Abstract
Population-based cancer registries (PBCR) participate in epidemiological surveillance and in the evaluation of cancer types by enabling analysis of incidence and survival data over time. The aim of this study was to examine overall survival (OS) in patients with colorectal cancer (CRC) by analyzing data from the Martinique population-based cancer registry between 1993 and 2012. All colorectal cancer cases diagnosed in Martinique between 1993 and 2012 were included. Characteristics of CRC patients were analyzed according to age subgroups, namely: <50 years, 50 to 74 years and over 75 years.
We recorded the following socio-demographic and clinical variables: year of diagnosis, age at diagnosis, sex, histology, zone of residence, and subsite of the cancer. Incidence of malignant neoplasms of the colon and rectum (ICD-10 C18–21) was extracted from the Martinique Cancer Registry database. Stage at diagnosis (localized: stage I–II, regional: stage III and metastatic stage: stage IV) were also analyzed for the 2008 to 2012 period.
A total of 2230 cases of incident invasive CRC were included during the study period (1993–2012): 1171 were women (52.5%); 1588 patients (71.2%) had colon cancer. Stage at diagnosis was evaluated in 779 patients (89.6%): 486/779 (62.4%) had stage III–IV at diagnosis, including 285 (36.6%) patients with metastases at diagnosis (stage IV). One-year, 5-year and 10-year OS for the study period 1993 to 2012 was 74.6%, 43.8% and 33.0% respectively. There was a statistical difference in overall survival according to gender (P = .0153), age at diagnosis (P < .001) and stage (P < .001).
Median OS was 2.0 years (95% CI [1.4–2.1]) in the stage III–IV group during the period 2008 to 2012, whereas it was unreached in the stage I–II group. Multivariable analysis confirmed that stage III–IV at diagnosis (hazard ratio (HR) = 3.70 [2.89–4.99]; P < .0001) and colon cancer (HR = 1.30 [1.01–1.69]; P = .04) were main prognostic factors for OS. Women had a HR of 0.78 [0.62–0.96], P = .02. CRC patients in the 50 to 74 years age group had a HR of 0.63 [0.50–0.80], P = .0001.
This study underlines the importance of structuring management of CRC cancer patients.
Keywords: cancer registry, colorectal cancer, Martinique, stage, survival
1. Introduction
Population-based cancer registries (PBCR) participate in epidemiological surveillance and in the evaluation of cancer types by enabling analysis of incidence and survival data over time. Continuous, exhaustive recording of all cancer diagnoses, studies of prognostic factors are performed to evaluate the efficacy of management by the healthcare system. For the Caribbean zone, data are sparse concerning countries that do not have a PBCR, and are based primarily on estimations and projections.[1,2]
Martinique is an administrative region of France comprising a single Department, and situated in the West Indies in the Caribbean ocean. Martinique has many unique features, such as its outlying geographical location and the fact that it is an island. The socio-cultural and demographic context is also specific to this region, with the result that multiple factors need to be taken into account when considering the determinants of health in the Martinique population. In total, there were 380,877 inhabitants in Martinique as of 1 January 2015, and a total of 806 deaths from cancer were recorded, corresponding to 26.4% of all deaths, with circulatory disease arriving in second position with 749 deaths (24.5% of all deaths).
In the Caribbean, Martinique has been a leader in the development of oncology through the implementation of a polyvalent and efficient healthcare system, enabling personalized management of overall healthcare for patients suffering from cancer. The volume of activity in medicine, surgery and obstetrics is lower, per head of population, in Martinique than in mainland France. The hospitalization rate varies from 12.4 to 14.1 hospital admissions per 100 inhabitants in the French overseas Departments, compared to an average 15.7 admissions per 100 inhabitants in metropolitan France. In 2015, the average length of stay in Martinique was slightly higher than in mainland France (respectively 6.2 vs 5.7 days).[3] The number of hospital admissions for cancer per 1000 inhabitants is 5.1 in Martinique, compared to 6.6 in mainland France. The density of medical professionals varies considerably between the French West Indies and mainland France, with on average 1 radiotherapy per 114,000 inhabitants in the French West Indies, compared to 1 for 68,000 in mainland France. Regarding medical oncologists, the contrast is even more striking, with one medical oncologist per 160,000 inhabitants in the French West Indies, compared to 1 for 57,000 in mainland France.[4,5]
At a regional level, the number of general practitioners in Martinique decreased between 2010 and 2014 from 83.2 per 100,000 inhabitants in 2010 to 80.5 in 2014. This rate is 25% lower than in metropolitan France (105.9 per 100,000 inhabitants), while the number of nurses was 1019/100,000 inhabitants in Martinique in 2014, compared to 943/100,000 in mainland France.
The University Hospital of Martinique (CHUM) has a capacity of 1426 beds and 120 additional places, with a total of 5200 staff members mobilized to guarantee high-quality care. The Oncology – Hematology – Urology division of the CHUM brings together the main facilities for the management of cancer in Martinique, and currently regroups all the medical, scientific and paramedical professionals in oncology. The recent acquisition of tomotherapy facilities has given the population access to innovative therapies, and enables the administration of higher doses of radiation to the tumor, with less irradiation of surrounding organs. This equipment offers access to efficacious therapies, with a minimum of adverse effects.
