Abstract
Background
Bacterial meningitis is a major cause of morbidity and mortality in sub-Saharan Africa. We analyzed data from the World Health Organization’s (WHO) Invasive Bacterial Vaccine-preventable Diseases Surveillance Network (2011–2016) to describe the epidemiology of laboratory-confirmed Streptococcus pneumoniae (Spn), Neisseria meningitidis, and Haemophilus influenzae meningitis within the WHO African Region. We also evaluated declines in vaccine-type pneumococcal meningitis following pneumococcal conjugate vaccine (PCV) introduction.
Methods
Reports of meningitis in children <5 years old from sentinel surveillance hospitals in 26 countries were classified as suspected, probable, or confirmed. Confirmed meningitis cases were analyzed by age group and subregion (South-East and West-Central). We described case fatality ratios (CFRs), pathogen distribution, and annual changes in serotype and serogroup, including changes in vaccine-type Spn meningitis following PCV introduction.
Results
Among 49 844 reported meningitis cases, 1670 (3.3%) were laboratory-confirmed. Spn (1007/1670 [60.3%]) was the most commonly detected pathogen; vaccine-type Spn meningitis cases declined over time. CFR was the highest for Spn meningitis: 12.9% (46/357) in the South-East subregion and 30.9% (89/288) in the West-Central subregion. Meningitis caused by N. meningitidis was more common in West-Central than South-East Africa (321/954 [33.6%] vs 110/716 [15.4%]; P < .0001). Haemophilus influenzae (232/1670 [13.9%]) was the least prevalent organism.
Conclusions
Spn was the most common cause of pediatric bacterial meningitis in the African region even after reported cases declined following PCV introduction. Sustaining robust surveillance is essential to monitor changes in pathogen distribution and to inform and guide vaccination policies.
Keywords: pediatric bacterial meningitis, sub-Saharan Africa, case fatality ratios, pneumococcal conjugate vaccine, PCV
We describe the epidemiology of meningitis cases due to Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae across 26 African countries participating in the World Health Organization’s Invasive Bacterial Vaccine-preventable Diseases Network, including trends in vaccine-type pneumococcal meningitis post–pneumococcal conjugate vaccine introduction.
Bacterial meningitis is a severe infection associated with high morbidity and mortality in young children, especially in low-income countries [1]. Sub-Saharan Africa has high rates of endemic and epidemic disease, accounting for the world’s greatest burden of meningitis due to Haemophilus influenzae type b (Hib), Neisseria meningitidis (Nm), and Streptococcus pneumoniae (Spn) [2–5].
Over the last decade, countries within the World Health Organization (WHO) African Region introduced vaccines targeting these organisms. Hib conjugate vaccine was first successfully introduced in The Gambia in 1997, followed by a substantial reduction in invasive Hib disease incidence [6]. Through financial support from Gavi, the Vaccine Alliance, all 47 countries within the WHO African Region have introduced Hib-containing vaccine as part of their national childhood Expanded Programme on Immunization (EPI) [7].
A mass vaccination campaign with meningococcal serogroup A conjugate vaccine (MACV, MenAfriVac®) was first implemented in Burkina Faso in 2010, resulting in a 99.8% decline in the risk of serogroup A meningitis [8]. MACV mass vaccination campaigns have since been conducted in 21 countries within the African “meningitis belt,” an area that spans from Ethiopia to Senegal [9]. Seven countries also introduced MACV into their routine childhood immunization schedules beginning in 2016 [4, 10, 11].
Currently, 39 of 47 (83%) countries in the African Region have introduced pneumococcal conjugate vaccine (PCV) into their routine EPI [7]. Post–PCV introduction studies in several African counties have shown significant declines in rates of invasive pneumococcal disease and severe pneumonia [12–16].
Robust disease surveillance systems are essential for guiding further vaccine introduction, monitoring vaccine impact, and describing changes in disease epidemiology over time. In 2001, the African Paediatric Bacterial Meningitis (PBM) surveillance network was initiated with the support of WHO and global immunization partners. This hospital-based sentinel surveillance system collected information on clinically suspected bacterial meningitis cases among children <5 years of age in 31 African countries [17]. In 2008, PBM became part of the global Invasive Bacterial Vaccine-preventable Diseases (IB-VPD) Surveillance Network with other WHO member states [18]. The objectives of the IB-VPD surveillance network include describing the epidemiology and estimating the burden of invasive bacterial vaccine-preventable diseases, establishing a surveillance platform to measure vaccine impact, and characterizing the circulating bacterial serotypes/serogroups among children <5 years old.
We used data collected as part of IB-VPD surveillance in the WHO African Region (2011–2016) to describe laboratory-confirmed meningitis among pediatric patients within the South-East and West-Central African subregions, including pathogen distribution, clinical outcomes, and trends in vaccine-type pneumococcal meningitis following PCV introduction.
METHODS
Thirty-one of 47 countries in the WHO African Region provided surveillance data for children <60 months old to the IB-VPD network from 2011 through 2016. We excluded neonates (<1 month old) because bacterial etiology of meningitis in this age group is different compared to that of older children and more commonly acquired during birth or hospitalization. Cases were identified as “suspected” based upon the presence of fever and meningeal signs; “probable” based upon cerebrospinal fluid (CSF) appearance, white blood cell count, protein, and glucose; and laboratory-confirmed if H. influenzae (Hi), Nm, or Spn was identified by a laboratory test in a child who met the suspected or probable case definition (Supplementary Figure 1). Countries (n = 5) with >50% of records missing data for the classification of suspected meningitis or with <10 suspected meningitis cases across all surveillance years were excluded.
Data Collection
Trained clinicians at each sentinel surveillance site were responsible for enrolling patients with suspected meningitis. For each patient, a standardized case investigation form containing information on patient demographics, clinical signs/symptoms, outcome, and laboratory results was completed. Data were entered into an Epi Info database and transferred on a monthly basis to Ministries of Health and to WHO Intercountry Support Teams. The Intercountry Support Teams were responsible for data aggregation across countries with subsequent sharing of the data with the WHO Regional Office.
