >1); I72 = seed mass increase at 72hr; and DT = dormancy type, where ND = non dormant and PD = physiological dormancy. The standard errors of means are shown in parentheses.
Seed traits: Embryo type, mass, viability and imbibition
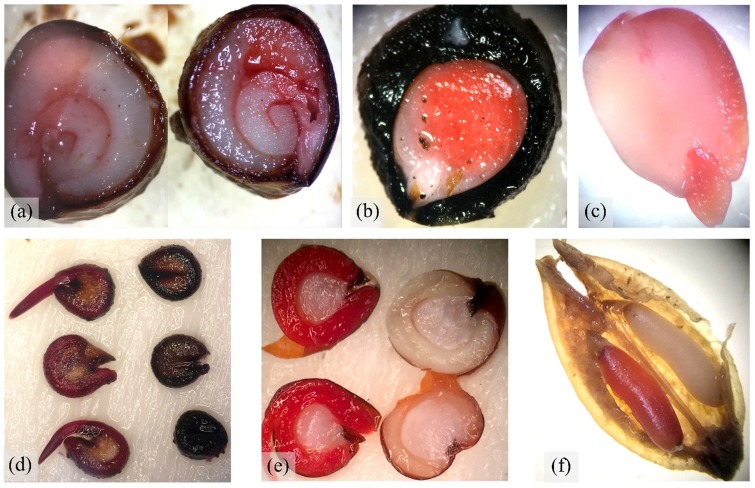
For each species, the ratio of embryo length to seed length (E:S) was calculated based on longitudinal dissections and measurements of 50 fully-imbibed seeds [44]. Embryo type was classified according to Martin [45]. Seed viability was assessed in a 1% solution of 2,3,5-triphenyl tetrazolium chloride (TZ), except for H. tephrosperma, for which seeds were incubated on moist filter paper at 30/20°C due to consistently poor TZ stain results; they gave a germination response of 100%. Embryos that only partially absorbed stain were scored as weakly viable (Fig 2A, 2B and 2C) and were classified as non-viable seeds. To ensure accurate TZ interpretations, results were frequently compared to tests for viability by germinating seeds at diurnal temperatures at 30/20°C, and according to the germination methods outlined below. Seed mass was determined using the mean of three replicates of 100 seeds, with results then divided by 100 to represent weight (g) per seed.
Fig 2.
Microscope images of TZ stained seeds for viability tests, including (a) A. oleifolius, (b) G. parviflora, (c) C. pauper, (d) M. pyramidata, e) A. rhagodioides, and (f) M. platycarpum. Weakly viable seeds were only partially stained and considered not viable (a-c). Seeds with viable embryos stained red (left in images d-f) and unviable embryos remained unstained (right-hand-side in d-f).
For imbibition tests, four replicates of 25 seeds were weighed, placed on moist filter paper, and incubated under three diurnal temperatures of 30/20°C, 25/15°C and 17/7°C, under a 12 hr light/dark schedule. Seeds were weighed when dry at beginning of experiment and, after 5 min on moist filter paper, seeds were removed and patted dry with a paper towel to absorb surface moisture, and weighed. To determine increases in seed weight, seeds were re-weighed at 10 min, 30 min and at 1, 2, 3, 6, 9, 24, 48, 72, 96, 120, 144, 168 and 192+ hrs. All bracts and seed coverings were removed for imbibition, germination and viability tests, but they were retained for the longevity experiment as this is how seeds are currently stored after collection. For one species (G. parviflora), additional tests were performed in an attempt to alleviate dormancy, including the move-along method (as described by Baskin & Baskin [46]) and soaking in boiling water and 90% H2SO4, both treatments for 1 min, 30 min and at 1, 4, 12, 24 and 48 hours.
Germination responses under diurnal temperatures
Prior to germination treatments, seeds of all species were surface sterilised by soaking in 1% sodium hypochlorite for one minute, then rinsed for 40 seconds with double distilled water. For each species, four replicates of 25 seeds each were used. Seeds were placed in 90-mm diameter petri dishes on filter paper moistened with distilled water and incubated at a 12/12-hr light/dark regime at daily alternating temperatures of 30/20°C, 25/15°C and 17/7°C). Seeds were incubated in cabinets (Thermoline Scientific, temperature and humidity cabinet, Model: TRISLH-495-1-s.d., Vol. 240, Sydney, Australia) under cool-white fluorescent lamps with a 40 μmol.m-2 photosynthetic photon flux. To determine the effects of gibberellic acid (a plant hormone herein referred to as GA3) on seed germination, species were incubated at 30/20°C and the filter paper was moistened with a 350 ppm GA3 solution. To prevent microbial outbreak and ensure constant hydration during germination tests, seeds were transferred to sterilised Petri dishes weekly, on new filter papers moistened with the same appropriate water/GA3 solutions. Seed germination (when the radicle emerged to at least half of seed size) was recorded daily for 30 days, or until germination ceased for four consecutive readings across all treatments. Dormancy type was classified according to Baskin and Baskin [46]. For two species that exhibited dormancy traits (G. parviflora and A. rhagodioides) an after-ripening treatment was applied by storing seeds for one year, under constant dark and air-conditioned temperatures between 10–20°C and 45–50% humidity. An additional four replicate plates at each diurnal temperature were included for both of these species using the after-ripened seeds. Seeds for the treatments with and without after-ripening were from the same seed lot.
Longevity
Seed longevity was assessed to understand the relationship between longevity and dormancy classification. Seeds of species in this study are currently collected for restoration purposes and stored at the study location in air-conditioned shipping containers at ~20°C, hence we tested the effects of these aging conditions on seed longevity. Seeds were manually cleaned to remove excess organic matter from the seed batch, and stored in paper bags, under constant dark and air-conditioned temperatures between 10–20°C and 45–50% humidity. Temperature and humidity of aging treatments were monitored and recorded once a fortnight for 24 months. To test for viability loss with aging, 100 seeds were extracted at 0, 3, 6, 12, 18 and 24 months, and TZ stained as per viability testing methods listed above. Due to seed shortages in A. oleifolius, only 60 seeds were tested for viability at each longevity test.
Data analysis
Imbibition was calculated using increase in seed weight after 72 hours:
where Wi is mean mass of imbibed seeds and Wd is mean mass of dry seeds [47]. Viability of seed batches was tested in the days prior to experiment, and Viability-Adjusted Germination (VAG, herein referred to as germination) was calculated using the following equation [48]:
For each replicate dish, the time to minimum germination (tmin) was taken as the first day that germination was observed, time to 50% germination (t50) was the first day that germination was recorded at ≥50%, and time to maximum germination (tmax) was the first day at which the maximum germination was recorded. Mean tmin, t50 and tmax were calculated from the four replicates of each species at each diurnal temperature.
