Abstract
The first breeding populations of Aedes aegypti (Linnaeus) were identified in California in 2013, and have since been detected in 13 counties. Recent studies suggest two introductions likely occurred, with genetically distinct populations in the central and southern regions of the state. Given the threat of dengue, chikungunya, and Zika virus transmission, it is imperative to understand if these populations harbor genes that could confer resistance to pyrethrin-based insecticides, known as pyrethroids, the most commonly used class of adulticides in the state. In 2017, the California Department of Public Health initiated a pesticide resistance screening program for Ae. aegypti to assess the presence of specific mutations on the sodium channel gene (V1016I and F1534C) associated with knockdown resistance to pyrethroids. Mosquitoes collected between 2015 and 2017 from 11 counties were screened for mutations using real-time polymerase chain reaction assays. Results revealed distinctly different resistance profiles between the central and southern regions. The central population displayed nearly fixed resistant mutations at both loci, whereas the southern population was more variable. The relative proportion of resistant alleles observed in sampled mosquitoes collected in southern California increased each year from 2015 through 2017, indicating potential increases in resistance across this region. The presence of these mutations indicates that these mosquitoes may be predisposed to surviving pyrethroid treatments. Additional biological and biochemical assays will help better elucidate the mechanisms underlying insecticide resistance in California Ae. aegypti and prompt the use of pesticides that are most effective at controlling these mosquitoes.
Keywords: Aedes aegypti, insecticide resistance, California, knockdown resistance
The yellow fever mosquito, Aedes aegypti, is the primary vector of arthropod-borne viruses that include dengue, yellow fever, chikungunya, and Zika (Pialoux et al. 2007, Bhatt et al. 2013, Petersen et al. 2016). These pathogens are a persistent threat to people in regions where Ae. aegypti is established, and with the recent and projected range expansion of Ae. aegypti (CDC 2018), risk of transmission of these viruses is also spreading. During the 2015–2016 Zika virus outbreak in the Americas, many urban areas in the southern and southeastern United States became acutely aware of this new threat (Grubaugh et al. 2017). Due to the lack of vaccine for Zika, chikungunya, and dengue viruses, mosquito control is the primary method utilized to minimize mosquito bite encounters, thereby decreasing the risk of infection (Morrison et al. 2008, Webster et al. 2009).
In California, Ae. aegypti were first detected in 2013, and enhanced surveillance revealed populations were well established in the surrounding urban area (Yoshimizu et al. 2016, Metzger et al. 2017). By the end of 2018, detections had been made in 222 cities and census designated areas in 13 counties, spanning a large portion of the urbanized central and southern regions of the state (CDPH 2018). Studies on the population genetics of Ae. aegypti in California have identified two genetically distinct populations: the ‘central’ population (San Mateo, Fresno, Madera, and Tulare counties) and the ‘southern’ population (Orange, San Diego, and Los Angeles counties), having likely originated in the South Central United States and Southwest United States/northern Mexico regions, respectively (Gloria-Soria et al. 2014, Pless et al. 2017). Though introduction and establishment of this invasive species was cause for concern given the number of travel-related cases of dengue and chikungunya in California each year (Porse et al. 2015), the outbreak of Zika virus beginning in 2015 brought the issue to the forefront.
In November 2015, the first cases of Zika virus were reported in California (Porse et al. 2018). The California Department of Public Health (CDPH) and local health departments investigated each case and determined that all cases were travel-associated. While no local Aedes mosquito-borne disease transmission has occurred in California to date, the possibility does exist. Small Zika virus outbreaks occurred in two other states in the continental United States with established Ae. aegypti populations: Florida and Texas (Grubaugh et al. 2017). Given the significant outcomes of Zika virus infection on pregnant women and their fetuses (World Health Organization 2016), it is imperative to develop preparedness plans in which adult mosquito control plays a primary role to halt local transmission.
