Abstract
Telomere length is a heritable marker of cellular age that is associated with morbidity and mortality. Poor sleep behaviors, which are also associated with adverse health events, may be related to leukocyte telomere length (LTL). We studied a subpopulation of 3,145 postmenopausal women (1,796 European-American (EA) and 1,349 African-American (AA)) enrolled in the Women’s Health Initiative in 1993–1998 with data on Southern blot-measured LTL and self-reported usual sleep duration and sleep disturbance. LTL-sleep associations were analyzed separately for duration and disturbance using weighted and confounder-adjusted linear regression models in the entire sample (AAs + EAs; adjusted for race/ethnicity) and in racial/ethnic strata, since LTL differs by ancestry. After adjustment for covariates, each additional daily hour of sleep beyond 5 hours, approximately, was associated with a 27-base-pair (95% confidence interval (CI): 6, 48) longer LTL in the entire sample. Associations between sleep duration and LTL were strongest among AAs (adjusted β = 37, 95% CI: 4, 70); a similar, nonsignificant association was observed for EAs (adjusted β = 20, 95% CI: −7, 48). Sleep disturbance was not associated with LTL in our study. Our models did not show departure from linearity (quadratic sleep terms: P ≥ 0.55). Our results suggest that longer sleep duration is associated with longer LTL in postmenopausal women.
Keywords: sleep, sleep disturbance, sleep duration, telomere length, Women’s Health Initiative
Telomeres are repetitive noncoding DNA structures at the ends of chromosomes that shorten with each cellular division (1). Although telomere shortening can be accelerated by inflammation, oxidative stress, and infection (2, 3), constitutive telomere length primarily indicates heritability (h2 = 70%) and early life experiences (4–9). Inherited telomere length is longer in females than in males (6), and telomere length is longer in persons of African ancestry than in those of European ancestry (10, 11). Dysfunctional telomere maintenance—often quantified by shorter telomere length—can result in chromosomal fusions and ultimately cell-cycle arrest or apoptosis (1, 12).
Short leukocyte telomere length (LTL) is associated with increased risks of mortality, cardiovascular disease, diabetes, and Alzheimer disease (1, 13–15). Associations between LTL and cancer risk have also been reported. Although retrospective studies tend to show that short LTL is associated with cancer risk (16), prospective and/or Mendelian randomization studies indicate that long LTL is associated with cancer risk (15). Research supports the involvement of environmental and lifestyle factors, such as obesity, inactivity, smoking, and perceived stress (17–22), in LTL.
Sleep is an essential physiological process that is restorative for the body and mind (23). Poor sleep characteristics are common among the elderly, and they include short sleep duration, poor sleep quality, insomnia, sleep apnea syndrome, and restless legs syndrome (23–25). Though inextricably related via sleep, these conditions reflect distinct phenotypes (26). Specifically, the sleep duration construct represents a combination of biological and lifestyle demands, while sleep quality describes a person’s subjective feeling of restedness, which is not necessarily dependent on duration (27–29). Several characterizations of disturbed sleep are associated with all-cause mortality, hypertension, cardiovascular disease, and cancer (23, 30).
Sleep and LTL are independently associated with aging and disease. Several forms of stress are associated with both sleep deprivation and LTL loss (9, 31–34), ranging from inflammation at the cellular level to disadvantaged backgrounds at the societal level (32, 35–38).
Although associations between sleep disturbances, partially modifiable behaviors, and short LTL are supported by the current literature (39–51), the studies are heterogeneous in design, demographic characteristics, and/or health status (24). We assessed whether sleep duration and sleep disturbance are associated with LTL in a sample of postmenopausal African-American (AA) and European-American (EA) women from the Women’s Health Initiative (WHI). We hypothesized that long LTL is associated with better sleep characteristics, namely longer sleep duration and less disturbed sleep.
METHODS
Study population
The WHI is a nationwide prospective study of postmenopausal women in the United States (52). Briefly, 161,808 women aged 50–79 years were enrolled in the WHI at 40 clinical centers between 1993 and 1998. Women joined one or more WHI clinical trials (2 hormone therapy trials, a dietary modification trial, and a calcium plus vitamin D trial) or the WHI Observational Study.
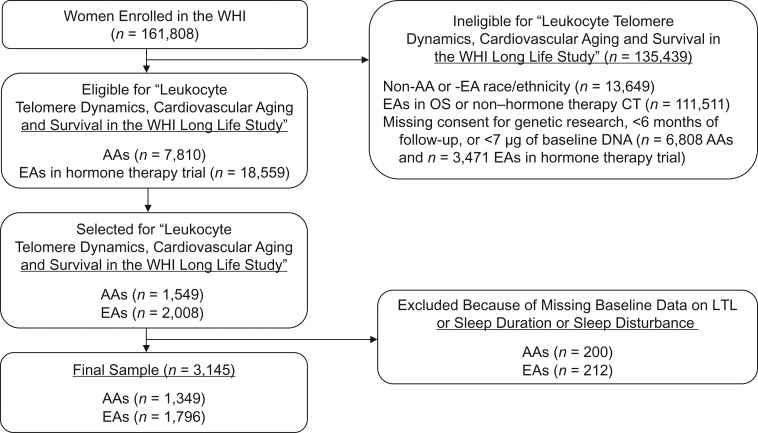
At the baseline examination, participants completed self-administered questionnaires on demographic characteristics, medical history, and lifestyle behaviors. A subset of 1,549 AA women from any WHI study (clinical trial or observational study; n = 14,618) and 2,008 EA women from the hormone therapy trials (n = 22,030) were selected for a substudy entitled “Leukocyte Telomere Dynamics, Cardiovascular Aging and Survival in the WHI Long Life Study” (53). Eligibility criteria included consent for genetic research, ≥6 months of follow-up, and availability of ≥7 μg of baseline DNA. The present study included women with baseline LTL measurements and complete data on baseline sleep duration and sleep disturbance. The final sample included 1,349 AAs and 1,796 EAs (Figure 1).
Figure 1.
