Abstract
Aims
Elevated apolipoprotein C-III (apoC-III) levels are associated with hypertriglyceridaemia and coronary heart disease. AKCEA-APOCIII-LRx is an N-acetyl galactosamine-conjugated antisense oligonucleotide targeted to the liver that selectively inhibits apoC-III protein synthesis.
Methods and results
The safety, tolerability, and efficacy of AKCEA-APOCIII-LRx was assessed in a double-blind, placebo-controlled, dose-escalation Phase 1/2a study in healthy volunteers (ages 18–65) with triglyceride levels ≥90 or ≥200 mg/dL. Single-dose cohorts were treated with 10, 30, 60, 90, and 120 mg subcutaneously (sc) and multiple-dose cohorts were treated with 15 and 30 mg weekly sc for 6 weeks or 60 mg every 4 weeks sc for 3 months. In the single-dose cohorts treated with 10, 30, 60, 90, or 120 mg of AKCEA-APOCIII-LRx, median reductions of 0, −42%, −73%, −81%, and −92% in apoC-III, and −12%, −7%, −42%, −73%, and −77% in triglycerides were observed 14 days after dosing. In multiple-dose cohorts of 15 and 30 mg weekly and 60 mg every 4 weeks, median reductions of −66%, −84%, and −89% in apoC-III, and −59%, −73%, and −66% in triglycerides were observed 1 week after the last dose. Significant reductions in total cholesterol, apolipoprotein B, non-high-density lipoprotein cholesterol (HDL-C), very low-density lipoprotein cholesterol, and increases in HDL-C were also observed. AKCEA-APOCIII-LRx was well tolerated with one injection site reaction of mild erythema, and no flu-like reactions, platelet count reductions, liver, or renal safety signals.
Conclusion
Treatment of hypertriglyceridaemic subjects with AKCEA-APOCIII-LRx results in a broad improvement in the atherogenic lipid profile with a favourable safety and tolerability profile. ClinicalTrials.gov Identifier: NCT02900027.
Keywords: Apolipoprotein C-III, Hypertriglyceridaemia, Antisense, Cardiovascular disease
Introduction
Hypertriglyceridaemia is a prevalent disorder whose incidence is increasing due to the global epidemics of obesity and diabetes mellitus.1 The largest category of hypertriglyceridaemic patients have modest elevations of triglycerides >2 mmol/L), due to a variety of common metabolic disturbances. Such elevations of triglycerides are accompanied by elevations of remnant cholesterol that promote atherogenesis and cardiovascular events.2,3 A smaller subset of patients with hypertriglyceridaemia have fasting chylomicronemia and triglyceride levels >10 mmol/L4 and are categorized as having familial chylomicronemia syndrome (FCS).5
A key regulator of plasma triglyceride-rich lipoprotein (TRL) metabolism is apolipoprotein C-III (apoC-III), a 79 amino acid glycoprotein synthesized principally in the liver and to a lesser extent in the intestine.6 Apolipoprotein C-III circulates on very low-density lipoprotein (VLDL), LDL, Lp(a), and HDL particles and can be present in multiple copies per particle.7,8 Apolipoprotein C-III plays a key role in determining serum triglyceride levels by two main mechanisms, by inhibiting LPL activity as well as by directly inhibiting hepatic uptake of TRL, thus leading to increased levels of chylomicrons and TRLs.9–11
RNA-targeted therapies represent a novel platform for drug discovery involving chemically modified oligonucleotides.12 Volanesorsen, a second-generation antisense oligonucleotide (ASO) targets hepatic APOC3 mRNA to inhibit apoC-III protein production, resulting in substantial reductions in plasma triglyceride levels.9,13,14 Furthermore, new targeting approaches of ASOs, using triantennary N-acetyl galactosamine (GalNAc3) modified ASOs that target the asialoglycoprotein receptor (ASGPR) in hepatocytes, allows similar efficacy untargeted ASO with 20–30-fold lower dosing, thus minimizing systemic exposure.15–18 In this Phase 1/2a study, we describe the safety, tolerability, and efficacy of a GalNAc3-modified ASO, AKCEA-APOCIII-LRx, which targets hepatic APOC3 mRNA, in otherwise healthy individuals with modest elevations of plasma triglyceride levels.
Methods
Detailed methods are presented in the Supplementary material online, Appendix.
Patient population and study design
The objectives of the study performed in healthy subjects with elevated triglycerides were the following: (i) to evaluate the safety and tolerability of single and multiple doses of AKCEA-APOCIII-LRx administered subcutaneously; (ii) to evaluate the pharmacokinetics of single and multiple subcutaneous doses of AKCEA-APOCIII-LRx; and (iii) to evaluate the effects of single and multiple doses of AKCEA-APOCIII-LRx on pharmacodynamics (PD) including plasma apoC-III, triglycerides, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), and very low-density lipoprotein cholesterol (VLDL-C).
AKCEA-APOCIII-LRx is a second-generation ASO drug targeted to human APOC3 mRNA. AKCEA-APOCIII-LRx contains the same nucleic acid sequence as the unconjugated volanesorsen,9 but additionally contains a triantennary N-acetyl galactosamine (GalNAc3) complex at the 5′ position attached via a proprietary linker. This Phase 1/2a, double blind, randomized, placebo-controlled, dose-escalation study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of AKCEA-APOCIII-LRx (ISIS 678354) administered subcutaneously to healthy male and female volunteers (age 18–65), with a body mass index (BMI) ≤35.0 kg/m2 and elevated triglycerides. The study design is summarized in Supplementary material online, Figure S1 and consisted of single and multiple dose cohorts. The single-dose study included five cohorts (N = 8 per cohort, total randomized six active: two placebo), using doses of 10, 30, 60, 90, and 120 mg. Subjects were required to have a fasting triglyceride level ≥90 mg/dL for the 10, 30, and 60 mg dose cohorts and ≥200 mg/dL for the 90 and 120 mg cohorts. For the multiple-dose study design all subjects were required to have a fasting triglyceride level ≥200 mg/dL. The doses were 15 and 30 mg for the weekly dosing cohorts (N = 8 per cohort, total randomized six active: two placebo) and 60 mg for the every 4 weeks dosing cohort (N = 10, total randomized six active: four placebo).
Results
Baseline characteristics of the study groups
A total of 40 subjects were enrolled in the single ascending dose (SAD) study, and 17 in the weekly and 10 subjects in the every 4 weeks multiple ascending dose (MAD) study. In the SAD cohorts, subjects in all dose groups had elevated BMI (>25 kg/m2), and subjects in the 90 mg and 120 mg dose group, due to inclusion criteria of triglycerides >200 mg/dL, had elevated triglyceride levels (Table 1). In the MAD cohorts, all dose groups also had elevated BMI and elevated triglycerides. The mean apoC-III plasma levels in both SAD and MAD cohorts ranged from 9 to 15 mg/dL. The lipid panels otherwise showed modest elevation of total cholesterol, non-HDL-C, LDL-C, and apoB.
Table 1.