Insecticide resistance in Ae. aegypti has been well documented in many parts of the world (Montella et al. 2007, Lima et al. 2011, Marcombe et al. 2012, Vontas et al. 2012, Smith et al. 2016). One of the most common chemical classes of pesticides used to control adult Ae. aeygpti are pyrethrin-based (e.g., pyrethroids) because they are relatively low in cost and toxicity to mammals (WHO 2014). Knockdown resistance (kdr) results from a nonsynonymous mutation occurring on the voltage-sensitive sodium channel (Vssc) transmembrane protein that prevents pyrethroid insecticides from attaching properly and causing mortality (Soderlund and Knipple 2003). There are several single nucleotide polymorphism mutations along the Vssc known to confer kdr-type resistance in Ae. aegypti. In the Americas, two of the most common mutations are substitutions occurring at codon 1016, resulting in an amino acid change of valine (V) to isoleucine (I) (V1016I), and at codon 1534, resulting in an amino acid change of phenylalanine (F) to cysteine (C) (F1534C) (Saavedra-Rodriguez et al. 2007). Analyses of adult Ae. aegypti from Fresno County, California, collected in 2013 indicated high levels of resistance to certain pyrethroid adulticides, as well as fixed resistant mutations at the 1016 locus (Cornel et al. 2016). Biological assays of the CLOVIS strain also indicated decreased susceptibility to sumithrin, pyrethrum, and permethrin (Cornel et al. 2016).
In 2017, CDPH initiated an Ae. aegypti pesticide resistance testing program designed to screen for the V1016I and F1534C mutations. Herein, we describe the kdr-type genetic profiles of Ae. aegypti collected from the central and southern infestation zones in California from 2015 through 2017. Using samples collected by multiple local vector control agencies, we determined the proportion of resistant alleles at the 1016 and 1534 loci. Due to the focal nature of Ae. aegypti (Harrington et al. 2005), the resistance frequencies were analyzed at both county and regional levels, and where possible, temporally. These results will help vector control agencies develop a plan to combat the spread of Ae. aegypti and effectively reduce the risk of local disease transmission.
Materials and Methods
Mosquito Samples 2015
Adult Ae. aegypti mosquitoes collected by local California vector control agencies were submitted to Yale University for genetic analysis and DNA was extracted as described in Pless et al. (2017). Aliquots of 80 µl of extracted DNA maintained in a cold chain to prevent degradation were provided to CDPH for pesticide resistance analysis.
Mosquito Samples 2016–2017
Adult Ae. aegypti mosquitoes were collected by local California vector control agencies from October 2016 through December 2017 using multiple sampling schemes, including CDC Autocidal Gravid Ovitraps (CDC-AGOs), BioGents Sentinel (BGS) traps, Encephalitis Vector Survey (EVS) traps, and backpack aspirators. Larval and pupal samples were also collected through source surveys, and raised to adults in the laboratory. Agencies stored adult mosquitoes individually in empty and dry 1.5 ml Eppendorf tubes at −80°C or in a dry ice chest. Where cold storage access was limited, mosquitoes were preserved in 70% ethanol. Mosquitoes were shipped to CDPH, maintaining the cold chain for all dry specimens.
Upon receipt, all adult mosquito samples were stored at −80°C prior to processing. Abdomens were removed from all dry, female samples that were collected in a live-trap or reared in a laboratory. These abdomens and all remaining mosquitoes were then stored in 70% ethanol at −20°C in preparation for extraction. The head and thoraces of viable mosquitoes were stored at −80°C for future analyses.
Genotyping Assays
DNA extractions of abdomens or whole mosquitoes were conducted using Qiagen DNeasy Blood and Tissue Kit per the manufacturer’s protocol (Qiagen, Hercules, CA). DNA samples were eluted to a final volume of 200 µl.