Loss of seed viability with aging was calculated as:
where is mean viability at day 0, and is mean viability at day of test. A Pearson test was used to test the correlation between seed weight and t50, longevity (P50) and seed fill, and between embryo type (ranked according to E:S) and t50. One-way ANOVAs were used to test the effects of diurnal temperatures on maximum germination and tmax. Shapiro-Wilk tests confirmed the normality of both maximum germination and t50 data prior to ANOVAs. Tukey HSD post-hoc tests were used to make multiple comparsions of means among diurnal temperature treatments. Welsh’s t-tests were performed to compare germination between water and GA3 treatents, and to test the effects of after-ripeing in A. rhagadioides.
A generalised linear model with binomial error and a probit link function was fitted to the seed longevity data (i.e. loss of viability over time), and thus fit the viability equation [49];
where v was the viability after p months in aging, σ is the standard deviation of the normal distribution of seed deaths in time, and Ki is the initial seed viability. An estimate of the time taken for seed viability to fall to 50% (P50) was calculated by solving for p when v = 50%. The Pearson test, Shapiro-Wilk tests, ANOVAs and GLMs were all conducted in R [50].
Results
The heaviest seeds had the longest germination times, hence there was a positive correlation between seed mas and t50 (S1 Fig; P = 0.034), albeit based on only six species with germination data. However, there was no correlation between seed mass and longevity (P50: R = 0.44, P = 0.28), between seed mass and seed fill (R = 0.23, P = 0.59), or between embryo type and t50 (R = -0.41, P = 0.42).
Viability and dormancy
Each species in the study showed high germination in at least one diurnal temperature treatment within two weeks and without pre-treatment, except for A. rhagodioides and G. parviflora. No germination was observed for G. parviflora at any of the diurnal temperatures tested, nor through further treatments to relieve dormancy (boiling water and H2SO4 soaks, GA3, after-ripening and the move-along method). Hence the dormancy characteristics of G. parviflora were not determined, and no further germination results for this species will be presented. Seed fill was high for all species, except M. platycarpum, which was also excluded from germination studies due to lack of viable seeds. Imbibition studies demonstrated that seeds of all species readily take up water within 72 hours of wetting (Table 1).
Effect of temperature on seed germination proportions
Temperature had no significant effect on maximum germination for C. pauper, M. sedifolia and A. oleifolius (P ≥ 0.2 across all temperatures). However, maximum germination was significantly lower at the warmest diurnal temperature of 30/20°C for M. pyramidata (P = 0.01) and to a lesser degree for H. tephrosperma (P = 0.045) and A. rhagodioides (P = 0.047; S1 Table).
Effect of temperature on seed germination rates
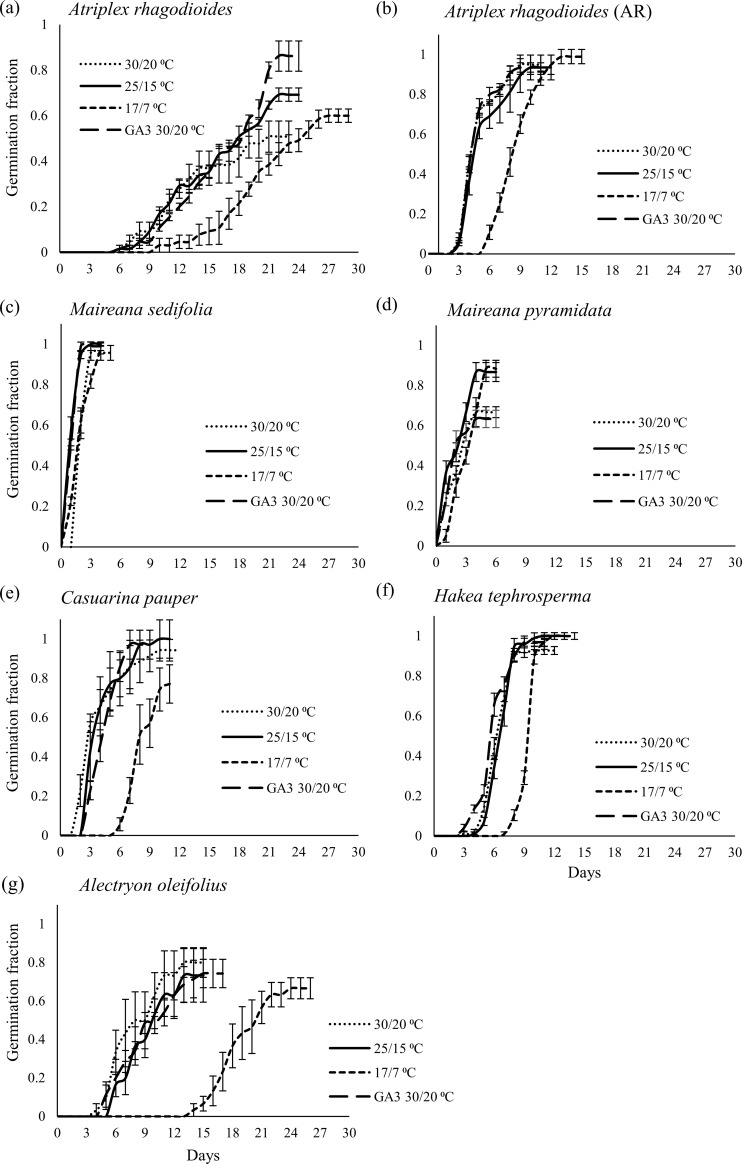
Non-dormant species germinated (tmin) rapidly and within six days in at least one diurnal temperature treatment (Fig 3). The two Maireana shrubs reached maximum germination quickest, within one to five days, while tree species were slower to germinate. Time to maximum germination (tmax) was significantly affected by temperature for all species except for C. pauper, with a general trend of decreasing germination times as diurnal temperatures increased (S2 Table). tmax was significantly longer in M. pyramidata and A. oleifolius at 17/7°C and 25/15°C than at 30/20°C (P ≤ 0.014). The species with germination times least affected by temperatures were H. tephrosperma which had significantly delayed germination only at the coldest temperature (17/7°C; P = 0.014), and C. pauper, which had no significant change in germination times across all temperatures (P = 0.25).