Selection of a subpopulation of participants from the Women’s Health Initiative (WHI) baseline cohort for a study of the association of sleep duration and sleep disturbance with leukocyte telomere length (LTL), United States, 1993–1998. AA, African American; CT, clinical trial; EA, European American; OS, observational study.
Participants provided written informed consent, and approval was received from the institutional review boards of all participating WHI study centers. The State University of New York at Buffalo institutional review board also approved this study.
LTL measurement
Peripheral blood was collected at the WHI baseline visit. DNA was extracted from buffy coat fractions using the 5-prime method (5 PRIME, Inc., Gaithersburg, Maryland) and stored at −80°C in the WHI Biorepository (Fisher BioServices, Rockville, Maryland) prior to processing.
The Center of Human Development and Aging laboratory at Rutgers University performed LTL measurement in batches of randomly selected samples over a period of 18 months. The laboratory was blinded to participant characteristics (53). DNA integrity was assessed visually after ethidium bromide-stained 1% agarose gel electrophoresis (200 V for 2 hours). To qualify for LTL measurement, the DNA had to appear as a single compact crown-shaped band that migrated in parallel with the other samples on the gel (53). Absolute LTL, in kilobases, was measured by Southern blot (54). Each sample was measured in duplicate on different gels, and the mean of 2 LTL measurements was used for analyses. Of the 3,547 DNA samples sent for LTL measurement, 17 (0.5%) had inadequate DNA and 292 (8.2%) were excluded because of poor DNA integrity or a bad smear. The average interassay coefficient of variation for the 3,238 successfully assayed blinded pair sets was 2.0% (53).
Collection of sleep data
At WHI baseline, participants answered 10 questions about their sleep behavior in the past month (55). The question on sleep duration was posed as, “About how many hours of sleep did you get on a typical night during the past 4 weeks?” Responses were provided in ordinal categories: ≤5, 6, 7, 8, 9, and ≥10 hours. Sleep duration was both treated continuously and dichotomized as sufficient (≥7 hours) versus insufficient (<7 hours) per the 2015 consensus statement of the American Academy of Sleep Medicine and Sleep Research Society (56).
The WHI Insomnia Rating Scale (WHIIRS) produces a validated sleep disturbance score representing insomnia symptoms determined by 5 of the 10 baseline sleep questions referenced above (55, 57). The WHIIRS score includes elements of sleep latency, sleep maintenance, early morning awakening, sleep latency following early wakening, and sleep quality; it does not include sleep duration. The WHIIRS sleep disturbance score ranges from 0 to 20, with higher scores reflecting more disturbed sleep. The WHIIRS sleep disturbance score was treated as continuous and as dichotomous (WHIIRS score ≥9) per the WHIIRS cutoff for insomnia determined by Levine et al. (57).
Covariates
Potentially confounding baseline variables were selected a priori on the basis of associations with sleep and LTL: age, race/ethnicity (hereafter called “race”), cigarette smoking (smoking status (current/former/never) and pack-years of smoking), physical activity (metabolic equivalent of task (MET)-hours/week), physical function, and socioeconomic status (SES; determined by education, household income, and neighborhood SES) (58, 59). Physical function scores ranging from 0 to 100, with higher values indicating a more favorable health state, were calculated for each participant using the 10-question Physical Function Scale from the RAND 36-Item Short Form Health Survey (60, 61). Neighborhood SES is a census-tract-level index score calculated using information on 6 SES variables on which data were collected in the 2000 US Census (62). Neighborhood SES scores ranged from 0 to 100 and were assigned on the basis of census tract; higher scores indicate more affluent tracts (63).
We also considered general female hormone use, hormone replacement therapy (current/former/never), duration of hormone therapy (never, <10 years, or ≥10 years), and age (years) at menopause. Further, we considered alcohol intake (current drinking (yes/no) and number of drinks per week), individual comorbidity (cancer other than nonmelanoma skin cancer, cardiovascular disease, hypertension, myocardial infarction, type 2 diabetes), daily caffeine intake (determined via food frequency questionnaire), current marital/partner status (partnered or not), social support score, use of sleep aids (yes/no), and snoring (yes, no, or did not know). The social support score was based on 9 items from the Medical Outcomes Study questionnaire (64) and ranged from 9 to 45, with higher scores indicating more social support (65). We also considered height, weight, waist:hip ratio, and waist circumference as measured by WHI personnel and body mass index (BMI), defined as weight (kg) divided by the square of height (m2) and categorized using World Health Organization classifications (66). We additionally considered a dichotomous variable representing depression status, measured using a short version of the Center for Epidemiologic Studies Depression Scale (67, 68). Finally, region of enrollment (Northeast, South, Midwest, or West), WHI cardiovascular substudy selection, batch and gel numbers, and a comorbidity indicator were also considered as covariates. The comorbidity indicator was based on indices created for other WHI studies (69, 70) and indicated the presence of one or more of the following conditions: stroke, cancer, diabetes, hip fracture, osteoarthritis, chronic obstructive pulmonary disease, frequent falls (>1 in the past year), and urinary incontinence.
Statistical analysis
We assessed descriptive statistics for the study variables by sufficient sleep duration status, insomnia status, and telomere length (dichotomized according to race-specific median LTL). We conducted bivariate analyses for these dichotomized variables using t tests for continuous covariates and χ2 tests for categorical covariates. We assessed age-adjusted and sample-weighted associations according to race for sleep characteristics and LTL using general linear model F tests.
We tested LTL-sleep associations using linear regression models with LTL as the continuous outcome variable and sleep characteristics (duration or disturbance) as predictors in separate models. We assessed departure from linearity, specifically a U-shaped association, using a quadratic sleep characteristic variable. All linear regression models included weights to account for WHI substudy sampling, calculated as the reciprocal of the participant’s sampling probability (ranging from 1.78 to 47.59). Results from all models were minimally adjusted for age and race, or age only in race-stratified models.
