Abstract
Poor outcomes of hepatocellular carcinomas (HCC) in chronic kidney disease (CKD) patients are well described. Transarterial therapy is the standard treatment for HCC, following which regular contrast-enhanced imaging for residual disease is recommended. CKD is considered a relative contraindication for transarterial therapy owing to renal failure.
This retrospective study investigated the outcomes of transarterial therapy in HCC patients with CKD. In total, 132 HCC patients who received transarterial therapy were enrolled, of whom 36 had CKD. Most CKD patients were elderly, with mean age of diagnosis of 69.7 ± 11.4 years. Hypertension (odds ratio [OR]; 5.06; 95% confidence interval [Cl]; 1.83–13.94), hepatitis C virus carrier rate (OR; 4.12, 95% CI; 1.13–14.99) and diabetes (OR; 3.62, 95% CI; 1.22–10.72) were significant predictors for CKD in HCC patients. Post therapy, the estimated glomerular filtration rate significantly decreased 13.7% from baseline in the CKD patients (P = .03). There were more post-therapy complications than in the non-CKD group, e.g. acute renal failure and sepsis (P < .01 vs P < .01). Overall survival in the CKD group was significantly poor (10.9 ± 8.5 vs 23.5 ± 16.3 months, P < .01).
The lower survival of CKD patients was unrelated to treatment modality or less contrast-enhanced imaging follow-up. Further research on patient care and factors leading to poor outcomes for CKD is needed.
Keywords: chronic kidney disease, hepatocellular carcinoma, transarterial therapy
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and remains the third leading cause of cancer-related death in the last decade.[1,2] Incidence rates of HCC vary widely between geographic regions and are highest in Eastern Asia and sub-Saharan Africa.[1,2] North and South America are low-incidence areas with fewer than five cases reported per 100,000 population per year.[2] The global mortality-to-incidence ratio for HCC is 0.95 and 5-year survival is only 6.9%.[2] In Taiwan, HCC is the second most common cause of cancer-related death and the standardized mortality rate was 22.3 in 100,000 people in 2016.[3] Liver cirrhosis and HCC are often comorbid at the time of diagnosis and it is well known that advanced cirrhosis may predispose a patient to renal insufficiency or chronic kidney disease (CKD).[4] HCC was associated with higher prevalence of CKD than any other cancer;[5] therefore, CKD is commonly seen in patients with HCC.
Multiple treatment options are available for HCC including curative resection, liver transplantation, radiofrequency ablation, transarterial therapy, radiotherapy, and treatment with systemically targeted agents. In patients with early-stage HCC, curative therapies such as resection or radiofrequency ablation can provides a potential cure; however, 60% to 70% of patients are not eligible for these treatments.[6] Transarterial therapy is the most common procedure for the treatment of unresectable HCC and can prolong the survival.[7] However, acute renal failure is a severe complication that occurs in HCC patients with preexisting CKD who undergo transarterial therapy.[8] Consequently, HCC patients with CKD may have limited treatment options when seeking effective therapy resulting in worsened outcomes.[9]
The principle of transarterial therapy is to obliterate the hepatic artery supply to the tumor and/or deliver drugs, thereby halting or slowing tumor progression. This procedure is an effective therapeutic option in unresectable HCC and liver metastases.[10] Until now, there have been fewer studies that specifically evaluated the outcome of transarterial therapy in HCC patients with CKD.
The primary aim of this study was to investigate the risk factors of CKD in patients with HCC. The secondary aim was to explore the impact of transarterial therapy for HCC patients with CKD by analyzing the changes in renal function, adverse events, and long-term survival.
2. Materials and methods
2.1. Study design
We retrospectively reviewed medical records of 474 patients who had an HCC diagnosis from January 2014 to December 2016. Of these, 339 patients were excluded because they did not receive transarterial therapy, received other treatments for HCC before transarterial therapy, or failed to maintain regular follow-up. Three patients with end-stage kidney disease on dialysis were excluded from this study due to the impact of dialysis on the residual kidney function. Therefore, a total of 132 patients were ultimately enrolled in this study. The data was obtained from MacKay Memorial Hospital, and all data was collected based on the first transarterial therapy session of the study patients. Demographic, clinical and laboratory data was collected and mortality rates were analyzed. This study was approved by the Institutional Review Board of Mackay Memorial Hospital (reference number: 19MMHIS029e). This was an anonymous observational study, so the need for informed consent was waived.