Identification of kdr-type Vssc mutations was conducted using melt-curve assays. For the V1016I mutation, the protocol described by Saavedra-Rodriguez et al. (2007) was slightly modified. The 21 µl reaction mixture contained 10 µl of iQ Syber Green Supermix (Bio-Rad, Hercules, CA), 2.5 µl of the valine forward primer (5′-GCGGGCAGGGCGGCGGGGGCGGGGCCACAAATTGTTTCCCACCCGCACCGG-3′), 2 µl of the isoleucine forward primer (5′-GCGGGCACAAATTGTTTCCCACCCGCACTGA-3′), 2 µl of the reverse primer (5′-TGATGAACCSGAATTGGACAAAAGC-3′), 3.5 µl PCR-grade water, and 1 µl of DNA template. All primer concentrations were 10 µM. Amplification consisted of 95°C for 3 min, followed by 35 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 30 s. The melt-curve protocol followed with 10 s each at 0.2°C increments between 65 and 95°C. Melt curves were generated by the CFX Manager Software Version 3.1 (Bio-Rad) in which homozygous susceptible individuals had a single peak at 86°C (V/V), heterozygous individuals had two peaks at 79°C and 86°C (V/I), and homozygous resistant individuals had a single peak at 79°C (I/I).
The F1534C mutation was identified using a slightly modified protocol described by Yanola et al. (2011). The 20 µl reaction mixture contained 10 µl of iQ Syber Green Supermix (Bio-Rad), 0.33 µl of the phenylalanine forward primer (5′-GCGGGCTCTACTTTGTGTTCTTCATCATATT-3′), 1 µl of the forward cysteine primer (5′-GCGGGCAGGGCGGCGGGGGCGGGGCCTCTACTTTGTGTTCTTCATCATGTG-3′), 1 µl of the reverse primer (5′-TCTGCTCGTTGAAGTTGTCGAT-3′), 5.67 µl PCR-grade water, and 2 µl of DNA template. All primer concentrations were 10 µM. Amplification consisted of 95°C for 3 min, followed by 35–40 cycles of 95°C for 10 s, 57°C for 10 s, and 72°C for 30 s. The melt-curve protocol followed with 5 s each at 0.5°C increments between 65 and 95°C. Melt curves were generated by the CFX Manager Software Version 3.1 (Bio-Rad) in which homozygous susceptible individuals had a single peak at 80°C (F/F), heterozygous individuals had two peaks at 80°C and 85°C (F/C), and homozygous resistant individuals had a single peak at 85°C (C/C).
Analysis
Analyses of the resistant and susceptible alleles for each mutation locus were conducted at two geographic levels: by region (central and southern) and by county. The frequency of each mutation in a given population was calculated using the following allelic frequency formula:
where Fp= the frequency of the resistant allele, p = resistant allele, q = susceptible allele, and n = total number of samples.
Geographic maps of allelic frequencies were created using the ggplot2, ggmap, maps, and mapdata libraries for the R statistical software package (Team and R Development Core Team 2016).
Results
Mosquito Samples
From 2015 through 2017, a total of 4,968 mosquitoes were submitted for testing from 11 California counties. Of these, 4,076 whole mosquitoes and 892 mosquito abdomens were tested. Conclusive results for the V1016I and F1534C assays were obtained from 4,852 and 4,870 samples, respectively (Table 1).
Table 1.