Baseline characteristics of subjects in the single and multiple ascending dose cohorts
| AKCEA-APOCIII-LRX, single-dose cohort | ||||||
|---|---|---|---|---|---|---|
| Pooled placebo (n = 10) | 10 mg (n = 6) | 30 mg (n = 6) | 60 mg (n = 6) | 90 mg (n = 6) | 120 mg (n = 6) | |
| Gender (male:female) | 3:7 | 4:2 | 4:2 | 5:1 | 4:2 | 3:3 |
| Age (years), mean (SD) | 54.3 (8.9) | 59.0 (5.5) | 50.5 (15.8) | 51.5 (10.0) | 48.8 (7.4) | 53.3 (14.1) |
| BMI (kg/m2), mean (SD) | 29.4 (2.5) | 29.8 (3.0) | 28.8 (4.0) | 30.5 (2.6) | 28.5 (4.0) | 27.5 (2.4) |
| Lipids and lipoproteins (mg/dL) | ||||||
| Apo CIII, mean (SD) | 10.4 (2.5) | 11.3 (2.3) | 8.5 (2.2) | 8.8 (3.6) | 12.4 (5.3) | 14.8 (1.7) |
| Apo CIII, median (IQR) | 10.5 (8.0–13.0) | 10.5 (9.5–13.0) | 7.5 (7.5–11.0) | 7.8 (7.0–9.5) | 11.8 (9.0–16.0) | 14.5 (14.0–16.0) |
| Triglycerides, mean (SD) | 134.7 (48.1) | 173.3 (67.3) | 127.3 (50.1) | 139.1 (87.8) | 245.4 (130.8) | 234.7 (86.6) |
| Triglycerides, median (IQR) | 118.8 (95.5–194.5) | 163.8 (144.5–234.5) | 108.8 (93.0–157.5) | 105.0 (93.5–121.0) | 192.8 (171.0–341.5) | 197.0 (173.5–318.5) |
| VLDL-C (direct), mean (SD) | 29.8 (13.3) | 35.9 (17.9) | 26.6 (5.1) | 30.9 (18.5) | 59.3 (29.2) | 55.4 (15.8) |
| Non-HDL-C, mean (SD) | 160.7 (24.2) | 173.2 (36.0) | 160.8 (31.3) | 160.4 (30.1) | 163.3 (33.7) | 198.4 (15.9) |
| Total cholesterol, mean (SD) | 213.2 (33.3) | 218.6 (43.7) | 205.6 (44.6) | 198.8 (21.9) | 203.8 (28.6) | 238.9 (22.3) |
| LDL-C (ultracentrifugation), mean (SD) | 131.0 (25.0) | 137.3 (41.5) | 134.2 (31.4) | 129.5 (25.1) | 104.0 (13.3) | 143.0 (28.3) |
| HDL-C (precipitation), mean (SD) | 52.5 (19.1) | 45.4 (12.5) | 44.8 (17.8) | 38.3 (9.1) | 40.5 (8.7) | 40.5 (11.9) |
| ApoB, mean (SD) | 106.9 (22.3) | ND | ND | ND | 99.2 (16.8) | 127.4 (12.7) |
| Lp(a) (nmol/L), mean (SD) | 17.0 (12.2) | ND | ND | ND | 34.8 (36.6) | 48.4 (46.0) |
| AKCEA-APOCIII-LRX, multiple-dose cohort | |||||
|---|---|---|---|---|---|
| Pooled placebo weekly (n = 4) | 15 mg/week (n = 6) | 30 mg/week (n = 7) | Placebo every 4 weeks (n = 4) | 60 mg/every 4 weeks (n = 6) | |
| Gender (male:female) | 2:2 | 4:2 | 7:0 | 2:2 | 3:3 |
| Age (years), mean (SD) | 53.0 (9.6) | 53.3 (8.0) | 41.9 (6.6) | 57.0 (5.0) | 49.8 (7.5) |
| BMI (kg/m2), mean (SD) | 27.5 (3.2) | 28.7 (4.7) | 30.6 (3.0) | 28.2 (3.3) | 27.7 (2.3) |
| Lipids and lipoproteins (mg/dL) | |||||
| Apo CIII, mean (SD) | 12.5 (2.8) | 14.4 (3.1) | 10.3 (3.2) | 12.8 (1.6) | 15.0 (7.2) |
| Apo CIII, median (IQR) | 11.5 (10.5–14.5) | 15.3 (11.5–17.0) | 10.0 (8.0–13.5) | 13.3 (11.8–13.8) | 12.0 (10.5–18.5) |
| Triglycerides, mean (SD) | 225.3 (96.2) | 223.2 (144.1) | 188.7 (73.3) | 190.8 (58.7) | 300.8 (232.6) |
| Triglycerides, median (IQR) | 210.5 (151.0–299.5) | 176.3 (107.5–307.5) | 155.5 (124.5–270.0) | 197.0 (140.8–240.8) | 225.3 (150.5–357.0) |
| VLDL-C (direct), mean (SD) | 45.9 (25.5) | 44.9 (20.1) | 39.3 (14.3) | 38.5 (20.2) | 53.4 (36.5) |
| Non-HDL-C, mean (SD) | 192.1 (46.9) | 172.8 (28.4) | 189.0 (33.9) | 211.1 (32.0) | 230.8 (72.6) |
| Total cholesterol, mean (SD) | 229.8 (40.5) | 225.4 (25.1) | 228.5 (33.7) | 257.1 (27.1) | 270.6 (73.3) |
| LDL-C (ultracentrifugation), mean (SD) | 146.3 (36.4) | 127.9 (21.1) | 149.7 (25.7) | 172.6 (32.7) | 177.4 (61.6) |
| HDL-C (precipitation), mean (SD) | 37.6 (10.3) | 52.6 (20.7) | 39.5 (8.5) | 46.0 (8.1) | 39.8 (17.7) |
| ApoB, mean (SD) | 118.8 (26.2) | 106.4 (20.1) | 117.2 (16.7) | 127.1 (15.3) | 144.2 (42.5) |
| Lp(a) (nmol/L), mean (SD) | 86.5 (78.3) | 32.4 (43.7) | 33.6 (23.8) | 17.8 (17.1) | 30.7 (30.4) |
ND, not determined.
Absolute and mean percent changes in lipids and lipoproteins in the single ascending dose cohorts
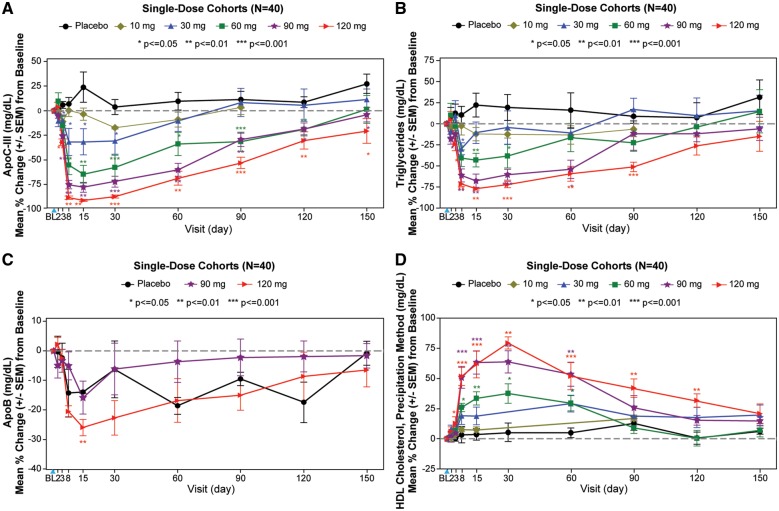
In the SAD cohorts of 10, 30, 60, 90, and 120 mg, median reductions of 0, −42%, −73%, −81%, and −92% in apoC-III, and −12%, −7%, −42%, −73%, and −77% in triglycerides were observed 14 days after dosing. Significant reductions were also noted in non-HDL-C in the 90 mg and 120 mg doses, and VLDL-C in the 60 mg, 90 mg, and 120 mg doses, and apoB in the 120 mg dose, and increases in HDL-C in the 60 mg, 90 mg, and 120 mg doses (Table 2).
