Abstract
Objectives:
G protein-coupled receptor 137 (GPR137) was reported to be associated with several cancers, but its role in bladder cancer has not been reported. The purpose of this study was to evaluate clinical significance of GPR137 in bladder cancer.
Methods:
The expressions of GPR137 in pathological tissues and corresponding normal tissues from bladder cancer patients were detected via quantitative real time polymerase chain reaction (qRT-PCR). Western blot was performed to detect GPR137 expression in bladder cancer tissues and adjacent normal tissues. Chi-Squared test analyzed the relationship between GPR137 expression and clinical features of bladder cancer patients. Additionally, Kaplan–Meier method was adopted in estimating overall survival of bladder cancer patients. Prognostic value of GPR137 was evaluated through Cox regression analysis.
Results:
The expression of GPR137 mRNA and protein in pathological tissues was significantly higher than that in adjacent normal tissues (P < .001). Moreover, similar result was found for bladder cancer patients and healthy controls (P < .001). And GPR137 expression was associated with tumor size (P = .006) and TNM stage (P = .012). The results of Kaplan–Meier analysis suggested that patients with high expression of GPR137 had shorter overall survival time than those with low expression (Log rank test, P = .001). Cox regression analysis indicated that GPR137 could act as an independent biomarker for bladder cancer prognosis (HR = 1.850, 95% CI = 1.272–2.689, P = .001).
Conclusion:
Abnormal expression of GPR137 is associated with bladder cancer and GPR137 is a potential biomarker for the therapy and prognosis of bladder cancer.
Keywords: biomarker, bladder cancer, G protein-coupled receptor 137
1. Introduction
Bladder cancer is one of the most common genitourinary malignancies with frequent recurrence and high mortality.[1,2] Currently, main diagnostic and surveillance strategies for bladder cancer depend on cystoscopy and urine cytology.[3] However, these techniques have some limitations and could not provide satisfactory outcomes.[4] Thus, exploiting novel biomarkers related to genetic alterations during tumorigenesis may greatly help to improve clinical outcomes of bladder cancer patients.
G-protein-coupled receptors (GPCRs) are the largest family of membrane proteins for signal transduction on cell surface.[5] There are approximately over 800 GPCRs in human genome, most of which share 7 homology transmembrane domains.[6,7] In order to activate specific heterotrimeric G proteins, GPCRs change the conformation of specific ligands via combining with the ligands. Then signal network will be activated, causing various cellular responses.[8] At the present, corresponding specific ligands for most GPCRs are known, but there are still over 100 GPCRs called orphan GPCRs (oGPCRs) whose ligands are unknown.[9] G protein-coupled receptor 137 (GPR137) is an oGPCR encoding gene, generally expressing in central nervous system (CNS), endocrine gland, thymus and lung.[10] It has been reported that GPCRs play crucial roles in tumor progression and metastasis.[11] Moreover, GPR137 was found to be associated with different cancers including gastric cancer,[12] pancreatic cancer,[13] colon cancer,[14] and malignant glioma.[15]GPR137 was highly expressed in clinical ovarian cancer tissues, and it played pro-oncogenic roles in the disease via regulating PI3K/AKT pathway.[16] But its function in bladder cancer has not been reported in previous studies.
In this study, we aimed to detect relative expression of GPR137 in pathological tissues and corresponding normal tissues from bladder cancer patients. In addition, the associations between the levels of GPR137 and clinical features were analyzed to estimate the impact of GPR137 on bladder cancer progression. We also expected to evaluate overall survival between patients showing different GPR137 levels and to assess prognostic value of GPR137 adopting Cox regression analysis.
2. Materials and methods
2.1. Patients and specimens
One hundred ten patients confirmed with bladder cancer through pathological and clinical diagnoses in First People's Hospital, Jining were included in this study. Pathological specimens and adjacent normal tissues were collected and stored in liquid nitrogen until application. None of the patients had received chemotherapy or radiotherapy before tissue samples were collected. 62 healthy controls were from physical examination center of the hospital. The study was approved by Ethics Committee of the hospital and all of the patients had signed written informed consents in advance. Bladder cancer tissues and adjacent normal tissues were obtained and immediately snap-frozen in liquid nitrogen and stored at −80°C. 2 ml peripheral venous blood from each of bladder cancer patients and healthy controls were put into blood collection tube with EDTA, centrifugated for supernate (serum), stored at −80°C. RNA extraction was conducted in a short time, stored at −80°C for quantitative analysis. The sampling was completed about 1 and a half years.