2.2. Diagnosis and variable definitions
HCC was diagnosed with alpha-fetoprotein and imaging studies such as ultrasonography, radio-contrast enhanced tri-phasic dynamic computed tomography and/or magnetic resonance imaging.[11] If an atypical vascular pattern of a liver tumor was observed on imaging modalities, a biopsy would be arranged for histological evidence of HCC.[11] Patients with underlying cirrhosis were evaluated based on the Child-Pugh classification. Staging of HCC was done based on the Barcelona Clinic Liver Cancer (BCLC) staging protocol.[12]
The baseline serum creatinine level was determined as the most recent creatinine measurement before each transarterial therapy. The estimated glomerular filtration rate (eGFR) was computed using the 4-variable modification of diet in renal disease study equation.[13] The patients were divided into 2 groups according to their eGFR. The first group was the non-CKD group, consisting of patients with eGFR ≥ 60 mL/min/1.73 m2. The second group was the CKD group, which included patients with eGFR < 60 mL/min/1.73 m2. Acute renal failure was defined as an increase in serum creatinine concentration of 0.5 mg/dL or 25% above the baseline within the first 7 days of therapy. Liver failure was defined as an increase in serum bilirubin level (≥ 2.0 mg/dL), increasing or newly developed ascites or hepatic encephalopathy within 2 weeks of therapy.
In this study, transarterial embolization and transarterial chemoembolization were both classified as transarterial therapy. The Seldinger technique of arterial embolization was carried out in our hospital. Superior mesenteric and celiac arteriograms were obtained by using the transfemoral approach, followed by common hepatic, proper hepatic, and right and left hepatic arteriograms by injecting a radiocontrast agent (OPTIRAY, 74%, 100 mL, Mallinckrodt). An infusion of Lipiodol (Laboratoire Guerbet, France) and/or Doxorubicin (Pfizer, USA) was administered into the feeder vessels. The amounts of doxorubicin (10 to 150 mg) and lipiodol (2 to 30 mL) were determined according to the size and number of the tumor(s). The degree of embolization was controlled by the operator until the tumor stain had disappeared.
Peri-procedural hydration and/or oral acetylcysteine was prescribed at the physicians’ discretion. After transarterial therapy, laboratory investigations including blood cell counts and biochemical tests were carried out within 1 week. Post-treatment follow-up including renal and liver function evaluation was carried out in 1 month and contrast-enhanced dynamic imaging was performed 1 to 2 months after the procedure.
2.3. Statistical analysis
Descriptive statistics for continuous variables were reported as mean ± standard deviation. Categorical variables were described using frequency distributions and reported as n (%). Student's t test was used to compare the means of normally distributed continuous variables. The chi-square or Fisher exact test was used to compare categorical variables. To investigate the risk factors for CKD in HCC patients, the logistic regression models were applied. The survival distributions of CKD and non-CKD patients were assessed using the Kaplan–Meier method, and the differences were evaluated using the log rank test. Statistical analysis was performed using the STATA statistical package (version 15.0; Stata, College Station, TX). All P values are two-sided and P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
There were 132 HCC patients who had received at least one session of transarterial therapy for initial diagnosis of HCC was enrolled. Among them, 36 HCC patients had CKD and received 58 sessions of therapy. The non-CKD group consisted of 96 patients who were treated with 245 sessions of therapy.
