Abstract
Background
We studied the association between human papillomavirus (HPV) viral load (VL) and HPV concordance.
Methods
The HITCH cohort study included young, heterosexual, recently formed, sexually active couples. Questionnaires and genital samples were collected at 0 and 4 months. Samples were tested for HPV DNA by polymerase chain reaction (PCR; Linear Array). VLs of HPV6/11/16/18/31/42/51 were quantified using type-specific real-time PCR. Correlations between VL and type-specific HPV prevalence and incidence were evaluated using multilevel, mixed-effects linear/logistic regression models.
Results
We included 492 couples. VLs were higher in penile than vaginal samples. VL at subsequent visits correlated significantly within men (r, 0.373), within women (r, 0.193), and within couples (r range: 0.303–0.328). Men with high VL had more type-specific persistent HPV infections (odds ratio [OR], 4.6 [95% confidence interval {CI}, 2.0–10.5]). High VL in men was associated with prevalent (OR, 5.3 [95% CI, 2.5–11.2]) and incident (OR, 6.7 [95% CI, 1.5–30.7]) type-specific HPV infections in their partner. Women’s VL was associated with type-specific HPV prevalence in their partner at the same (OR, 5.9) and subsequent (OR, 4.7) visit.
Conclusions
Persistent HPV infections have limited VL fluctuations. VL between sex partners are correlated and seem predictive of transmission episodes.
Keywords: HPV, HPV6, HPV11, HPV16, HPV18, human papillomavirus, infection transmission, sexually transmitted infections, viral load
In this Canadian prospective cohort study of young, recently formed, heterosexual couples, persistent infections of human papillomavirus had limited fluctuations in viral load. Viral loads between sex partners were correlated and seemed predictive of transmission episodes.
Human papillomavirus (HPV) is the most prevalent sexually transmitted infection [1]. It is estimated that nearly 80% of sexually active individuals will have a genital HPV infection at least once in their lifetime [1]. Persistent infection with HPV is necessary for the development of cervical cancer and anogenital warts, and is an important cause of oral and other anogenital cancers. Approximately 5% of the worldwide tumor burden is estimated to be attributed to HPV [2].
Various strategies have been or are being implemented to prevent and control HPV-related diseases, such as condom use, HPV vaccination, and cervical cancer screening. Knowledge of the risk and dynamics of HPV transmission is instrumental to model the impact of such strategies at the population level. HPV transmission is mediated by past and current sexual behavior, susceptibility of the sexual partner, and viral factors; much about determinants for susceptibility and infectivity of HPV remains insufficiently understood [3]. Vaginal intercourse is considered the most efficient route of HPV transmission. The cumulative incidence of genital HPV infections is generally higher in individuals who had their first vaginal intercourse at a younger age and those who have had a higher number of sexual partners [3–7]. Most transmission occurs early in the relationship [6, 8–12]. Viral load, which reflects the productivity of DNA replication within the affected epithelium and consequent viral shedding, may play a major role in HPV infectivity [3]. Within individuals, viral loads are thought to predict HPV persistence and clearance [13–15]. A Dutch research group studied HPV viral loads in heterosexual couples in which the women had cervical intraepithelial neoplasia (CIN); HPV concordance increased with higher viral loads [16]. Furthermore, HPV-related penile lesions were more frequent and severe and had higher viral loads in men whose sexual partner had CIN compared with a control population [17]. Another study suggested that HPV viral load was associated with the incidence of new type-specific HPV infections in heterosexual partners [18]. However, this study used a semiquantitative method to determine viral load, measuring the intensity of genotype-specific bands on the Roche HPV Linear Array. Overall, couples in these aforementioned studies were primarily engaged in long-term, stable partnerships. Considering that most HPV transmission events occur at an earlier stage after the onset of a sexual relationship, it is essential to study HPV transmission in a longitudinal cohort of newly formed couples who frequently have genital samples tested for HPV.
