Abstract
Rationale:
Primary granulocytic sarcoma of the breast is a rare and poor-prognosis malignancy. Clinicians do not have sufficient knowledge of this disease and often misdirect it as other soft tissue sarcomas or inflammation.
Patient concerns:
A 42-year-old female presented with a self-discovered asymptomatic growing and palpable right breast mass that had been present for 4 months.
Diagnoses:
The patient was diagnosed as primary myeloid sarcoma.
Interventions:
The patient received modified radical mastectomy in the right breast and sentinel lymph node biopsy. Pathological diagnosis is primary granulocytic sarcoma. Then the patient accepted acute myeloid leukemia-induction chemotherapy.
Outcomes:
The follow-up of this patient has no evidence of disease progression or spread during 1 year.
Lessons:
Granulocytic sarcoma in the breast tissue is rare. But it still should be considered in the differential diagnosis of any tumor in the breast. The present study discusses comprehensively the clinical and pathological characteristics to improve the understanding of myeloid sarcoma.
Keywords: acute myeloid leukemia, breast neoplasms, chloroma, granulocytic sarcoma, myeloid sarcoma, treatment
1. Introduction
Granulocytic sarcoma (GS) is a malignant neoplasm of myeloid origin that locates outside the bone marrow. The tumor is also being termed as chloroma, extraordinary myeloid tumor, or myeloid sarcoma (MS), which generally present with a green color due to the presence of myeloperoxidase (MPO).[1] The incidence of GS is only 2% to 14% of all acute myeloid leukemia cases. Granulocytic sarcoma can be developed in any area of the body, and the most common sites of GS are the bones, lymph nodes, soft tissues, and skin.[2,3] But primary GS of the breast is rare, the clinical characteristics and diagnosis of which are typical and challenging.[4–6] Here, we report a case of GS in the breast without evidence of hematological features of myeloid malignancy in order to improve understanding of the disease further and provide a reference for standardized and individualized clinical treatments.
2. Case report
A 42-year-old Asian female hospitalized to our Hospital in November 17, 2017, with a self-discovered asymptomatic growing and palpable right breast mass that had been present for 4 months. Physical examinations showed a 2 cm × 2 cm mass located in the lower outer quadrant, and a mobile swollen axillary lymph gland approximately 2 cm in diameter. Her blood routine reviews were as follows: WBC count was 8.09 × 109/L (normal range, 3.5–9.5 ×109/L); with 76.6% neutrophils (normal range, 40–75%); 19.4% lymphocytes (normal range, 20–50%); 3.3% monocytes (normal range, 3–10%); 0.5% eosinophils (normal range, 0.7–8%); and 0.2% basophils (normal range, 0–1%). Her RBC count was 4.31 ×1012/L (normal range, 3.8–5.1 × 1012/L); hemoglobin was 108 g/L (normal range, 110–150 g/L). And her platelet count was 219 ×109/L (normal range, 125–350 × 109/L). Serum biochemical parameters were well within normal limits.
Ultrasound scan of the breast (Fig. 1) revealed a single, irregular, ill-defined margin hypothetic area measuring approximately 2 cm × 1.8 cm, located in 8 o’clock positions, corresponding to the known palpable mass. On the right axillary presented a well-defined hypothesis lesion about 2 cm × 0.7 cm, which could be considered to lymph node. Molybdenum target mammography (Fig. 1) showed a mass without calcification, located in the outer quadrant. Breast magnetic resonance imaging (MRI) (Fig. 2) showed an intumescent mass over the outer quadrant of the right breast coalesce with axillary lymph node. The lump was hyperactive on T1-weighted and T2-weighted images, suggesting malignancy. Dotted lesions were scattered all over bilateral breasts. The computed tomography (CT) scan revealed a proliferative inflammatory lesion at the right lung. Cardiac ultrasound reminded tricuspid regurgitation and left ventricular diastolic.
Figure 1.