We assessed confounding by the aforementioned variables using a difference-based approach (≥10% change in sleep characteristic β coefficient) from minimally adjusted models. We determined a uniform set of confounders for each sleep duration model and sleep disturbance model separately, since sleep duration and sleep disturbance reflect distinct phenotypes (28). The final adjusted model for sleep duration included minimal adjustments plus LTL assay batch, BMI, annual household income, pack-years of cigarette smoking, physical function score, and sleep aid use. The final adjusted model for sleep disturbance included minimal adjustments plus LTL batch, BMI, cardiovascular disease status, comorbidity, depression, education, income, marital/partner status, neighborhood SES, pack-years of cigarette smoking, physical function score, sleep aid use, social support score, and enrollment region. Multicollinearity was evaluated using tolerance values less than 0.10; no variables met this threshold. We assessed effect modification by race in our final models using an interaction P value threshold of ≤0.10.
We report base-pair (bp) LTL differences and 95% confidence intervals from minimally adjusted and adjusted models for sleep duration and sleep disturbance, with sleep characteristics treated as continuous and dichotomous variables. All analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). A 2-sided P value threshold of <0.05 was used to determine statistical significance.
RESULTS
Our sample included AA (42.9%) and EA (57.1%) women with an average age of 64.0 (standard deviation (SD), 7.1) years. Overall, 76.8% of our sample reported education beyond high school, and the mean BMI was 29.6 (SD, 6.1). LTL values were normally distributed, with a mean of 6.96 (SD, 0.62) kilobases. Sleep durations of ≤5, 6, 7, 8, 9, and ≥10 hours per night were reported for 11.6%, 31.5%, 33.9%, 18.8%, 3.6%, and 0.6% of participants, respectively. Table 1 shows data on select baseline characteristics according to sufficiency of sleep duration (defined as ≥7 hours/night). Compared with women reporting insufficient sleep (<7 hours/night), women with sufficient sleep duration were older, more likely to be EA, had lower BMI, higher physical activity, and less depression, and were more likely to have smoked ≥20 pack-years, be moderate drinkers, and currently married/partnered and to have enrolled in the western United States. Our AA sample was younger at baseline, more likely to be obese, to have lower SES, and to have more pack-years of smoking, and less likely to report sleep aid use in the past 4 weeks compared with EAs.
Table 1.
Baseline Characteristics of a Subpopulation of Women’s Health Initiative Participants According to Sufficiency of Sleep Duration, United States, 1993–1998
| Characteristic | Sufficient Sleep (≥7 hours/night) (n = 1,788) | Insufficient Sleep (<7 hours/night) (n = 1,357) | P Valuea | ||||
|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Age, years | 64.66 (6.95) | 63.08 (7.25) | <0.01 | ||||
| Race/ethnicity | <0.01 | ||||||
| African-American | 603 | 33.7 | 746 | 55.0 | |||
| European-American | 1,185 | 66.3 | 611 | 45.0 | |||
| Educationb | 0.28 | ||||||
| Less than high school diploma | 93 | 5.2 | 82 | 6.0 | |||
| High school diploma | 302 | 16.9 | 248 | 18.3 | |||
| Some college or associate’s degree | 695 | 38.9 | 531 | 39.1 | |||
| College graduation or more | 692 | 38.7 | 483 | 35.6 | |||
| Body mass indexc | 29.12 (5.86) | 30.36 (6.33) | <0.01 | ||||
| <25 | 477 | 26.7 | 275 | 20.3 | <0.01 | ||
| 25–29 | 610 | 34.1 | 459 | 33.8 | |||
| ≥30 | 691 | 38.6 | 612 | 45.1 | |||
| Cigarette smokingd, pack-years | 0.05 | ||||||
| 0 (never smoker) | 895 | 50.1 | 666 | 49.1 | |||
| <5 | 113 | 6.3 | 103 | 7.6 | |||
| 5–20 | 481 | 26.9 | 397 | 29.3 | |||
| ≥20 | 252 | 14.1 | 154 | 11.3 | |||
| Alcohol intakee | <0.01 | ||||||
| Nondrinker | 218 | 12.2 | 155 | 11.4 | |||
| Past drinker | 363 | 20.3 | 330 | 24.3 | |||
| Light drinker (<1 drink/week) | 595 | 33.3 | 511 | 37.7 | |||
| Moderate drinker (≥1 drink/week) | 604 | 33.8 | 352 | 25.9 | |||
| Sleep aid userf | 322 | 18.0 | 329 | 24.2 | <0.01 | ||
| Depressedg | 335 | 18.7 | 336 | 24.8 | <0.01 | ||
| Cardiovascular diseaseh | 247 | 13.8 | 196 | 14.4 | 0.77 | ||
| Comorbidity indexi | 1,455 | 81.4 | 1,098 | 80.9 | 0.74 | ||
| Physical functionj | 81.04 (19.49) | 77.89 (21.92) | <0.01 | ||||
| Married/partneredk | 1,105 | 61.8 | 725 | 53.4 | <0.01 | ||
| Social support scorel | 36.58 (7.10) | 35.08 (7.69) | <0.01 | ||||
| Region of enrollment | 0.03 | ||||||
| Northeast | 392 | 21.9 | 311 | 22.9 | |||
| South | 501 | 28.0 | 425 | 31.3 | |||
| Midwest | 520 | 29.1 | 388 | 28.6 | |||
| West | 375 | 21.0 | 233 | 17.2 | |||
| Annual household incomem | 0.27 | ||||||
| <$10,000 | 81 | 4.5 | 78 | 5.7 | |||
| $10,000–$19,999 | 243 | 13.6 | 214 | 15.8 | |||
| $20,000–$34,999 | 479 | 26.8 | 359 | 26.5 | |||
| $35,000–$49,999 | 365 | 20.4 | 269 | 19.8 | |||
| $50,000–$74,999 | 326 | 18.2 | 223 | 16.4 | |||
| ≥$75,000 | 203 | 11.4 | 153 | 11.3 | |||
| Neighborhood SESn | 73.15 (10.34) | 69.96 (11.72) | <0.01 | ||||
| Insomnia (WHIIRS score ≥9) | 359 | 20.1 | 595 | 43.8 | <0.01 | ||
Abbreviations: SD, standard deviation; SES, socioeconomic status; WHIIRS, Women’s Health Initiative Insomnia Rating Scale.