Table 2.
Absolute and mean percent changes in lipids and lipoproteins in the single and multiple ascending dose cohorts
| AKCEA-APOCIII-LRX, single-dose cohort | ||||||
|---|---|---|---|---|---|---|
| Pooled placebo (n = 10) | 10 mg (n = 6) | 30 mg (n = 6) | 60 mg (n = 6) | 90 mg (n = 6) | 120 mg (n = 6) | |
| Apo CIII | ||||||
| Baseline, mean (SD) | 10.4 (2.5) | 11.3 (2.3) | 8.5 (2.2) | 8.8 (3.6) | 12.4 (5.3) | 14.8 (1.7) |
| Day 15, mean (SD) | 13.0 (6.1) | 10.7 (1.6) | 5.5 (2.2) | 3.2 (2.1) | 2.7 (1.8) | 1.3 (0.5) |
| Percent change, mean (SD) | 23.9 (48.9) | −3.6 (14.9) | −31.7 (32.5) | −64.7 (21.7) | −77.9 (12.3) | −91.2 (2.5) |
| 95% CI for mean | −11.1 to 58.8 | −19.2 to 12.1 | −65.8 to 2.4 | −87.5 to −41.9 | −90.7 to −65.0 | −93.8 to −88.6 |
| Percent change, median (IQR) | 11.0 (4.0 to 23.8) | 0.0 (−15.8 to 10.0) | −41.7 (−56.5 to −6.7) | −72.8 (−80.0 to −47.4) | −81.4 (−85.4 to −77.8) | −92.4 (−93.1 to −88.6) |
| P-value | 0.113 | 0.042 | 0.006 | 0.006 | 0.006 | |
| Triglycerides | ||||||
| Baseline, mean (SD) | 134.7 (48.1) | 173.3 (67.3) | 127.3 (50.1) | 139.1 (87.8) | 245.4 (130.8) | 234.7 (86.6) |
| Day 15, mean (SD) | 174.2 (118.0) | 147.7 (51.7) | 104.2 (33.7) | 70.0 (22.9) | 68.2 (31.5) | 52.0 (11.9) |
| Percent change, mean (SD) | 22.2 (44.5) | −12.2 (19.5) | −10.6 (30.9) | −43.0 (19.7) | −67.5 (19.0) | −76.9 (3.7) |
| 95% CI for mean | −9.6 to 54.1 | −32.6 to 8.2 | −43.1 to 21.9 | −63.8 to −22.3 | −87.5 to −47.6 | −80.7 to −73.0 |
| Percent change, median (IQR) | 9.6 (0.0 to 36.0) | −11.6 (−23.7 to −7.5) | −7.3 (−21.4 to 7.9) | −42.3 (−57.0 to −29.4) | −73.3 (−80.3 to −59.8) | −77.1 (−77.8 to −74.6) |
| P-value | 0.052 | 0.137 | 0.007 | 0.006 | 0.006 | |
| VLDL-C (direct) | ||||||
| Baseline, mean (SD) | 29.8 (13.3) | 35.9 (17.9) | 26.6 (5.1) | 30.9 (18.5) | 59.3 (29.2) | 55.4 (15.8) |
| Day 15, mean (SD) | 31.4 (21.1) | 24.8 (11.8) | 25.0 (9.9) | 11.0 (6.6) | 11.0 (6.7) | 17.0 (5.9) |
| Percent change, mean (SD) | 5.2 (48.5) | −23.4 (31.4) | −2.0 (41.9) | −65.0 (10.6) | −81.2 (9.1) | −68.0 (11.5) |
| 95% CI for mean | −29.5 to 39.9 | −56.3 to 9.6 | −45.9 to 41.9 | −76.1 to −53.8 | −90.8 to −71.6 | −80.0 to −55.9 |
| Percent change, median (IQR) | 0.1 (−41.8 to 41.7) | −30.9 (−51.9 to 0.0) | −3.0 (−21.4 to 25.5) | −64.5 (−68.4 to −56.7) | −81.5 (−89.5 to −72.9) | −66.8 (−72.0 to −60.0) |
| P-value | 0.101 | 0.674 | <0.001 | <0.001 | <0.001 | |
| Non-HDL-C | ||||||
| Baseline, mean (SD) | 160.7 (24.2) | 173.2 (36.0) | 160.8 (31.3) | 160.4 (30.1) | 163.3 (33.7) | 198.4 (15.9) |
| Day 15, mean (SD) | 151.0 (39.3) | 164.3 (32.5) | 148.5 (26.5) | 143.3 (39.5) | 123.8 (37.1) | 147.8 (22.9) |
| Percent change, mean (SD) | −6.4 (16.9) | −4.5 (10.0) | −6.3 (17.2) | −11.5 (13.1) | −24.4 (15.2) | −25.6 (9.2) |
| 95% CI for mean | −18.5 to 5.7 | −15.0 to 5.9 | −24.4 to 11.7 | −25.3 to 2.3 | −40.4 to −8.5 | −35.3 to −16.0 |
| Percent change, median (IQR) | −12.5 (−17.4 to −3.4) | −6.3 (−10.1 to 1.3) | −7.8 (−22.2 to 0.0) | −6.7 (−18.8 to −5.3) | −25.7 (−29.3 to −22.1) | −24.3 (−33.8 to −23.6) |
| P-value | 0.273 | 0.957 | 0.957 | 0.042 | 0.022 | |
| Total cholesterol | ||||||
| Baseline, mean (SD) | 213.2 (33.3) | 218.6 (43.7) | 205.6 (44.6) | 198.8 (21.9) | 203.8 (28.6) | 238.9 (22.3) |
| Day 15, mean (SD) | 204.6 (40.0) | 213.0 (38.0) | 201.2 (34.6) | 194.0 (29.9) | 188.3 (34.7) | 211.0 (22.3) |
| Percent change, mean (SD) | −3.7 (14.8) | −2.0 (7.7) | −0.5 (15.5) | −2.6 (7.7) | −7.5 (12.6) | −11.5 (8.0) |
| 95% CI for mean | −14.3 to 6.9 | −10.0 to 6.0 | −16.8 to 15.7 | −10.7 to 5.4 | −20.7 to 5.8 | −19.8 to −3.1 |
| Percent change, median (IQR) | −8.1 (−11.9 to −2.2) | −3.4 (−5.7 to 4.2) | −5.6 (−11.5 to 6.0) | −0.5 (−5.2 to 1.1) | −8.9 (−13.1 to −5.6) | −11.2 (−17.7 to −7.7) |
| P-value | 0.231 | 0.560 | 0.319 | 0.633 | 0.273 | |
| LDL-C (ultracentrifugation) | ||||||
| Baseline, mean (SD) | 131.0 (25.0) | 137.3 (41.5) | 134.2 (31.4) | 129.5 (25.1) | 104.0 (13.3) | 143.0 (28.3) |
| Day 15, mean (SD) | 119.6 (22.6) | 139.5 (33.4) | 123.5 (32.2) | 132.3 (36.6) | 112.8 (31.6) | 130.8 (24.3) |