Allelic frequencies of the V1016I and F1534C mutations by region of the state and year (central—Fresno, Kern, Madera, Merced, and Tulare counties; southern—Imperial, Los Angeles, Orange, Riverside, San Bernardino, and San Diego counties), California, 2015 through 2017
| Region | Year | V1016I | F1534C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SS | SR | RR | Resistance frequency | Total | SS | SR | RR | Resistance frequency | ||
| Central | 2015 | 42 | 0 | 0 | 42 | 100.0% | 44 | 0 | 0 | 44 | 100.0% |
| 2016 | 160 | 0 | 0 | 160 | 100.0% | 161 | 0 | 0 | 161 | 100.0% | |
| 2017 | 1,047 | 0 | 14 | 1,027 | 98.8% | 1,047 | 0 | 0 | 1,047 | 100.0% | |
| 2015–2017 | 1,249 | 0 | 14 | 1,229 | 99.0% | 1,252 | 0 | 0 | 1,252 | 100.0% | |
| Southern | 2015 | 143 | 58 | 65 | 20 | 36.7% | 145 | 56 | 53 | 36 | 43.1% |
| 2016 | 212 | 43 | 97 | 72 | 56.8% | 214 | 27 | 66 | 121 | 72.0% | |
| 2017 | 3,248 | 538 | 1,370 | 1,339 | 62.3% | 3,259 | 197 | 477 | 2,585 | 86.6% | |
| 2015–2017 | 3,603 | 639 | 1,532 | 1,431 | 61.0% | 3,618 | 280 | 596 | 2,742 | 84.0% |
SS, indicates homozygous susceptible; SR, heterozygous; RR, homozygous resistant individuals
Analysis
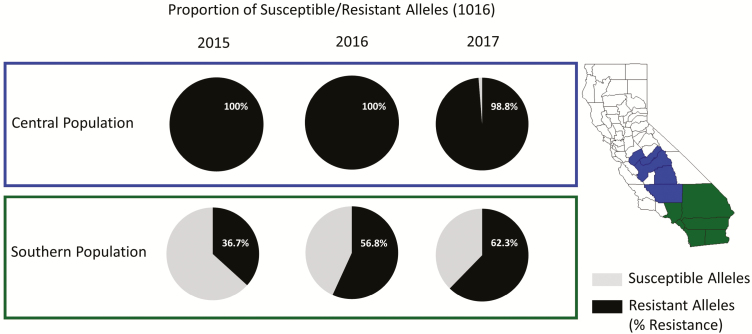
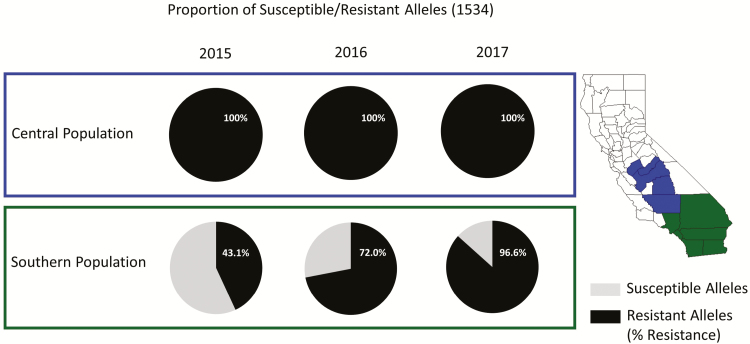
California counties were divided into two populations: central (Fresno, Kern, Madera, Merced, and Tulare counties) and southern (Imperial, Los Angeles, Orange, Riverside, San Bernardino, and San Diego counties) based on the analysis by Pless et al. (2017). The frequency of the resistant genotypes for the V1016I and F1534C mutations in the central population is nearly fixed at 100% (Figs. 1 and 2). Of the 1,249 mosquitoes tested from 2015 through 2017, only 14 contained a susceptible allele for the 1016, and no samples were homozygous susceptible. For the 1534 mutation, no susceptible alleles were identified in the 1,252 samples tested (Table 1).
Fig. 1.
Proportions of the susceptible and resistant alleles of the 1016 locus mutation at a regional-level by year.
Fig. 2.
Proportions of the susceptible and resistant alleles of the 1534 locus mutation at a regional-level by year.