Laboratory Methods
From most patients with suspected meningitis, a CSF specimen was collected and processed by the sentinel site laboratory for cell count, glucose and protein concentrations, Gram stain, and bacterial culture (when supplies were available). Some laboratories performed rapid diagnostic tests, such as immunochromatographic test for Spn and bacterial latex agglutination testing for Hi, Nm, and Spn.
Most countries within the South-East and West-Central subregions sent CSF specimens to 1 of 2 regional reference laboratories (RRLs): the National Institute for Communicable Diseases (NICD) in South Africa serving the South-East subregion, and the Medical Research Council Unit (MRC) in The Gambia serving the West-Central subregion. Both RRLs perform real-time polymerase chain reaction (PCR) for detection of Hi, Nm, and Spn, with serotyping (Hi, Spn) or serogrouping (Nm) of positive samples using real-time and/or conventional PCR as previously described [19–23]. Different cycle threshold cutoff values were used by each RRL to define positive, negative, and inconclusive results. The MRC used a cycle threshold (Ct) cutoff for pathogen gene detection (Hi = hpd; Nm = ctra or sodC; Spn = lytA) of ≤36, whereas NICD used a Ct value ≤35. PCR detection of the human RNaseP gene was used by both RRLs to confirm true-negative PCR results, with both laboratories defining negative results as inconclusive with an RNaseP Ct value ≥36.
When available, isolates were also sent to the RRLs for confirmation and serotyping/serogrouping by latex agglutination (MRC) or Quellung reaction (NICD).
For patients in whom laboratory results across testing modalities identified >1 pathogen, the PCR result was considered the gold standard and was reported. If >1 pathogen was detected by PCR, contamination was suspected and the result was excluded. For samples not tested by PCR, CSF culture results were prioritized over rapid diagnostic tests.
RRLs submitted all testing results back to the sentinel sites and intercountry support teams. Sentinel sites were responsible for updating RRL sample results into their Epi Info database using unique sample identifiers.
Statistical Analysis
We analyzed data using SAS version 9.4 software (SAS Institute, Cary, North Carolina). Confirmed meningitis cases were stratified by subregion, age group (1–12 months and 13–59 months), and pathogen. We calculated case fatality ratios (CFRs) for confirmed meningitis cases using data from countries that reported outcome data for ≥5 confirmed cases per age group during 2011–2016.
Annual numbers of Hi and Nm meningitis cases for each subregion, by serotype and serogroup status, respectively, were analyzed for countries that reported data across all surveillance years and had available serogroup/serotype data.
Vaccine-type pneumococcal meningitis was defined as that caused by serotypes included in the 10-valent PCV (PCV10) (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F) or the 13-valent PCV (PCV13; including PCV10 serotypes plus 3, 6A, and 19A). Additionally, serotypes that are indistinguishable from PCV10 serotypes by PCR (6A/6B, 7F/7A, 9V/9A, 18C/18F/18B/18A) were classified as PCV10 vaccine serotypes. Annual numbers of overall and vaccine-type Spn meningitis cases were analyzed by subregion for countries that introduced PCV during the surveillance period and met the following criteria: reported Spn serotype data; had data for all surveillance years; had ≥65% PCV coverage for at least 2 consecutive years based upon WHO/United Nations International Children's Emergency Funds estimates; and had at least 2 years of available data following vaccine introduction [24].
We stratified countries meeting the above inclusion criteria by PCV formulation (ie, PCV10 or PCV13). The Cochran-Armitage test for trend was used to evaluate changes in the annual percentage of PCV10 and PCV13 Spn meningitis cases among all Spn meningitis cases with a reported serotype. To confirm observed trends, sensitivity analyses were performed; we excluded data from the United Republic of Tanzania and Ghana because <12 confirmed pneumococcal meningitis cases were serotyped across all surveillance years.
Fisher exact test was used to compare categorical variables. A 2-tailed P value of <.05 was considered statistically significant for all statistical tests.
RESULTS
Descriptive Epidemiology of Meningitis Cases in the African Region
We included 26 of the 31 WHO African Region member states participating in the IB-VPD surveillance network during 2011–2016. These encompassed 51 sentinel surveillance sites, including 14 located within the African meningitis belt [4] (Table 1). Of the 49 844 patients with suspected meningitis, 38 919 (78.1%) met the “suspected” case definition, 5994 (12.0%) did not meet this definition, and 4931 (9.9%) were missing data needed to determine suspected status based upon the case definition (Figure 1). A lack of recorded fever was the main reason that patients did not meet the suspected meningitis case definition. Overall, 48 284 (96.9%) patients with suspected meningitis had a lumbar puncture performed. Of those, 1670 (3.5%) had laboratory-confirmed Hi, Nm, or Spn meningitis; 51.4% of the cases were confirmed by PCR and 39.1% by culture (Supplementary Figure 2). Among suspected meningitis cases that did not meet the laboratory-confirmed case definition but had both another pathogen detected and named within the database (n = 155), the most common organisms isolated included Staphylococcus aureus (21.9%), Streptococcus species (18.7%), Salmonella species (12.9%), Pseudomonas species (10.3%), and Klebsiella species (7.7%).
Table 1.