a Calculated using χ2 tests for categorical variables and t tests for continuous variables.
b Six women with sufficient sleep and 13 women with insufficient sleep were missing data on education.
c Calculated as weight (kg)/height (m)2. Ten women with sufficient sleep and 11 women with insufficient sleep were missing data on body mass index.
d Forty-seven women with sufficient sleep and 37 women with insufficient sleep were missing data on pack-years of smoking.
e Eight women with sufficient sleep and 9 women with insufficient sleep were missing data on alcohol intake.
f Four women with sufficient sleep and 2 women with insufficient sleep were missing data on use of sleep aids.
g Depression was defined as a short Center for Epidemiologic Studies Depression Scale score greater than or equal to 0.09. Forty-three women with sufficient sleep and 37 women with insufficient sleep were missing data on depression.
h One hundred and four women with sufficient sleep and 61 women with insufficient sleep were missing data on cardiovascular disease.
i Defined as the presence of 1 or more of the following conditions: stroke, cancer, diabetes, hip fracture, osteoarthritis, chronic obstructive pulmonary disease, frequent falls, and urinary incontinence.
j Defined using the RAND 36-Item Short Form Health Survey (60, 61). Possible scores ranged from 0 to 100, with higher values indicating a more favorable health state. Thirty-one women with sufficient sleep and 28 women with insufficient sleep were missing data on physical function.
k Seven women with sufficient sleep and 4 women with insufficient sleep were missing data on marital status.
l Based on 9 items from the Medical Outcomes Study questionnaire (64). Possible scores ranged from 9 to 45, with higher scores indicating more social support (65). Thirty-four women with sufficient sleep and 44 women with insufficient sleep were missing data on social support score.
m Ninety-one women with sufficient sleep and 61 women with insufficient sleep were missing data on household income.
n A census-tract-level index score calculated using information on 6 SES variables assessed in the 2000 US Census (62). Possible scores ranged from 0 to 100; higher scores indicate more affluent census tracts (63). One hundred and seventy-four women with sufficient sleep and 188 women with insufficient sleep were missing data on neighborhood SES.
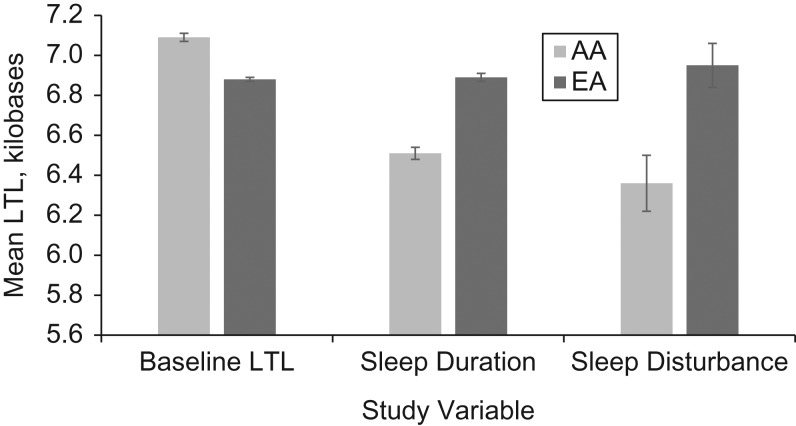
Sample-weighted and age-adjusted mean sleep duration, sleep disturbance, and LTL data are shown by race in Figure 2. AAs reported shorter continuous sleep duration (mean = 6.51 (standard error (SE), 0.03) hours/night) than EAs (mean = 6.89 (SE, 0.02) hours/night). AAs had a lower sleep disturbance score (mean = 6.36 (SE, 0.14)) than EAs (mean = 6.95 (SE, 0.11)). Mean LTL was 214 bp longer in AAs (mean = 7.09 (SE, 0.02) kilobases) than in EAs (mean = 6.88 (SE, 0.01) kilobases; P < 0.001). In the entire AA + EA sample, with adjustment for race, each 1-year increase in age was associated with 23-bp (SE, 1.4; P < 0.001) shorter LTL, on average. We did not observe evidence of race as an effect modifier (adjusted interaction: P = 0.64 for sleep duration and P = 0.71 for sleep disturbance); therefore, we report results for the entire sample (race-adjusted) and according to racial strata.
Figure 2.
Age-adjusted and sample-weighted mean values for baseline leukocyte telomere length (LTL), sleep duration (ordinal hours per night; range, 5–10), and sleep disturbance (WHIIRS score; range, 0–20), by race (African-American (AA) or European-American (EA)), in a subpopulation of participants from the Women’s Health Initiative, United States, 1993–1998. Bars, standard errors. WHIIRS, Women’s Health Initiative Insomnia Risk Scale.