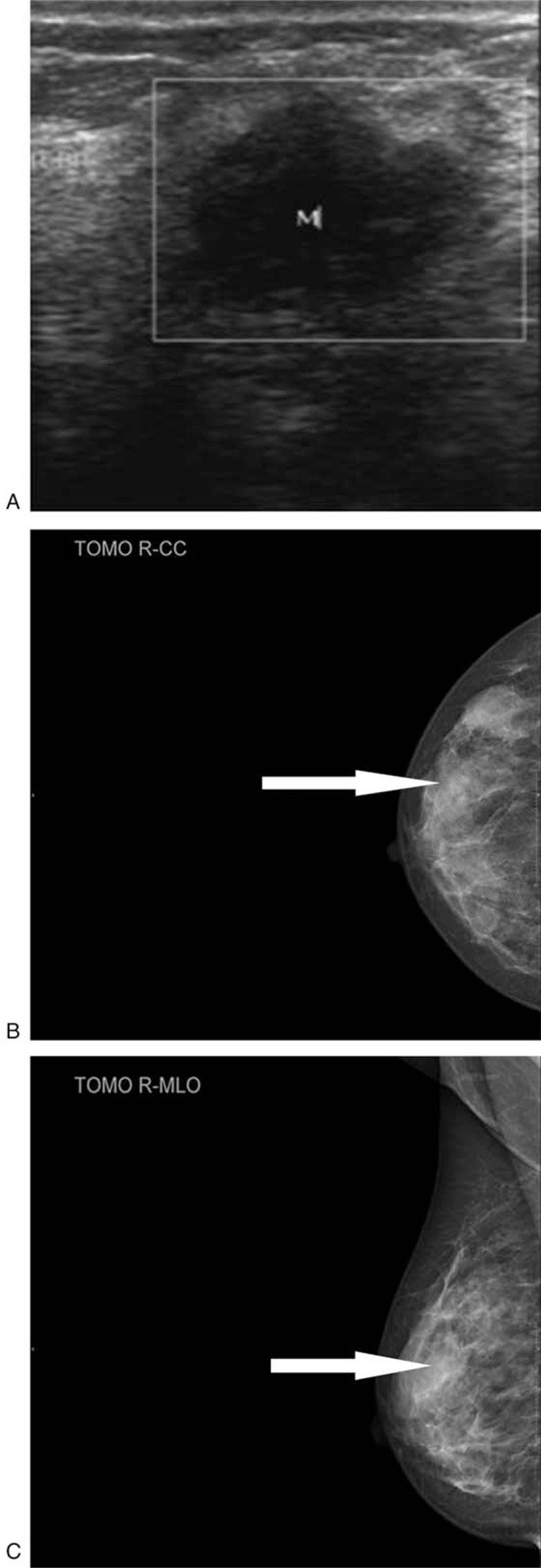
Ultrasound scan and Molybdenum target mammography image: (A) ultrasound scan of the breast revealed a single, irregular, ill-defined margin hypothetic area measuring approximately cm 1.8 cm, located in 8 o’clock positions. B, C, Molybdenum target mammography showed a mass without calcification located in the up outer quadrant of the right breast.
Figure 2.
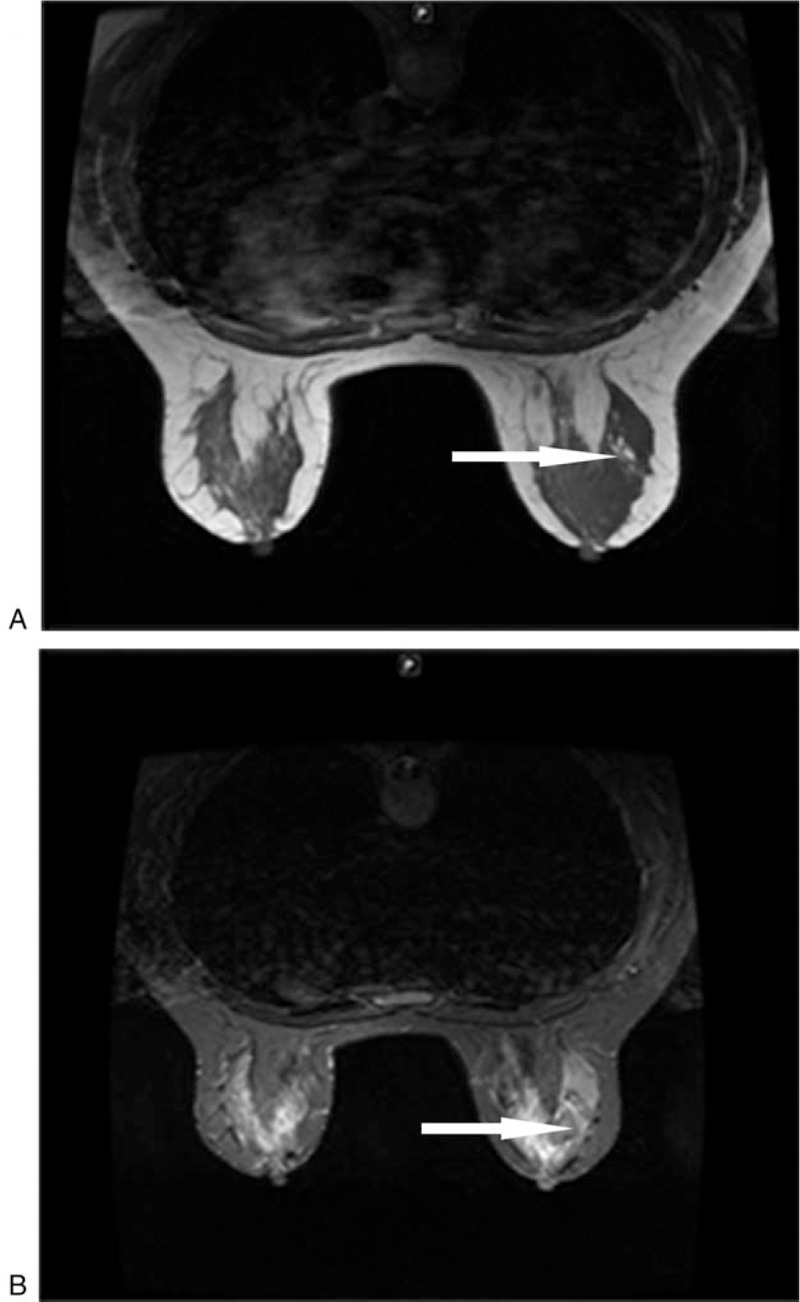
Breast magnetic resonance imaging (MRI) showed an intumescent mass over the outer quadrant of the right breast coalesce with axillary lymph node. The lump was hyperactive on (A) T1-weighted and (B) T2-weighted images, suggesting malignancy. Dotted lesions were scattered all over bilateral breasts.
The patient accepted modified radical mastectomy in the right breast and sentinel lymph node biopsy (SLNB) at November 20, 2017. Intraoperative frozen section was diagnosed as invasive carcinoma. Paraffin section diagnosis is GS of the healthy breast, all lymph glands have no tumor involved. Immune-histochemical indicated (Fig. 3): MPO(+), LCA(+), BCL-2(+), C-myc(+), ER(−), PR(−), cerbB2(−), CK5/6(−), ECad(−), CK(−), P120(−), CD20(−), PAX5(−), CD3(−), CD5(−), BCL-6(−), CD10(−), MUM-1(−), Ki-67 60%. Bone marrow aspirate and biopsy have no abnormal performance.
Figure 3.
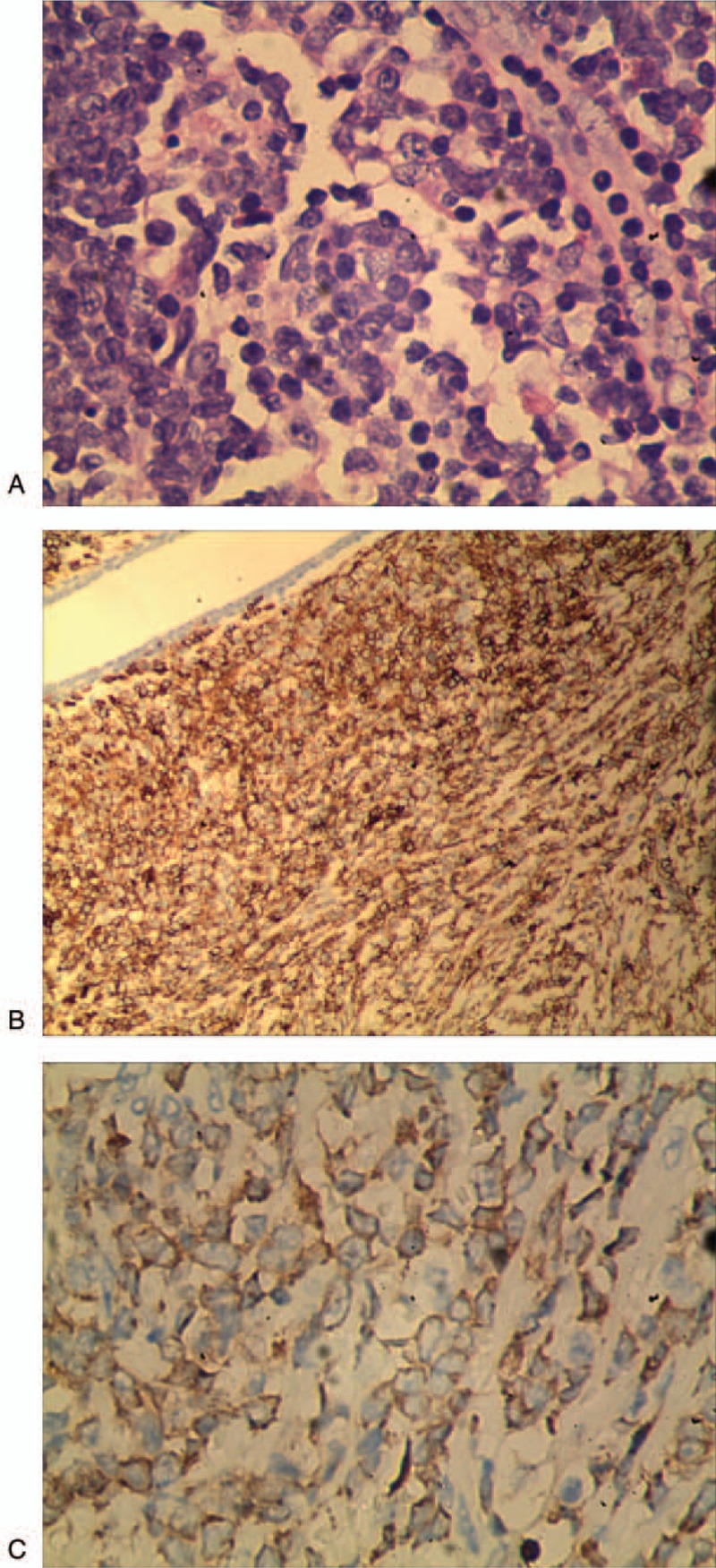