Description of World Health Organization African Region Countries Included in the Analysis of the Invasive Bacterial Vaccine-preventable Diseases Surveillance System
| Country | Subregion | No. of Surveillance Sites | No. of Surveillance Sites Within the Meningitis Belt [4] | Annual Birth Cohorta | Years of Available Data | Year of Hib Introduction | Year of First MACV National Prevention Campaign | Year of PCV Introduction (Formulation) | No. of Suspected Meningitis Cases Reported | No. of CSF Specimens Testedb |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopia | SE | 3 | 3 | 3 256 400 | 2011–2016 | 2007 | 2013 | 2011 (PCV10) | 3810 | 3808 |
| Lesotho | SE | 2 | 0 | 61 400 | 2011–2016 | 2008 | … | 2015 (PCV13) | 468 | 303 |
| Madagascar | SE | 1 | 0 | 825 100 | 2011–2016 | 2008 | … | 2012 (PCV10) | 2907 | 2599 |
| Malawic | SE | 1 | 0 | 664 000 | 2011–2012 | 2002 | … | 2011 (PCV13) | 106 | 102 |
| Mozambique | SE | 3 | 0 | 1 123 000 | 2011, 2013–2016 | 2009 | … | 2013 (PCV10) | 816 | 749 |
| Rwanda | SE | 2 | 0 | 370 700 | 2011–2016 | 2002 | … | 2009 (PCV13) | 308 | 280 |
| Kingdom of Eswatini (formerly Swaziland) | SE | 2 | 0 | 38 700 | 2011–2016 | 2009 | … | 2014 (PCV13) | 432 | 257 |
| Uganda | SE | 3 | 0 | 1 748 500 | 2011–2016 | 2002 | … | 2013 (PCV10) | 3920 | 3700 |
| United Republic of Tanzania | SE | 3 | 0 | 2 122 000 | 2011–2016 | 2009 | … | 2012 (PCV13) | 590 | 515 |
| Zambia | SE | 1 | 0 | 632 600 | 2011–2016 | 2004 | … | 2013 (PCV10) | 2082 | 1427 |
| Zimbabwe | SE | 1 | 0 | 535 300 | 2011–2016 | 2008 | … | 2012 (PCV13) | 2820 | 2788 |
| Angolac | WC | 1 | 0 | 1 204 400 | 2012–2016 | 2006 | … | 2013 (PCV13) | 1574 | 1297 |
| Benin | WC | 3 | 3 | 402 600 | 2011–2016 | 2005 | 2012 | 2011 (PCV13) | 8437 | 2751 |
| Burkina Fasoc | WC | 1 | 1 | 725 700 | 2011–2016 | 2006 | 2010 | 2013 (PCV13) | 1379 | 1374 |
| Burundic | WC | 1 | 0 | 444 600 | 2011–2016 | 2004 | … | 2011 (PCV13) | 323 | 35 |
| Cameroon | WC | 1 | 0 | 850 800 | 2011–2016 | 2009 | 2011 | 2011 (PCV13) | 4263 | 559 |
| CARc | WC | 1 | 0 | 164 000 | 2011–2014, 2016 | 2008 | … | 2011 (PCV13) | 935 | 711 |
| Cote d’Ivoire | WC | 2 | 1 | 872 800 | 2011, 2013–2016 | 2009 | 2014 | 2014 (PCV13) | 1931 | 1928 |
| DRC | WC | 3 | 0 | 3 328 900 | 2011–2016 | 2009 | … | 2012 (PCV13) | 1373 | 1109 |
| Gambiad | WC | 1 | 1 | 80 500 | 2011–2016 | 1997 | 2013 | 2011c (PCV13) | 287 | 255 |
| Ghana | WC | 2 | 0 | 875 700 | 2011–2016 | 2002 | 2012 | 2012 (PCV13) | 2464 | 2444 |
| Niger | WC | 5 | 5 | 995 100 | 2011–2016 | 2008 | 2010 | 2014 (PCV13) | 1844 | 1363 |
| Nigeria | WC | 5 | 1 | 7 232 600 | 2011–2016 | 2012 | 2011 | 2014 (PCV10) | 3259 | 3068 |
| Senegal | WC | 1 | 1 | 548 600 | 2011–2016 | 2005 | 2012 | 2013 (PCV13) | 639 | 637 |
| Sierra Leone | WC | 1 | 0 | 258 800 | 2011–2014, 2016 | 2007 | … | 2011 (PCV13) | 259 | 216 |
| Togo | WC | 1 | 0 | 258 800 | 2011–2016 | 2008 | 2014 | 2014 (PCV13) | 2618 | 2550 |
| Total | … | 51 | 14 | … | … | … | … | … | 49 844 | 36 825 |
Abbreviations: CAR, Central African Republic; CSF, cerebrospinal fluid; DRC, Democratic Republic of Congo; Hib, conjugate Haemophilus influenzae type B vaccine; MACV, meningococcal serogroup A conjugate vaccine; PCV, pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; SE, South-East; WC, West-Central.
aWorld Bank population statistics by country, 2016 (https://data.worldbank.org/indicator/sp.dyn.cbrt.in), Estimates rounded to nearest 100.
bSpecimens tested by at least 1 laboratory method (polymerase chain reaction [PCR], culture, latex agglutination, immunochromatographic test).
cThese countries do not send samples to the regional reference laboratories for confirmatory testing by PCR and serotyping.
dThe Gambia introduced 7-valent PCV in 2009.
Figure 1.
Bacterial meningitis case classification, 2011–2016. Abbreviation: LP, lumbar puncture. Percentage of cases that did not meet probable criteria due to missing data: a1.9%, b1.1%, c1.3%. dNeisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae in addition to other potential pathogens identified by culture or latex agglutination test. eDetection of N. meningitidis, S. pneumoniae, and H. influenzae by polymerase chain reaction, culture, or rapid diagnostic tests (latex agglutination or immunochromatographic test).
Among the 1670 patients with laboratory-confirmed meningitis, 1581 (94.7%) were hospitalized and 189 (11.3%) died.
Descriptive Epidemiology of Confirmed Meningitis Cases in the South-East and West-Central Subregions
During 2011–2016, 3.9% (716/18 259) of suspected meningitis cases in the South-East subregion and 3.0% (954/31 585) in the West-Central subregion were laboratory-confirmed (Table 2). Spn was the most common pathogen detected in the South-East (499/716 [69.7%]) and West-Central (508/954 [53.2%]) subregions. However, Nm meningitis was more prevalent in the West-Central compared to the South-East subregion (321/954 [33.6%] vs 110/716 [15.4%]; P < .0001).