Sleep duration and LTL
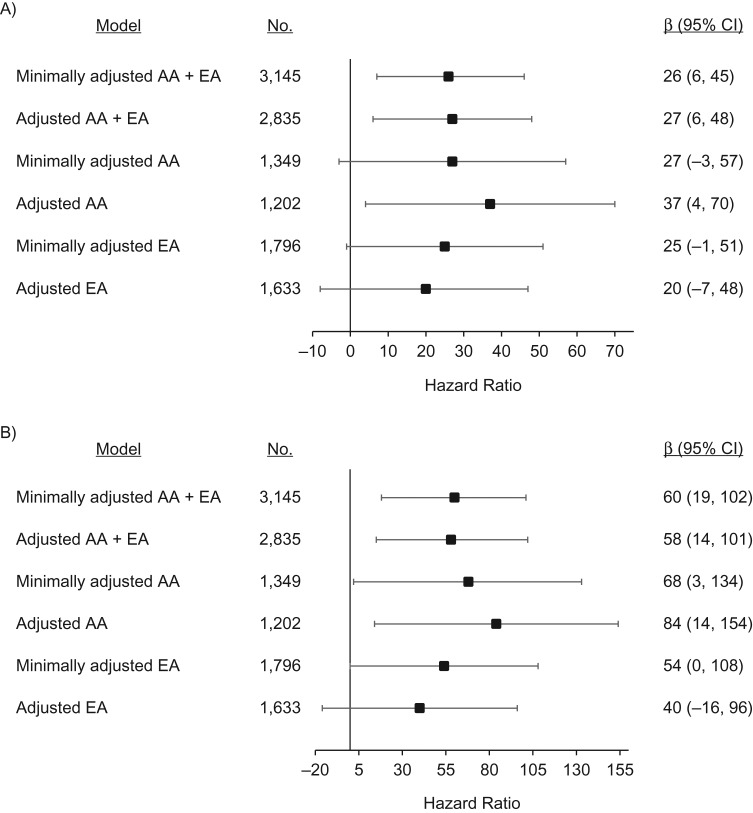
We observed statistically significant positive linear associations between continuous sleep duration and LTL in AA + EA models and among AAs only. Each unit increase in sleep duration, approximately each hour in excess of 5 hours, was associated with 27-bp (95% confidence interval (CI): 6, 48) longer LTL in adjusted AA + EA models (Figure 3A). Each unit increase in sleep duration was associated with 37-bp (95% CI: 4, 70) longer LTL among AAs after covariate adjustment. The magnitude and direction of the associations were similar but nonsignificant for EAs (adjusted β = 20, 95% CI: −7, 48).
Figure 3.
Associations of leukocyte telomere length (LTL) with continuous sleep duration (hours/night) and dichotomized sleep duration (sufficient (≥7 hours/night) vs. insufficient (<7 hours/night)) at baseline in a subpopulation of participants from the Women’s Health Initiative, United States, 1993–1998. Minimally adjusted models adjusted for age (and race in the AA + EA model). Adjusted models included minimal adjustments plus LTL batch, body mass index (weight (kg)/height (m)2), annual household income, use of sleep aids, pack-years of cigarette smoking, and physical function. A) Change in mean baseline LTL (β), in base pairs, per additional unit of sleep duration (approximately 1 hour); B) change in mean baseline LTL (β), in base pairs, among sufficient sleepers compared with insufficient sleepers. Bars, 95% confidence intervals (CIs). AA, African-American; EA, European-American.
Dichotomous sleep duration results were similar. We observed statistically significant associations between sufficient sleep duration (≥7 hours/night) and LTL in AA + EA models and among AAs only (Figure 3B). In adjusted models, sufficient sleep was associated with 58-bp (95% CI: 14, 101) longer LTL in the AA + EA model and 84-bp (95% CI: 14, 154) longer LTL among AAs. The magnitude and direction of associations were similar but nonsignificant for EAs (adjusted β = 40, 95% CI: −16, 96).
Sleep disturbance and LTL
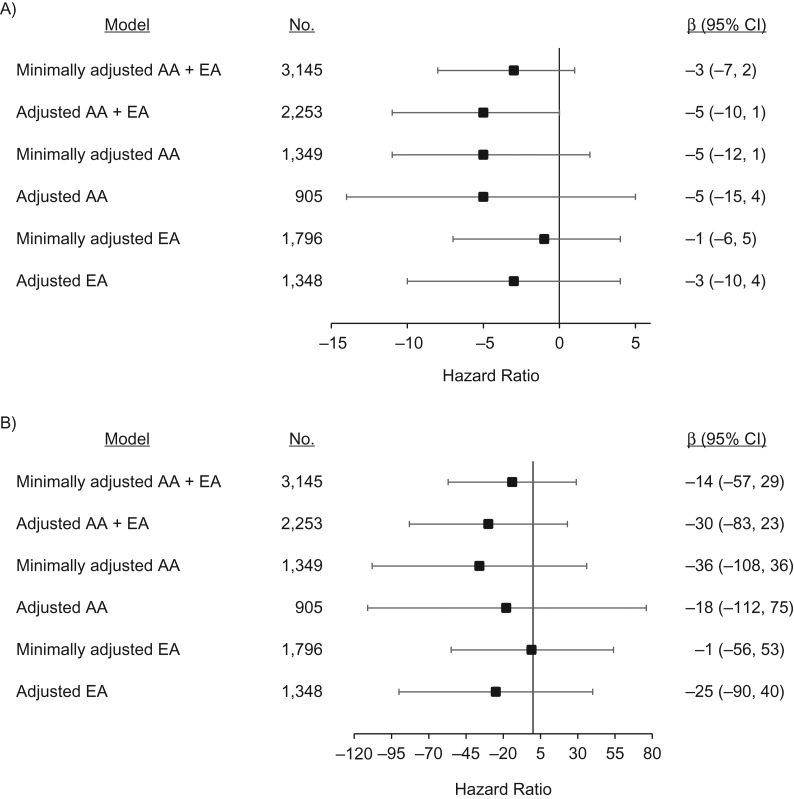
Each unit increase in sleep disturbance score was associated with a non–statistically significant 5-bp (95% CI: −10, 1) shorter LTL in the adjusted AA + EA model (Figure 4A). Racial stratum-specific results for sleep disturbance and LTL were directionally similar and not statistically significant for either continuous sleep disturbance score (Figure 4A) or dichotomous insomnia status (Figure 4B).
Figure 4.
Associations of leukocyte telomere length (LTL) with continuous WHIIRS sleep disturbance score (range, 0–20) and dichotomized WHIIRS sleep disturbance score (insomnia (WHIIRS score ≥9) vs. no insomnia (WHIIRS score <9)) at baseline in a subpopulation of participants from the Women’s Health Initiative, United States, 1993–1998. Minimally adjusted models adjusted for age (and race in the AA + EA model). Adjusted models included minimal adjustments plus LTL batch, body mass index (weight (kg)/height (m)2), cardiovascular disease status, comorbidity, depression status, education, annual household income, marital/partner status, neighborhood socioeconomic status, pack-years of cigarette smoking, physical function score, use of sleep aids, social support score, and enrollment region. A) Change in mean baseline LTL (β), in base pairs, per additional unit of WHIIRS sleep disturbance score; B) change in mean baseline LTL (β), in base pairs, for women with insomnia compared with women without insomnia. Bars, 95% confidence intervals (CIs). AA, African-American; EA, European-American; WHIIRS, Women’s Health Initiative Insomnia Risk Scale.