The pathological appearance of breast granulocytic sarcoma showed diffuse infiltration growth, most cells were large, the nucleus was vacuolated, the nucleoli were obvious, and a few cells were renal nuclei. A, Hematoxylin and eosin stain (magnification, ×400). B, Leukocyte common antigen (LCA) was decisive (magnification, ×400). C, Myeloperoxidase reactivity in tumor cells was strongly positive (magnification, ×400).
Postoperatively, the patient received 2 cycles of IA regimen chemotherapy composed of inhibition and cytosine arabinoside. Then she started on HA regimen chemotherapy, which includes Homoharringtonine and cytosine arabinoside. Currently the woman achieved complete remission. We advised her to accept the bone marrow transplant. But the patient refused. The follow-up of this patient has no evidence of disease progression or spread during 1 year. In the future, the patient will receive ultrasound scan of the breast, chest X-ray and bone marrow aspirate and biopsy every 3 months.
3. Discussion
Granulocytic sarcoma (GS) is an uncommon extracellular tumor comprised of immature granulocytic cells. This type of malignancy frequently occurs during the course of active hematological disease, including acute or chronic leukemia and myeloproliferative disorder, or occurs during remission from leukemia.[7–10] Granulocytic c sarcoma of the breast more often was found in patients with an established history of a myeloid leukemia.[11] Primary granulocytic sarcoma (PGS) of the breast without a relevant history of hematological abnormality, is rarely in clinical.[12] These tumors are usually clinically indistinguishable from other breast tumors. Patients with PGS of the breast, like in our case, most presented with a palpable mass that can be either painful or painless and involved in unilateral breast, but it also can involve in bilateral involvement. Some patients may have skin involvement and/ or enlarged axillary lymph nodes. However, patients usually exhibit no other associated symptoms, such as nipple retraction or discharge.[11,13–15]
The diagnosis of PGS in the breast is normally a challenge, and misdiagnosis is usually high. A third study of primary GS diagnosed by the MD Anderson Cancer Center reported that almost 75% patients were middle-aged.[16] Misdiagnosis may particularly occur in those poorly differentiated tumors and in patients whom presenting with isolated disease. The most common medications are as large-cell non-Hodgkin lymphoma, lymphoblastic lymphoma, undifferentiated carcinoma, lobular carcinoma of the breast (especially infiltrating lobular carcinoma), extraordinary hematopoiesis, and inflammation.[17] Therefore, how to differentiate lymph sarcoma is a difficult problem to be resolved clinically.