Table 2.
Confirmeda Pediatric Bacterial Meningitis Cases by Subregion, Age Group, and Pathogen
| Subregion and Age Group | No. of CSF Specimens Testedb | No. of Confirmed Cases | Confirmed Hi Cases (% of Confirmed Cases) | Confirmed Nm Cases (% of Confirmed Cases) | Confirmed Spn Cases (% of Confirmed Cases) |
|---|---|---|---|---|---|
| South-Eastc (n = 18 259 total suspected meningitis cases) | |||||
| 1–12 mo | 9620 | 409 | 63 (15.4) | 34 (8.3) | 312 (76.2) |
| 13–59 mo | 6908 | 307 | 44 (14.3) | 76 (24.8) | 187 (60.9) |
| Total | 16 528 | 716 | 107 (14.9) | 110 (15.4) | 499 (69.7) |
| West-Centrald (n = 31 585 total suspected meningitis cases) | |||||
| 1–12 mo | 8518 | 495 | 80 (16.2) | 133 (26.9) | 282 (57.0) |
| 13–59 mo | 11 779 | 459 | 45 (9.8) | 188 (41.0) | 226 (49.2) |
| Total | 20 297 | 954 | 125 (13.1) | 321 (33.6) | 508 (53.2) |
| Totals of both regions | 36 825 | 1670 | 232 (13.9) | 431 (25.8) | 1007 (60.3) |
Abbreviations: CSF, cerebrospinal fluid; Hi, Haemophilus influenzae; Nm, Neisseria meningitidis; Spn, Streptococcus pneumoniae.
aConfirmed cases refer to suspected or probable bacterial meningitis with laboratory evidence of Hi, Nm, or Spn.
bSpecimens tested by at least 1 laboratory method (polymerase chain reaction, culture, latex agglutination, immunochromatographic test).
cSouth-East subregion countries: Ethiopia, Lesotho, Madagascar, Malawi, Mozambique, Rwanda, Kingdom of Eswatini (formerly Swaziland), Uganda, United Republic of Tanzania, Zambia, Zimbabwe.
dWest-Central subregion countries: Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Cote d’Ivoire, Democratic Republic of Congo, Gambia, Ghana, Niger, Nigeria, Senegal, Sierra Leone, Togo.
Among laboratory-confirmed cases, outcome data were reported for 68.0% in the South-East and 59.0% in the West-Central subregions. The overall CFR was higher among cases in the West-Central vs the South-East subregion (132/563 [23.4%] vs 54/487 [11.0%]; P < .0001) (Table 3). CFR was highest among those infected with Spn in both regions, with higher Spn-specific CFR in the West-Central than the South-East (89/288 [30.9%] vs 46/357 [12.8%]; P < .0001) subregion.
Table 3.
Case Fatality Ratios for Confirmed Casesa,b by Subregionc, Age Group, and Pathogen
| Subregion and Age Group | Total | Hi | Nm | Spn | ||||
|---|---|---|---|---|---|---|---|---|
| Deaths | CFR, % (no./No.) | Deaths | CFR, % (no./No.) | Deaths | CFR, % (no./No.) | Deaths | CFR, % (no./No.) | |
| South-Eastd | ||||||||
| 1–12 mo | 41 | 14.0 (41/292) | 5 | 11.6 (5/43) | 1 | 5.0 (1/20) | 35 | 15.3 (35/229) |
| 13–59 mo | 13 | 6.7 (13/195) | 1 | 3.3 (1/30) | 1 | 2.7 (1/37) | 11 | 8.6 (11/128) |
| Total | 54 | 11.1 (54/487) | 6 | 8.2 (6/73) | 2 | 3.5 (2/57) | 46 | 12.9 (46/357) |
| West-Centrale | ||||||||
| 1–12 mo | 59 | 20.8 (59/284) | 7 | 20.0 (7/35) | 9 | 9.8 (9/92) | 43 | 27.4 (43/157) |
| 13–59 mo | 73 | 26.2 (73/279) | 4 | 12.9 (4/31) | 23 | 19.7 (23/117) | 46 | 35.1 (46/131) |
| Total | 132 | 23.4 (132/563) | 11 | 16.7 (11/66) | 32 | 15.3 (32/209) | 89 | 30.9 (89/288) |
| Totals of both regions | 186 | 17.7 (186/1050) | 17 | 12.2 (17/139) | 34 | 12.8 (34/266) | 135 | 20.9 (135/645) |
Abbreviations: CFR, case fatality ratio; Hi, Haemophilus influenzae; Nm, Neisseria meningitidis; Spn, Streptococcus pneumoniae.
aConfirmed cases refer to suspected or probable bacterial meningitis with laboratory evidence of Spn, Nm, or Hi.
bCases were only included if outcome was known.
cCountries that reported <5 confirmed cases with outcome data by age group across all surveillance years were excluded: 1–12 months: United Republic of Tanzania; 13–59 months: Mozambique, Kingdom of Eswatini (formerly Swaziland).
dSouth-East subregion countries: Ethiopia, Lesotho, Madagascar, Malawi, Mozambique, Rwanda, Kingdom of Eswatin (formerly Swaziland), Uganda, United Republic of Tanzania, Zambia, Zimbabwe.
eWest-Central subregion countries: Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Cote d’Ivoire, Democratic Republic of Congo, Gambia, Ghana, Niger, Nigeria, Senegal, Sierra Leone, Togo.