| Percent change, mean (SD) | −7.7 (14.0) | 3.4 (11.3) | −7.3 (19.0) | 1.8 (18.9) | 7.6 (23.1) | −7.1 (17.0) |
| 95% CI for mean | −17.7 to 2.3 | −8.5 to 15.2 | −27.2 to 12.6 | −18.0 to 21.6 | −16.6 to 31.9 | −25.0 to 10.8 |
| Percent change, median (IQR) | −9.7 (−12.2 to −6.0) | 6.7 (−10.2, 11.3) | −4.4 (−22.3 to 8.0) | 2.9 (−9.3 to 17.4) | −4.1 (−9.7 to 32.2) | −9.5 (−17.4 to 0.0) |
| P-value | 0.220 | 0.964 | 0.291 | 0.093 | 0.945 | |
| ApoB | ||||||
| Baseline, mean (SD) | 106.9 (22.3) | ND | ND | ND | 99.2 (16.8) | 127.4 (12.7) |
| Day 15, mean (SD) | 92.0 (19.1) | ND | ND | ND | 84.7 (25.2) | 94.7 (14.9) |
| Percent change, mean (SD) | −13.9 (0.9) | −15.9 (13.6) | −26.0 (6.8) | |||
| 95% CI for mean | −15.4 to −12.4 | −30.2 to −1.6 | −33.1 to −18.9 | |||
| Percent change, median (IQR) | −14.1 (−14.5 to −13.3) | −13.0 (−26.9 to −4.5) | −25.1 (−32.8 to −22.6) | |||
| P-value | 1.000 | 0.010 | ||||
| HDL-C (precipitation) | ||||||
| Baseline, mean (SD) | 52.5 (19.1) | 45.4 (12.5) | 44.8 (17.8) | 38.3 (9.1) | 40.5 (8.7) | 40.5 (11.9) |
| Day 15, mean (SD) | 53.6 (19.2) | 48.7 (13.2) | 52.7 (20.6) | 50.7 (10.8) | 64.5 (5.6) | 63.2 (11.3) |
| Percent change, mean (SD) | 3.7 (14.9) | 7.3 (6.4) | 18.9 (17.9) | 33.5 (13.3) | 63.3 (23.2) | 61.7 (27.5) |
| 95% CI for mean | −7.0 to 14.4 | 0.5 to 14.1 | 0.2 to 37.7 | 19.6 to 47.5 | 38.9 to 87.7 | 32.8 to 90.6 |
| Percent change, median (IQR) | 4.0 (−7.8 to 9.1) | 7.2 (1.7 to 9.7) | 13.9 (7.3 to 29.7) | 32.2 (23.8 to 44.8) | 60.6 (43.8 to 79.7) | 72.4 (38.3 to 77.5) |
| P-value | 0.702 | 0.112 | 0.003 | <0.001 | <0.001 | |
| Lp(a) (nmol/L) | ||||||
| Baseline, mean (SD) | 17.0 (12.2) | ND | ND | ND | 34.8 (36.6) | 48.4 (46.0) |
| Day 15, mean (SD) | 13.5 (12.0) | ND | ND | ND | 33.8 (34.2) | 48.8 (52.8) |
| Percent change, mean (SD) | −32.6 (33.0) | 0.7 (26.6) | −13.5 (25.9) | |||
| 95% CI for mean | −85.2 to 19.9 | −27.3 to 28.6 | −40.8 to 13.7 | |||
| Percent change, median (IQR) | −18.8 (−50.9 to −14.4) | −8.7 (−17.7 to 9.1) | −2.8 (−36.4 to 0.0) | |||
| P-value | 0.186 | 0.233 | ||||
| AKCEA-APOCIII-LRX, multiple-dose cohort | |||||
|---|---|---|---|---|---|
| Pooled placebo weekly (n = 4) | 15 mg/week (n = 6) | 30 mg/week (n = 7) | Placebo every 4 weeks (n = 4) | 60 mg/every 4 weeks (n = 6) | |
| Apo CIII | |||||
| Baseline, mean (SD) | 12.5 (2.8) | 14.4 (3.1) | 10.3 (3.2) | 12.8 (1.6) | 15.0 (7.2) |
| 1 week after last dosea, mean (SD) | 14.3 (2.9) | 5.3 (3.3) | 1.5 (0.5) | 13.0 (1.8) | 3.2 (3.8) |
| Percent change, mean (SD) | 15.9 (21.8) | −65.4 (17.8) | −84.3 (6.4) | 2.4 (12.3) | −83.1 (10.9) |
| 95% CI for mean | −18.7 to 50.5 | −84.1 to −46.8 | −91.0 to −77.7 | −17.2 to 22.0 | −96.7 to −69.5 |
| Percent change, median (IQR) | 14.3 (2.6 to 29.1) | −66.2 (−73.9 to −58.8) | −83.5 (−88.9 to −81.8) | 4.2 (−5.3 to 10.1) | −88.9 (−89.2 to −82.6) |
| P-value | 0.010 | 0.005 | 0.016 | ||
| Triglycerides | |||||
| Baseline, mean (SD) | 225.3 (96.2) | 223.2 (144.1) | 188.7 (73.3) | 190.8 (58.7) | 300.8 (232.6) |
| 1 week after last dosea, mean (SD) | 254.5 (108.8) | 73.5 (25.5) | 53.3 (6.8) | 183.8 (55.8) | 82.8 (29.0) |
| Percent change, mean (SD) | 18.4 (38.6) | −60.7 (13.3) | −70.5 (9.5) | 2.9 (44.4) | −64.6 (16.2) |
| 95% CI for mean | −43.0 to 79.8 | −74.7 to −46.8 | −80.4 to −60.5 | −67.6 to 73.5 | −84.7 to −44.5 |
| Percent change, median (IQR) | 17.9 (−13.1 to 49.9) | −59.1 (−63.3 to −54.3) | −72.7 (−77.2 to −61.3) | −10.0 (−26.7 to 32.5) | −66.4 (−72.8 to −54.8) |
| P-value | 0.010 | 0.010 | 0.016 | ||
| VLDL-C (direct) | |||||
| Baseline, mean (SD) | 45.9 (25.5) | 44.9 (20.1) | 39.3 (14.3) | 38.5 (20.2) | 53.4 (36.5) |
| 1 week after last dosea, mean (SD) | 44.3 (17.5) | 13.7 (10.2) | 10.2 (2.3) | 39.5 (13.5) | 21.6 (5.4) |
| Percent change, mean (SD) | 6.0 (40.4) | −70.9 (15.1) | −72.9 (7.7) | 13.6 (50.8) | −40.1 (47.3) |
| 95% CI for mean | −58.2 to 70.3 | −86.8 to −55.0 | −81.0 to −64.7 | −67.2 to 94.5 | −98.8 to 18.7 |
| Percent change, median (IQR) | −4.0 (−22.5 to 34.6) | −79.1 (−81.1 to −57.2) | −75.6 (−78.6 to −70.4) | −4.3 (−18.0 to 45.3) | −50.0 (−66.9 to −48.8) |