Sensitivity analysis
We examined the influence of enrollment in the WHI hormone therapy trials on our observed associations, as women enrolled in the hormone therapy trials had more favorable health profiles on average than women enrolled in the WHI Observational Study because of the additional eligibility criteria in the hormone therapy trials. In the sample of AAs enrolled in the hormone therapy trials (n = 305 in crude models and n = 280 in adjusted models), we observed statistical significance for 76-bp (95% CI: 8, 145; P = 0.03) longer LTL per unit increase in sleep duration. For sleep disturbance in the AA hormone therapy subset, we observed 23-bp (95% CI: −43, −3; P = 0.03) shorter LTL per unit increase in the sleep disturbance score. In the AA + EA models, the adjusted regression coefficients for the sleep duration–LTL and sleep disturbance–LTL models were similar in size and statistical significance.
DISCUSSION
We observed a positive linear association between sleep duration and telomere length in a large national sample of postmenopausal AA and EA women. Participants with sufficient sleep (≥7 hours/night) had telomeres that were 58 bp longer, on average, than those with insufficient sleep (<7 hours/night). According to the 23-bp difference per year of age observed in our study sample, our results suggested that women who did not achieve sufficient sleep duration had an LTL equivalent to that of women in our population who were more than 2 years older, on average, than women of the same age who reported sufficient sleep. We did not observe statistically significant associations between sleep disturbance and LTL.
Our results corroborate the current literature on sleep and telomere length (39–51, 71) yet expand it in new ways. To date, 9 studies have assessed sleep duration and 10 studies have assessed some metric of sleep quality, often by means of the Pittsburgh Sleep Quality Index or dichotomized measures of insomnia, in relationship to LTL. As we observed in our study, shorter sleep duration is most commonly linearly associated with shorter LTL (39, 40, 42, 44, 48, 51), though a U-shaped association was observed in a single study (43). Quadratic continuous sleep terms (adjusted sleep duration P values of 0.55, 0.86, and 0.64 and adjusted sleep disturbance P values of 0.95, 0.85, and 0.97 for AAs + EAs, AAs, and EAs, respectively) did not support a U-shaped association in our study. Our ability to observe a nonlinear relationship may have been limited, since only 3.9% of AAs and 4.3% of EAs reported sleeping more than 8 hours per night in our sample.
To our knowledge, our study is the first to report on sleep-LTL associations in a large sample of healthy AAs, where we had the potential to shed new light on literature inconsistences, particularly among older women. In contrast to the results of our study, an association between sleep duration and LTL was observed in males but not females in the Whitehall II Study (average age = 63.3 years) (42), an association was observed among women aged <50 years but not those aged ≥50 years in the Nurses’ Health Study (39), and no association was observed for healthy San Francisco Bay Area women with an average age of 57.5 years (41). These 3 studies analyzed LTL assayed via polymerase chain reaction, which measures relative telomere length as opposed to absolute telomere length in kilobases, which is obtained by means of the gold-standard Southern blot assay for LTL. Polymerase chain reaction methods have larger measurement error than the Southern blot technique (11, 72), which may have biased associations among older women toward the null (24). Differences in the distribution of sleep durations may also have prevented investigators in previous studies from observing sleep-LTL associations. Although sleep quality is represented by different constructs across studies, poor sleep quality has generally been associated with shorter LTL (40, 41, 43, 45, 47–50); however, researchers in other studies have similarly reported null sleep disturbance–LTL associations (44, 71).
The majority of our study population was enrolled in the WHI hormone therapy trials, and therefore preexisting illness is an unlikely explanation for the associations observed. We assessed baseline comorbid conditions and a comorbidity indicator as potential confounders and adjusted our models as needed. The 8-item comorbidity indicator was not a confounder of the sleep duration models, nor was the 10-item comorbidity index, which also included cardiovascular disease and depression status. We observed confounding by the 8-item comorbidity indicator in the sleep disturbance models, but not the 10-item indicator, since cardiovascular disease and depression were independently identified as confounders in sleep disturbance models. Perceived social support has been shown to act as a buffer to stress (71) and to be associated with both poor sleep quality and short LTL (40, 41); however, the Medical Outcomes Study-based social support score was not a confounder of sleep-LTL associations in our study.
Covariates identified in association with LTL in this study replicate those reported in the literature for many demographic and lifestyle factors, including age, BMI, race, income, education, marital/partner status, and smoking (47, 73, 74). Although depression is more commonly associated with short telomeres (75), our results were consistent with those of a recent study that found an association between depression and long telomeres (74). Notably, both our study and another study that found an opposing-direction LTL-depression association employed a variant of the self-report-based Center for Epidemiologic Studies Depression Scale (68).
Our study had several strengths. Given the well-documented association of longer LTL with AA race as compared with EA race, we were able to present our results both by race and for the entire study sample after adjustment for race, since effect modification was not apparent in our analyses. We leveraged existing data on sleep, LTL, and covariates, which allowed us to control for demographic, lifestyle, and molecular quality control variables. Since LTL is a putative marker of biological age and sleep concerns are especially evident in elderly populations (50), we benefitted from the relevance of our study questions to the age group of this sample. To our knowledge, this was the first study to make use of the validated WHIIRS sleep disturbance score in reference to LTL. Finally, our gold-standard Southern blot-assayed LTL measurements were performed on blood samples collected and handled using strict and well-controlled protocols, per the WHI.