Follow-up investigation lasted about 5 years and all patients participated in the investigation. Follow-up data and clinical features were collected in a database. Overall survival was used for estimating clinical influence of GPR137 on bladder cancer.
2.2. RNA extraction and qRT-PCR
Total RNA was separately extracted from pathological tissues, corresponding normal tissues and serum samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacture's instruction. Residual DNA in total RNA was dealt with DNase. UV absorbance was used to detect the concentration of total RNA (A260/A280). The quality of total RNA was tested adopting 1% agarose gel electrophoresis.
Fluorescence quantitative real-time PCR (qRT-PCR) was used to detect relative expression of GPR137 for pathological tissues and corresponding normal tissues, as well as for serum from bladder patients and healthy controls. PrimeScript RT reagent kit (Takara, China) was used to obtain complementary DNA (cDNA) and qRT-PCR was carried out with SYBR Green assay (Takara, China). GAPDH was internal control and primer sequences used in this study were listed in Table 1. Data analysis was performed through 2−ΔΔCt method. The integrity of extracted RNA was detected via 1% agarose gel electrophoresis. The concentration and purity of extracted RNA were detected through ultraviolet spectrophotometer, and extracted RNA samples were stored in −80°C refrigerator.
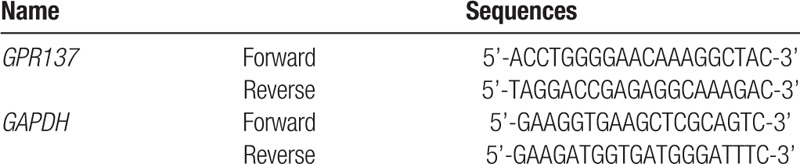
Table 1.
The sequences of the primers used in this study.
2.3. Immunohistochemistry
Paraffin-embedded tissue sections (formalin fixed) was cut into 4-μm and put into 65°C oven for deparaffinization, antigen retrieval for 60 minutes. It was blocked by goat serum and sections were incubated overnight with primary antibody (1:100 dilution) at 4°C. After treatment with biotinylated secondary antibody, antibody complex was detected employing avidin-biotin-peroxidase complex solution and visualized using DAB, hematoxylin stain.
The staining of tissue sections were observed and recorded by 2 pathologists. Staining ranges contained: <5%, 0 point; 5% to 25%, 1 point; 26% to 50%, 2 points; 51% to 75%, 3 points; >75%, 4 points. Staining intensities were as follows: nonstaining, 0 point; light yellow, 1 point; brownish yellow, 2 points and brown, 3 points. Final scores were the sum of scores for staining range and staining intensity. And scores ≤3 stood for low expression while those >3 for high expression.
2.4. Western blot analysis
Total protein from tissues was isolated using RIPA lysis buffer (Beyotime, Nantong, China) and quantified using a BCA assay kit (Pierce, Rockford, IL, USA). A total of 200 μg of protein was separated by SDS-PAGE and transferred onto PVDF membranes (Schleicher &Schuell GmbH, Dassel, Germany). After incubating with 5% skim milk for 1 hour at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. Then, the membranes were incubated with corresponding secondary antibodies at room temperature for 1 hour. Protein expression levels were normalized by β-actin. The intensity of protein expression was quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.5. Statistical analysis
The comparison of GPR137 expression between pathological samples and normal tissue samples were operated through Student t test and the results were shown as mean ± SD. The relationship between GPR137 expression and clinical features in bladder cancer patients was estimated by Chi-Squared test. Chi-Squared test is the degree of deviation between the actual observation value and the theoretical inference value of statistical samples. The deviation between the actual observation value and the theoretical inference value determines the size of the Chi-Squared value. If the Chi-Squared value is larger, the deviation between the 2 values is larger; conversely, the deviation between the 2 values is smaller. If the 2 values are completely equal, the Chi-Squared value is 0, indicating that the theoretical value is completely consistent. Kaplan–Meier method, also known as product-limit estimate, is one of the most commonly used survival analysis methods. It is mainly suitable for estimating survival rate and drawing survival curve for non-grouped survival data. Kaplan–Meier method was applied in overall survival analysis and prognostic significance of GPR137 was evaluated by Cox regression analysis. All statistical analyses were performed in SPSS 18.0 software and SigmaPlot software was used for drawing. P < .05 was considered as significant level.