Imaging tests, including mammography and ultrasound, are sometimes difficult to distinguish GS from other similar tumors.[18] Due to the fact that it usually appears as a noncalcified low-echo lump with unequal size and boundary blur in imaging examination, GS is prone to be misdirected as a malignant breast tumor.[15,19,20] Magnetic resonance imaging (MRI) has the virtue of distinguishing benign and malignant tumors, but not for the diagnosis of GS. In this case, the t1-weighted image and the t2-weighted image of the lesion tissue are displayed as a penetrating intensity tumor, suggesting a malignant tumor compared to the breast substance.
At present, the ability to get an accurate diagnosis of GS depends largely on the additional histological and immunohistochemically findings within the tumor. Immunohistochemistry, flow cytometry, fluorescence in situ hybridization, and other molecular techniques can improve the diagnostic accuracy. Many features can aid in proving the diagnosis, such as myeloperoxidase (MPO), CD34, CD43, CD117, and CD68.[16,21] The immunohistochemical detection of MPO-positive cells is helpful to confirm the final diagnosis. The expression levels of MPO-positive cells in GS as reported by Mourad et al and Pileri et al were 66% and 83.6%, respectively.[4,22] Cytokeratin staining may exclude an epithelial-based tumor and use of myeloid MPO staining would have suggested a hematologic tumor origin. Specific CD markers can also be handy, such as CD117 and CD68, CD43 is positive in most of the cases, and 75% are reactive for CD45.[23,24] Negative B- and T-cell markers (CD45, CD20, UCHL-1, CD3, and CD30) would further exclude the diagnosis of lymphoma. Several genetic anomalies have been associated with GS, such as t(8; 21) and in(16), which result in AML1/ETO (RUNX1/RUNX1T1) and CBF/MYH11 gene fusions respectively.[4,25,26] Account for the rarely rise of PGS in the breast, randomized controlled trials had been limited. Therapy for GS of breast is currently still in controversy. There is no definitive consensus regarding the optimal treatment of primary extracellular GS. However, most studies have concluded that all patients with GS should receive standard systemic chemotherapy followed by mastectomy or lumpectomy.[21,26–28] Imrie et al[29] reported that the overall survival of chemotherapy-treated patients was longer compared with those who did do not receive chemotherapy. In our case, patient received mastectomy and systemic chemotherapy, and there was not any local recurrence identified in the breast half a year later.
From the above, GS in the breast tissue is rare. However, it still needs to be considered in the differential diagnosis of any tumor in the breast. As in our case, the patient has no systemic signs of the disease and the immunological examination is hard to distinguish GS from other tumors. Histology then plays an important part in the diagnosis. The optimal treatment for PGS is still controversial. A simple bacteriological diagnosis of GS is insufficient to decide systemic treatment strategies. The present studies pointed out that lumpectomy combined with systemic chemotherapy results in a good outcome for patients with GS of the breast. However, it is not the right most favorable treatment strategy for all GS patients. In the targeted treatment era, attempt should be made to define the chromosomal abnormality to guide treatment and stratify prognosis. Multicenter controlled trials are needed to further refine management decisions and investigate the role of novel targeted therapies.
Author contributions
Conceptualization: Lei Gu.
Project administration: Yonghui Luo.
Writing – original draft: Hengyu Wu, Lei Liu, Lei Gu.
Writing – review & editing: Lei Liu.
Footnotes
Abbreviations: CT = computed tomography, GS = granulocytic sarcoma, MPO = myeloperoxidase, MRI = magnetic resonance imaging, MS = myeloid sarcoma, PGS = primary granulocytic sarcoma, SLNB = sentinel lymph node biopsy.
This research received no specific grant from any funding agency in the public, commercial, or non-for-profit sectors.
Written informed consent was issued by the patient for publication of this case report and accompanied images.
The authors have no conflicts of interest to disclose.