| P-value | 0.010 | 0.010 | 0.111 | ||
| Non-HDL-C | |||||
| Baseline, mean (SD) | 192.1 (46.9) | 172.8 (28.4) | 189.0 (33.9) | 211.1 (32.0) | 230.8 (72.6) |
| 1 week after last dosea, mean (SD) | 190.5 (45.8) | 137.5 (40.9) | 133.2 (36.8) | 213.0 (22.4) | 163.4 (40.2) |
| Percent change, mean (SD) | −0.5 (8.9) | −21.8 (14.4) | −29.6 (11.2) | 1.6 (8.2) | −30.7 (7.3) |
| 95% CI for mean | −14.7 to 13.6 | −36.9 to −6.7 | −41.4 to −17.9 | −11.4 to 14.6 | −39.8 to −21.5 |
| Percent change, median (IQR) | −1.9 (−6.7 to 5.7) | −23.3 (−28.6 to −11.2) | −28.2 (−40.4 to −21.6) | 3.5 (−4.9 to 8.2) | −29.0 (−35.8 to −26.7) |
| P-value | 0.038 | 0.010 | 0.016 | ||
| Total cholesterol | |||||
| Baseline, mean (SD) | 229.8 (40.5) | 225.4 (25.1) | 228.5 (33.7) | 257.1 (27.1) | 270.6 (73.3) |
| 1 week after last dosea, mean (SD) | 229.5 (44.6) | 213.3 (27.9) | 190.2 (41.5) | 261.5 (15.0) | 232.2 (46.9) |
| Percent change, mean (SD) | −0.2 (5.9) | −5.3 (8.2) | −15.8 (10.7) | 2.2 (7.5) | −16.7 (5.7) |
| 95% CI for mean | −9.6 to 9.2 | −13.9 to 3.3 | −27.0 to −4.6 | −9.7 to 14.1 | −23.8 to −9.6 |
| Percent change, median (IQR) | −0.1 (−5.2 to 4.8) | −7.6 (−10.1 to −3.2) | −13.2 (−28.0 to −10.1) | 1.7 (−3.4 to 7.9) | −18.0 (−19.3 to −11.8) |
| P-value | 0.352 | 0.038 | 0.016 | ||
| LDL-C (ultracentrifugation) | |||||
| Baseline, mean (SD) | 146.3 (36.4) | 127.9 (21.1) | 149.7 (25.7) | 172.6 (32.7) | 177.4 (61.6) |
| 1 week after last dosea, mean (SD) | 146.3 (44.5) | 123.8 (32.9) | 123.0 (35.3) | 173.5 (26.5) | 141.8 (39.7) |
| Percent change, mean (SD) | −0.8 (10.3) | −2.8 (26.1) | −17.0 (17.7) | 1.2 (5.9) | −21.6 (15.1) |
| 95% CI for mean | −17.1 to 15.6 | −30.2 to 24.6 | −35.6 to 1.5 | −8.1 to 10.6 | −40.4 to −2.8 |
| Percent change, median (IQR) | −1.0 (−8.9 to 7.3) | −12.1 (−19.0 to 26.3) | −14.3 (−37.3 to −4.2) | −1.2 (−2.5 to 4.9) | −26.2 (−33.0 to −15.8) |
| P-value | 0.610 | 0.257 | 0.111 | ||
| ApoB | |||||
| Baseline, mean (SD) | 118.8 (26.2) | 106.4 (20.1) | 117.2 (16.7) | 127.1 (15.3) | 144.2 (42.5) |
| 1 week after last dosea, mean (SD) | 121.8 (25.6) | 91.0 (23.7) | 86.3 (22.5) | 125.0 (11.5) | 102.0 (22.6) |
| Percent change, mean (SD) | 3.2 (10.9) | −15.4 (10.5) | −26.4 (12.9) | −1.3 (5.7) | −30.2 (5.8) |
| 95% CI for mean | −14.1 to 20.6 | −26.5 to −4.4 | −39.9 to −12.9 | −10.4 to 7.8 | −37.5 to −23.0 |
| Percent change, median (IQR) | 2.3 (−4.6 to 11.1) | −14.1 (−21.3 to −11.7) | −27.2 (−34.5 to −15.6) | 0.1 (−5.6 to 3.0) | −27.7 (−30.0 to −27.3) |
| P-value | 0.038 | 0.010 | 0.016 | ||
| HDL-C (precipitation) | |||||
| Baseline, mean (SD) | 37.6 (10.3) | 52.6 (20.7) | 39.5 (8.5) | 46.0 (8.1) | 39.8 (17.7) |
| 1 week after last dosea, mean (SD) | 39.0 (12.3) | 75.8 (24.6) | 57.0 (8.6) | 48.5 (9.6) | 68.8 (22.0) |
| Percent change, mean (SD) | 3.4 (16.8) | 49.6 (19.7) | 55.6 (30.2) | 6.1 (14.8) | 75.8 (50.4) |
| 95% CI for mean | −23.3 to 30.1 | 28.9 to 70.3 | 23.8 to 87.3 | −17.4 to 29.6 | 13.3 to 138.4 |
| Percent change, median (IQR) | 0.0 (−8.5 to 15.3) | 43.8 (33.9 to 60.9) | 66.0 (20.0 to 80.6) | 6.3 (−5.6 to 17.7) | 80.0 (35.9 to 86.5) |
| P-value | 0.010 | 0.038 | 0.016 | ||
| Lp(a), nmol/L | |||||
| Baseline, mean (SD) | 86.5 (78.3) | 32.4 (43.7) | 33.6 (23.8) | 17.8 (17.1) | 30.7 (30.4) |
| 1 week after last dosea, mean (SD) | 89.0 (82.1) | 34.5 (46.6) | 30.3 (22.0) | 16.0 (13.0) | 15.4 (12.3) |
| Percent change, mean (SD) | 73.2 (151.8) | −0.1 (48.0) | −10.6 (18.7) | 12.8 (32.1) | −26.9 (21.1) |
| 95% CI for mean | −168.4 to 314.8 | −50.5 to 50.4 | −30.3 to 9.0 | −38.3 to 63.9 | −53.1 to −0.7 |
| Percent change, median (IQR) | 6.6 (−9.8 to 156.2) | 6.8 (−50.0 to 19.3) | −9.2 (−23.7 to −6.3) | 11.6 (−13.0 to 38.6) | −17.6 (−25.5 to −16.0) |
| P-value | 0.762 | 0.257 | 0.111 | ||
The data was compared between AKCEA-APOCIII-LRx treatments and placebo using one-way analysis of variance or Wilcoxon rank sum test.
ND, not determined.
Day 43 weekly dosing cohorts; Day 92 every 4 weeks dosing cohort.