3. Results
3.1. Relative expression of GPR137 in bladder cancer patients
Total RNA extracted through 1.1% agarose gel electrophoresis (Fig. 1A) showed that the 28S rRNA and 18S rRNA bands were clear and brightness ratio was close to 2:1, indicating that RNA was not degraded and relatively intact. QRT-PCR was used to detect relative expression of GPR137 in pathological tissues and normal tissues from the bladder cancer patients as well as for serum from the patients and healthy controls. Relative expression level in pathological tissues was 1.83 ± 0.33, while the figure for corresponding normal tissues was 1 ± 0.09 (Fig. 1B), showing significant difference between them (P < .001). Similarly, the expression of serum GPR137 in bladder cancer patients was also significantly higher than that in healthy controls (P < .001, Fig. 1C). We also analyzed the expression of GPR137 in patients at different stages, and found that the expression of GPR137 at stages I-II and III-IV was significantly increased compared with that in adjacent normal tissues (P < .001), but there was no significant difference in the expression of GPR137 between stages I-II and III-IV (P > .5 Fig. 1D). Besides, Western bloting was used to detect the expression of GPR137 in bladder cancer tissues and adjacent normal tissues. Compared with adjacent normal tissues, bladder cancer tissues showed significantly increased GPR137 expression. (P < .001, Fig. 2).
Figure 1.
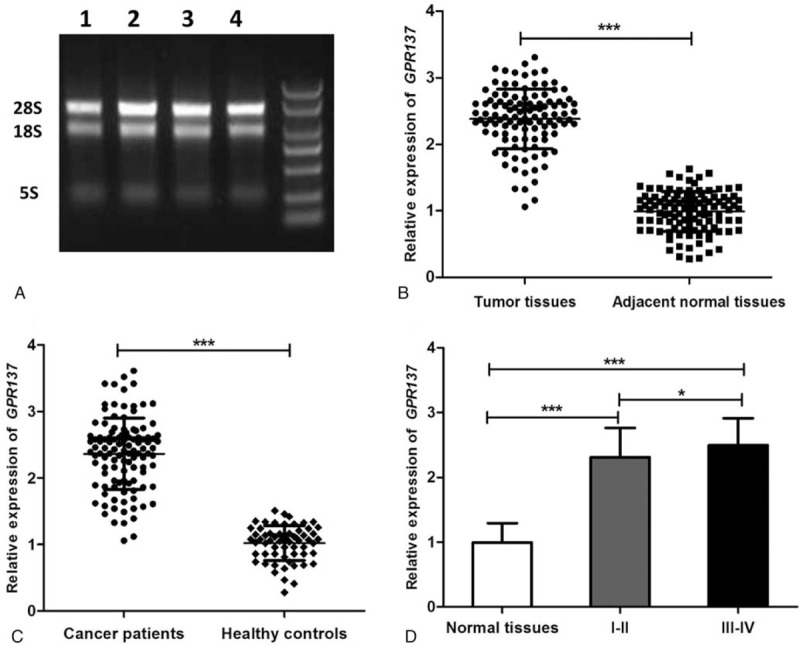
(A) Agarose gel electrophoresis of total RNA. (B) The expression of GPR137 in patients with bladder cancer was significantly higher than that in adjacent normal tissues at mRNA level. (C) The expression of GPR137 in bladder cancer patients at different TNM stages (I-II and III-IV) as well as in normal tissues. ∗∗∗ P < .001 and ∗ P < .05 represented significant difference between the compared 2 groups.
Figure 2.