Colorectal cancer (CRC) is the third most common malignant neoplasm and the fourth main cause of cancer death in the world, with nearly 1.8 million new cases and 881,000 deaths in 2018.[6,7]
The epidemiology of cancer in the French West-Indies (FWI) differs from that of mainland France, but also from that of other countries in the Caribbean and the Americas.
Since the 1990s, the epidemiological profile of cancer has been in transition. In men, prostate, colorectal and stomach cancers are the 3 most frequent localizations, while in women, the most frequent tumor sites are breast, colorectal and stomach. Overall, the population of metropolitan France is proportionally more affected by cancers with poor prognosis (e.g., lung cancer) than the population the overseas territories such as Martinique. Conversely, the populations of the French West Indies and French Guiana have higher rates of cancers with better prognosis, such as prostate cancers.
CRC ranks as the second most frequent cancer in both men and women in Martinique and Guadeloupe, and 4th and 3rd most frequent in men and women respectively in French Guyana.[8] With an incidence rate considerably higher than that observed in South America, incidence rates for colorectal cancer are lower in the FWI than in mainland France.[9,10] In the Latin America and the Caribbean, the world-standardized incidence rate of CRC in men is 18.4/100,000, and 15.5/100,000 in women.[6] In Martinique, CRC has incidence rates of 26.9/100,000 in men and 20.4/100,000 in women; these rates are lower than those observed in mainland France, where the incidence rates are respectively 37.8/100,000 and 24.4/100,000 in men and women. In terms of mortality, the rates are 9.2/100,000 and 7.2/100,000 in men and women respectively in Latin America and the Caribbean,[6] respectively 10.8/100,000 and 7.8/100,000 in Martinique; and 12.7/100,000 in men and 7.5/100,000 in women in mainland France.[11]
CRC screening is available in most countries worldwide and participation rates are highly variable. The main screening tests are the guaiac fecal occult blood test and the fecal immunochemical test.
In Martinique, a national organized screening program for CRC has been implemented since 2008 and targets the general population of persons at risk aged 50 to 74 years.[12] Cancer registries represent a unique opportunity to evaluate health policy implementation in practice, perform etiological studies and survival analysis. In view of the burden of CRC in the Caribbean, and the need to broaden our knowledge of CRC patterns in our region, the importance of this study resides in the analysis of cancer registry data, to improve our understanding of the epidemiological characteristics of CRC in terms of survival and stage at diagnosis. The aim of this study was therefore to examine overall survival in patients with CRC using data from the Martinique population-based cancer registry, between 1993 and 2012.
2. Methods
2.1. Population and design
We performed a retrospective study on all incident cases of invasive colorectal cancer diagnosed in Martinique between January 1, 1993 and December 31, 2012. Data were recorded in the PBCR database of Martinique in strict conformity with the international standards laid down by the International Agency for Research on Cancer,[13] the French FRANCIM network, and the European Network of Cancer Registries. Registry procedures were approved by the French National authority for the protection of privacy and personal data.
2.2. Data collection
Data were extracted anonymously using the International Classification of Diseases version 10 (ICD-10) codes and the International Classification of Diseases for Oncology.[14] Population data were available from the French census bureau for the study period. Characteristics of CRC patients were analyzed according to age subgroups, namely: <50 years, 50 to 74 years and over 75 years.
We recorded the following socio-demographic and clinical variables: year of diagnosis, age at diagnosis, sex, histology, zone of residence and subsite of the cancer. Incidence of malignant neoplasms of the colon and rectum (ICD-10 C18–21) was extracted from the Martinique Cancer Registry database.
Stage at diagnosis (localized: stage I–II, regional: stage III and metastatic stage: stage IV) was also analyzed for the 2008 to 2012 period.
Data regarding CRC screening were obtained from the French Public Health Agency. Indicators for participation in the screening program were calculated by sliding 2-year periods, reflecting the bi-annual frequency of invitations to participate in the program. The reference population for the calculation of participation rates was based on the population size projections from the French National Institute of Statistics and Economic Studies (Institut national de la statistique et des études économiques, INSEE)[15] to ensure homogeneity of estimations across Departments.[12]
Vital status (dead/alive) was obtained according to a standardized procedure, namely by consulting the National Directory for the Identification of Natural Persons (Répertoire national d’identification des personnes physiques, RNIPP maintained by the INSEE), after also consulting the death registries in the town hall of the locality where the person lived, and of the locality where the person was born, and medical files were also used to update information. Missing data on vital status were analyzed.
In Martinique, there are no Death Certificate Only registrations, because reliable data regarding deaths for patients residing in Martinique are obtained from the French epidemiological center on medical causes of death from the French National Institute of Health and Medical Research (CépiDc, Inserm: http://www.cepidc.inserm.fr/site4/), ensuring completeness of death information.
In view of the high quality of the database of the Martinique Cancer Registry, the registry data are published regularly at an international scale through the International Agency for Research on Cancer (Cancer incidence in Five Continents) since 1981.