The temporal relationships of changes in apoC-III, triglycerides, apoB, and HDL-C are shown in Figure1A–D. Most of the effects occurred by Day 8, with nadirs or peaks at Day 15 and then reversions to baseline by days 120–150.
Figure 1.
Single ascending dose cohorts. The graphs display the mean percent change (±SEM) in apoC-III (A), triglycerides (B), apoB (C), and HDL-C (D) in the single dose cohorts. The blue arrowhead represents the timing of the dose. The data were compared between AKCEA-APOCIII-LRx treatments and placebo using one-way analysis of variance or Wilcoxon rank sum test. Subjects were required to have a fasting triglyceride level ≥90 mg/dL for the 10, 30, and 60 mg dose cohorts and ≥200 mg/dL for the 90 and 120 mg cohorts.
Absolute and mean percent changes in lipids and lipoproteins in the multiple ascending dose cohorts
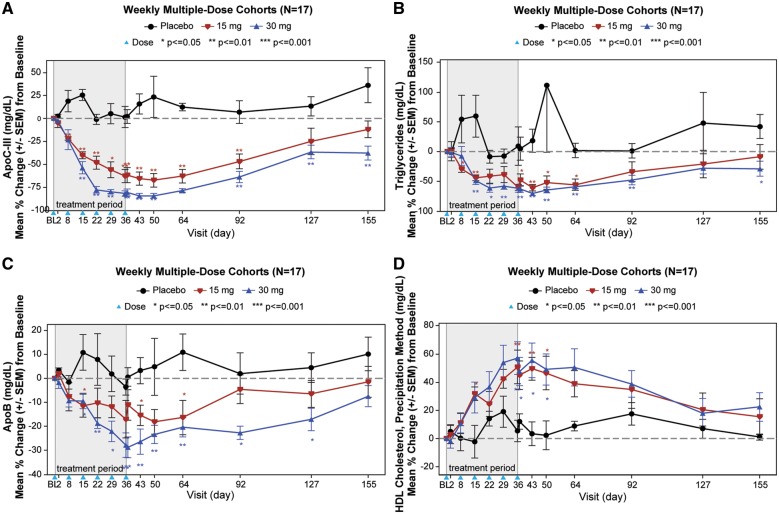
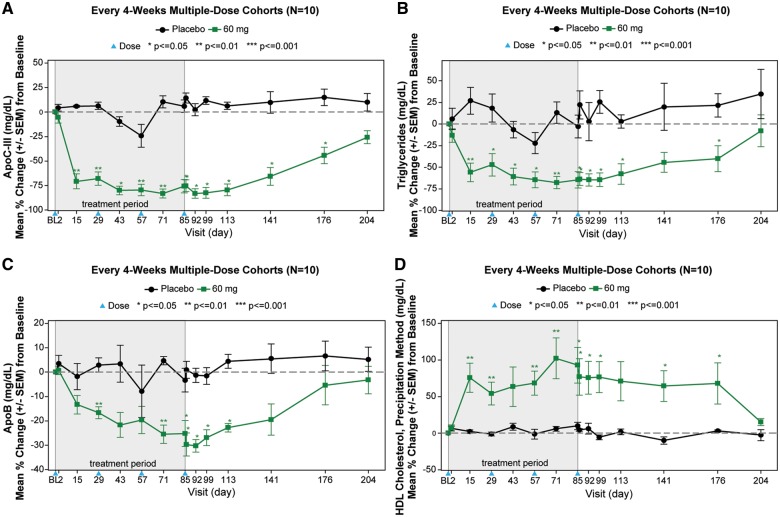
In MAD cohorts of 15 and 30 mg weekly and 60 mg every 4 weeks, median reductions of −66%, −84%, and −89% in apoC-III, and −59%, −73%, and −66% in triglycerides were observed 1 week after the last dose. Significant reductions in total cholesterol, apoB, non-HDL-C, VLDL-C, and increases in HDL-C were also observed in the 15 mg/week, 30 mg/week, and 60 mg/every 4 weeks groups (Table 2).
The temporal relationships of changes in apoC-III, triglycerides, apoB, and HDL-C are shown in Figures2A–D and 3A–D. Most of the nadirs or peaks occurred by days 36–50 in the weekly cohorts and days 85–99 in the every 4 weeks cohort, with return to baseline approximately 4 months after the last dose.
Figure 2.
Multiple ascending dose cohorts. The graphs display the mean percent change (±SEM) in apoC-III (A), triglycerides (B), apoB (C), and HDL-C (D) in the multiple dose cohorts. The blue arrowhead represents the timing of the doses. The data were compared between AKCEA-APOCIII-LRx treatments and placebo using one-way analysis of variance or Wilcoxon rank sum test. Subjects were required to have a fasting triglyceride level ≥200 mg/dL.
Figure 3.
Every 4-week dosing multiple dose cohorts. The graphs display the mean percent change (±SEM) in apoC-III (A), triglycerides (B), apoB (C), and HDL-C (D) in the every 4-week multiple dose cohorts. The blue arrowhead represents the timing of the doses. The data were compared between AKCEA-APOCIII-LRx treatments and placebo using one-way analysis of variance or Wilcoxon rank sum test. Subjects were required to have a fasting triglyceride level ≥200 mg/dL.
We also performed a mixed model analysis as a sensitivity analysis and these results are included in Supplementary material online, Table S3.
Safety and tolerability
AKCEA-APOCIII-LRx was well tolerated as either a single subcutaneous injection or multiple subcutaneous injections. There were no deaths, serious adverse events, or treatment-emergent adverse events (TEAEs) leading to discontinuation of study or treatment in any of the AKCEA-APOCIII-LRx treatment cohorts. There was one injection site reaction of mild erythema at the injection site following the third injection (Day 15) in a subject in the 15-mg weekly dosing cohort and no flu-like reactions in any subjects. No subject met criteria for any stopping rules for discontinuation of Study Drug. There were no clinically relevant changes in urinalysis, chemistry, or haematology, and in particular, there were no changes in platelet counts. No changes were observed in vital sign measurements or electrocardiogram findings, and no treatment-related cardiac toxicity, renal, or liver safety signals were reported.
The number of subjects experiencing TEAEs, and number of events, is provided in Supplementary material online, Table S1. Headache {2 of 30 subjects, 6.7% [95% confidence interval (CI) 0.8–22.1%]} was the only event experienced by more than one subject receiving AKCEA-APOCIII-LRx in the single-dose cohorts (one subject each in the 10 and 90 mg cohorts), and in the in the weekly multiple dose cohorts (two subjects in the 15 mg cohort). All adverse events in subjects receiving AKCEA-APOCIII-LR in the single-dose and weekly multiple-dose cohorts were classified as mild and all resolved.