Annual Numbers of Hi, Nm, and Spn Meningitis Cases by Serotype/Serogroup
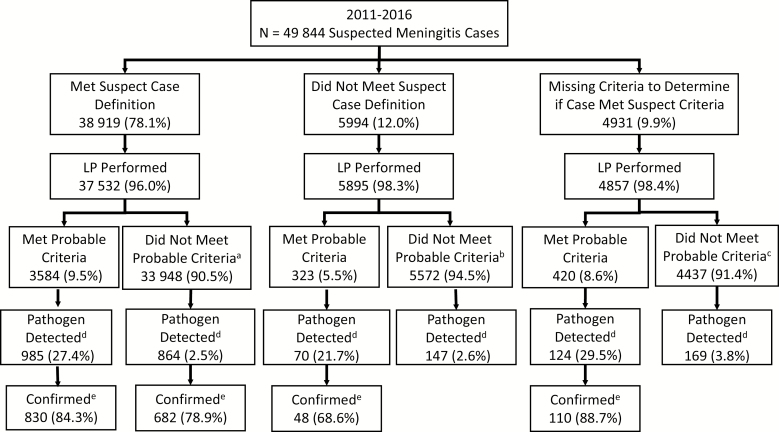
No clear trend in the number of Hi meningitis cases over time was noted in either subregion. Among those with serotype data available, Hib remained the most common serotype; 76.8% (53/69) of Hi cases in the South-East and 44.1% (19/43) of those in the West-Central subregions were Hib (Figure 2A and 2B). At least 1 case of Hib was reported from 14 of the 15 countries included in this analysis.
Figure 2.
Annual number of confirmed Haemophilus influenzae meningitis cases by serotype and African subregion. A, Total number of confirmed cases reported by country in South-East Africa: Ethiopia (n = 10), Lesotho (n = 8), Madagascar (n = 5), Kingdom of Eswatini (formerly Swaziland; n = 3), Uganda (n = 36), Zambia (n = 21), and Zimbabwe (n = 3). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Malawi, Mozambique); did not report any H. influenzae serotype data (Malawi, Rwanda, United Republic of Tanzania). B, Number of confirmed cases reported by country in West-Central Africa: Benin (n = 28), Cameroon (n = 15), Gambia (n = 5), Ghana (n = 2), Niger (n = 8), Nigeria (n = 17), Senegal (n = 7), Togo (n = 14). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Angola, Central African Republic, Cote d’Ivoire, Sierra Leone); did not report any H. influenzae serotype data (Angola, Burkina Faso, Central African Republic, Democratic Republic of Congo). Abbreviations: Hia, Haemophilus influenzae type a; Hib, Haemophilus influenzae type b; Hic, Haemophilus influenzae type c; Hie, Haemophilus influenzae type e.
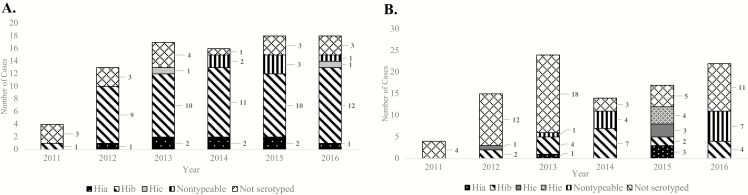
Annual number of Nm meningitis cases in the South-East increased through 2015 and then substantially declined in 2016 whereas in the West-Central subregion, case numbers decreased in 2013 and then remained stable (Figure 3A and 3B). Among Nm meningitis cases with serogroup data available, serogroup W was the most common (90.2% [65/72] in the South-East, 54.8% [34/62] in the West-Central subregion). In 2016, serogroup C cases in the West-Central subregion increased. No confirmed cases of serogroup A were identified in the surveillance sentinel sites in the West-Central subregion, although 4 cases were identified in the South-East.
Figure 3.
Annual number of confirmed Neisseria meningitidis meningitis cases by serogroup and African subregion. A, Number of confirmed cases reported by country in South-East Africa: Ethiopia (n = 20), Lesotho (n = 3), Rwanda (n = 5), United Republic of Tanzania (n = 1), Uganda (n = 17), Zambia (n = 37), and Zimbabwe (n = 1). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Malawi, Mozambique); did not report any N. meningitidis serogroup data (Malawi, Madagascar, Kingdom of Eswatini [formerly Swaziland]). B, Number of confirmed cases reported by country in West-Central Africa: Benin (n = 35), Gambia (n = 16), Ghana (n = 12), Niger (n = 82), Nigeria (n = 29), Senegal (n = 26). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Angola, Burkina Faso, Central African Republic, Cote d’Ivoire, Sierra Leone); did not report any N. meningitidis serogroup data (Angola, Burundi, Central African Republic, Democratic Republic of Congo, Togo). Abbreviations: NmA, Neisseria meningitidis serogroup A; NmB, Neisseria meningitidis serogroup B; NmW, Neisseria meningitidis serogroup W; NmX, Neisseria meningitidis serogroup X; NmY, Neisseria meningitidis serogroup Y.
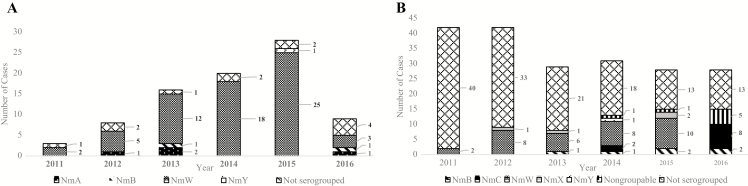
Spn meningitis case numbers were highest in 2012 in the South-East and in 2011 in the West-Central followed by an apparent downward trend in both subregions (Figure 4A and 4B). Spn was the most frequently detected pathogen in both subregions over the entire surveillance period. The number of Spn meningitis cases due to the unique PCV13 serotypes (3, 6A, 19A) remained low for both subregions throughout the surveillance period. Among Spn meningitis cases serotyped in the South-East (n = 307) and West-Central (n = 93) subregions, serotype 1 (n = 43 [14.0%]) and serotype 14 (n = 10 [10.8%]) were most prevalent. In the South-East, serotype 1 declined after 2013 whereas serotype 14 decreased after 2011 in the West-Central subregion (data not shown).
Figure 4.