Fig 3. Cumulative germination (mean ± se) across diurnal temperatures: Seeds were incubated at 30/20°C, 25/15°C and 17/7°C for 30 days.
Data also includes results from seeds incubated at 30/20°C, with the addition of a growth stimulant (GA3).
Germination responses to GA3 and after-ripening
The only species with a positive germination response to GA3 treatments was A. rhagodioides (P = 0.010; P > 0.5 for all other species). A. rhagodioides achieved tmax in nine days with after-ripened seed compared to twenty days with fresh seed (Fig 3B and S2 Table). Similarly, for A. rhagodioides maximum germination of after-ripened seed was significantly higher than for fresh seed (P ≤ 0.04 for all temperatures), and it eliminated the effect of GA3 treatments (P = 0.36) and widened temperature ranges for maximum germination, indicating dormancy loss.
Seed longevity
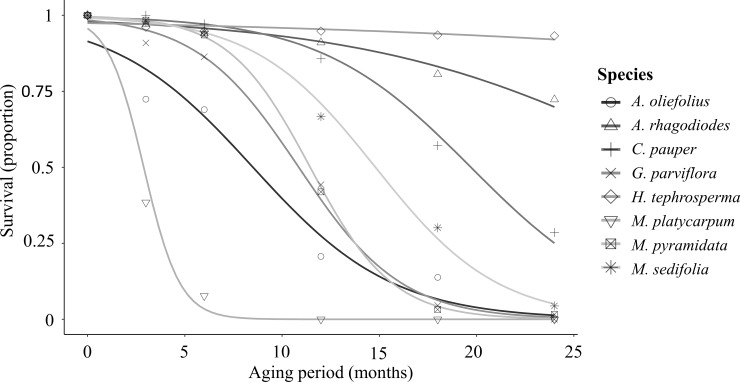
Myoporum platycarpum was the only species to exhibit characteristics of recalcitrant seeds, meaning they do not survive desiccation or aging. Seeds of M. platycarpum were freshly collected and most had no embryo, and of the few that had an embryo most were non-viable. Seed freshly picked from adult trees showed only 13% viability, which fell to half that within one month of aging, and was close to 0% viability within six months of aging (Fig 4). Most species showed a substantial decline in viability (>50%) within 12 months of aging, with the exception of H. tephrosperma, A. rhagodioides and C. pauper. These three species experienced less than 20% decline in viability within 12 months. The only species to show a decline in seed viability of <10% during 24 months of aging was H. tephrosperma. Seed longevity, or P50, for each species was (in order of longest to shortest lived, in months): H. tephrosperma, 84.1; A. rhagodioides, 32.0; C. pauper, 19.7; M. sedifolia, 14.7; M. pyramidata, 11.5; G. parviflora, 10.7; A. oleifolius, 8.7; M. platycarpum, 3.0.
Fig 4. Loss of seed viability with aging.
Seed age at beginning of experiment are show in Table 1.
Discussion
Arid species in this study were generally categorised by two types of adaptive strategies to facilitate seed germination in sporadic rainfall: 1) rapid germination across a wide range of diurnal temperatures, or 2) dormancy and/or long-lived seeds to temporarily delay, or stagger, germination. Our study demonstrates that rapid germination is a common, alternative and important strategy in seeds from arid zone species, which allows seeds to capitalise on sporadic rainfall. Seeds of three species (M. pyramidata, A. rhagodioides and H. tephrosperma) had significantly higher germination at cooler temperatures, than at 30/20°C, hence avoid germination when evaporation rates are highest [51]. Germination inhibition at hottest temperatures is considered an important germination strategy for seeds with dormancy or longevity in this study. We also demonstrated the tendency for seed weight and embryo type to be correlated to germination rate of the species in our study. Conversely, seed mass was unrelated to longevity and seed fill. We recommend further seed-mass germination studies, using a greater suite of indigenous species from similar bioregions, to better understand the role of seed morphology in prescribing germination behaviour.
Rapid germination across wide diurnal temperatures
All non-dormant seeds of species in this study exhibited rapid germination rates, which suggests that rapid germination is particularly well suited to this environment and may be at a selective advantage over other seed germination strategies. For temperate species, seeds that germinate in less than four days are considered to have rapid germination [27] although, for annual species, rapid germination means germinating within 24 hours [52]. Considering all species in this study are slow-growing, perennial, woody species, we herein refer to rapid germination as species that germinate (tmin) within 4–6 days. All species, except for dormant seeds of G. parviflora and A. rhagodioides, commenced germination within six days upon wetting and displayed 50% germination in ≤9 days at the highest temperatures tested. Rapid germinating species are able to take advantage of single rainfall events, whereas slow germinators require several rainfall events persisting across days [39], which rarely occurs in the arid zone studied here [51]. All species achieved >50% germination across all temperatures tested, suggesting they have wide thermal ranges for germination. Wide thermal ranges for germination allow seeds to take advantage of stochastic rainfall events that occur during any season. While this may enable opportunistic plant recruitment, additional studies should quantify hydrothermal niches for seed germination to further understand recruitment timing across different seasons.
Germination strategies, particularly those that affect the timing of germination such as dormancy, may be important determinants of population dynamics in arid ecosystems. For example, vegetation classification in this arid zone prior to disturbance was Belah-Rosewood Woodland and Belah-Bluebush Woodland [53], of which the dominant species include the non-dormant, fast germinators C. pauper and Maireana shrubs. The two species that have seeds with physiological dormancy, A. rhagodioides and G. parviflora, and non-dormant seeds of A. oleifolius, had the slowest germination speeds, are less dominant [54] and appear as scattered individuals throughout the landscape [53]. Although the age of seeds in this study varied and pre-storage components may have contributed to aging, longevity or dormancy loss in some species, reports of non-dormancy here are consistent with the findings of Callister [55] for all species, except M. platycarpum. Further studies are required to confirm dormancy in fresh seeds of C. pauper, H. tephrosperma and A. oleifolius. The lack of seeding events in these species during this study (potentially due to drought conditions) suggests that acquiring the quantities of seed required for their restoration may become more challenging under climate change.
Rapid germination appears to be unrelated to other morphological seed traits, except for seed mass and embryo type. All seeds in this study had fully developed embryos without an endosperm, or with embryos that are coiled and larger than the seed, and these are traits that are generally thought to indicate a rapid germination strategy [28, 29]. Germination for seeds with peripheral embryos (including Atriplex and Maireana) is typically very fast because it involves merely the uncoiling of the spiral embryo upon imbibition, which ruptures the seed coat [56]. Many such species with peripheral embryos inhabit high-stress environments, where the rapid exploitation of temporarily favourable conditions for germination is more important [40]. Our results showed that the size and development of embryos was not a consistent predictor of germination strategy.