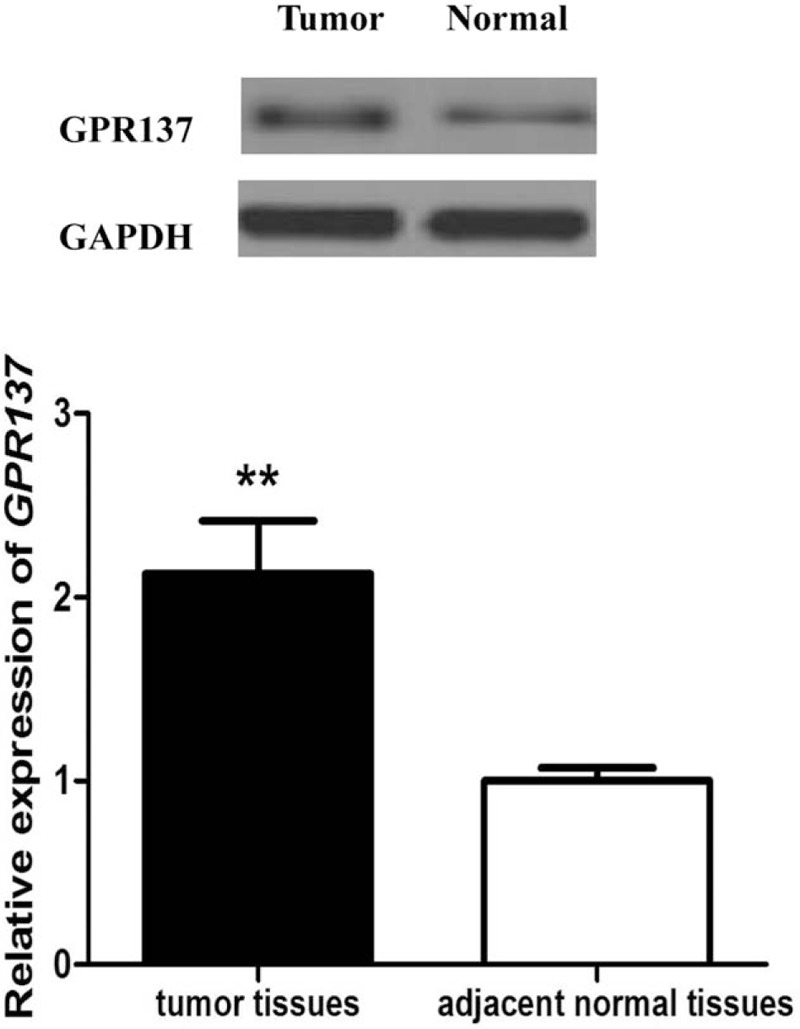
The expression of GPR137 in patients with bladder cancer was significantly higher than that in adjacent normal tissues at protein level. ∗∗∗ P < .001 represented significant difference between the compared 2 groups.
IHC results showed that in bladder cancer tissue sections, GPR137 expression was significantly strong and the percentage of positive staining cells was as high as 80.9% (89/110). But GPR137 expression in normal tissues was weak and the proportion of positive staining cells was only 30.9% (34/110). The difference was significant between bladder cancer tissues and normal tissues (P < .05).
3.2. Relationship between GPR137 expression and clinical features
The clinical features of bladder cancer patients were showed in Table 2. A total of 142 bladder cancer patients were divided into 2 groups: low- and high- expression groups according to the median of GPR137 expression so as to describe the association between GPR137 expression and clinical features. Chi-square method was used to analyze the relationship and the results showed that the expression of GPR137 was associated with tumor size (P = .006) and TNM stage (P = .012), but not with gender, age, multiplicity, histogical grade, or lymph node metastasis (P > .05). Moreover, based on gender and age, we carefully analyzed expression differences of GPR137 among bladder cancer patients and found that the expression of GPR137 in male patients was significantly higher than that in female ones (P = .023, Fig. 3), but no significance dissimilarity in the levels was observed after stratification analysis by age (P = .810).
Table 2.
The association between GPR137 expression and clinical features of bladder cancer patients.
Figure 3.
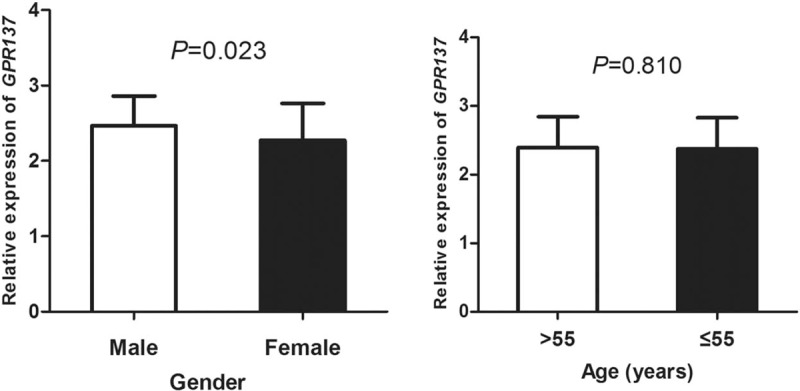
The expression of GPR137 among bladder cancer patients stratified by gender and age. The results showed that GPR137 expression in male patients was significantly higher than that in female patients (P = .023).