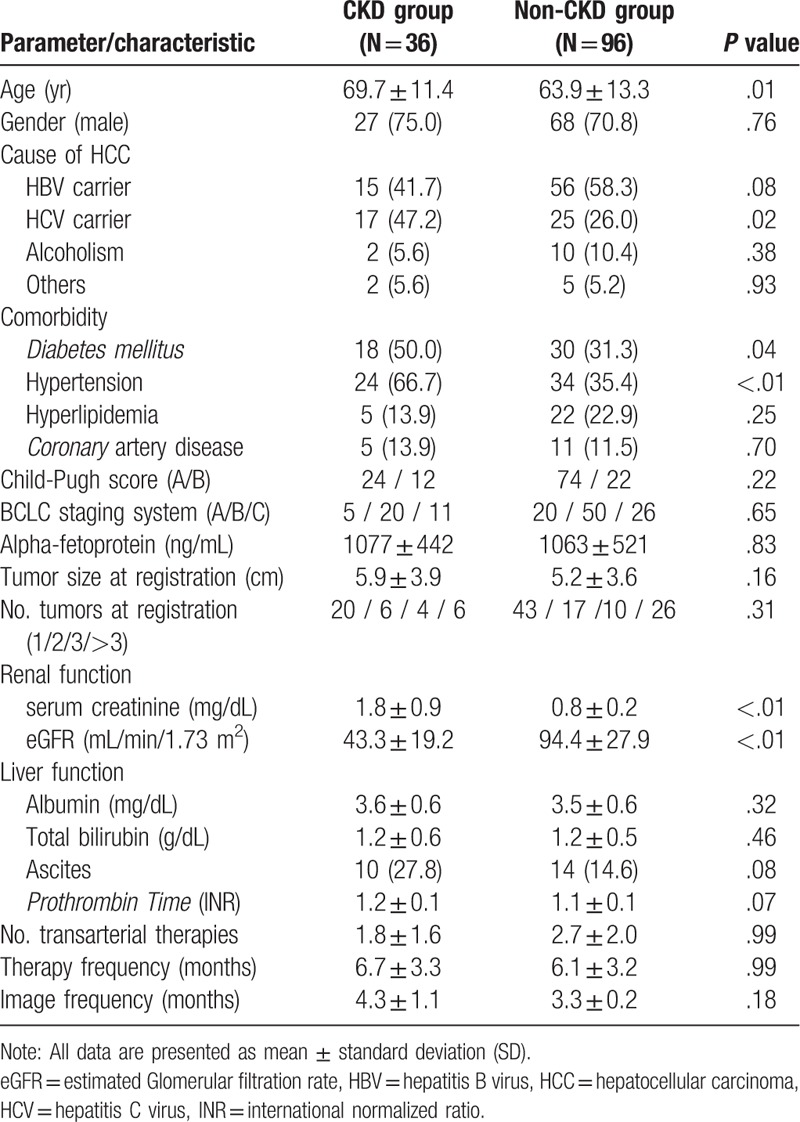
The mean age at entry was 65 ± 13 years, and 92 (72%) patients were male. The age of the CKD patients was higher than that of the non-CKD patients (P = .01). The CKD group had a higher hepatitis C virus (HCV) carrier rate than non-CKD group (P = .02) relating to the cause of HCC. It also had higher comorbidities of hypertension (P < .01) and diabetes (P = .04) (Table 1). There was no statistical difference in these 2 groups for the tumor burden of Alpha-fetoprotein, BCLC classification, and tumor size and numbers (Table 1).
Table 1.
Clinical features of HCC patients in CKD and non-CKD groups.
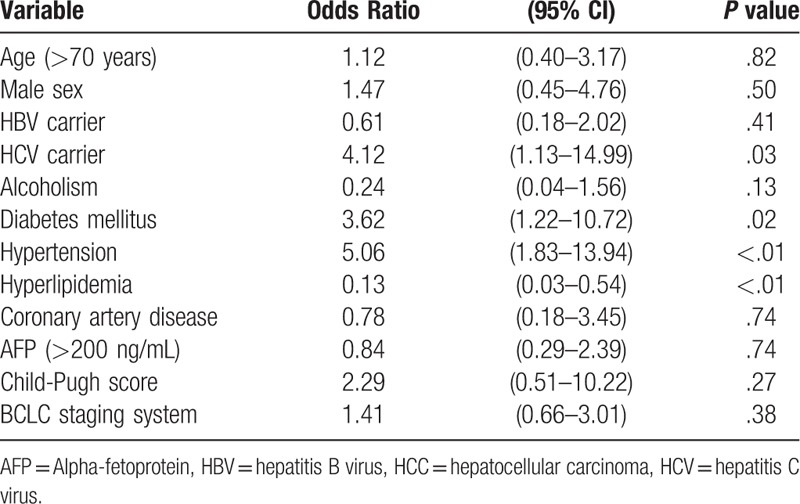
The multivariable logistic regression model indicated that hypertension, HCV carrier and diabetes mellitus were independently factors associated with CKD in HCC patients and the odds ratio (OR) were 5.06, 4.12, and 3.62. (Table 2). Having a previous history of hyperlipidemia was a protective factor (OR = 0.13).