We used data from the HPV Infection and Transmission Among Couples Through Heterosexual Activity (HITCH) study cohort to study the correlation between HPV viral loads, and concordance within approximately 500 young, recently formed heterosexual couples [8, 9, 19–23]. We previously reported high HPV incidence, prevalence, and type-specific concordance of infections among these couples, confirming frequent HPV transmission early in sexual relationships [8, 19]. Thus, data from HITCH provide an excellent source to study determinants of HPV transmission and persistence. In the current study, we examined the association between genital HPV viral loads and HPV infection concordance in sexually active couples, hypothesizing that higher genital viral loads would be associated with an increased incidence and prevalence of the same HPV type in sexual partners, and a decreased clearance.
METHODS
Study Population
The HITCH cohort study has been described previously [8, 9, 19–24]. In brief, HITCH enrolled >500 heterosexual couples between May 2005 and February 2011, consisting of young women (primarily aged 18–24 years) attending university in Montreal (Canada), and their male partners (aged ≥18 years). Couples were eligible if they reported having had their first vaginal sex together within 6 months before enrollment. Couples were excluded if the woman was pregnant or planned to become pregnant in the next 24 months, if she did not have an intact uterus, or if she had a history of cervical lesions/cancer. Women were followed for 2 years during 6 visits, whereas men were followed for at least 4 months during 2 visits. At baseline and during follow-up, participants filled out web-based questionnaires on demographics; socioeconomic status; smoking; sexual behavior; and medical, contraceptive, reproductive, and sexual history. During clinic visits, nurses collected penile specimens, while women self-collected vaginal swabs. To minimize contamination of genital specimens through recent sexual contact, participants were asked to abstain from intercourse 24 hours before sample collection. Participation was completely voluntary; all subjects provided written informed consent. HITCH procedures and documents were approved by the ethical review committees at McGill University, Concordia University, and the Centre Hospitalier de l’Université de Montréal, and are annually renewed at McGill University. HITCH follows all national laws and major international guidelines regarding studies involving human subjects.
Genital Specimen Processing
Genital specimens were tested by polymerase chain reaction (PCR) based on amplification of a 450 base pair segment of the HPV L1 capsid gene for 36 mucosal HPV genotypes using the Linear Array HPV genotyping assay (Roche Molecular Systems), as described previously [25]. Concomitantly, a β-globin DNA sequence was amplified to verify specimen adequacy.
Viral loads of HPV types 6, 11, 16, 18, 31, 42, and 51 were measured in positive samples from the first and second visits using type-specific real-time PCR assays. HPV types 6, 11, 16, and 18 were selected as these HPV infections can be prevented with the quadrivalent vaccine. Selection of HPV-31 and -51 was based on their oncogenicity and/or high prevalence in HITCH, and HPV-42 was selected as a nononcogenic type with a high prevalence in the HITCH cohort [19]. HPV-positive samples were screened for the presence of PCR inhibitors by amplification of an internal control, as described previously [26]. The presence of PCR inhibitors was suspected when 1000 copies of the internal control generated a signal corresponding to <700 copies. Samples containing inhibitory activity were diluted 10-fold and further tested. Samples and diluted samples free of inhibiting activity were then tested in duplicate in a Light Cycler PCR and detection system (Roche Molecular Systems) for quantification of HPV types that tested positive in the Linear Array. Cycling parameters and primer sequences are described per HPV type in Supplementary Table 1. Cycle thresholds obtained for each sample were compared to those of a titration curve obtained by serial 10-fold dilutions of HPV-16 DNA plasmid in 75 ng of human genomic DNA (Roche Diagnostics) in 10 mM Tris-HCl (pH 8.2). Viral loads were calculated by dividing the number of HPV DNA copies by the total number of cells, which was estimated by quantitation of β-globin by real-time PCR, as described previously [27]. Some uncircumcised men had 2 penile samples per visit as glans and shaft samples were collected separately; in those cases, the sum of viral copies were divided by the total number of cells in both samples to determine the viral load.