2.3. Statistical analysis
Patient characteristics are described as mean (±standard deviation) for quantitative variables, and as number (percentage) for qualitative variables. Comparisons were performed using the Student t or Chi square tests, as appropriate. For all analyses, a P value < .05 was considered statistically significant. We measured survival as the time from the date of diagnosis until the date of death, regardless of the cause, or loss to follow-up, or censoring on 7th November 2015. The log-rank test was used to assess the statistical differences in the observed survival curves by each categorical variable, namely: gender, subsite, age group, geographical zone and stage at diagnosis. A multivariable Cox model was built to identify independent prognostic factors for overall survival. Variables with a P value < .20 in the univariate analysis were included in the multivariable analysis. A P value < .05 was considered statistically significant. All analyses were performed using SAS version 9.2. (SAS Institute Inc., Cary, NC).
According to French legislation, cancer data were previously rendered anonymous with codes. The Martinique Cancer Registry database was approved by the French National authority for the protection of privacy and personal data (Commission Nationale Informatique et Libertés, CNIL No. 987 001). Additional approval from ethical committees was not required since our study did not involve direct patient contact.
3. Results
A total of 2230 cases of incident invasive CRC were included during the study period (1993–2012); 1171 occurred in women (52.5%); 1588 patients (71.2%) had colon cancer.
In total, the study comprised 1182 CRC patients aged 50 to 74 years old (53.0% of CRC patients), 275 patients were aged <50 years and 773 were aged over 75 years (respectively 12.3% and 34.7% of all CRC patients). The mean age at diagnosis was 67.0 ± 13.5 years in men and 67.6 ± 14.8 years in women (P = .33) for the overall study period from 1993 to 2012.
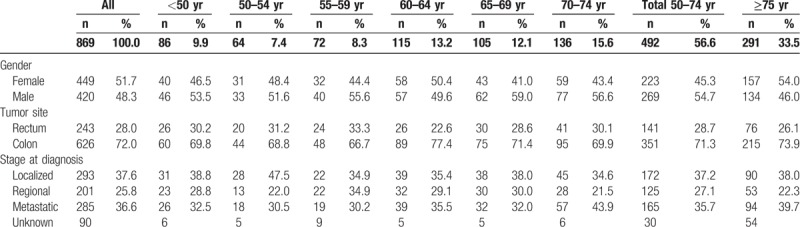
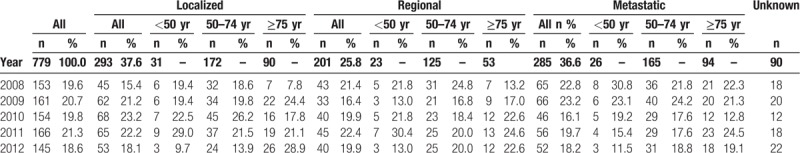
Table 1 summarizes the demographic characteristics, tumor location (colon or rectum) and stage at diagnosis by age group for the 2008 to 2012 period. Stage at diagnosis was evaluated in 779 patients (89.6%); 486 (62.4%) had stage III–IV at diagnosis, including 285 (36.6%) patients with metastases at diagnosis (stage IV).
Table 1.
Characteristics of colorectal cancer patients according to age group in Martinique, 2008 to 2012.
The distribution of cancer cases by age group was also analyzed by 5-year period of analysis, to assess the impact on survival of the introduction of the screening program in Martinique in 2008 (Fig. 1). Participation rates by sex and period of screening were also analyzed and are presented in Figure 2. Table 2 details the participation rates in organized screening for CRC in Martinique for the period 2009 to 2012. Participation was higher in women than in men across all periods, with rates ranging from 23% in men to 35% in women over the last period (2011–2012).
Figure 1.
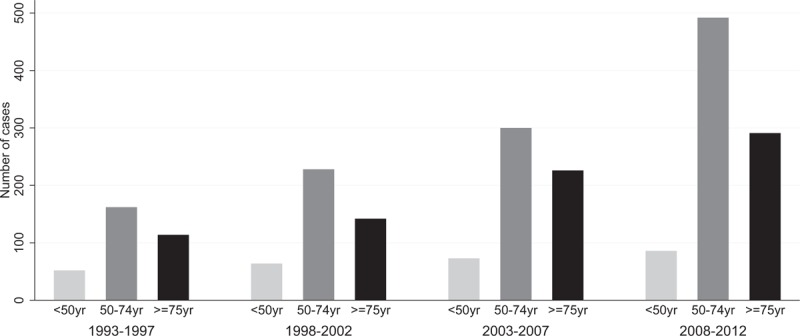
Distribution of cancer cases stratified by age group at diagnosis and 5-year period of analysis (1993–2012), Martinique.
Figure 2.