The species in this study with the fastest germination rates (C. pauper, M. sedifolia and M. pyramidata), are dominant species from the region, and produced small seeds that were easier to obtain due to frequent and prolific seeding events. High seed production requires high maternal input but the risk of population crashes are mediated because these species are less dependent on high seed survival rates [1]. Other studies report strong evidence for survival advantages associated with larger seeds under stressful conditions [57–59] but, considering the variable size of arid seeds and the trade-off associated to increased seed production in small seeded species, survival advantages of large-seeded species does not appear large enough to counterbalance the advantage of small-seeded species during seed production [11, 60, 61]. We found that small seeded species were amongst the fastest to germinate, however the negative relationship between germination speed and seed size was weak, suggesting that seed size may not always be a reliable proxy for germination rate. Future studies are required to test a larger number of species of a greater order of magnitude of seed mass variation, prior to making general assumptions about the relationship between seed mass, germination rate and success.
While the germination responses of M. platycarpum were not classified in this study, Callister [55] shows that it germinates to 50% within 4–5 days following wetting, consistent with other non-dormant trees species in this study. Callister et al. [62] suggests that seed-dormancy, not seed viability, is the key factor limiting germination in M. platycarpum. In contrast, as observed in our study, examining embryo firmness and colour through dissection microscopy is not an appropriate method to assess seed viability in M. platycarpum because TZ results show that healthy looking embryo are often dead (Fig 2F). Alternative viability testing, by assessing seed metabolic rate, may provide more accurate viability assessment (see results in [63]) and should be considered for future testing. Our results suggest that seeds of M. platycarpum are non-dormant and that seed viability and short-lived seeds are the key factors inhibiting germination.
Low prevalence of seed dormancy
Most seeds of plants from the arid zone have dormancy traits that enable germination to coincide with periods of highest water availability [46, 64], yet only two species in this study (A. rhagodioides and G. parviflora) showed dormancy traits. These two species presented physiological dormancy traits as they readily imbibe water and have fully developed embryos, but failed to germinate to maximum potential within thirty days without treatment [46]. A. rhagodioides also showed a positive response to GA3 before after-ripening. The dormancy cues of G. parviflora remain unresolved as all treatments failed to relieve dormancy, and there was a lack of viable seed available for further germination treatments. Most species from the same family as G. parviflora, the Rutaceae family, produce seeds with physiological dormancy and are commonly associated with complex germination requirements [46, 65–67]. Fewer species showed signs of dormancy than was expected. There is potential that due to the age of seeds used for this study, some of the study species may have exhibited dormancy traits as fresh seed. We have classified four such species (A. oleifolius, C. pauper, H. tephrosperma and M. pyramidata) as non-dormant, and we do not believe there would be significant levels of dormancy in the fresh seed of these species. Callister [55] provides supporting evidence for this assertion for C. pauper and A. oleifolius. Local nurseries (including The Seeds of South Australia Database) and seed practitioners also support the claims of non-dormancy in these species (I. Sluiter, A. Quamby, T. Langdon, pers. comm. 2018), and there is evidence of non-dormancy in congeneric species within the genus [46]. Traits that increase the likelihood of germination coinciding with periods of highest water availability may be less important in environments with unpredictable wetting pulses.
Delayed germination through dormancy has been observed in many arid zone species [30–32]. Similarly, for A. rhagodioides, a small proportion of seeds germinate upon maturity, or upon seed dispersal, but germination rate and proportion improve as seeds age. This suggests that seeds can remain in the soil or canopy for years and stagger germination across seasons, which spreads the risk of recruitment failure through time and increases the probably that favourable conditions for seedling establishment will occur during the lifespan of a seed cohort [30, 68]. Physiological dormancy in A. rhagodioides was relieved through a period of after-ripening, a trait also reported in other Atriplex species [69, 70]. After-ripening also enables a wider temporal window for germination which suggests that germination opportunities increase as seeds after-ripen. Developing a short-term soil seedbank, before the onset of suitable rainfall events, is an important adaptation in response to the unpredictability of resource availability in arid ecosystems. However, A. rhagodioides and G. parviflora were the only dormant species in this study, and non-dormant and prolifically-seeding trees are the most dominant species of the region [71]. This again suggests that in arid zones without distinct wet seasons, playing it safe through dormancy may be less important than previously assumed.
The literature on bet-hedging predominately investigates annual species with short life-cycles (i.e. [72, 73]), whereas this study focuses on long-lived perennial species. Bet-hedging is more likely in annuals because the consequences of death of entire seed cohorts are greater [72, 73]. Comparative studies indicate that seed dormancy is higher in annuals than in perennials [74–76], although very few studies investigate bet-hedging in perennial shrubs [76, 77], and no such studies exist for arid tree species. Perennial species (including C. pauper, M. platycarpum and M. sedifolia) can have many flowering and seeding events throughout their lifespan. Thus, losing an entire seed cohort is of little consequence, reducing the selection pressure to maintain a soil seedbank [78, 79]. Additionally, C. pauper [80], H. tephrosperma [80], M. platycarpum [81] and A. oliefolius [82] have the capacity to regenerate via root suckers, which may further mediate the impact of losing entire seed cohorts for consecutive years, and possibly negate the need for dormancy. Our study supports the notion that perenniality selects against seed dormancy [74, 83, 84] however, we only tested dormancy in eight species and studies from other arid zones report dormancy in many perennial species that seed frequently. Therefore, we recommend further research testing the prevalence of dormancy for long-lived species that fruit prolifically.
The ability to delay seed release, through serotiny, may further mediate the likelihood of dormancy and bet-hedging in H. tephrosperma that has non-dormant seeds with exceptional seed longevity. Through delayed seed release, seeds of H. tephrosperma can likely persist is the canopy for many years following seed maturity, as reported in congeneric Hakea species [85]. Serotiny levels in H. tephrosperma have not been investigated to-date, although only one occurrence of seed release was observed in populations from the study site during the three years of this study (personal observation). However, the level of serotiny in Hakea is positively related to follicle mass [85], and hence the large and woody follicles of H. tephrospherma suggest it is strongly serotinous. While serotiny in Proteaceae is considered beneficial to surviving in fire-prone landscapes, it is also observed in fire-free landscapes [86] or, like this study site, where small bushfires occur at interdecadal scales. We suggest that seed release in H. tephrosperma is triggered by seasonal temperatures and/or humidity, rather than fire, and is a crucial survival strategy for this species because it is non-dormant and flowers infrequently when rainfall is limited.