3.3. Overall survival analysis and prognostic value of GPR137
All participants were followed up for 5 years and Kaplan–Meier method was performed to analyze overall survival of bladder cancer patients with different levels of GPR137. Patients in low expression group had the relatively longer overall survival time than that in high expression group and there was a remarkable difference between the 2 groups (Log rank test: P = .001, Fig. 4)
Figure 4.
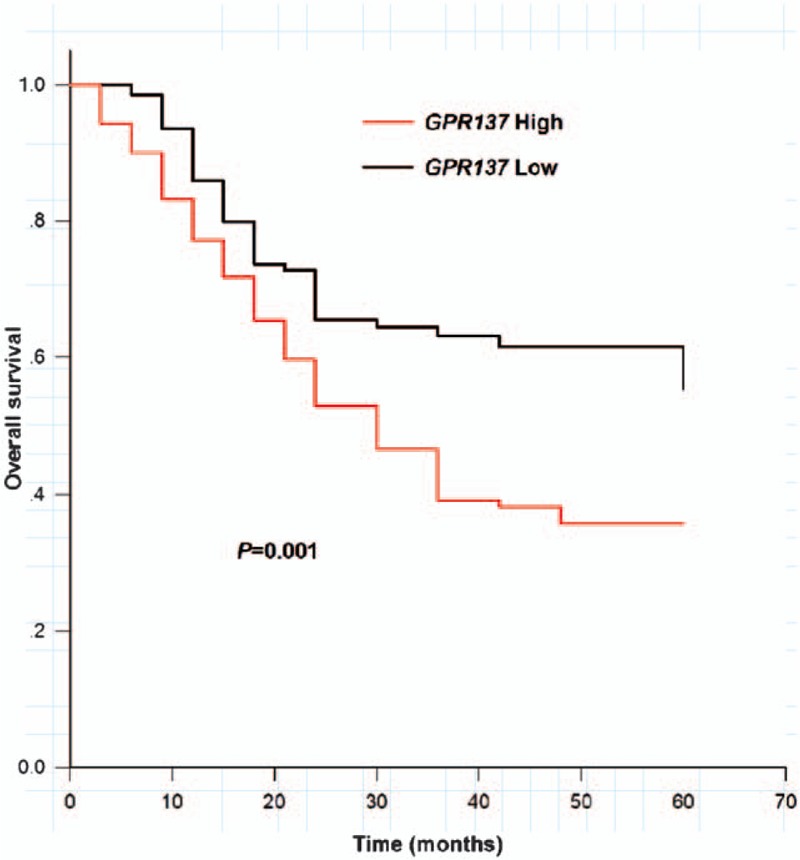
Overall survival of bladder cancer patients with high GPR137 expression (red line) and low expression (black line) (Log rank test, P = .001).
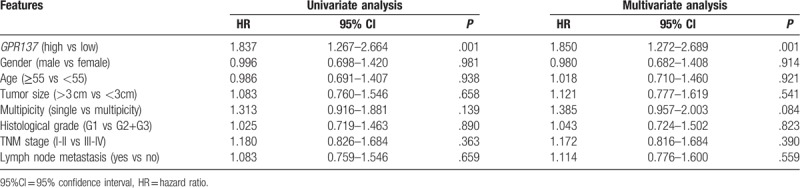
Cox regression analysis was used to estimate the prognostic value of GPR137 and the results were shown in Table 3. Accordingly, GPR137 over-expression was significantly associated with poor prognosis of bladder cancer patients (P = .001) and GPR137 could act as an independent biomarker for bladder cancer prognosis (HR = 1.850, 95% CI = 1.272–2.689, P = .001).
Table 3.
Univariate and multivariate analysis of GPR137 and clinical features in bladder cancer patients.