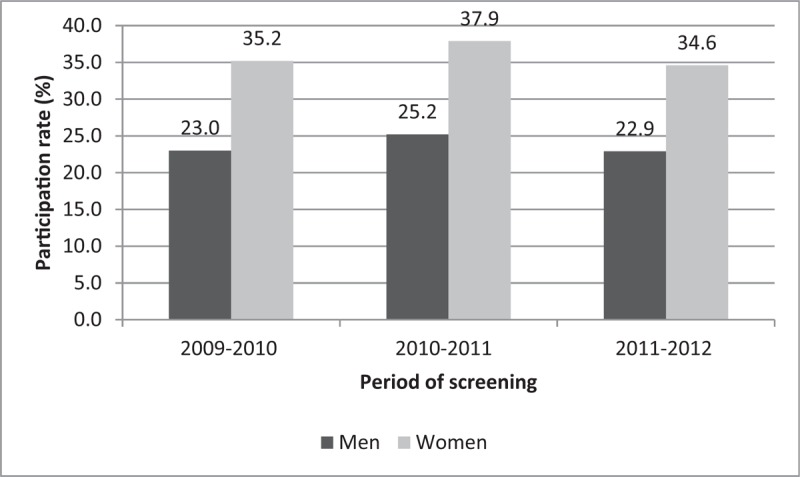
Participation rates in colorectal cancer screening, 2009 to 2012, Martinique. Source: Santé Publique France – 01/03/2013 – participation rates are based on population projections from the INSEE (central scenario) for the period 2007 to 2042.
Table 2.
Participation rates based on data from the regional center for the management of the organized screening program for colorectal cancer, 2009 to 2012, Martinique.
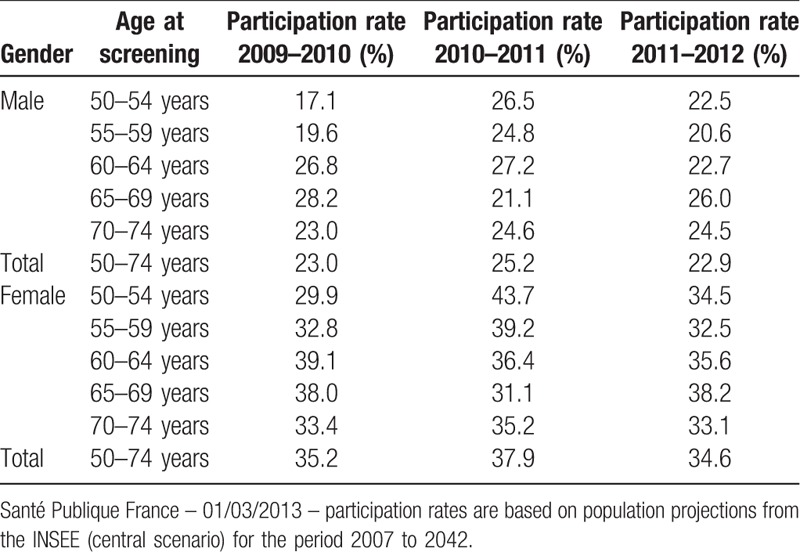
Table 3 presents the stage at diagnosis according to the year of diagnosis, for the period 2008 to 2012.
Table 3.
Characteristics of colorectal cancer patients and stage at diagnosis per year in Martinique, 2008 to 2012.
3.1. Overall survival (OS)
From 1993 to 2012, 1-year OS was 74.6%, while 5-year and 10-year OS was 43.8% and 33.0% respectively (Table 4). Median OS was 3.7 years (95% CI [3.2–4.0]). During the 1993 – 2012 period, five-year OS in men was 31.7% [27.7–35.8] and 38.6% [34.6–42.6] in women. There was a statistically significant difference in OS according to age at diagnosis (P < .001) and gender (P = .0394), with significantly worse median OS in men (3.4 years vs 3.9 years in women). No significant difference was observed according to primary tumor site or zone of residence.
Table 4.
Overall survival (95% confidence interval) in patients with colorectal cancer in Martinique, 1993 to 2012.
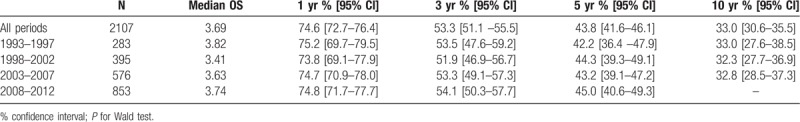
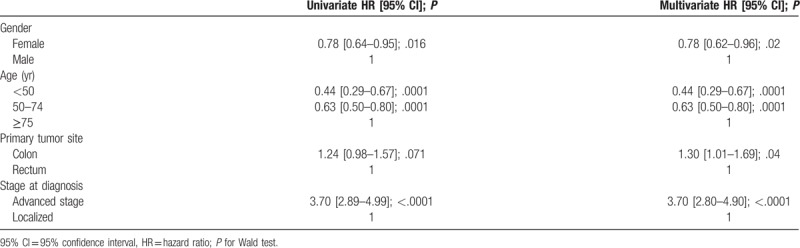
Table 5 presents OS of CRC patients stratified by sex, age at diagnosis, tumor site, zone of residence and stage at diagnosis for the period from 2008 to 2012. There was a statistically significant difference in OS by gender (P = .0153), age at diagnosis (P < .001) and stage (P < .001). Table 6 presents OS by stage at diagnosis and age at diagnosis. Figure 3 presents Kaplan–Meier curves of colorectal cancer-specific survival across age groups at diagnosis between 1993 and 2012. Figure 4 presents OS by stage at diagnosis between 2008 and 2012. The median OS was 2.0 years (95% CI [1.4–2.1]) in the stage III-IV group for the period 2008 to 2012, whereas it was unreached in the stage I–II group (Fig. 4). The prognostic factors for OS among CRC patients are shown in Table 7 for 2008 to 2012. Multivariable analysis confirmed that stage III–IV at diagnosis (hazard ratio (HR) = 3.70 [2.89–4.99]; P < .0001) and colon cancer (HR = 1.30 [1.01–1.69]; P = .04) were the main prognostic factors for OS. Women had a HR of 0.78 [0.62–0.96], P = .02. CRC patients in the 50 to 74 year age group had a HR of 0.63 [0.50–0.80], P = .0001 (Table 7).