Low serotiny and bet-hedging (with the exception of H. tephrosperma and A. rhagodioides) in species in this study suggest a strong selection for rapid flowering and seed dispersal in response to rainfall. For example, the two Maireana species in this study can flower at any time of the year, but usually do so following large rain events [79, 87]. Furthermore, their seeds are encircled by a papery wing—a trait adapted for dispersal away from the adult plant by wind soon after it has matured, or when dehiscing. The two non-dormant tree species (C. pauper and M. platycarpum) also flower prolifically, have seeds that are rapidly dispersed by wind, and have no apparent adaptations for serotiny or seed banking. Conversely, fruiting in A. rhagodioides and H. tephrosperma may not occur every year, but they have the capacity to recharge their seed bank through flowering and fruiting during favourable periods. Thus, because dispersal ability, serotiny and bet-hedging all function to avoid the risk of seedling emergence during unfavourable conditions, they may substitute for one another so that selection for one may weaken selection for the other strategy [88–90]. For species that fruit irregularly with no obvious adaptations for delaying seed release and germination (A. oliefolius and G. parviflora), there will be limited recruitment opportunities with increasing aridity and rainfall variability. As such, we have concerns for the persistence of these species under climate change.
Seed longevity is an alternative strategy to spread the risk of reproductive failure across time, and may further reduce the requirement for dormancy and bet-hedging [75, 83]. Seed longevity is also critical for species with dormancy traits, or serotinous species with canopy-stored seed, because seeds may need to survive multiple seasons. Our results are consistent with other studies that have found serotinous species typically have orthodox seeds that are long-lived [91], or tolerant to desiccation. However, longevity results in this study may only reflect seed-aging behaviour during ex situ storage and further studies should test seed persistence in situ to understand soil-seed banking dynamics. Species from arid environments are more likely to have comparatively long-lived seeds than those from cooler, wetter ecosystems [92]. Nonetheless, most species in this study had significantly higher longevity than expected [91–93] and we suggest that high seed longevity is a key adaptation to cope with the unpredictability of rainfall at the study site. Perennial plants that invest in seed longevity as a survival strategy increase their probability of encountering conditions favorable for seedling establishment [94, 95], and our results support the notion that longevity can negate the need for dormancy. In summary, a perennial life history and long-lived seed together reduce the likelihood of bet-hedging for the species in this system, where dominant perennial plants appear to invest less in seed dormancy and bet-hedging, and instead select for seed longevity and increases to offspring numbers.
Conclusion
We discuss seed morphology and physiology, and the germination behaviour of seeds that may facilitate seed survival and growth in arid zones. Most species exhibit rapid germination across wide diurnal temperatures, often producing prolific quantities of seed, which enables species to take advantage of rainfall events that fall across all seasons. Fewer species tend to avoid unfavourable conditions by delaying germination through seed dormancy and possibly bet-hedging. High seed longevity under ex situ aging was observed in most species included in this study. While seed dormancy is an important survival strategy for an arid seed, rapid germination and high seed production rates may be alternative regeneration traits and have a crucial role in explaining population dynamics in arid ecosystems with unpredictable rainfall. In such systems, the benefits of rapid germination may outweigh those associated with delayed germination through dormancy, and the population structure of remnant ecosystems in the region (which are dominated by rapid germinators) may be a testament to this.
Supporting information
(TIF)
Maximum germination is also shown for the GA3 treatment (when diurnal temperature is 30/20°C), and after the after-ripening treatment for A. rhagodioides. Letters indicate the results of Tukey pairwise comparisons among the three diurnal temperature treatments. Treatments that share a letter are not significantly different from each other. For the GA3 and after-ripening treatments, asterisks represent significant differences compared to the control treatment. For the ‘GA3 + after-ripening’ treatment, the comparison is to the GA3 only treatment (n.s. = not significant; * 0.05 > p > 0.01; ** 0.01 > p > 0.001; p < 0.001).
(DOCX)
Atriplex rhagodioides (AR) refer to seeds rendered non-dormant through a 12 month after-ripening.
(DOCX)
Acknowledgments
We wish to acknowledge the environmental team at Tronox Mining for instrumental support and access to land and to Kings Park Science for trainings in seed-based technologies. Finally we thank Dr Ian Sluiter and Tim Zweirsen for sharing insights to seed-based restoration approaches in arid zones and support for the project.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by Tronox Mining (https://www.tronox.com) and received by CD as part of a PhD project aimed at improving restoration outcomes from seeding efforts. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Larson J, Funk J. Regeneration: an overlooked aspect of trait-based plant community assembly models. Journal of Ecology. 2016;104: 1284–98. [Google Scholar]
- 2.Guo Q, Brown J, Valone T, Kachman S. Constraints of seed size on plant distribution and abundance. Ecology. 2000;81: 2149–55. [Google Scholar]
- 3.Wagner F, Rossi V, Baraloto C, Bonal D, Stahl C, Hérault B. Are Commonly Measured Functional Traits Involved in Tropical Tree Responses to Climate? International Journal of Ecology. 2014;2014: 1–10. [Google Scholar]
- 4.Kleyer M, Bekker R, Knevel I, Bakker J, Thompson K, Sonnenschein M, et al. The LEDA Traitbase: A database of life‐history traits of the Northwest European flora. Journal of Ecology. 2008;96: 1266–74. [Google Scholar]
- 5.Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, et al. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany. 2013;61: 167–234. [Google Scholar]
- 6.Saatkamp A, Cochrane A, Commander L, Guja L, Jimenez-Alfaro B, Larson J, et al. A research agenda for seed-trait functional ecology. New Phytol. 2019;221: 1764–75. 10.1111/nph.15502 [DOI] [PubMed] [Google Scholar]
- 7.Kleyer M, Minden V. Why functional ecology should consider all plant organs: An allocation-based perspective. Basic and Applied Ecology. 2015;16: 1–9. [Google Scholar]
- 8.Jiménez‐Alfaro B, Silveira F, Fidelis A, Poschlod P, Commander L. Seed germination traits can contribute better to plant community ecology. Journal of Vegetation Science. 2016;27: 637–45. [Google Scholar]
- 9.Pierce S, Bottinelli A, Bassani I, Ceriani R, Cerabolini B. How well do seed production traits correlate with leaf traits, whole-plant traits and plant ecological strategies? Plant Ecology. 2014;215: 1351–9. [Google Scholar]