Annual number of confirmed Streptococcus pneumoniae meningitis cases by vaccine-associated serotype and African subregion. A, Number of confirmed cases reported by country in South-East Africa: Ethiopia (n = 42), Madagascar (n = 81), Kingdom of Eswatini (formerly Swaziland, n = 6), United Republic of Tanzania (n = 6), Uganda (n = 134), Zambia (n = 94), Zimbabwe (n = 31). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Malawi, Mozambique); pneumococcal conjugate vaccine (PCV) not introduced during the surveillance period (Rwanda); at least 2 years of data not available following vaccine introduction (Lesotho); did not report any S. pneumoniae serotyping data (Malawi). B, Number of confirmed cases reported by country in West-Central Africa: Benin (n = 67), Cameroon (n = 57), Ghana (n = 19), Senegal (n = 47), Togo (n = 35). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Angola, Central African Republic, Cote d’Ivoire, Sierra Leone); ≤65% PCV coverage for at least 2 consecutive years (Niger, Nigeria); PCV not introduced during the surveillance period (Gambia); did not report any S. pneumoniae serotyping data (Angola, Burkina Faso, Burundi, Central African Republic, Democratic Republic of Congo). Thirteen-valent PCV unique serotypes include 3, 6A, and 19A. Abbreviations: PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
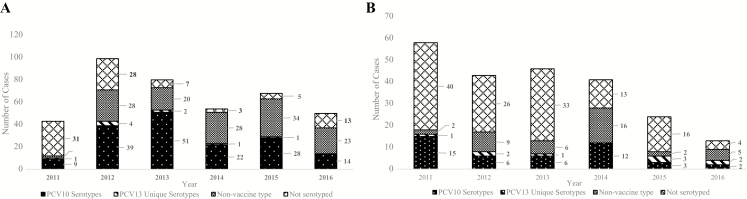
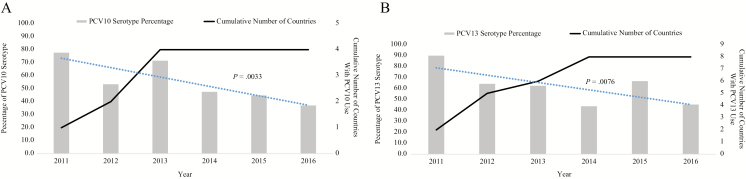
Four countries included in the analysis introduced PCV10 during 2011–2013 and 8 countries introduced PCV13 during 2011–2014 (Figure 5A and 5B). For countries that introduced PCV10, the percentage of vaccine-type meningitis cases decreased from 77.8% (7/9) in 2011 to 37.1% (13/35) in 2016 (P = .0033). For countries that introduced PCV13, the percentage of vaccine-type meningitis cases decreased from 90.0% (18/20) in 2011 to 45.5% (5/11) in 2016 (P = .0076). The sensitivity analysis revealed similar results.
Figure 5.
Annual proportion of confirmed Streptococcus pneumoniae meningitis cases due to vaccine serotypes by pneumococcal conjugate vaccine (PCV) formulation, 2011–2016. A, Proportion of confirmed S. pneumoniae meningitis cases due to 10-valent PCV (PCV10) serotypes in countries that introduced PCV10 (n = 4), by year. Of the 6 countries that introduced PCV10, 4 contributed confirmed cases for this analysis: Ethiopia (n = 22), Madagascar (n = 66), Uganda (n = 106), and Zambia (n = 76). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Mozambique); ≤65% PCV coverage for at least 2 consecutive years (Nigeria). The percentage of PCV10 serotypes was determined by the number of reported PCV10 serotypes/all reported serotype results in a year: 2011 (7/9); 2012 (31/58); 2013 (48/68); 2014 (20/42); 2015 (26/58); 2016 (13/35). B, Proportion of confirmed S. pneumoniae meningitis cases due to 13-valent PCV (PCV13) serotypes in countries that introduced PCV13 (n = 8), by year. Of 20 countries that introduced PCV13, 8 were included in this analysis: Benin (n = 20), Cameroon (n = 17), Ghana (n = 8), Senegal (n = 26), Kingdom of Eswatini (formerly Swaziland, n = 3), Togo (n = 22), United Republic of Tanzania (n = 2), and Zimbabwe (n = 21). Confirmed cases from the following countries were excluded: data unavailable for all surveillance years (Angola, Central African Republic, Cote d’Ivoire, Malawi, Sierra Leone); at least 2 years of data not available following vaccine introduction (Lesotho); ≤65% PCV coverage for at least 2 consecutive years (Niger); PCV not introduced during the surveillance period (Gambia, Rwanda); and/or did not report any S. pneumoniae serotyping data (Angola, Burkina Faso, Burundi, Central African Republic, Democratic Republic of Congo, Malawi). The percentage of PCV13 serotypes was determined by the number of reported PCV13 serotypes/all reported serotype results in a year: 2011(18/20); 2012 (18/28); 2013 (10/16); 2014 (14/32); 2015 (8/12); 2016 (5/11). Dashed blue line indicates the trend line. Abbreviations: PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
DISCUSSION
The IB-VPD surveillance network provides a system for monitoring Hi, Nm, and Spn meningitis infections in children <5 years old across the WHO African Region and highlights the differences and similarities in disease patterns within subregions.
We observed a decline in the proportion of laboratory-confirmed, vaccine-type Spn meningitis in countries that had serotyping data, continuously high PCV coverage, and data available throughout the surveillance period. All of the countries introduced PCV, and all but 2 (Nigeria and Niger) had ≥65% 3-dose vaccine coverage for 2 consecutive years [24]. This decline in vaccine-type Spn meningitis following PCV introduction suggest vaccine effect and are consistent with declines in the burden of pneumococcal disease observed in other countries after PCV introduction [5].
Spn serotypes 1 and 14 were the most prevalent serotypes in the South-East and West-Central subregions, respectively. Both serotypes are associated with invasive pneumococcal disease and are included in PCV10 and PCV13 [25]. In both subregions, the number of cases caused by these serotypes declined over time. Interestingly, serotype 1 was responsible for recent meningitis outbreaks in Ghana despite PCV13 introduction in infants; the highest attack rate was among people ≥5 years old [26, 27]. This could suggest that the vaccine is not providing the desired herd protection (indirect effect) for serotype 1 or that more time is needed to realize the full effects of the vaccination programs. Because the IB-VPD network only collects data among children aged <5 years, we were unable to estimate PCV indirect effects.