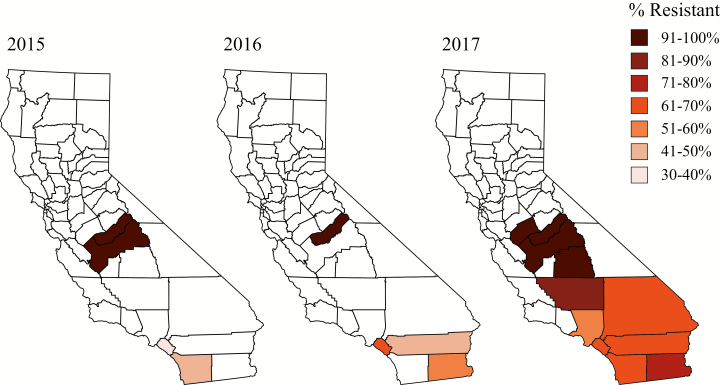
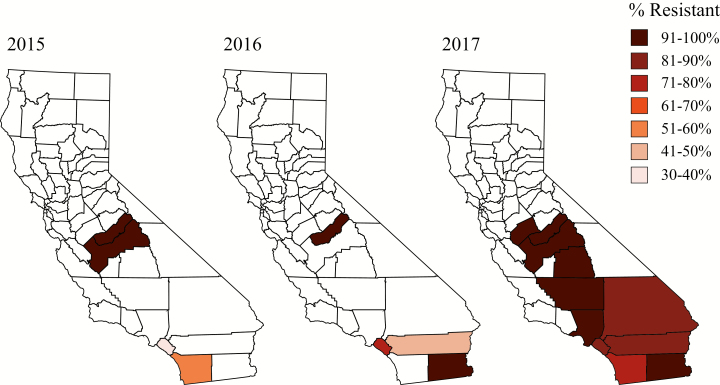
The resistance allele profile in the southern population of the state differed from the central population. In 2015, the frequency of the resistant allele was less than 50% in the southern population for both V1016I and F1534C (36.71 and 43.10%, respectively; Table 1, Figs. 1 and 2). The regional resistant allele percentages increased each year, reaching as high as 62.32 and 86.64%, respectively, in 2017 (Table 1, Figs. 1 and 2). Of the counties included in the southern region population, Orange County was the only one with three consecutive years of data, showing that even on a smaller county level, the frequency of the resistant alleles increased over time. From 2015 through 2017, the percentage of resistant alleles increased by nearly 2-fold for the 1016 mutation locus, and 2.5-fold for the 1534 mutation locus (Table 2, Figs. 3 and 4).
Table 2.
Allelic frequencies of the V1016I and F1534C mutations by region (central and southern), year, and county, California, 2015 through 2017
| Region | County | V1016I | F1534C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SS | SR | RR | Resistance Frequency | Total | SS | SR | RR | Resistance Frequency | |||
| 2015 | Central | Fresno | 20 | 0 | 0 | 20 | 100.0% | 20 | 0 | 0 | 20 | 100.0% |
| Central | Madera | 22 | 0 | 0 | 22 | 100.0% | 24 | 0 | 0 | 24 | 100.0% | |
| Southern | Orange | 89 | 45 | 30 | 14 | 32.6% | 91 | 45 | 32 | 14 | 33.0% | |
| Southern | San Diego | 54 | 13 | 35 | 6 | 43.5% | 54 | 11 | 21 | 22 | 60.2% | |
| 2016 | Central | Madera | 160 | 0 | 0 | 160 | 100.0% | 161 | 0 | 0 | 161 | 100.0% |
| Southern | Imperial | 40 | 8 | 16 | 16 | 60.0% | 42 | 1 | 1 | 40 | 96.4% | |
| Southern | Orange | 127 | 18 | 64 | 45 | 60.6% | 127 | 9 | 51 | 67 | 72.8% | |
| Southern | Riverside | 45 | 17 | 17 | 11 | 43.3% | 45 | 17 | 14 | 14 | 46.7% | |
| 2017 | Central | Fresno | 511 | 0 | 7 | 504 | 99.3% | 513 | 0 | 0 | 513 | 100.0% |
| Central | Kern | 5 | 0 | 1 | 4 | 90.0% | 5 | 0 | 0 | 5 | 100.0% | |
| Central | Madera | 403 | 0 | 0 | 403 | 100.0% | 401 | 0 | 0 | 401 | 100.0% | |
| Central | Merced | 57 | 0 | 0 | 51 | 100.0% | 57 | 0 | 0 | 57 | 100.0% | |
| Central | Tulare | 71 | 0 | 6 | 65 | 95.8% | 71 | 0 | 0 | 71 | 100.0% | |
| Southern | Imperial | 33 | 0 | 15 | 18 | 77.3% | 37 | 1 | 0 | 36 | 97.3% | |
| Southern | Los Angeles | 1,123 | 207 | 513 | 403 | 58.7% | 1,119 | 27 | 139 | 953 | 91.4% | |
| Southern | Orange | 141 | 23 | 65 | 52 | 60.6% | 141 | 11 | 31 | 99 | 81.2% | |
| Southern | Riverside | 1,093 | 199 | 453 | 441 | 61.1% | 1,101 | 113 | 169 | 819 | 82.1% | |
| Southern | San Bernardino | 719 | 82 | 270 | 367 | 69.8% | 722 | 28 | 101 | 593 | 89.1% | |
| Southern | San Diego | 139 | 27 | 54 | 58 | 61.2% | 139 | 17 | 37 | 85 | 74.5% |
Fig. 3.