Table 2.
Multivariate analysis of risk factors of CKD in patients with HCC.
3.2. Transarterial therapy and adverse events
Transarterial chemoembolization was performed less in the CKD group than the non-CKD group (P < .01) (Table 3). There was no difference in dose of administered drugs in both groups. The CKD group had a higher rate of acute renal failure and sepsis compared to non-CKD group (P < .01 and P < .01, respectively). Acute renal failure occurred in 20.7% treatment sessions for CKD patients, and in 5.3% treatment sessions for non-CKD patients (P < .01). One non-CKD patient received temporary hemodialysis for renal failure. There were no differences in the adverse effect of liver failure and gastrointestinal bleeding. The CKD group had a statistically significant higher mortality rate than the non-CKD group after therapy (P < .01)
Table 3.
Transarterial therapy and adverse events in CKD and non-CKD groups.
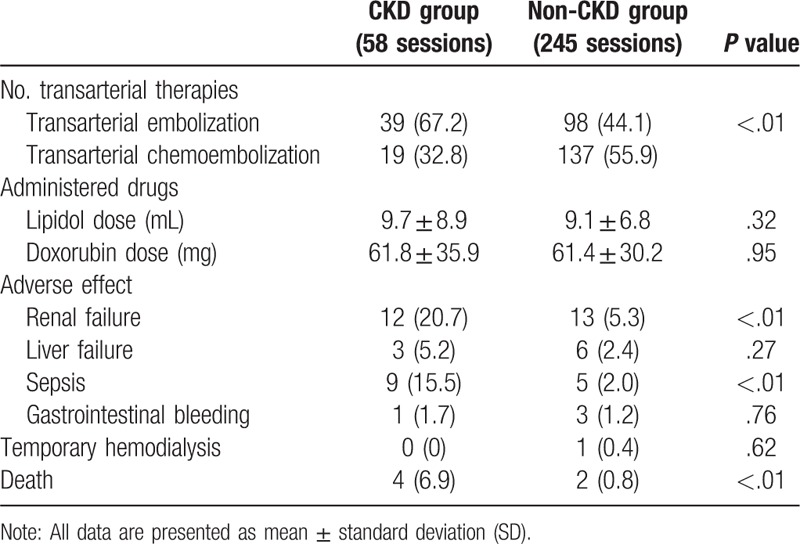
Figure 1 shows the impact of transarterial therapy on renal function: the eGFR in CKD group decreased 13.7% from baseline after therapy and this change was found to be statically significant. In the non-CKD group, the eGFR decreased 2.2% from baseline without significant changes after treatment. One month after the procedure, renal function returned to the baseline in both groups.
Figure 1.
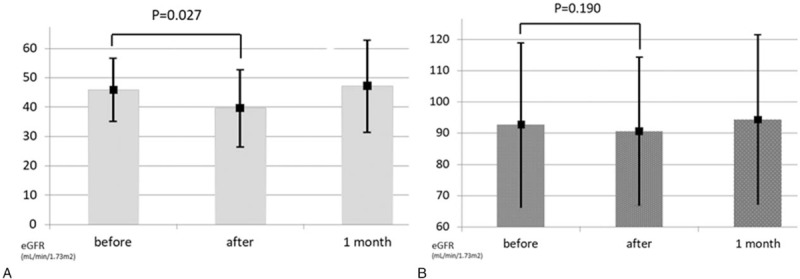
Change of eGFR before and after transarterial therapy, and 1 month post-therapy in CKD (A) and non-CKD group (B). CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate.