- 10.Huang Z, Liu S, Bradford K, Huxman T, Venable L. The contribution of germination functional traits to population dynamics of a desert plant community. Ecology. 2016;97: 250–61. 10.1890/15-0744.1 [DOI] [PubMed] [Google Scholar]
- 11.Moles A, Westoby M. Seedling survival and seed size: a synthesis of the literature. Journal of Ecology. 2004;92: 372–83. [Google Scholar]
- 12.Norden N, Daws M, Antoine C, Gonzalez M, Garwood N, Chave J. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct Ecology. 2008;23: 203–10. [Google Scholar]
- 13.Gomaa N, Picó F. Seed germination, seedling traits, and seed bank of the tree Moringa peregrina (Moringaceae) in a hyper‐arid environment. American Journal of Botany. 2011;98: 1024–30. 10.3732/ajb.1000051 [DOI] [PubMed] [Google Scholar]
- 14.Westoby M, Jurado E, Leishman M. Comparative evolutionary ecology of seed size. Trends in Ecology and Evolution 1992;7: 368–72. 10.1016/0169-5347(92)90006-W [DOI] [PubMed] [Google Scholar]
- 15.Baraloto C, Forget P, D. G. Seed mass, seedling size and neotropical tree seedling establishment. Journal of Ecology. 2005;93: 1156–66. [Google Scholar]
- 16.Westoby M, Falster D, Moles A, Vesk P, Wright I. Plant ecological strategies: Some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33: 125–59. [Google Scholar]
- 17.Saatkamp A, Poschlod P, Venable D. The functional role of soil seed banks in natural communities In: Gallagher R, editor. Seeds—the ecology of regeneration in plant communities. 3 ed Wallingford, UK: CABI; 2014. p. 263–94. [Google Scholar]
- 18.Long R, Gorecki M, Renton M, Scott J, Colville L, Goggin D, et al. The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews. 2015;90: 31–59. 10.1111/brv.12095 [DOI] [PubMed] [Google Scholar]
- 19.Donohue K. Seeds and seasons: interpreting germination timing in the field. Seed Science Research. 2005;15: 175–87. [Google Scholar]
- 20.Simons A, Johnston M. Variation in seed traits of Lobelia inflata (Campanulaceae): sources and fitness consequences. American Journal of Botany. 2000;87: 124–32. [PubMed] [Google Scholar]
- 21.Hoyle G, Steadman K, Good R, McIntosh E, Galea L, Nicotra A. Seed germination strategies: an evolutionary trajectory independent of vegetative functional traits. Frontiers in Plant Science. 2015;6 (Oct): 1–13. 10.3389/fpls.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Shamsi N, El-Keblawy A, Mosa K, Navarro A. Drought tolerance and germination response to light and temperature for seeds of saline and non-saline habitats of the habitat-indifferent desert halophyte Suaeda vermiculata. Acta Physiologiae Plantarum. 2018;40: 1–13. [Google Scholar]
- 23.Zeng Y, Wang Y, Zhang J. Is reduced seed germination due to water limitation a special survival strategy used by xerophytes in arid dunes? Journal of Arid Environments. 2010;74: 508–11. [Google Scholar]
- 24.Choinski J, Tuohy J. Effect of water potential and temperature on the germination of 4 species of African savanna trees. Annals of Botany. 1991;68: 227‐33. [Google Scholar]
- 25.Salazar A, Goldstein G, Franco A, Miralles-Wilhelm F. Timing of seed dispersal and dormancy, rather than persistent soil seed-banks, control seedling recruitment of woody plants in Neotropical savannas. Seed Science Research. 2011;21: 103–16. [Google Scholar]
- 26.Poschlod P, Abedi M, Bartelheimer M, Drobnik J, Rosbakh S, Saatkamp A. Seed ecology and assembly rules in plant communities In: van der Maarel E, Franklin J, editors. Vegetation Ecology. 2nd ed Hoboken: Wiley-Blackwell; 2013. p. 164–202. [Google Scholar]
- 27.Grime J, Mason G, Curtis A. A comparative study of germination characteristics in a local flora. Journal of Ecology. 1981;69: 1017–59. [Google Scholar]
- 28.Vivrette N. Distribution and ecological significance of seed-embryo types in Mediterranean climates in California, Chile, and Australia In: Arroyo M, Zedler P, Fox M, editors. Ecology and Biogeography of Mediterranean Ecosystems in Chile, California and Australia. New York, USA: Springer Verlag; 1995. p. 274–88. [Google Scholar]
- 29.Vandelook F, Janssens S, Probert R. Relative embryo length as an adaptation to habitat and life cycle in Apiaceae. New Phytologist. 2012;195: 479–87. 10.1111/j.1469-8137.2012.04172.x [DOI] [PubMed] [Google Scholar]
- 30.Gremer J, Kimball S, Venable D. Within and among year germination in Sonoran Desert winter annuals: bet hedging and predictive germination in a variable environment. Ecology Letters. 2016;19: 1209–18. 10.1111/ele.12655 [DOI] [PubMed] [Google Scholar]
- 31.Lewandrowski W, Erickson T, Dalziell E, Stevens J. Ecological niche and bet-hedging strategies for Triodia (R.Br.) seed germination. Annals of Botany. 2018;121: 367–75. 10.1093/aob/mcx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Commander L, Golos P, Miller B, Merritt D. Seed germination traits of desert perennials. Plant Ecology. 2017;218: 1077–91. [Google Scholar]
- 33.Tielbörger K, Petruů M, Lampei C. Bet‐hedging germination in annual plants: a sound empirical test of the theoretical foundations. Oikos. 2012;121: 1860–8. [Google Scholar]
- 34.Baskin C, Baskin J. Classification, biogeograhpy, and phylogenetic relationships of seed dormancy In: Smith R, Dickie J, Linington S, Pritchard H, Probert R, editors. Seed conservation: turning science into practice. London: The Royal Botanic Gardens, Kew; 2003. p. 518–44. [Google Scholar]
- 35.Volis S, Bohrer G. Joint evolution of seed traits along an aridity gradient: Seed size and dormancy are not two substitutable evolutionary traits in temporally heterogeneous environment. New Phytologist. 2013;197: 655–67. 10.1111/nph.12024 [DOI] [PubMed] [Google Scholar]
- 36.Brown J, Venable D. Evolutionary ecology of seed-bank annuals in temporally varying environments. American Naturalist. 1986;127: 31–47. [Google Scholar]
- 37.Harel D, Holzapfel C, Sternberg M. Seed mass and dormancy of annual plant populations and communities decreases with aridity and rainfall predictability. Basic and Applied Ecology. 2011;12: 674–84. [Google Scholar]
- 38.Bochet E, García-Fayos P, Alborch B, Tormo J. Soil water availability effects on seed germination account for species segregation in semiarid roadslopes. Plant and Soil. 2007;295: 179–91. [Google Scholar]
- 39.Jurado E, Westoby M. Germination biology of selected central Australian plants. Australian Journal of Ecology. 1992;17: 341–8. [Google Scholar]
- 40.Parsons R. Incidence and ecology of very fast germination. Seed Science Research. 2012;22: 161–7. [Google Scholar]
- 41.Chesson P, Gebauer R, Schwinning S, Huntly N, Wiegand K, Ernest M, et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141: 236–53. 10.1007/s00442-004-1551-1 [DOI] [PubMed] [Google Scholar]
- 42.Sluiter I, Schultz N. Rehabilitation report on 2017 monitoring of revegetation at the Ginkgo Mineral Sands Mine. Merbein, VIC: Ogyris Pty. Ltd. and Cristal Mining Australia Ltd, 2017. [Google Scholar]
- 43.BOM. Monthly rainfall and temperature data: Pooncarie Mail Agency: Commonwealth of Australia, Bureau of Meteorology; 2018 [12 Oct 2018]. Available from: http://www.bom.gov.au/climate/data/.