Table 5.
Overall survival in patients with colorectal cancer in Martinique, 2008 to 2012.
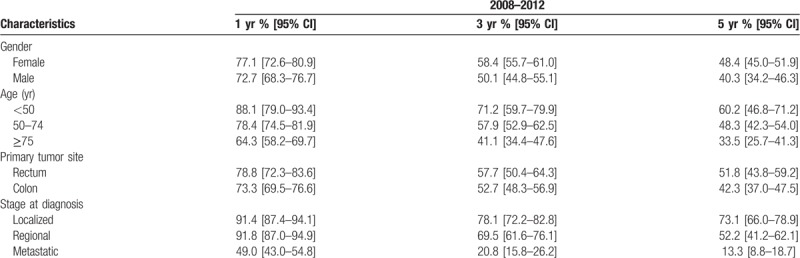
Table 6.
Overall survival in patients with colorectal cancer in Martinique, according to age at diagnosis and stage at diagnosis, 2008 to 2012.
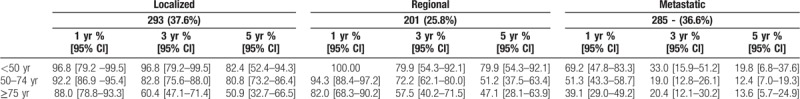
Figure 3.
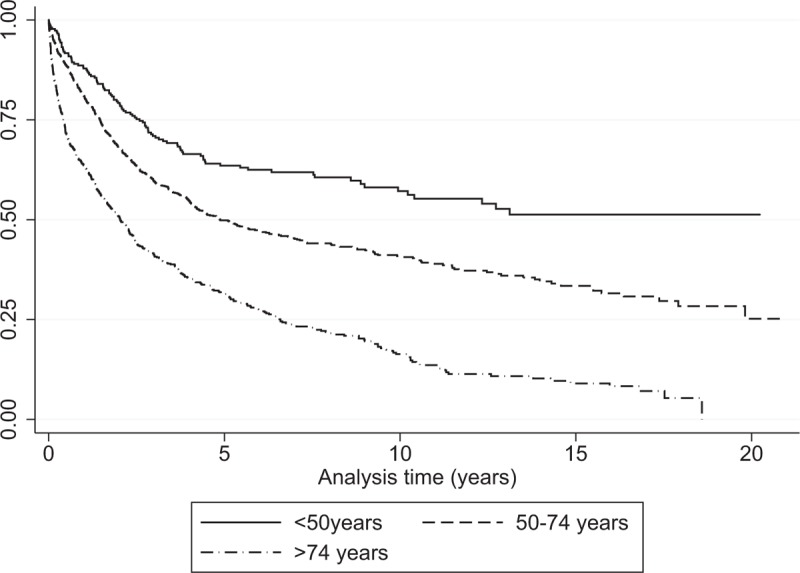
Kaplan–Meier curves of colorectal cancer-specific survival across age groups at diagnosis, 1993–2012, Martinique.
Figure 4.
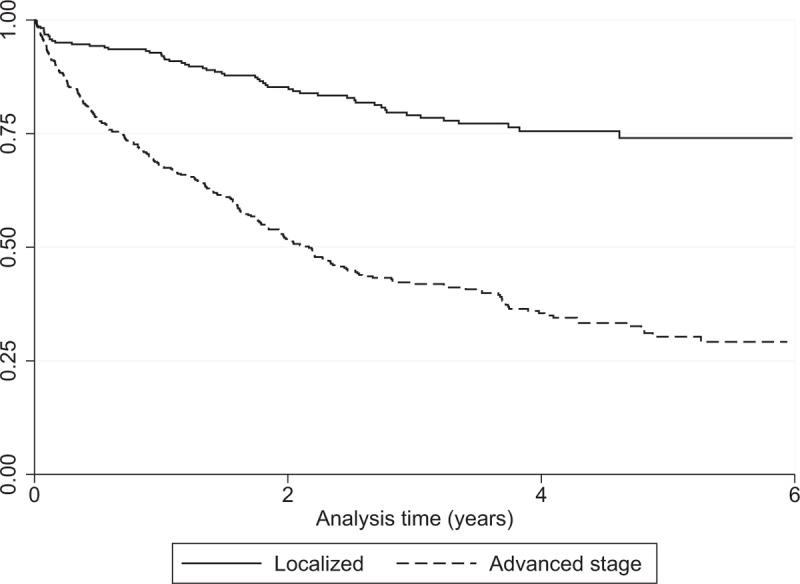
Overall survival of patients diagnosed with CRC in Martinique during 2008 to 2012 stratified by stage at diagnosis (Kaplan–Meier). CRC = colorectal cancer.