3.3. Prognosis and outcome
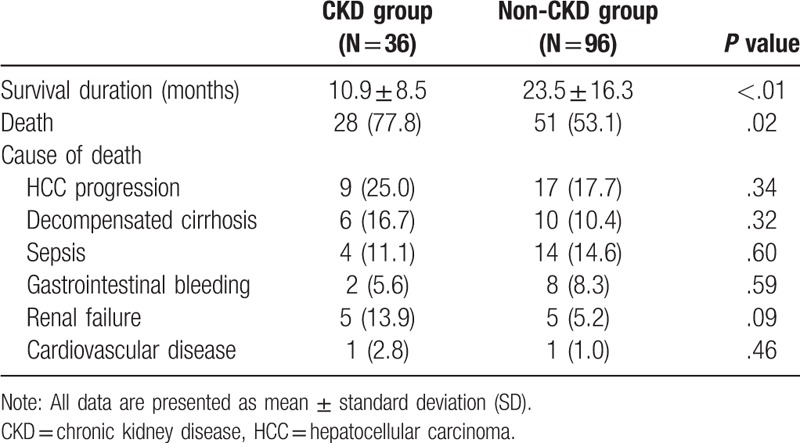
The CKD group had a lower survival rate compared to the non-CKD group (P < .01) (Table 4). During the observation period, 28 (77.8%) CKD patients and 51 (53.1%) non-CKD patients died (P = .02). The most common cause of death was progression of HCC, followed by sepsis and decompensated cirrhosis (Table 4). There was no significant difference in the cause of mortality in the both groups.
Table 4.
Outcome of HCC in transarterial therapy in CKD and non-CKD groups.
There was a significant difference in the survival rate from initial diagnosis of HCC to patient death between CKD and non-CKD patients (P < .01) (Fig. 2). The 1-year and 2-year survival rates were 45.6% and 20.7% in the CKD group and 81.2% and 63.7% in the non-CKD group.
Figure 2.
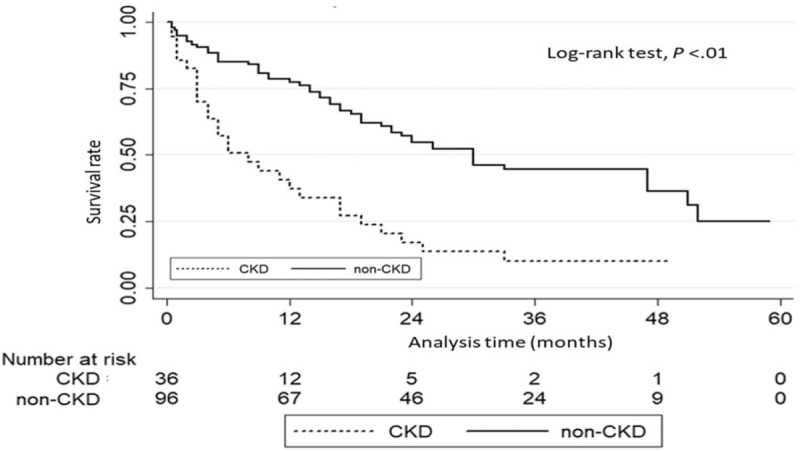
Survival analysis in HCC patients with and without CKD receiving transarterial therapy. CKD = chronic kidney disease, HCC = hepatocellular carcinoma.
4. Discussion
This study demonstrated that the risk factors for CKD in HCC patients are hypertension, diabetes, and HCV carrier. Transarterial therapy for HCC patients with CKD had a significant influence on renal function and increased mortality. Even with aggressive transarterial therapy and follow-up imaging, CKD patients still had inferior outcome and prognosis of HCC.