References
- [1].Campidelli C, Agostinelli C, Stitson R, et al. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol 2009;132:426. [DOI] [PubMed] [Google Scholar]
- [2].Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact 2010;184:16–20. [DOI] [PubMed] [Google Scholar]
- [3].Lakhani S, Ellis I, Schnitt S, et al. World Health Organization Classification of Tumours of the breast. 2012. [Google Scholar]
- [4].Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340–50. [DOI] [PubMed] [Google Scholar]
- [5].Yilmaz AF, Saydam G, Sahin F, et al. Granulocytic sarcoma: a systematic review. Am J Blood Res 2013;3:265. [PMC free article] [PubMed] [Google Scholar]
- [6].Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood 2011;118:3785–93. [DOI] [PubMed] [Google Scholar]
- [7].Collins C, Knoderer H. Central nervous system involvement at the time of presentation in acute promyelocytic leukemia. Pediatr Blood Cancer 2010;54:603–5. [DOI] [PubMed] [Google Scholar]
- [8].Colović N, Colović M, Cemerikić V, et al. Granulocytic sarcoma of the brain in a patient with acute myeloid leukemia. Acta Chir Iugosl 2004;51:129–31. [PubMed] [Google Scholar]
- [9].Cervantes GM, Cayci Z. Intracranial CNS manifestations of myeloid sarcoma in patients with acute myeloid leukemia: review of the literature and three case reports from the author's institution. J Clin Med 2015;4:1102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cho SF, Liu TC, Chang CS. Isolated central nervous system relapse presenting as myeloid sarcoma of acute myeloid leukemia after allogeneic peripheral blood stem cell transplantation. Ann Hematol 2013;92:133–5. [DOI] [PubMed] [Google Scholar]
- [11].Valbuena JR, Admirand JH, Gualco G, et al. Myeloid sarcoma involving the breast. Arch Pathol Lab Med 2005;129:32–8. [DOI] [PubMed] [Google Scholar]
- [12].Huang XE, Li YJ, Zhou XD. Granulocytic sarcoma of the breast: a case report. Oncol Lett 2015;10:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cunningham I. A clinical review of breast involvement in acute leukemia. Leuk Lymphoma 2006;47:2517–26. [DOI] [PubMed] [Google Scholar]
- [14].Surov A, Wienke A, Abbas J. Breast leukemia: an update. Acta Radiol 2012;53:261–6. [DOI] [PubMed] [Google Scholar]
- [15].Thachil J, Richards RM, Copeland G. Granulocytic sarcoma—a rare presentation of a breast lump. Ann R Coll Surg Engl 2007;89:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meis JM, Butler JJ, Osborne BM, et al. Granulocytic sarcoma in nonleukemic patients. Cancer 1986;58:2697. [DOI] [PubMed] [Google Scholar]
- [17].Ngu IW, Sinclair EC, Greenaway S, et al. Unusual presentation of granulocytic sarcoma in the breast: a case report and review of the literature. Diagn Cytopathol 2001;24:53. [DOI] [PubMed] [Google Scholar]
- [18].Basara I, Orguc S. Giant breast involvement in acute lymphoblastic leukemia: MRI findings. J Breast Cancer 2012;15:258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kinoshita T, Yokokawa M, Yashiro N. Multicentric granulocytic sarcoma of the breast: mammographic, sonographic, and MR findings. Clin Imag 2006;30:271–4. [DOI] [PubMed] [Google Scholar]
- [20].Barloon TJ, Young DC, Bass SH. Multicentric granulocytic sarcoma (chloroma) of the breast: mammographic findings. AJR Am J Roentgenol 1993;161:963–4. [DOI] [PubMed] [Google Scholar]
- [21].Markoc F, Bozdogan N, Yükrük FA, et al. Granulocytic sarcomas: difficulties in diagnosis. Tumori 2010;96:149. [DOI] [PubMed] [Google Scholar]
- [22].Mourad W, Kfoury H, Al HH. The value of CD34, myeloperoxidase and chloroacetate esterase (Leder) stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med 2001;21:287–91. [DOI] [PubMed] [Google Scholar]
- [23].Traweek ST, Arber DA, Rappaport H, et al. Extramedullary myeloid cell tumors. An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol 1993;17:1011–9. [DOI] [PubMed] [Google Scholar]
- [24].Chen J, Rd YR, Abbondanzo SL, et al. Aguilera NS. c-Kit (CD117) reactivity in extramedullary myeloid tumor/granulocytic sarcoma. Arch Pathol Lab Med 2001;125:1448–52. [DOI] [PubMed] [Google Scholar]
- [25].Tallman MS, Hakimian D, Shaw JM, et al. Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol 1993;11:690–7. [DOI] [PubMed] [Google Scholar]
- [26].Tsimberidou AM, Kantarjian HM, Estey E, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 2003;17:1100–3. [DOI] [PubMed] [Google Scholar]
- [27].Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma 2006;47:2527–41. [DOI] [PubMed] [Google Scholar]
- [28].Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 2002;94:1739–46. [DOI] [PubMed] [Google Scholar]
- [29].Imrie KR, Kovacs MJ, Selby D, et al. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med 1995;123:351. [DOI] [PubMed] [Google Scholar]