Table 7.
Prognostic factors for colorectal cancer survival in Martinique, 2008 to 2012 (N = 869).
4. Discussion
In this study of CRC from registry data, we recorded a total 2230 cases of incident invasive CRC over a period of almost 20 years. More than half occurred in women. Among 779 patients for whom the stage at diagnosis was available, 62.4% had stage III–IV cancer at diagnosis. Overall survival declined from 74.6% at one year, to 43.8% at five years, and only 33% at 10 years. There was a statistical difference in overall survival according to gender, age and stage at diagnosis.
The incidence of CRC is high in developed countries.[1] In France, the incidence rate for CRC is comparable to the average of the 28 European Union countries.[1] On average, 22,828 men and 19,174 women were diagnosed with CRC each year between 2007 and 2016 in mainland France, accounting for 12% of incident cancer cases in men and women. CRC is one of the three most common cancers, and its incidence declined between 2005 and 2012.[16] Mortality from CRC has been declining steadily since the 1980s.[16] CRC was responsible for almost 17,000 deaths per year in mainland France over the period 2007 to 2014, representing 10% of cancer-related deaths in men and 12% in women.
In the regions of Guadeloupe, Martinique, and French Guyana, world-standardized incidence rates are similar, and remain lower than the national average; mortality from CRC is also lower than nationally. Regarding the incidence of colon cancer, a key finding is the continuously increasing standardized rate in Martinique,[17] whereas rates have been stable in mainland France since the early 2000s, and have been reportedly declining in for more than 20 years in the United States and the United Kingdom. These results underling the need to establish partnerships with existing cancer surveillance structures in the Caribbean in order to perform larger-scale studies, study the clinical characteristics of CRC, and identify risk factors for the discordant trends observed here as compared to mainland France.
The risk factors for CRC can be grouped into genetic and environmental factors. The features of a western lifestyle that impact the risk of CRC include diet (regular consumption of red meat or transformed meat products, low consumption of dietary fiber and antioxidant vitamins), alcohol and tobacco consumption, sedentarity and obesity. Individual factors that predispose to CRC include a personal or family history of adenoma or CRC, chronic inflammatory bowel disease, and age greater than 50 years.[18–20] Patients in Martinique have different culture, diet, medical care systems and genetic predisposition compared to Europeans. These specificities deserve to be investigated in further studies in the Caribbean, in order to better understand the burden of cancer in our region.
In the Caribbean, main high-quality cancer data are provided by countries with PBCR, such as Puerto-Rico, Cuba, Guadeloupe, French Guyana and Martinique. Our study showed that the 1 to 5-year survival rate was 75% and 44%, respectively. The median of survival was 3.7 years. The log-rank test showed that there was a statistically significant association between survival and both age at diagnosis and gender; elderly CRC patients aged >74 years had worse survival.
The survival rate is the best index for evaluation of the effectiveness of healthcare, diagnostic, and curative interventions in CRC patients. In our study, 5-year OS increased from 43.8% for the whole period from 1993 to 2012, to 45.0% during the latest period (2008–2012). Median OS for patients with metastatic CRC is currently estimated at around 30 months.[21,22]
In a study of data from French cancer registries for the period 1989 to 2010, the OS for the period 2005 to 2010, at 5 years after diagnosis, was 51% in men and 52% in women.[23] In Martinique, analysis by sex showed that survival was lower in men (40.3%) than in women (48.4%). Net survival at 5 years for individuals diagnosed between 2005 and 2010 in mainland France is 60% in both sexes.[24] Analysis by age group showed lower survival rates in Martinique in those aged over 75 years, at 36% in mainland France compared to 33.5% in Martinique. OS ranged from 65% in those aged 55 to 65, to 60% in those aged 65 to 74, and 48.3% in the 50 to 74 years age group in Martinique. Overall, it can be seen that for similar periods of analysis, survival rates are lower in Martinique.
Analysis by tumor site revealed that survival is greater in patients with rectal cancer (51.8%) compared to those with colon cancer (42.3%). Although both these survival rates are lower than those observed in mainland France (respectively 52% and 50% for rectal and colon cancer), this discrepancy can likely be explained by progress in terms of management, opportunities for adjuvant treatment to surgery, or by a reduction in surgical mortality.