4. Discussion
Biomarkers for cancer prognosis could optimize treatment decisions and clinical outcomes for patients. Genetic alterations associated with tumor progression might be potential biomarkers for prognosis. Increasing studies have discussed molecular prognostic biomarkers for bladder cancer. Research showed that hsa-miR-325 was a functional tumor suppressor in bladder cancer and can be an interesting molecular candidate for target therapy in treating this disease.[17] Zhang et al demonstrated that the expression of RNA-binding protein SAM68 was increased in muscle invasive bladder cancer (MIBC), which appeared to be a potential predictor for the disease.[18]MicroRNA-214 was reported to act as a tumor-suppressor in bladder cancer and might be a novel indicator for its prognosis.[19]
GPCRs have been reported to take part in cell proliferation, survival and motility, and their functions in tumor growth, angiogenesis, and metastasis also attract increasing attentions.[11]GPCR48 was reported to promote prostate cancer cells’ proliferation, thus providing a plausible therapeutic target for the malignancy treatment.[20] Smith et al found that GPR30 was preferentially expressed in high risk epithelial ovarian cancer, relating to low survival rates of the patients.[21] Moreover, over expression of GPR161 was detected in breast cancer and a previous study indicated that the gene was an important regulator and a potential drug target in triple-negative breast cancer.[22] Therefore, investigating the mechanism and function of GPCRs in tumorigenesis might provide an opportunity for cancer prevention and treatment.
In order to investigate clinical effects of GPR137 on bladder cancer, we detected its levels in pathological tissues and corresponding normal tissues from bladder cancer patients. The results suggested that the expression of GPR137 was different between pathological tissues and normal tissues. A further study indicated that the expression of GPR137 was associated with tumor size and TNM stage. We also found that GPR137 expression in bladder cancer patients at stages I-II and III-IV was significantly increased compared with adjacent normal tissues. Moreover, GPR137 expression was also significantly higher in patients at stage III-IV than those at stage I-II. The results suggested that GPR137 might influence the progression of bladder cancer. These findings suggested that GPR137 might be an oncogene in bladder cancer. Du et al indicated that GPR137 was associated with the proliferation of bladder cancer cells in vitro.[23] Knockdown of GPR137 in pancreatic cancer cells could inhibit cancer cell growth and induce apoptosis.[13] In medulloblastoma, GPR137 also acted as an oncogene and its knockdown could disturb cell cycle progression.[24]
In this study, overall survival and Cox regression analyses were used to estimate prognostic value of GPR137 in bladder cancer. The patients with high expression of GPR137 had lower survival rate than those with low expression. And the results of Cox regression analysis suggested that GPR137 might be an independent biomarker for bladder cancer prognosis. Therefore, we inferred that the function of oGPCRs and their regulatory mechanism in human cancer may improve the outcomes of cancer patients. For example, GPR37, 1 of oGPCRs, was proved to act as tumor-suppressor in hepatocellular carcinoma and associate with patients’ survival.[25] Wu et al indicated that GPR48 could serve as a prognostic biomarker and a therapeutic target in resectable colorectal cancer.[26]GPR110 was considered as an oncogene in both lung and prostrate cancers and could be a therapeutic candidate and disease marker in both of the cancers.[27]
In conclusion, the expression of GPR137 in patients with bladder cancer was significantly higher than that in adjacent normal tissues at both mRNA and protein levels. And the expression of GPR137 was associated with tumor size. In addition, bladder cancer patients with different GPR137 expressions experienced varied clinical outcomes, specifically, high expression was associated with low survival rate. GPR137 may be a valuable indicator for bladder cancer prognosis and a potential therapeutic target for the disease.
Author contributions
Conceptualization: Feng Zhong, Chao Wang.
Data curation: Feng Zhong, Beibei Sun.
Formal analysis: Feng Zhong.
Funding acquisition: Jianlei Lu, Beibei Sun.
Investigation: Beibei Sun.
Methodology: Feng Zhong, Beibei Sun.
Writing – original draft: Jianlei Lu, Chao Wang.
Writing – review & editing: Jianlei Lu, Chao Wang.
Footnotes
Abbreviations: cDNA = complementary DNA, CNS = central nervous system, GPCRs = G-protein-coupled receptors, GPR137 = G protein-coupled receptor 137, MIBC = muscle invasive bladder cancer, oGPCRs = orphan GPCRs, qRT-PCR = quantitative real-time polymerase chain reaction.
JL and FZ are co-first author. BS and CW contributed equally to this work.
The authors have no conflicts of interests to disclose.