County-level frequencies of the resistant allele of the 1016 mutation locus by year.
Fig. 4.
County-level frequencies of the resistant allele of the 1534 mutation locus by year.
Discussion
The introduction and establishment of Ae. aegypti in California have led to an increased risk of local transmission of arboviruses in the state (Metzger et al. 2017). Travel-associated cases of chikungunya, dengue, and Zika viruses have resulted in local outbreaks of disease in other states and U.S. territories (Florida, Texas, and Puerto Rico) with established populations of this mosquito species (Ramos et al. 2008, Kendrick et al. 2014, Rey 2014, Baud et al. 2017, Grubaugh et al. 2017). In response to this new threat, CDPH and local vector control agencies have developed invasive Aedes mosquito surveillance and response plans that recommend the use of adulticides in the event of local disease transmission.
Understanding the resistance profiles of Ae. aegypti populations in the state is imperative for selecting and deploying appropriate pesticides in the event of local disease transmission. If, for instance, a population is highly resistant to most pyrethroid insecticides, effective control will require the use of an alternative class of insecticide such as organophosphates. The results of these two kdr assays indicate that such measures may need to be taken, particularly in the Central Valley region of the state. The extremely high and fixed resistant mutations observed in the central population could result in control failure if pyrethroid insecticides were to be used to control adult mosquitoes during a local transmission event. Although the kdr resistance profiles for the southern population show a high percentage of susceptible alleles remaining, the proportion of resistant alleles for both the V1016I and F1534C loci have increased steadily since 2015 and will need to be continually monitored. At the county level, Orange County clearly demonstrates that these resistance profiles can change rapidly over time. This knowledge supports the ongoing implementation by local vector control agencies of integrated vector management methods, including pesticide rotation and source reduction, to prevent further resistance from developing.
Additional research is essential to evaluate the efficacy of commonly used adulticides against Ae. aegypti in California. Biological assays, such as bottle bioassays and outdoor cage trials that challenge live mosquitoes against diagnostic and label doses of insecticides, are needed to determine whether functional resistance is present in local mosquito populations. Unfortunately, it can be difficult to collect the large numbers of mosquitoes required for these types of assays. Biochemical assays focusing on enzymatic activity in adult female mosquitoes could reveal metabolic mechanisms behind resistance in local Ae. aegypti populations. The data obtained from these molecular assays, in conjunction with data from the field, biochemical assays, and bottle bioassays, will provide vector control agencies with comprehensive information on the pesticide resistance profile of local Ae. aegypti populations. Such information will help ensure that the pesticides selected for adult mosquito control are effective in preventing or interrupting local transmission of dengue, chikungunya, or Zika viruses in California.