Our study was not without limitations, as it employed a cross-sectional design and therefore no causal relationship between sleep and LTL can be inferred. Although LTL tends to track over the life span (76), sleep patterns vary with time and with age (77). We relied on subjective sleep measures, which may be influenced by mood and memory (78), as opposed to objective sleep assessed via actigraphy or polysomnography (44, 48, 71). The cardiovascular event-based selection of the WHI substudy sample is another limitation. We addressed this concern by applying sample weights throughout our analyses and by assessing the potential impact of comorbidity in our models. LTL provides information on average telomere length across all white blood cells in a blood sample, so we were unable to address or exclude specific cell types (40, 50). As with any observational study, other unassessed covariates may have influenced our results.
Longer LTL was observed in postmenopausal AA and EA women who reported longer sleep duration, while no significant association with LTL was observed for sleep disturbance. Our results suggest that sleep duration, a partially modifiable risk factor for adverse health outcomes, may be important for maintaining proper telomere maintenance machinery and thereby promote general health and healthy aging. Alternatively, it is possible that persons with shorter telomeres are more likely to have lifestyles that include shorter sleep schedules. Additional research on the physiological mechanism(s) connecting sleep and LTL is needed to understand the underlying biology and any potentially causal relationships that may have contributed to the associations we observed herein. Longitudinal investigations may provide additional insight into the complex relationship between sleep duration and telomere length in the context of chronic disease.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, State University of New York at Buffalo, Buffalo, New York (Laurie Grieshober, Jean Wactawski-Wende, Lina Mu, Jing Nie, Heather M. Ochs-Balcom); Department of Population Health Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah (Laurie Grieshober); Department of Biostatistics, School of Public Health and Health Professions, State University of New York at Buffalo, Buffalo, New York (Rachael Hageman Blair); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Jingmin Liu, Cara L. Carty, Alex P. Reiner); Program in Public Health, Department of Family, Population, and Preventive Medicine, Stony Brook University, Stony Brook, New York (Lauren Hale); Division of Research, Kaiser Permanente Northern California, Oakland, California (Candyce H. Kroenke); Division of Epidemiology, Department of Family Medicine and Public Health, School of Medicine, University of California, San Diego, La Jolla, California (Andrea Z. LaCroix); and Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Alex P. Reiner).
This research was supported by the National Institutes of Health under National Cancer Institute award R25CA113951 and National Center for Advancing Translational Science award TL1TR002540. The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN268201300007C.
We thank the WHI investigators and staff for their dedication and the study participants for making the program possible.
This article was prepared in collaboration with the WHI investigators and has been reviewed and/or approved by them. A listing of WHI investigators can be found at http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
Conflict of interest: none declared.
Abbreviations
- AA
African American
- BMI
body mass index
- bp
base pairs
- CI
confidence interval
- EA
European American
- LTL
leukocyte telomere length
- SD
standard deviation
- SE
standard error
- SES
socioeconomic status
- WHI
Women’s Health Initiative
- WHIIRS
Women’s Health Initiative Insomnia Rating Scale
REFERENCES
- 1. Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280(14):3180–3193. [DOI] [PubMed] [Google Scholar]
- 2. Cohen S, Janicki-Deverts D, Turner RB, et al. . Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA. 2013;309(7):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PLoS One. 2008;3(5):e2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puterman E, Gemmill A, Karasek D, et al. . Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci U S A. 2016;113(42):E6335–E6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hjelmborg JB, Dalgård C, Möller S, et al. . The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Factor-Litvak P, Susser E, Kezios K, et al. . Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137(4):e20153927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741):20160436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graakjaer J, Londono-Vallejo JA, Christensen K, et al. . The pattern of chromosome-specific variations in telomere length in humans shows signs of heritability and is maintained through life. Ann N Y Acad Sci. 2006;1067:311–316. [DOI] [PubMed] [Google Scholar]
- 9. Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms. A critical survey of the evidence base, research methodology and basic biology. Bioessays. 2012;34(11):943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunt SC, Chen W, Gardner JP, et al. . Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elbers CC, Garcia ME, Kimura M, et al. . Comparison between Southern blots and qPCR analysis of leukocyte telomere length in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2014;69(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Mar V, Zhou W, et al. . Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13(18):2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haycock PC, Heydon EE, Kaptoge S, et al. . Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forero DA, González-Giraldo Y, López-Quintero C, et al. . Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2016;71(8):1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Telomeres Mendelian Randomization Collaboration, Haycock PC, Burgess S, et al. . Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017;3(5):636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu X, Han W, Xue W, et al. . The association between telomere length and cancer risk in population studies. Sci Rep. 2016;6:22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Broer L, Codd V, Nyholt DR, et al. . Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett JH, Iles MM, Dunning AM, et al. . Telomere length and common disease: study design and analytical challenges. Hum Genet. 2015;134(7):679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du M, Prescott J, Cornelis MC, et al. . Genetic predisposition to higher body mass index or type 2 diabetes and leukocyte telomere length in the Nurses’ Health Study. PLoS One. 2013;8(2):e52240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valdes AM, Andrew T, Gardner JP, et al. . Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. [DOI] [PubMed] [Google Scholar]
- 21. Mathur MB, Epel E, Kind S, et al. . Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun. 2016;54:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shadyab AH, Macera CA, Shaffer RA, et al. . Associations of accelerometer-measured and self-reported sedentary time with leukocyte telomere length in older women. Am J Epidemiol. 2017;185(3):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64(10):1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tempaku PF, Mazzotti DR, Tufik S. Telomere length as a marker of sleep loss and sleep disturbances: a potential link between sleep and cellular senescence. Sleep Med. 2015;16(5):559–563. [DOI] [PubMed] [Google Scholar]
- 25. Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:Article 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bin YS. Is sleep quality more important than sleep duration for public health? Sleep. 2016;39(9):1629–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lallukka T, Sivertsen B, Kronholm E, et al. . Association of sleep duration and sleep quality with the physical, social, and emotional functioning among Australian adults. Sleep Health. 2018;4(2):194–200. [DOI] [PubMed] [Google Scholar]