The well-known risk factors of CKD are advanced age, diabetes, hypertension, hyperlipidemia, coronary heart disease, recurrent kidney infection, glomerulonephritis, and abnormal kidney structure.[14] HCV infection and cirrhosis are also recognized causes of CKD and are associated with reduced survival in the CKD population.[14,15] The prevalence rate of CKD is 12.8% among cancer patients, and kidney cancer has the highest prevalence of CKD, followed by liver cancer (17.6%).[5] Transarterial therapy improves survival in cirrhotic patients with unresectable HCC.[7] However, liver cirrhosis is an important risk factor for acute renal failure during therapy because it is associated with diminished perfusion to the kidneys from peripheral vasodilatation and decreased production of compensatory vasodilators from the kidneys.[16]
Acute deterioration in renal function due to contrast media administration is also a common complication after transarterial therapy in those receiving it intravenously.[8,17] The mechanism of contrast-induced nephropathy (CIN) is a combination of renal vasoconstriction and direct contrast toxicity to the renal tubules mediated via reactive oxygen species.[17] Many risk factors are associated with CIN, such as CKD, diabetes, age > 70 years, concurrent administration of nephrotoxic drugs, congestive heart failure, hypertension, and anemia.[18] Among these factors, CKD is the most important.[18] Periprocedural hydration, prophylactic hemodialysis, agents of acetylcysteine, and ascorbic acid are recommended after contrast exposure in high-risk patients.[18] Current studies show temporary prophylactic hemodialysis is ineffective, or even harmful for CKD stage 3 populations, but hemodialysis has favorable effects for patients with stage 4/5 CKD.[18]
In this study, transarterial chemoembolization is performed less often in CKD patients, due to a belief among physicians that it can cause kidney injury. Doxorubicin, the key chemotherapeutic drug for HCC treatment, has toxic effects on organs such as the heart and liver, which may lead to modulation of blood supply to the kidney and alter xenobiotic detoxification processes, consequently contributing to nephropathy.[19] One study revealed that transcatheter arterial chemoembolization is feasible in CKD patients by instituting periprocedural hemodialysis.[20] CKD is well-known to be associated with increased all-cause mortality.[21] Another study has shown that, with regard to other risk factors related to mortality, there was no statistically significant difference in cancer patients with diabetes or hypertension. Therefore, CKD is a strong independent prognostic factor for mortality among cancer patients.[5] Our result consistently showed an inferior outcome in HCC patients with CKD.
Due to the lack of evidence-based guidelines, CKD has been regarded as a relative contraindication for transarterial therapy by many clinicians in the past.[22] However, there is no underuse of necessary diagnostic imaging or intervention in real-world practice. Therefore, it is not the difference in treatment modalities and management that affects the higher mortality in HCC patients with CKD. Thus, the higher mortality in CKD patients might have other causes. CKD is associated with higher inflammatory factors, endothelial dysfunction, and enhanced coagulation, which increase the risk of infection.[5] On the other hand, malnutrition and frailty in CKD individuals could make them more likely to die from acute illness and suffer from more toxicities after treatments.[5]
Our study has several limitations. First, it was retrospective in nature, had a small sample size, and carried a probable patient selection bias inherent to a tertiary medical center. Second, there was no standard protocol of peri-procedural hydration and not each patient collaborated with the nephrologists, which may have caused the poor outcome and higher mortality in CKD patients. Finally, most patients in the CKD group had eGFR between 15 and 60 mL/min/1.73 m2. Therefore, those patients with coexisting severe renal dysfunction were not analyzed.
5. Conclusions
Our study shows that renal failure and sepsis are common adverse events following transarterial therapy for HCC patients with CKD. The inferior survival of CKD patients is not related to less frequent imaging follow-up or therapy. Future studies are warranted on a larger number of such patients to assess additional factors that may predict poorer outcomes.
Acknowledgments
The authors would like to thank all gastroenterology and hepatology faculty of MacKay Memorial Hospital for excellent clinical assistance and care.
Author contributions
Conceptualization: Wei-Chen Lin.
Data curation: Wei-Chen Lin, Chen-Wang Chang.
Formal analysis: Wei-Chen Lin, Chen-Wang Chang.
Investigation: Ching-Wei Chang, Tsang-En Wang.
Methodology: Chen-Wang Chang, Horng-Yuan Wang.
Project administration: Ming-Jen Chen.
Resources: Ching-Wei Chang, Tsang-En Wang.
Supervision: Ming-Jen Chen, Horng-Yuan Wang.