Low numbers of Hi meningitis cases were observed over the surveillance period in both subregions, and Hib accounted for the largest proportion of Hi meningitis cases. All but 1 country introduced conjugate Hib vaccine without a booster dose prior to the beginning of our surveillance period. Hib vaccine is effective and the low number of Hi meningitis cases without an increase over time is encouraging. However, continued Hib surveillance is necessary, as vaccine failures with conjugate Hib have been documented and may be more common in countries with a high prevalence of human immunodeficiency virus (HIV) [28].
Neisseria meningitidis was more common in the West-Central than in the South-East subregion. There was a notable lack of serogroup A in the West-Central subregion, but a high prevalence of serogroup W and an increase in serogroup C, a serogroup associated with recent outbreaks [29, 30]. The South-East subregion had 4 cases of Nm serogroup A: 3 from Ethiopia (2012, 2013, 2016) confirmed by PCR, and 1 from Lesotho (2013) confirmed by culture. Lesotho has not conducted a MACV campaign. Ethiopia conducted a mass MACV campaign in a phased approach from 2013 to 2015 targeting the age group 1–29 years [31]. The patient vaccination status and province of residence for the NmA cases reported is unknown. Several IB-VPD surveillance sites in the West-Central subregion are in countries within the meningitis belt, areas associated with hyperendemic meningococcal disease with periodic epidemics in the dry season (December–June) [4, 10]. However, since the introduction of MACV there has been a shift from a predominance of serogroup A Nm meningitis to non-A serogroup Nm and Spn meningitis within these areas [26, 30, 32, 33]. There have also been increasing reports of non-A serogroup Nm and Spn outbreaks from areas outside the classic meningitis belt, such as in Liberia and central Ghana [27, 29].
Bacterial meningitis in Africa has been associated with high mortality. These data further demonstrate the high mortality of bacterial meningitis, with CFRs ranging from 12.2% for Hi to 20.9% for Spn. Additionally, we saw that the overall and pathogen-specific CFRs were higher in the West-Central than in the South-East subregion. While our data cannot provide a direct explanation for this finding, greater health disparities may exist for some countries of the West-Central subregion compared to countries in the South-East, which could influence access to healthcare and thus CFR. For instance, the West-Central subregion has demonstrated high maternal mortality, patient dissatisfaction with healthcare facility services, and a need for improved testing and treatment services for people living with HIV—all of which signify challenges within the existing healthcare infrastructure [34–36].
The CFR (12.8%) for Spn in the South-East subregion was lower than previously reported from a PCV introduction study in South Africa (33%) [16, 37]. However, given that countries with a mature PCV vaccination program, such as the United States, have an estimated Spn meningitis CFR of 8%, this value may not be that surprising in the post-PCV era [38]. Our CFR estimates in both subregions are limited by the number of records with reported outcomes. This could result in our CFRs being either an over- or underestimation of the true ratio.
Our data have several other limitations. First, we may have underestimated the number of suspected meningitis cases as we excluded patients without data available to determine whether or not a suspected case definition was met. Second, we excluded 5 countries due to data quality issues. For the remaining countries, missing data limited the determination of suspected or probable bacterial meningitis in some situations. Third, not all samples were sent to the RRLs for PCR testing, which resulted in a large proportion of confirmed cases without available serotyping/serogroup data. Fourth, because this is a sentinel surveillance system involving large tertiary hospitals, the catchment population is difficult to determine and incidence rates could not be calculated. Therefore, we were only able to evaluate the number of cases and proportion of vaccine-type disease over time under the assumption that the catchment populations have remained stable. Fifth, some countries were excluded from trend analyses because they did not report data consistently throughout the surveillance period. Last, by characterizing the PCR indistinguishable Spn serotypes as PCV10 vaccine type, we could be overestimating vaccine serotype and underestimating vaccine impact as this assumes that predominant serotypes within these groups are vaccine-type.
Despite these limitations, the IB-VPD surveillance network is a large surveillance system that can be used to monitor disease in children <5 years of age across the WHO African region. This report is the first comprehensive analysis of IB-VPD meningitis data after widespread Hib, MACV, and PCV introductions in Africa. As the individual countries continue to strengthen data quality and reporting, this system has the potential to not only continue to monitor vaccine impact but to also inform future vaccine policies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge all of the surveillance officers, laboratory personnel, data managers, hospitals, and Ministries of Health that are part of the African Paediatric Bacterial Meningitis surveillance network. The authors also acknowledge the laboratory staff from the regional and global regional reference laboratories the WHO data managers, and to Matthew Westercamp at the Centers for Disease Control and Prevention (CDC) for his help with data management and analyses.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization (WHO), the CDC and the Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine.
Financial support. Financial support for sentinel site surveillance was provided by Gavi, the Vaccine Alliance, through a grant to the WHO and Ministries of Health of the 31 countries participating in the African Paediatric Bacterial Meningitis Surveillance Network.
Supplement sponsorship. This supplement was supported with funds from Gavi, the Vaccine Alliance through The World Health Organization and the CDC Foundation, and The Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine.
Potential conflicts of interest. A. v. G. received grants from the WHO Regional Office for Africa during the conduct of the study and has received grants and other fees from Pfizer and Sanofi, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis 2010; 10:32–42. [DOI] [PubMed] [Google Scholar]
- 2. O’Brien KL, Wolfson LJ, Watt JP, et al. . Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893–902. [DOI] [PubMed] [Google Scholar]
- 3. Watt JP, Wolfson LJ, O’Brien KL, et al. . Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009; 374:903–11. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. CDC yellow book 2018: health information for international travel. New York: Oxford University Press, 2017. [Google Scholar]
- 5. Wahl B, O’Brien KL, Greenbaum A, et al. . Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howie SR, Oluwalana C, Secka O, et al. . The effectiveness of conjugate Haemophilus influenzae type B vaccine in The Gambia 14 years after introduction. Clin Infect Dis 2013; 57:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Vaccine Access Center View-hub data visualization platform. Available at: https://view-hub.org. Accessed 28 July 2018.