- 28. Jarrin DC, McGrath JJ, Drake CL. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes (Lond). 2013;37(4):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom Med. 2004;66(2):239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen X, Wu Y, Zhang D. Nighttime sleep duration, 24-hour sleep duration and risk of all-cause mortality among adults: a meta-analysis of prospective cohort studies. Sci Rep. 2016;6:21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Starkweather AR, Alhaeeri AA, Montpetit A, et al. . An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014;63(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchell C, Hobcraft J, McLanahan SS, et al. . Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci U S A. 2014;111(16):5944–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carroll JE, Diez-Roux AV, Adler NE, et al. . Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the Multi-Ethnic Study of Atherosclerosis (MESA). Brain Behav Immun. 2013;28:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shalev I, Entringer S, Wadhwa PD, et al. . Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore PJ, Adler NE, Williams DR, et al. . Socioeconomic status and health: the role of sleep. Psychosom Med. 2002;64(2):337–344. [DOI] [PubMed] [Google Scholar]
- 36. Bagley EJ, Kelly RJ, Buckhalt JA, et al. . What keeps low-SES children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. 2015;16(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ordway MR, Sadler LS, Canapari CA, et al. . Sleep, biological stress, and health among toddlers living in socioeconomically disadvantaged homes: a research protocol. Res Nurs Health. 2017;40(6):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doering JJ. The physical and social environment of sleep in socioeconomically disadvantaged postpartum women. J Obstet Gynecol Neonatal Nurs. 2013;42(1):E33–E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liang G, Schernhammer E, Qi L, et al. . Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS One. 2011;6(8):e23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prather AA, Gurfein B, Moran P, et al. . Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav Immun. 2015;47:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prather AA, Puterman E, Lin J, et al. . Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackowska M, Hamer M, Carvalho LA, et al. . Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study. PLoS One. 2012;7(10):e47292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cribbet MR, Carlisle M, Cawthon RM, et al. . Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep. 2014;37(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee KA, Gay C, Humphreys J, et al. . Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. Sleep. 2014;37(1):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwon AM, Baik I, Thomas RJ, et al. . The association between leukocyte telomere lengths and sleep instability based on cardiopulmonary coupling analysis. Sleep Breath. 2015;19(3):963–968. [DOI] [PubMed] [Google Scholar]
- 46. Révész D, Milaneschi Y, Terpstra EM, et al. . Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology. 2016;67:153–162. [DOI] [PubMed] [Google Scholar]
- 47. Puterman E, Lin J, Krauss J, et al. . Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol Psychiatry. 2015;20(4):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tempaku PF, Mazzotti DR, Hirotsu C, et al. . The effect of the severity of obstructive sleep apnea syndrome on telomere length. Oncotarget. 2016;7(43):69216–69224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garland SN, Palmer C, Donelson M, et al. . A nested case-controlled comparison of telomere length and psychological functioning in breast cancer survivors with and without insomnia symptoms. Rejuvenation Res. 2014;17(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carroll JE, Esquivel S, Goldberg A, et al. . Insomnia and telomere length in older adults. Sleep. 2016;39(3):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. James S, McLanahan S, Brooks-Gunn J, et al. . Sleep duration and telomere length in children. J Pediatr. 2017;187:247–252.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 53. Carty CL, Kooperberg C, Liu J, et al. . Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol. 2015;35(10):2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kimura M, Stone RC, Hunt SC, et al. . Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5(9):1596–1607. [DOI] [PubMed] [Google Scholar]
- 55. Levine DW, Kaplan RM, Kripke DF, et al. . Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. [DOI] [PubMed] [Google Scholar]
- 56. Watson NF, Badr MS, Belenky G, et al. . Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levine DW, Kripke DF, Kaplan RM, et al. . Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. [DOI] [PubMed] [Google Scholar]
- 58. Lee DC, Im JA, Kim JH, et al. . Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46(4):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Toffol E, Kalleinen N, Urrila AS, et al. . The relationship between mood and sleep in different female reproductive states. BMC Psychiatry. 2014;14:Article 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. [DOI] [PubMed] [Google Scholar]
- 61. Bohannon RW, DePasquale L. Physical Functioning Scale of the Short-Form (SF) 36: internal consistency and validity with older adults. J Geriatr Phys Ther. 2010;33(1):16–18. [PubMed] [Google Scholar]
- 62. Dubowitz T, Heron M, Bird CE, et al. . Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican-Americans in the United States. Am J Clin Nutr. 2008;87(6):1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dubowitz T, Ghosh-Dastidar M, Eibner C, et al. . The Women’s Health Initiative: the food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring). 2012;20(4):862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 65. Kroenke CH, Michael Y, Tindle H, et al. . Social networks, social support and burden in relationships, and mortality after breast cancer diagnosis. Breast Cancer Res Treat. 2012;133(1):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 67. Tuunainen A, Langer RD, Klauber MR, et al. . Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103(2–3):261–270. [DOI] [PubMed] [Google Scholar]
- 68. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 69. Rillamas-Sun E, LaCroix AZ, Bell CL, et al. . The impact of multimorbidity and coronary disease comorbidity on physical function in women aged 80 years and older: the Women’s Health Initiative. J Gerontol A Biol Sci Med Sci. 2016;71(suppl 1):S54–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. . Accelerometer-measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc. 2018;66(5):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Puterman E, Epel ES, Lin J, et al. . Multisystem resiliency moderates the major depression-telomere length association: findings from the Heart and Soul Study. Brain Behav Immun. 2013;33:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Montpetit AJ, Alhareeri AA, Montpetit M, et al. . Telomere length: a review of methods for measurement. Nurs Res. 2014;63(4):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Needham BL, Adler N, Gregorich S, et al. . Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med. 2013;85:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lynch SM, Peek MK, Mitra N, et al. . Race, ethnicity, psychosocial factors, and telomere length in a multicenter setting. PLoS One. 2016;11(1):e0146723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Darrow SM, Verhoeven JE, Révész D, et al. . The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78(7):776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Benetos A, Kark JD, Susser E, et al. . Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. [DOI] [PubMed] [Google Scholar]
- 78. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(suppl 1):S10–S17. [DOI] [PubMed] [Google Scholar]