In the every 4-week multiple-dose cohort, the majority of TEAEs were mild. There were three moderate severity TEAEs and one severe TEAE in subjects receiving AKCEA-APOCIII-LRx, and all resolved. One subject experienced one event of blood creatine phosphokinase increased considered to be severe and not related to Study Drug, and one subject experienced one event of blood creatinine increased considered to be moderate in severity that was an isolated, not confirmed elevation with no other changes in renal labs, and not considered related to Study Drug. Two of six subjects [33.3% (95% CI 4.3–77.7%)], receiving AKCEA-APOCIII-LRx every 4 weeks, each experienced multiple events of both alanine aminotransferase (ALT) increased (one event in each subject of moderate severity) and single events of aspartate aminotransferase (AST) increased. These events were considered possibly related to Study Drug and were the only events experienced by more than one subject receiving AKCEA-APOCIII-LRx in the every 4 weeks multiple-dose cohort. Both subjects had prior history of AST and ALT elevations suggesting a medical history that may have contributed to the observed liver enzyme elevations during the study. Also, other dose cohorts with similar cumulative doses, the 120 mg single dose and 30 mg weekly dose, were not associated with increases in AST or ALT.
Pharmacokinetic data
The pharmacokinetic data and interpretation are shown Supplementary material online, Table S2.
Discussion
This study demonstrated that AKCEA-APOCIII-LRx, a GalNAc modified ASO targeting APOC3 mRNA, significantly decreases plasma apoC-III and triglyceride levels in subjects with hypertriglyceridaemia. Additionally, it promoted a favourable lipid profile in significantly lowering total cholesterol, apoB, VLDL-C, and non-HDL-C levels and also increasing HDL-C (take home figure). AKCEA-APOCIII-LRx was well tolerated with no significant adverse events at the injection site, or significant effects on liver or renal function, or platelet count.
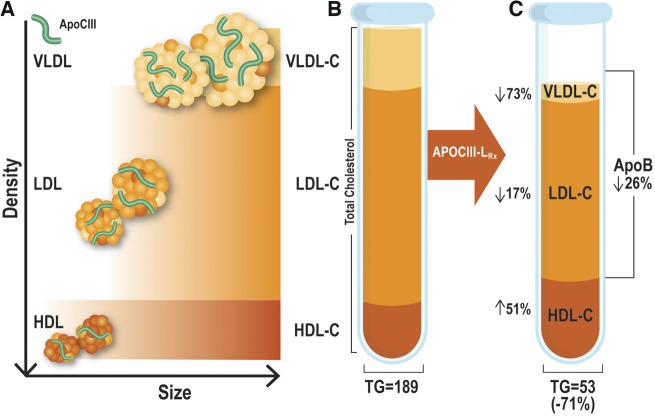
Take home figure.
Panel (A) represents the size and density patterns of HDL, LDL, and VLDL particles. Each of the particles is shown containing variable amounts of apoC-III. The relative proportion and locations of total cholesterol, HDL-C, LDL-C, and VLDL-C, and apoB are shown in a stylized tube of ultracentrifugally prepared lipoproteins (B). The effect of APOCIII-LRx on these lipoproteins are shown on the right (C) with significant reductions in triglycerides, VLDL-C, LDL-C, and increases in HDL-C. IDL and chylomicrons are not shown in this figure as the subjects were fasting. The numbers in the illustration are derived from the 30 mg/weekly dose measured at 1 week after the last dose.
The evidence for APOC3 as a target for cardiovascular disease (CVD) risk reduction was suggested by genome-wide significance studies showing that individuals with loss of function mutations exhibited reduced plasma triglyceride levels, reduced coronary heart disease, and increased longevity.19,20 In aggregate, these studies have shown that loss of function variations in the APOC3 gene are associated with approximately 40% reduction in plasma triglycerides, which in turn is manifested by a similar reduction in CVD events. These observations are supported by epidemiological studies2,21 and sub-studies from randomized clinical trials3 showing that baseline elevated triglycerides or persistently elevated triglycerides following lipid-lowering therapies are associated with significant risk for first or secondary CVD events.
The efficacy of AKCEA-APOCIII-LRx in the current study is in line with prior studies with volanesorsen, showing reductions in apoC-III protein levels by 70–80% and reductions in triglycerides by 60–70% with the highest doses.9,13 Furthermore, both agents significantly reduced VLDL-C and non-HDL-C and increased HDL-C. In FCS patients, who have extremely low LDL-C levels, volanesorsen tends to have an LDL-C raising effect with increases back towards a ‘normal’ level in context of significant reduction in non-HDL cholesterol.9 However, whereas volanesorsen had a neutral effect in total plasma apoB in patients on no other lipid-modifying therapy or slight reduction in patients on fibrates,13 AKCEA-APOCIII-LRx had significant reductions in total apoB levels and an overall more favourable lipid profile. The differences in changes in apoB in these studies likely reflects the aetiology and underlying extent of triglyceride elevation, with the higher the triglyceride the lower the reduction in total apoB with APOC3 mRNA inhibition.
There are some differences in targeting triglycerides with fish oils/omega-3 fatty acids vs. apoC-III. Fish oils contain a mixture of eicosapentaenoic acid and docosahexaenoic acid, have modest effects on plasma triglycerides and tend to raise LDL-C, which may mitigate clinical benefit. Pure preparations of omega-3 fatty acids containing only eicosapentaenoic acid tend to be LDL-C neutral.22,23 In contrast, inhibiting APOC3 mRNA in this study was associated not only with a more potent triglyceride reduction compared with fish oils/omega-3 fatty acids, but also had a significant reduction in all apoB-containing atherogenic lipoproteins. Whether the more potent triglyceride reduction and favourable effect on all apoB containing lipoproteins leads to improved clinical outcomes awaits to be determined in future clinical studies.
AKCEA-APOCIII-LRx adds to the favourable clinical safety and efficacy experience noted with GalNac-modified ASOs, such as to apolipoprotein(a)16 and angiopoietin like-315 as well as six additional GalNac-modified drugs that have completed Phase 1 trials.12 Modifying ASOs with GalNAc allows specific targeting of the ASO to hepatocytes so that for similar hepatocyte exposure, reduced exposure to both non-parenchymal liver cells and systemic exposure is feasible. Each of these GalNAc-modified ASOs has demonstrated a similar or higher efficacy than their respective parent compounds but at 15–30-fold lower systemic doses. Although a formal analysis is not feasible due to study design differences, a comparison of dose–response of AKCEA-APOCIII-LRx and non-GalNac ASO volanesorsen in human data derived from a prior Phase 1 study14 showed that AKCEA-APOCIII-LRx has at least 15× higher potency based on ED50 (weekly dose that produced 50% of maximum effect) in reducing fasting serum apoC-III and TGs. In turn, the clinical experience to date of using lower doses of ASOs has demonstrated an improvement in early experience safety and tolerability.24 An observational study in FCS has suggested that these patients have greater than expected spontaneous fluctuations in platelet count.25 However, the incidence of thrombocytopenia in FCS subjects was higher in those treated with volanesorsen compared with those on placebo.26 In the current relatively small, short-term study using AKCEA-APOCIII-LRx in patients without FCS, no significant declines in platelet count were noted.