Acknowledgments
We thank Jaron Smith and Nicholas Ledesma for laboratory support of this project, Allyx Nicolici for her assistance with the maps, and the Powell laboratory at Yale University for providing mosquito DNA. This work would not have been possible without the continued cooperation of several vector control agencies in California, including Consolidated Mosquito Abatement District, Coachella Valley Mosquito and Vector Control District, Delano Mosquito Abatement District, Delta Vector Control District, Fresno Mosquito and Vector Control District, Greater Los Angeles County Vector Control District, Kern Mosquito and Vector Control District, Long Beach Vector Control Program, Madera County Mosquito and Vector Control District, Merced County Mosquito Abatement District, Northwest Mosquito and Vector Control District, Orange County Mosquito and Vector Control Program, Riverside County Environmental Health Vector Control Program, San Bernardino County Mosquito and Vector Control Program, San Diego County Department of Environmental Health Vector Control Program, San Gabriel Valley Mosquito and Vector Control Program, Tulare Mosquito Abatement District, and West Valley Mosquito and Vector Control District. This publication was supported by the Epidemiology & Laboratory Capacity Cooperative Agreement Number 6 NU50CK000410-05, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
References Cited
- Baud D., Gubler D. J., Schaub B., Lanteri M. C., and Musso D.. . 2017. An update on Zika virus infection. Lancet. 390: 2099–2109. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., Drake J. M., Brownstein J. S., Hoen A. G., Sankoh O., . et al. 2013. The global distribution and burden of dengue. Nature. 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2018. Estimated range of Aedes aegypti and Aedes albopictus in the US. Available from https://www.cdc.gov/zika/vector/range.html
- CDPH 2018. Aedes aegypti and Aedes albopictuas mosquitoes. Available from https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Aedes-aegypti-and-Aedes-albopictus-mosquitoes.aspx
- Cornel, A. J., J. Holeman, C. C. Nieman, Y. Lee, C. Smith, M. Amorino, K. K. Brisco, R. Barrera, G. C. Lanzaro and F. S. Mulligan III. Surveillance, insecticide resistance and control of an invasive Aedes aegypti (Diptera: Culicidae) population in California. F1000Research. 5: 194. 2016 doi: 10.12688/f1000research.8107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloria-Soria A., Brown J. E., Kramer V., Hardstone Yoshimizu M., and Powell J. R.. . 2014. Origin of the dengue fever mosquito, Aedes aegypti, in California. Plos Negl. Trop. Dis. 8: e3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh N. D., Ladner J. T., Kraemer M. U. G., Dudas G., Tan A. L., Gangavarapu K., Wiley M. R., White S., Thézé J., Magnani D. M., . et al. 2017. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 546: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. C., Scott T. W., Lerdthusnee K., Coleman R. C., Costero A., Clark G. G., Jones J. J., Kitthawee S., Kittayapong P., Sithiprasasna R., . et al. 2005. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 72: 209–220. [PubMed] [Google Scholar]
- Kendrick K., Stanek D., and Blackmore C.; Centers for Disease Control and Prevention (CDC) 2014. Notes from the field: transmission of chikungunya virus in the continental United States–Florida, 2014. MMWR. Morb. Mortal. Wkly. Rep. 63: 1137. [PMC free article] [PubMed] [Google Scholar]
- Lima E. P., Paiva M. H. S., De Araujo A. P., Da Silva E. V. G., Da Silva U. M., De Oliveira L. N., Santana A. E. G., Barbosa C. N., De Paiva Neto C. C., Goulart M. O., . et al. 2011. Insecticide resistance in Aedes aegypti populations from Ceara, Brazil. Parasites and Vectors 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcombe S., Mathieu R. B., Pocquet N., Riaz M. A., Poupardin R., Sélior S., Darriet F., Reynaud S., Yébakima A., Corbel V., . et al. 2012. Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PLoS One 7: e30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M. E., Yoshimizu M. H., Padgett K. A., Hu R., Kramer V. L., and Ritchie S.. . 2017. Detection and establishment of Aedes Aegypti and Aedes albopictus (Diptera: Culicidae) Mosquitoes in California, 2011–2015. J. Med. Entomol. 54: 533–543. [DOI] [PubMed] [Google Scholar]