- 8. Novak RT, Kambou JL, Diomandé FV, et al. . Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis 2012; 12:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The World Health Organization. Meningococcal meningitis Available at: http://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis. Accessed 11 November 2018.
- 10. Lapeyssonnie L. Cerebrospinal meningitis in Africa. Bull World Health Organ 1963; 28:1–114. [PMC free article] [PubMed] [Google Scholar]
- 11. Meningitis vaccine project Available at: http://www.meningvax.org/nextcampaigns.html. Accessed 11 November 2018.
- 12. Cutts FT, Zaman SM, Enwere G, et al. . Gambian Pneumococcal Vaccine Trial Group Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365:1139–46. [DOI] [PubMed] [Google Scholar]
- 13. Mackenzie GA, Hill PC, Jeffries DJ, et al. . Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect Dis 2016; 16:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackenzie GA, Hill PC, Sahito SM, et al. . Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies. Lancet Infect Dis 2017; 17:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammitt LL, Akech DO, Morpeth SC, et al. . Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014; 2:e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Mollendorf C, Tempia S, von Gottberg A, et al. . Estimated severe pneumococcal disease cases and deaths before and after pneumococcal conjugate vaccine introduction in children younger than 5 years of age in South Africa. PLoS One 2017; 12:e0179905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Pediatric bacterial meningitis surveillance—African region, 2002–2008. MMWR Morb Mortal Wkly Rep 2009; 58:493–7. [PubMed] [Google Scholar]
- 18. Murray J, Agócs M, Serhan F, et al. . Centers for Disease Control and Prevention Global invasive bacterial vaccine-preventable diseases surveillance—2008-2014. MMWR Morb Mortal Wkly Rep 2014; 63:1159–62. [PMC free article] [PubMed] [Google Scholar]
- 19. Pimenta FC, Roundtree A, Soysal A, et al. . Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 2013; 51:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Mair R, Hatcher C, et al. . Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real-time PCR assay to detect Haemophilus influenzae. Int J Med Microbiol 2011; 301:303–9. [DOI] [PubMed] [Google Scholar]
- 21. Dolan Thomas J, Hatcher CP, Satterfield DA, et al. . sodC-based real-time PCR for detection of Neisseria meningitidis. PLoS One 2011; 6:e19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mothershed EA, Sacchi CT, Whitney AM, et al. . Use of real-time PCR to resolve slide agglutination discrepancies in serogroup identification of Neisseria meningitidis. J Clin Microbiol 2004; 42:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dias CA, Teixeira LM, Carvalho Mda G, Beall B. Sequential multiplex PCR for determining capsular serotypes of pneumococci recovered from Brazilian children. J Med Microbiol 2007; 56:1185–8. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization. WHO-UNICEF estimates of PCV3 coverage Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepcv3.html. Accessed 28 June 2018.
- 25. Hausdorff WP. The roles of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine 2007; 25:2406–12. [DOI] [PubMed] [Google Scholar]
- 26. Aku FY, Lessa FC, Asiedu-Bekoe F, et al. . Meningitis outbreak caused by vaccine-preventable bacterial pathogens—northern Ghana, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwambana-Adams BA, Asiedu-Bekoe F, Sarkodie B, et al. . An outbreak of pneumococcal meningitis among older children (≥5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect Dis 2016; 16:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mangtani P, Mulholland K, Madhi SA, Edmond K, O’Loughlin R, Hajjeh R. Haemophilus influenzae type b disease in HIV-infected children: a review of the disease epidemiology and effectiveness of Hib conjugate vaccines. Vaccine 2010; 28:1677–83. [DOI] [PubMed] [Google Scholar]
- 29. Patel JC, George J, Vuong J, et al. . Rapid laboratory identification of Neisseria meningitidis serogroup C as the cause of an outbreak—Liberia, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:1144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nnadi C, Oladejo J, Yennan S, et al. . Large outbreak of Neisseria meningitidis serogroup C—Nigeria, December 2016–June 2017. MMWR Morb Mortal Wkly Rep 2017; 66:1352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Federal Ministry of Health. Ethiopia national expanded programme on immunization 2015. Available at: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Ethiopia/ethiop_cmyp_latest_revised_may_12_2015.pdf. Accessed 28 July 2018.
- 32. Lingani C, Bergeron-Caron C, Stuart JM, et al. . Meningococcal meningitis surveillance in the African meningitis belt, 2004–2013. Clin Infect Dis 2015; 61(Suppl 5):S410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kambire D, Soeters HM, Ouedraogo-Traore R, et al. . Nationwide trends in bacterial meningitis before the introduction of 13-valent pneumococcal conjugate vaccine—Burkina Faso, 2011–2013. PLoS One 2016; 11:e0166384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. United Nations Population Fund. Regional demographic profiles compared: West and Central Africa’s position 2016. Available at: http://wcaro.unfpa.org/sites/default/files/pub-pdf/UNFPA_WB_Comparison_ENG_20161114.pdf. Accessed 28 July 2018.
- 35. World Health Organization. Health systems in Africa: community perception and perspective, the report of a multi-country study 2012. Available at: www.afro.who.int/sites/default/files/.../english---health_systems_in_africa---2012.pdf. Accessed 28 July 2018.
- 36. Médecins Sans Frontières. Out of focus 2016. Available at: https://www.msf.org/sites/msf.org/files/2016_04_hiv_report_eng.pd. Accessed 28 July 2018.
- 37. Gordon SB, Walsh AL, Chaponda M, et al. . Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis 2000; 31:53–7. [DOI] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed Washington DC: Public Health Foundation, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.