Targeting plasma apoC-III proteins therapeutically may be applicable to a variety of disorders where TRLs and remnant cholesterol are elevated and are not adequately addressed by current triglyceride-lowering agents, fibrates, fish oil/omega-3 fatty acids, or niacin. For example, a large population of patients have multifactorial hypertriglyceridaemia due to combinations of genetic and metabolic abnormalities that lead to impaired LPL activity, enhanced hepatic production of VLDL, and impaired clearance of TRL. Additionally, following statin therapy in patients with CVD, up to 40% of patients have persistent elevation of triglycerides despite optimally controlled LDL-C. There are no currently approved drugs specifically to address modest elevations of triglycerides in the range of 150–500 mg/dL. Prior studies using fish oil/omega-3 fatty acids or fibrates, in the context of statin therapy background, have failed in their primary endpoints, although post hoc analyses suggest that subgroups of patients with modest elevations of triglycerides may derive benefit.27 The REDUCE-IT trial reported topline that administration of 4 g of icosapent ethyl resulted in a significant reduction in major adverse cardiac events in subjects with median baseline triglyceride levels of 216.7 mg/dL and controlled LDL-C on stable statin dose.23 However, the benefit was unrelated to baseline plasma triglyceride level, suggesting that the effect was unrelated to triglyceride lowering per se. The ongoing STRENGTH and PROMINENT trials will further answer the question whether modestly reducing elevations of triglycerides (180–500 mg/dL) with omega-3 fatty acids or potent fibrates will lead to lower risk of CVD events.
A limitation of this study is that the study subjects were otherwise healthy volunteers with variable elevation of triglyceride levels, which may not fully represent the efficacy that might be seen in patients with highly elevated triglyceride levels. A Phase 2 trial with AKCEA-APOCIII-LRX in patients with prior history of CVD and triglycerides ≥200 mg/dL is ongoing and will provide data for dosing and extent of triglyceride lowering in patients with pre-existing CVD (NCT03385239, Study of ISIS 678354 (AKCEA-APOCIII-LRx) in patients with hypertriglyceridaemia and established CVD], as well as additional safety and tolerability data in a larger dataset.
In conclusion, significant reduction of apoC-III, triglycerides, and other atherogenic lipoproteins can be achieved with AKCEA-APOCIII-LRx, a hepatocyte-targeted inhibitor of apoC3 mRNA.
Supplementary Material
Acknowledgements
We thank Wanda Sullivan from Ionis Pharmaceuticals for generation of the artwork.
Funding
This work was supported by the Ionis Pharmaceuticals.
Conflict of interest: V.J.A., S.X., S.G.H., R.S.G., and S.T. are employees of Ionis Pharmaceuticals. E.H. and L.D. are employees of Akcea Therapeutics. S.T. and J.L.W. are co-inventors and receive royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins and are co-founders of Oxitope, Inc. S.T. is a consultant to Boston Heart Diagnostics. J.L.W. is a consultant to Ionis Pharmaceuticals. S.T. has a dual appointment at UCSD and Ionis Pharmaceuticals.
See page 2797 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz321)
References
- 1. Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Boren J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A, Watts GF, Wiklund O; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nordestgaard BG, Varbo A.. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG.. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267–2275. [DOI] [PubMed] [Google Scholar]
- 4. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 5. Hegele RA, Berberich AJ, Ban MR, Wang J, Digenio A, Alexander VJ, D'Erasmo L, Arca M, Jones A, Bruckert E, Stroes ES, Bergeron J, Civeira F, Witztum JL, Gaudet D.. Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. J Clin Lipidol 2018;12:920–927 e4. [DOI] [PubMed] [Google Scholar]
- 6. Norata GD, Tsimikas S, Pirillo A, Catapano AL.. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci 2015;36:675–687. [DOI] [PubMed] [Google Scholar]
- 7. Yang X, Lee SR, Choi YS, Alexander VJ, Digenio A, Yang Q, Miller YI, Witztum JL, Tsimikas S.. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J Lipid Res 2016;57:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjonneland A, Polak JF, Rimm EB, Overvad K, McClelland RL, Sacks FM.. High-density lipoprotein subspecies defined by presence of apolipoprotein C-III and incident coronary heart disease in four cohorts. Circulation 2018;137:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL.. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014;371:2200–2206. [DOI] [PubMed] [Google Scholar]
- 10. Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, Thacker BE, Basu D, Lee RG, Mullick AE, Graham MJ, Goldberg IJ, Crooke RM, Witztum JL, Esko JD.. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest 2016;126:2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wyler von Ballmoos MC, Haring B, Sacks FM.. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J Clin Lipidol 2015;9:498–510. [DOI] [PubMed] [Google Scholar]
- 12. Crooke ST, Witztum JL, Bennett CF, Baker BF.. RNA-targeted therapeutics. Cell Metab 2018;27:714–739. [DOI] [PubMed] [Google Scholar]
- 13. Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, Crooke RM, Witztum JL, Brunzell JD, Kastelein JJ.. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med 2015;373:438–447. [DOI] [PubMed] [Google Scholar]
- 14. Graham MJ, Lee RG, Bell TA 3rd, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, Baker BF, Burkey J, Crooke ST, Crooke RM.. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 2013;112:1479–1490. [DOI] [PubMed] [Google Scholar]
- 15. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S.. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 16. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S.. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–2253. [DOI] [PubMed] [Google Scholar]
- 17. Yu RZ, Graham MJ, Post N, Riney S, Zanardi T, Hall S, Burkey J, Shemesh CS, Prakash TP, Seth PP, Swayze EE, Geary RS, Wang Y, Henry S.. Disposition and pharmacology of a GalNAc3-conjugated ASO targeting human lipoprotein (a) in mice. Mol Ther Nucleic Acids 2016;5:e317.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu RZ, Gunawan R, Post N, Zanardi T, Hall S, Burkey J, Kim TW, Graham MJ, Prakash TP, Seth PP, Swayze EE, Geary RS, Henry SP, Wang Y.. Disposition and pharmacokinetics of a GalNAc3-conjugated antisense oligonucleotide targeting human lipoprotein (a) in monkeys. Nucleic Acid Ther 2016;26:372–380. [DOI] [PubMed] [Google Scholar]
- 19. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR.. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008;322:1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A.. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 21. Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG.. Remnant cholesterol as a causal risk factor for ischemic heart disease. Journal of the American College of Cardiology 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 22. Ballantyne CM, Braeckman RA, Bays HE, Kastelein JJ, Otvos JD, Stirtan WG, Doyle RT Jr, Soni PN, Juliano RA.. Effects of icosapent ethyl on lipoprotein particle concentration and size in statin-treated patients with persistent high triglycerides (the ANCHOR Study). J Clin Lipidol 2015;9:377–383. [DOI] [PubMed] [Google Scholar]
- 23. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 24. Crooke ST, Baker BF, Xia S, Yu RZ, Viney NJ, Wang Y, Tsimikas S, Geary RS.. Integrated assessment of the clinical performance of galnac3-conjugated 2'-o-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucleic Acid Ther 2019;29:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaudet D, Baass A, Tremblay K, Brisson D, Laflamme N, Paquette M, Dufour R, Bergeron J.. Natural history (up to 15 years) of platelet count in 84 patients with familial hyperchylomicronemia due to lipoprotein lipase deficiency. J Clin Lipidol 2017;11:797–798. [Google Scholar]
- 26. Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A KRW, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civiera F, Hemphill L, Tsimikas S, Blom DJ, O’Dea L, Bruckert E. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019 (accepted for publication). [DOI] [PubMed]
- 27. Ginsberg HN. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) lipid trial: what we learn from subgroup analyses. Diabetes Care 2011;34 Suppl 2:S107–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.