References
- [1].Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl 2008;12–20. [DOI] [PubMed] [Google Scholar]
- [2].Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009;374:239–49. [DOI] [PubMed] [Google Scholar]
- [3].Vrooman OP, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53:909–16. [DOI] [PubMed] [Google Scholar]
- [4].Wang J, Zhang X, Wang L, et al. Downregulation of urinary cell-free microRNA-214 as a diagnostic and prognostic biomarker in bladder cancer. J Surg Oncol 2015;111:992–9. [DOI] [PubMed] [Google Scholar]
- [5].Vanti WB, Nguyen T, Cheng R, et al. Novel human G-protein-coupled receptors. Biochem Biophys Res Commun 2003;305:67–71. [DOI] [PubMed] [Google Scholar]
- [6].Lazarczyk M, Matyja E, Lipkowski A. Substance P and its receptors – a potential target for novel medicines in malignant brain tumour therapies (mini-review). Folia Neuropathologica/Assoc Polish Neuropathol Med Res Centre Polish Acad Sci 2007;45:99–107. [PubMed] [Google Scholar]
- [7].Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002;3:639–50. [DOI] [PubMed] [Google Scholar]
- [8].Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 2009;459:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoshida M, Miyazato M, Kangawa K. Orphan GPCRs and methods for identifying their ligands. Methods Enzymol 2012;514:33–44. [DOI] [PubMed] [Google Scholar]
- [10].Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 2008;135:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007;7:79–94. [DOI] [PubMed] [Google Scholar]
- [12].Wang Z, Zhang H, Wang J, et al. RNA interference-mediated silencing of G protein-coupled receptor 137 inhibits human gastric cancer cell growth. Mol Med Rep 2015;11:2578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cui X, Liu Y, Wang B, et al. Knockdown of GPR137 by RNAi inhibits pancreatic cancer cell growth and induces apoptosis. Biotechnol Appl Biochem 2015;62:861–7. [DOI] [PubMed] [Google Scholar]
- [14].Zhang K, Shen Z, Liang X, et al. Down-regulation of GPR137 expression inhibits proliferation of colon cancer cells. Biotechnol Appl Biochem 2014;46:935–41. [DOI] [PubMed] [Google Scholar]
- [15].Zong G, Wang H, Li J, et al. Inhibition of GPR137 expression reduces the proliferation and colony formation of malignant glioma cells. Neurol Sci 2014;35:1707–14. [DOI] [PubMed] [Google Scholar]
- [16].Zhang LQ, Yang SQ, Qu XD, et al. GRP137 promotes cell proliferation and metastasis through regulation of the PI3K/AKT pathway in human ovarian cancer. Tumori 2018;104:330–7. [DOI] [PubMed] [Google Scholar]
- [17].Lin T, Zhou S, Gao H, et al. MicroRNA-325 is a potential biomarker and tumor regulator in human bladder cancer. Technol Cancer Res Treat t 2018;17:1533033818790536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Z, Yu C, Li Y, et al. Utility of SAM68 in the progression and prognosis for bladder cancer. BMC cancer 2015;15:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang J, Zhang X, Wang L, et al. MicroRNA-214 suppresses oncogenesis and exerts impact on prognosis by targeting PDRG1 in bladder cancer. PloS one 2015;10:e0118086. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [20].Liang F, Yue J, Wang J, et al. GPCR48/LGR4 promotes tumorigenesis of prostate cancer via PI3K/Akt signaling pathway. Med Oncol 2015;32:49. [DOI] [PubMed] [Google Scholar]
- [21].Smith HO, Arias-Pulido H, Kuo DY, et al. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol 2009;114:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Feigin ME, Xue B, Hammell MC, et al. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci USA 2014;111:4191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Du Y, Bi W, Zhang F, et al. G-protein-coupled receptor 137 accelerates proliferation of urinary bladder cancer cells in vitro. Biotechnol Appl Biochem 2015;62:855–60. [DOI] [PubMed] [Google Scholar]
- [24].Wang C, Liang Q, Chen G, et al. Inhibition of GPR137 suppresses proliferation of medulloblastoma cells in vitro. Biotechnol Appl Biochem 2015;62:868–73. [DOI] [PubMed] [Google Scholar]
- [25].Liu F, Zhu C, Huang X, et al. A low level of GPR37 is associated with human hepatocellular carcinoma progression and poor patient survival. Pathol Res Pract 2014;210:885–92. [DOI] [PubMed] [Google Scholar]
- [26].Wu J, Xie N, Xie K, et al. GPR48, a poor prognostic factor, promotes tumor metastasis and activates beta-catenin/TCF signaling in colorectal cancer. Carcinogenesis 2013;34:2861–9. [DOI] [PubMed] [Google Scholar]
- [27].Lum AM, Wang BB, Beck-Engeser GB, et al. Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer. BMC Cancer 2010;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]