- Montella I. R., Martins A. J., Viana-Medeiros P. F., Lima J. B., Braga I. A., and Valle D.. . 2007. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am. J. Trop. Med. Hyg. 77: 467–477. [PubMed] [Google Scholar]
- Morrison A. C., Zielinski-Gutierrez E., Scott T. W., and Rosenberg R.. . 2008. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 5: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. R., Jamieson D. J., and Honein M. A.. . 2016. Zika Virus. N. Engl. J. Med. 375: 294–295. [DOI] [PubMed] [Google Scholar]
- Pialoux G., Gaüzère B. A., Jauréguiberry S., and Strobel M.. . 2007. Chikungunya, an epidemic arbovirosis. Lancet. Infect. Dis. 7: 319–327. [DOI] [PubMed] [Google Scholar]
- Pless E., Gloria-Soria A., Evans B. R., Kramer V., Bolling B. G., Tabachnick W. J., and Powell J. R.. . 2017. Multiple introductions of the dengue vector, Aedes aegypti, into California. Plos Negl. Trop. Dis. 11: e0005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porse C. C., Kramer V., Yoshimizu M. H., Metzger M., Hu R., Padgett K., and Vugia D. J.. . 2015. Public health response to Aedes aegypti and Ae. albopictus mosquitoes invading California, USA. Emerg. Infect. Dis. 21: 1827–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porse C. C., Messenger S., Vugia D. J., Jilek W., Salas M., Watt J., and Kramer V.. . 2018. Travel-associated Zika cases and threat of local transmission during global outbreak, California, USA. Emerg. Infect. Dis. 24: 1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M. M., Mohammed H., Zielinski-Gutierrez E., Hayden M. H., Lopez J. L. R., Fournier M., Trujillo A. R., Burton R., Brunkard J. M., Anaya-Lopez L., . et al. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am. J. Trop. Med. Hyg. 78: 364–369. [PubMed] [Google Scholar]
- Rey J. R. 2014. Dengue in Florida (USA). Insects. 5: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K., Urdaneta-Marquez L., Rajatileka S., Moulton M., Flores A. E., Fernandez-Salas I., Bisset J., Rodriguez M., McCall P. J., Donnelly M. J., . et al. 2007. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol. 16: 785–798. [DOI] [PubMed] [Google Scholar]
- Smith L. B., Kasai S., and Scott J. G.. . 2016. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 133: 1–12. [DOI] [PubMed] [Google Scholar]
- Soderlund D. M., and Knipple D. C.. . 2003. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem. Mol. Biol. 33: 563–577. [DOI] [PubMed] [Google Scholar]
- Team, R. D. C., and R. R Development Core Team 2016. R: a language and environment for statistical computing. R Found. Stat. Comput, Vienna, Austria. [Google Scholar]
- Vontas J., Kioulos E., Pavlidi N., Morou E., della Torre A., and Ranson H.. . 2012. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 104: 126–131. [Google Scholar]
- Webster D. P., Farrar J., and Rowland-Jones S.. . 2009. Progress towards a dengue vaccine. Lancet. Infect. Dis. 9: 678–687. [DOI] [PubMed] [Google Scholar]
- WHO 2014. Malaria World report 2013. World Health Organization, France. [Google Scholar]
- World Health Organization 2016. Zika virus situation report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Yanola J., Somboon P., Walton C., Nachaiwieng W., Somwang P., and Prapanthadara L. A.. . 2011. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop. Med. Int. Health 16: 501–509. [DOI] [PubMed] [Google Scholar]
- Yoshimizu M. H., Padgett K. A., Metzger M. E., Feiszli T., Irby L., Garcia M., Scalzo A., Mulligan S., Holeman J., Gay R., . et al. 2016. The initial detection and establishment of invasive Aedes aegypti in California, 2013. Proc. Mosq. Vector Control Assoc. Calif. 84: 153–159. [Google Scholar]