- 44.Baskin C, Baskin J. A revision of Martin's seed classification system, with particular reference to his dwarf-seed type. Seed Science Research. 2007;17: 11–20. [Google Scholar]
- 45.Martin A. The comparative internal morphology of seeds. American Midland Naturalist. 1946;36: 513–660. [Google Scholar]
- 46.Baskin C, Baskin J. Seeds: Ecology, biogeography and evolution of dormancy and germination 2nd ed San Diego, USA: Academic Press; 2014. [Google Scholar]
- 47.Turner S, Merritt D, Ridley E, Commander L, Baskin J, Baskin C, et al. Ecophysiology of Seed Dormancy in the Australian Endemic Species Acanthocarpus preissii (Dasypogonaceae). Annals of Botany. 2006;98: 1137–44. 10.1093/aob/mcl203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweedman L, Merritt D. Australian Seeds–a guide to their collection, identification and biology Collingwood, Victoria: CSIRO Publishing; 2006. [Google Scholar]
- 49.Ellis R, Roberts H. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45: 13–30. [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 51.Environdata. WeatherMation Live: Historical data from Gingko weather station Warwick, Qld: Environdata; 2018 [15 February 2019]. Available from: https://www.weathermation.net.au/.
- 52.Gutterman Y. Regeneration of plants in arid ecosystems resulting from patch disturbance. Dordrecht, Kluwer: Springer; 2001. [Google Scholar]
- 53.Ogyris. Preliminary report on the flora of the Ginkgo Sand Mine Prospect near Pooncarie, Southwest New South Wales. Ogyris Ecological Research, Mildura VIC: Prepared for BeMaX Resources NL., 2000. [Google Scholar]
- 54.Read I. The Bush. A guide to the vegetated landscapes of Australia Frenchs Forest, NSW: Reed Books; 1987. [Google Scholar]
- 55.Callister K. Casuarina pauper (belah) woodlands of Northwest Victoria: Monitoring and regeneration Mt Helen, Victoria: University of Ballarat; 2004. [Google Scholar]
- 56.Wallace A, Rhoads W, Frolich E. Germination behaviour of Salsola as influenced by temperature, moisture, depth of planting and gamma irradiation. Agronomy Journal. 1968;60: 76–8. [Google Scholar]
- 57.Leishman M, Westoby M. The role of seed size in seedling establishment in dry soil conditions—experimental evidence from semi-arid species. Journal of Ecology. 1994;82: 249–58. [Google Scholar]
- 58.Bergholz K, Jeltsch F, Weiss L, Pottek J, Geißler K, Ristow M. Fertilization affects the establishment ability of species differing in seed mass via direct nutrient addition and indirect competition effects. Oikos. 2015;124: 1547–54. [Google Scholar]
- 59.Lebrija-Trejos E, Reich P, Hernández A, Wright S. Species with greater seed mass are more tolerant of conspecific neighbours: A key driver of early survival and future abundances in a tropical forest. Ecology Letters. 2016;19: 1071–80. 10.1111/ele.12643 [DOI] [PubMed] [Google Scholar]
- 60.Moles A. Being John Harper: Using evolutionary ideas to improve understanding of global patterns in plant traits. Journal of Ecology. 2018;106: 1–18. [Google Scholar]
- 61.Moles A, Hodson D, Webb C. Seed size and shape and persistence in the soil in the New Zealand flora. Oikos. 2000;89: 541–5. [Google Scholar]
- 62.Callister K, Florentine S, Westbrooke M. An investigation of the soil seedbank and seed germination of perennial species in Belah (Casuarina Pauper) woodlands in north-west Victoria. Australian Journal of Botany. 2018;66: 202–12. [Google Scholar]
- 63.Dalziell E, Tomlinson S. Reduced metabolic rate indicates declining viability in seed collections: an experimental proof-of-concept. Conservation Physiology. 2017;5 Available from: 10.1093/conphys/cox058. [DOI] [Google Scholar]
- 64.Bell D. The process of germination in Australian species. Australian Journal of Botany. 1999;47: 475–517. [Google Scholar]
- 65.Ooi M. Dormancy classification and potential dormancy-breaking cues for shrub species from fire-prone south-eastern Australia Adkins S, Ashmore S& Navie S eds Seeds: Biology, Development and Ecology. Oxfordshire, UK: CAB International; 2007. p. 205–16. [Google Scholar]
- 66.Auld T. The ecology of the Rutaceae in the Sydney region of south-eastern Australia: poorly known ecology of a neglected family. Cunninghamia. 2001;7: 213–39. [Google Scholar]
- 67.Martyn A, Seed L, Ooi M, Offord C. Seed fill, viability and germination of NSW species in the family Rutaceae. Cunninghamia. 2009;11: 203–12. [Google Scholar]
- 68.Fan B, Zhou Y, Ma Q, Yu Q, Zhao C, Sun K. The bet-hedging strategies for seedling emergence of Calligonum mongolicum to adapt to the extreme desert environments in northwestern China. Frontiers in Plant Science. 2018;9:[1167 p.]. Available from: 10.3389/fpls.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyer S, Carlson S, Garvin S. Seed germination regulation and field seed bank carryover in shadscale (Atriplex confertifolia: Chenopodiaceae). Journal of Arid Environments. 1998;38: 255–67. [Google Scholar]
- 70.Meyer S. Atriplex L. In: Bonner F, Karrfalt R, Nisley R, editors. The Woody Plant Seed Manual Agriculture Handbook 727 Part II—Specific Handling Methods and Data for 236 Genera. USA: United States Department of Agriculture and Forest Service; 2008. p. 283–90. [Google Scholar]