When compared with the national average, lower socioeconomic status and greater income inequalities are observed in the FWI.[25] The differences in reported survival rates may be due to the stage of disease at the time of diagnosis or different methods of diagnosis. Stage at diagnosis is considered as a prognostic factor that is associated with a lower survival rate.[26] In our study, stage was evaluated in 779 patients (89.6%); 486 (62.4%) had stage III–IV at diagnosis, including 285 (36.6%) patients with metastases at diagnosis (stage IV). Results from a recent study in Puerto Rico found that CRC patients had more advanced stage and worse survival compared with Non-Government Health Plan patients.[27]
CRC develops according in an adenoma-carcinoma sequence that can develop over periods of up to several years. The time to appearance of clinical symptoms represents a temporal window of opportunity for the detection of adenoma and early forms of disease. In France, organized screening for CRC in populations at average risk has been implemented since 2007. Screening for adults aged ≥50 is highly effective and should remain a public health priority in efforts to reduce CRC mortality. Low adherence to CRC screening is an issue, despite the existence of guidelines. Although recommended for healthy, asymptomatic adults aged 50 to 74 years, only 22.9% of men and 34.6% of women participated in organized screening in 2011 to 2012 in Martinique.
Organized screening for CRC was introduced in 2008 in Martinique and Guadeloupe; and in 2009 in French Guyana. Participation rates varied from 25.1%, 31.8% and 17.5% in Guadeloupe, Martinique and French Guyana respectively in 2010 to 2011, to 34.9%, 26.2% and 14.4% respectively for the period 2013 to 2014.[28]
In our study, we observed that the distribution of stage changed with the introduction of organized screening for CRC. After a phase with a steady increase (2009–2010), then stabilization in 2011, the percentage of cancer cases declined to 18.6% in 2012. Analysis by stage at diagnosis showed a substantial increase in the proportion of cancers diagnosed at localized stage. Conversely, the proportion of cancers that were metastatic at diagnosis declined over time from 22.8% to 18.2%. These results suggest a positive impact of organized screening programs, through the discovery of cancers at earlier stages. The attendant reduction in the detection of aggressive stage cancers could be explained by earlier identification of these patients, and increased awareness among the general public. The impact of organized screening on OS is, however, more difficult to interpret, due to the low number of deaths in each subgroup of stage at diagnosis.
The limitation of this study includes limitation in the available data regarding screening process, pattern of care of colorectal cancer, treatments performed, and reports of multidisciplinary meetings.
We have added the data available based on the variables that are generally recorded as standard in international cancer registries. The results focus mainly on the stage at the time of diagnosis, which is a major predictive factor for the therapeutic strategy. The variables we included – although limited – are essential for any analysis of the factors related to mortality. Additional variables about the circumstances of diagnosis or screening would certainly enrich the dataset, but are unfortunately not available in our study.
Further studies taking account of clinical and biological factors as well as associated comorbidities are warranted to corroborate these findings.
5. Conclusion
This study provides important information about the epidemiological of CRC in Martinique, particularly overall survival and stage at diagnosis, in a region where incidence and mortality rates are on the increase as compared to France as a whole. The island context, and the characteristics of CRC management in this specific population are factors that need to be taken into account in further studies of CRC in Martinique. Future perspectives for additional avenues of research include studies of the clinical and biological characteristics, as well as research into the determinants of access to organized screening in this region.
Acknowledgments
The authors thank FRANCIM Network, Santé Publique France, INCa, AMREC, CHU Martinique and all those who contributed to the recording of cancer data in the registries: the Oncology Hematology Urology Pathology Division of the University Hospital of Martinique, the laboratories and departments of anatomy, cytology, and pathology; the departments of medical informatics of the public and private hospitals; the local offices of the national social security service; and general practitioners and specialists. We thank Fiona Ecarnot, MSc (EA3920, University Hospital Besancon, France) for editorial assistance.
Author contributions
Conceptualization: Clarisse Joachim.
Data curation: Clarisse Joachim, Audrey Pomier.
Formal analysis: Clarisse Joachim, Jonathan Macni.
Investigation: Jonathan Macni, Audrey Pomier.
Methodology: Clarisse Joachim, Moustapha Drame, Vincent Vinh-Hung.
Project administration: Clarisse Joachim, Patrick Escarmant, Jacqueline Vernique-Baudin, Vincent Vinh-Hung.
Software: Clarisse Joachim, Jonathan Macni.
Supervision: Clarisse Joachim, Jacqueline Vernique-Baudin, Vincent Vinh-Hung.
Validation: Clarisse Joachim, Jonathan Macni, Moustapha Drame, Audrey Pomier, Jacqueline Vernique-Baudin, Vincent Vinh-Hung.
Visualization: Clarisse Joachim.
Writing – original draft: Clarisse Joachim, Vincent Vinh-Hung.
Writing – review & editing: Clarisse Joachim, Jonathan Macni, Moustapha Drame, Audrey Pomier, Patrick Escarmant, Jacqueline Vernique-Baudin, Vincent Vinh-Hung.
Clarisse JOACHIM orcid: 0000-0002-2967-7205.