Validation: Ching-Wei Chang, Tsang-En Wang, Ming-Jen Chen, Horng-Yuan Wang.
Writing – original draft: Wei-Chen Lin.
Writing – review & editing: Horng-Yuan Wang.
Wei-Chen Lin orcid: 0000-0002-8142-538X.
Footnotes
Abbreviations: BCLC = Barcelona Clinic Liver Cancer, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, HCC = hepatocellular carcinoma, HCV = hepatitis C virus.
The authors have no conflicts of interest to disclose.
References
- [1].Turdean S, Gurzu S, Turcu M, et al. Current data in clinicopathological characteristics of primary hepatic tumors. Rom J Morphol Embryol 2012;53:719–24. [PubMed] [Google Scholar]
- [2].Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC Epidemiology). J Gastrointest Canc 2017;48:238. [DOI] [PubMed] [Google Scholar]
- [3].Health Promotion Administration, Ministry of Health and Welfare, Republic of China. Annual report of cancer registration; 2016. [cited 2018 Dec 25]. Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=10227 Accessed December 25, 2018. [Google Scholar]
- [4].Fede G, D’Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol 2012;56:810–8. [DOI] [PubMed] [Google Scholar]
- [5].Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol 2011;33:121–30. [DOI] [PubMed] [Google Scholar]
- [6].Pleguezuelo M, Marelli L, Misseri M, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2008;8:1623–41. [DOI] [PubMed] [Google Scholar]
- [7].Park J, Chung HC, Lee JS, et al. Acute kidney injury after transarterial chemoembolization for hepatocellular carcinoma: a retrospective analysis. Blood Purif 2008;26:454–9. [DOI] [PubMed] [Google Scholar]
- [8].Hsu CY, Huang YH, Su CW, et al. Transarterial chemoembolization in patients with hepatocellular carcinoma and renal insufficiency. J Clin Gastroenterol 2010;44:e171–7. [DOI] [PubMed] [Google Scholar]
- [9].Lee CH, Hsieh SY, Lin JL, et al. Hepatocellular carcinoma in patients with chronic kidney disease. World J Gastroenterol 2013;19:2466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suciu BA, Gurzu S, Marginean L, et al. Significant shrinkage of multifocal liver metastases and long-term survival in a patient with rectal cancer, after trans-arterial chemoembolization (TACE): a case report. Medicine 2015;94:e1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bruix J, Sherman M, Llovet JM. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference – European Association for the Study of the Liver. J Hepatol 2001;35:421–30. [DOI] [PubMed] [Google Scholar]
- [12].Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006;44:723–31. [DOI] [PubMed] [Google Scholar]
- [13].Mula-Abed WA. Estimated Glomerular Filtration Rate (eGFR): a serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med J 2012;27:339–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen YC, Chiou WY, Hung SK, et al. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol 2013;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perico N, Cattaneo D, Bikbov B, et al. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol 2009;4:207–20. [DOI] [PubMed] [Google Scholar]
- [16].Huo TI, Wu JC, Lee PC, et al. Incidence and risk factors for acute renal failure in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a prospective study. Liver Int 2004;24:210–5. [DOI] [PubMed] [Google Scholar]
- [17].Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010;45:188–95. [DOI] [PubMed] [Google Scholar]
- [18].Hung YM, Lin SL, Hung SY, et al. Preventing radiocontrast-induced nephropathy in chronic kidney disease patients undergoing coronary angiography. World J Cardiol 2012;4:157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Injac R, Boskovic M, Perse M, et al. Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60(OH)24 via suppression of oxidative stress. Pharmacol Rep 2008;60:742–9. [PubMed] [Google Scholar]
- [20].Watanabe M, Shibuya A, Minamino T, et al. Benefits and problems of transarterial therapy in patients with hepatocellular carcinoma and chronic kidney disease. J Vasc Interv Radiol 2014;25:1947–55. [DOI] [PubMed] [Google Scholar]
- [21].Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–47. [DOI] [PubMed] [Google Scholar]
- [22].Jansen MC, van Hillegersberg R, Chamuleau RA, et al. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol 2005;31:331–47. [DOI] [PubMed] [Google Scholar]