- 71.Sluiter I, Sluiter K. Pre-clearance vegetation and soils report of land at Cristal Mining Australia Ltd Murray-Darling Basin Sites: Snapper Mine–Autumn 2015. Ogyris Pty. Ltd. and Cristal Mining Australia Ltd, 2015. [Google Scholar]
- 72.Gremer J, Venable D. Bet hedging in desert winter annual plants: optimal germination strategies in a variable environment. Ecology Letters. 2014;17: 380–7. 10.1111/ele.12241 [DOI] [PubMed] [Google Scholar]
- 73.Venable D. Bet hedging in a guild of desert annuals. Ecology. 2007;88: 1086–90. 10.1890/06-1495 [DOI] [PubMed] [Google Scholar]
- 74.de Waal C, Anderson B, Ellis A. Dispersal, dormancy and life-history tradeoffs at the individual, population and species levels in southern African Asteraceae. New Phytologist. 2016;210: 356–65. 10.1111/nph.13744 [DOI] [PubMed] [Google Scholar]
- 75.Rees M. Trade-offs among dispersal strategies in British plants. Nature. 1993;366: 150–2. [Google Scholar]
- 76.Thompson K, Bakker J, Bekker R, Hodgson J. Ecological correlates of seed persistence in soil in the north‐west European flora. Journal of Ecology. 1998;86: 163–9. [Google Scholar]
- 77.Letnic M, Dickman C, Gayle M. Bet‐hedging and germination in the Australian arid zone shrub Acacia ligulata Austral Ecology. 2000;25: 368–74. [Google Scholar]
- 78.Auld T. Soil seedbank patterns of four trees and shrubs from arid Australia. Journal of Arid Environments. 1995;29: 33–45. [Google Scholar]
- 79.Wotton N. Aspects of the autecology of the Pearl Bluebush, Mairenana Sedifolia Adelaide, SA: University of Adelaide; 1993. [Google Scholar]
- 80.Murdoch F. Restoration ecology in the semi-arid woodlands of North-west Victoria: Federation University, Ballarat, VIC; 2005. [Google Scholar]
- 81.Chesterfield C, Parsons R. Regeneration of three tree species in arid South-eastern Australia. Australian Journal of Botany. 1985;33: 715–32. [Google Scholar]
- 82.Govt of SA. Significant flora fact sheet: Bullock Bush, Rosewood, Alectryon oliefolius. 2010. [19 August 2019]; Available from: https://www.naturalresources.sa.gov.au/aridlands/plants-and-animals/native-plants-and-animals/native-plants. [Google Scholar]
- 83.Tuljapurkar S. Delayed reproduction and fitness in variable environments. Proceedings of the National Academy of Sciences, USA. 1990;87: 1139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rees M. Delayed germination of seeds: a look at the effects of adult longevity, the timing of reproduction, and population age/stage structure. American Naturalist. 1994;144: 43–64. [Google Scholar]
- 85.Groom P, Lamont B. Fruit‐seed relations in Hakea: Serotinous species invest more dry matter in predispersal seed protection. Australian Journal of Ecology. 1997;22: 352–5. [Google Scholar]
- 86.Bradshaw S, Dixon K, Hopper S, Lambers H, Turner S. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends in Plant Science. 2011;16: 69–76. 10.1016/j.tplants.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 87.Hall E, Specht R, Eardley C. Regeneration of the vegetation on Koonamore Vegetation Reserve, 1926–1962. Australian Journal of Botany. 1964;12. [Google Scholar]
- 88.Snyder R. Multiple risk reduction mechanisms: can dormancy substitute for dispersal? Ecology Letters. 2006;9: 1106–14. 10.1111/j.1461-0248.2006.00962.x [DOI] [PubMed] [Google Scholar]
- 89.Siewert W, Tielbörger K. Dispersal-dormancy relationships in annual plants: putting model predictions to the test. American Naturalist. 2010;176: 490–500. 10.1086/656271 [DOI] [PubMed] [Google Scholar]
- 90.Klinkhamer P, de Jong T, Metz J, Val J. Life history tactics of annual organisms: the joint effects of dispersal and delayed germination. Theoretical Population Biology. 1987;32: 127–56. [Google Scholar]
- 91.Merritt D, Martyn A, Ainsley P, Young R, Seed L, Thorpe M, et al. A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodiversity and Conservation. 2014;23: 1081–104. [Google Scholar]
- 92.Probert R, Daws M, Hay F. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104: 57–69. 10.1093/aob/mcp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merritt D. Seed longevity of Australian species. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation. 2014;22: 8–10. [Google Scholar]
- 94.Zeineddine M, Jansen V. To age, to die: parity, evolutionary tracking and Cole’s paradox. Evolution. 2009;63: 1498–507. 10.1111/j.1558-5646.2009.00630.x [DOI] [PubMed] [Google Scholar]
- 95.Ehrlén J, Van Groenendael J. The trade-off between dispersability and longevity: an important aspect of plant species diversity. Applied Vegetation Science. 1998;1: 29–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Maximum germination is also shown for the GA3 treatment (when diurnal temperature is 30/20°C), and after the after-ripening treatment for A. rhagodioides. Letters indicate the results of Tukey pairwise comparisons among the three diurnal temperature treatments. Treatments that share a letter are not significantly different from each other. For the GA3 and after-ripening treatments, asterisks represent significant differences compared to the control treatment. For the ‘GA3 + after-ripening’ treatment, the comparison is to the GA3 only treatment (n.s. = not significant; * 0.05 > p > 0.01; ** 0.01 > p > 0.001; p < 0.001).
(DOCX)
Atriplex rhagodioides (AR) refer to seeds rendered non-dormant through a 12 month after-ripening.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.