Footnotes
Abbreviations: CHUM = University Hospital of Martinique, CNIL = Commission Nationale Informatique et Libertés, CRC = colorectal cancer, FWI = French West-Indies, HR = Hazard ratio, INSEE = Institut national de la statistique et des études économiques, OS = overall survival, PBCR = Population-based cancer registries, RNIPP = Répertoire national d’identification des personnes physiques.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J EM, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. 2012. No. 11. Available at: http://globocan.iarc.fr Accessed February 6, 2015. [DOI] [PubMed] [Google Scholar]
- [3].DREES. Les établissements de santé dans les départements et régions d’outre-mer: activité et capacités. 2017. [Google Scholar]
- [4].Activité de cancérologie pour les résidents des départements et régions d’outre-mer, période 2011–2014, Fiche d’analyse, collection Les Données, INCa, juin 2019. [Google Scholar]
- [5].Daniel Sicart, 2014, « Les professions de santé au 1er janvier 2014 », Document de travail, Série Statistiques, no. 189, Drees, juin. [Google Scholar]
- [6].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [7].Araghi M, Soerjomataram I, Jenkins M, et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer 2018. [DOI] [PubMed] [Google Scholar]
- [8].Epidémiologie des cancers aux Antilles Guyane: Focus sur quatre principales localisations. Bulletin de veille sanitaire BVS 2013; http://www.invs.sante.fr/content/download/80307/292551/version/41/file/bvs_ag_2013_08-09.pdf, No. 8–9/Octobre –Novembre 2013. [Google Scholar]
- [9].Forman D BF, Brewster DH, Gombe Mbalawa C, et al., eds. Cancer Incidence in Five Continents, Lyon, IARC. Vol Vol X2013. Available at: http://ci5.iarc.fr Accessed February 6, 2015. [Google Scholar]
- [10].INCa. Les cancers en France, Les Données, INCa, édition 2015. 2016. Available at: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers-en-France-Edition-2015 Accessed October 2, 2017. [Google Scholar]
- [11].Joachim-Contaret C, Véronique-Baudin J, Macni J, et al. Estimations régionales et départementales d’incidence et de mortalité par cancers en France, 2007–2016. Martinique. Saint-Maurice: Santé publique France; 2019. [Google Scholar]
- [12].Annexe IV à l’arrêté du 29 septembre 2006 relatif aux programmes de dépistage des cancers – JORF du 21 décembre 2006, 2018. Available at: https://www.legifrance.gouv.fr/eli/arrete/2006/9/29/SANP0623877A/jo Accessed July 18, 2019. [Google Scholar]
- [13].Globocan I. Fact Sheets by Cancer. Available at: http://gco.iarc.fr/today/fact-sheets-cancers Accessed July 18, 2019. [Google Scholar]
- [14].Fritz A PC JA, et al. International classification of diseases for oncology: ICD-O. 3rd ed. World Health Organization; 2000. Available at: http://www.who.int/classifications/icd/adaptations/oncology/en/ Accessed July 18, 2019. [Google Scholar]
- [15].INSEE. Insee - Santé - Taux de mortalité prématurée en 2009–2011. [Google Scholar]
- [16].Binder-Foucard F, Bossard N, Delafosse P, et al. Cancer incidence and mortality in France over the 1980–2012 period: solid tumors. Rev d’epidemiol sante pub 2014;62:95–108. [DOI] [PubMed] [Google Scholar]
- [17].Joachim C, Véronique-Baudin J, Razanakaivo M, et al. Trends in colorectal cancer in the Caribbean: a population-based study in Martinique. Rev d’epidemiol sante pub 2017;65:181–8. [DOI] [PubMed] [Google Scholar]
- [18].Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43. e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control: CCC 2013;24:1207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vieira AR, Abar L, Chan DSM, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann OncoV 28 2017. 1788–802. [DOI] [PubMed] [Google Scholar]
- [21].Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- [22].Lopez RI, Castro JL, Cedeno H, et al. Consensus on management of metastatic colorectal cancer in Central America and the Caribbean: San Jose, Costa Rica. ESMO Open 2018;3:e000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anne Cowppli-Bony ZU, Laurent Remontet, Anne-Valérie Guizard, et al. Survie des personnes atteintes de cancer en France métropolitaine 1989–2013. Etude à partir des registres des cancers du réseau Francim. Vol Partie 1 – Tumeurs solides 2016. [Google Scholar]
- [24].Cowppli-Bony A, Uhry Z, Remontet L, et al. Survie des personnes atteintes de cancer en France métropolitaine, 1989–2013. Partie 1 – Tumeurs solides. Saint-Maurice: Institut de veille sanitaire; 2016. [Google Scholar]
- [25].Menvielle G, Dugas J, Richard JB, et al. Socioeconomic and healthcare use-related determinants of cervical, breast and colorectal cancer screening practice in the French West Indies. Eur J Cancer Prev: Off J Eur Cancer Prev Organ (ECP) 2018;27:269–73. [DOI] [PubMed] [Google Scholar]
- [26].Pruitt SL, Harzke AJ, Davidson NO, et al. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control: CCC 2013;24:961–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ortiz-Ortiz KJ, Ramírez-García R, Cruz-Correa M, et al. Effects of type of health insurance coverage on colorectal cancer survival in Puerto Rico: a population-based study. PLoS One 2014;9:e9674605/05 01/01/received 04/10/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].France Sp. Evaluation des programmes de dépistage des cancers. 2018. Available at: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-chroniques-et-traumatismes/Cancers/Evaluation-des-programmes-de-depistage-des-cancers/Evaluation-du-programme-de-depistage-du-cancer-colorectal Accessed March 13, 2019. [Google Scholar]