Statistical Analyses
As viral load distributions were skewed to the right, viral loads were described using the median, geometric mean, tolerance interval, and range. The tolerance interval was calculated using the formula 10^((∑(log10(x))/ n) ± log10 SD), in which ∑(log10(x))/n is equal to the mean of the log10-transformed viral loads and log10 SD is the standard deviation of the log10-transformed viral loads. Because of data skewness, correlations were evaluated using log10-transformed viral loads. The normal distribution of log-transformed viral loads was evaluated using the Shapiro–Wilk test. We drew box plots following Tukey principles to assess the distribution of viral loads by HPV type for the first visit, second visit, and both visits combined [28]. For the distribution of viral loads in both visits combined, each individual contributed one measurement: if an individual was positive for an HPV type at both visits, we averaged his/her log10-transformed type-specific viral loads. Kruskal–Wallis tests were used to evaluate whether viral loads differed significantly between HPV types.
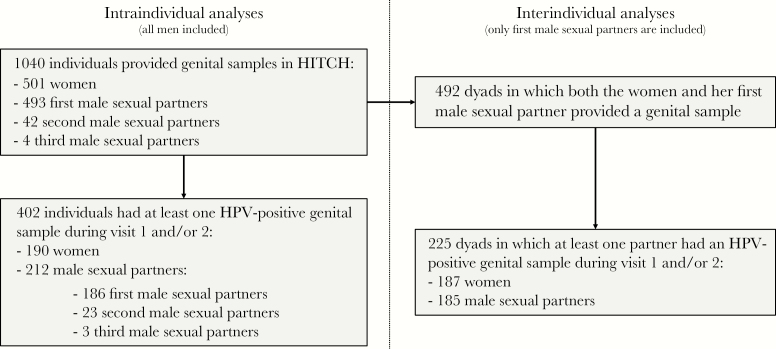
Type-specific HPV viral loads were compared within individuals at 2 consecutive visits (intraindividual analyses) and between sexual partners either at the same visit or at 2 consecutive visits (interindividual analyses; Figure 1). For intraindividual analyses, samples were excluded if the 2 visits were >9 months apart (n = 26 for women, n = 37 for men). Interindividual analyses were restricted to couples who reported being sexually active at both visits, and to women and their first male sexual partners; that is, if a woman had a second male sexual partner during HITCH, his genital samples were analyzed for intraindividual analyses but not for interindividual analyses. Furthermore, interindividual, same-visit analyses were only conducted in couples who visited within 7 days from each other. Similar to intraindividual analyses, interindividual between-visit analyses were restricted to couples with a second visit occurring within 9 months from the first.
Figure 1.
Study flowchart of patients from the HPV Infection and Transmission Among Couples Through Heterosexual Activity (HITCH) cohort study. Intraindividual analyses consisted of all analyses within men or within women only (ie, no data from the heterosexual partner was needed), which included evaluation of the viral load distribution in men or women, the correlation of human papillomavirus (HPV) viral loads within individuals at visit 1 and 2, and the association between viral loads and HPV prevalence or clearance at the next visit within individuals. Interindividual analyses included all analyses that involved data from both partners of a heterosexual couple.
To evaluate the correlation between HPV viral loads in partners and/or at subsequent visits, correlation coefficients were calculated using multilevel mixed-effects linear regression, adjusting for repeated measurements (ie, multiple HPV types within the same individual/couple). The magnitude of the association of type-specific viral loads with HPV prevalence, incidence, and clearance was assessed by estimating odds ratios (ORs) and their respective 95% confidence intervals (CIs) using multilevel mixed-effects logistic regression. For these models, HPV-positive individuals were categorized into type-, sex-, and visit-specific viral load tertiles (low/medium/high). For example, a man with an HPV-16–positive genital sample at visit 1 was considered to have a low viral load if his viral load was below the 33rd percentile of all detected viral loads for HPV-16 in male genital samples at baseline. HPV prevalence was defined as the number of HPV-positive individuals divided by the total number of individuals of the same sex tested for the same HPV type. HPV incidence was defined as the percentage of type- and sex-specific HPV-negative individuals who tested positive at the next visit. HPV clearance was defined as the percentage of type- and sex-specific HPV-positive individuals who tested negative at the next visit.
All statistical analyses were performed using Stata version 15.1 software (StataCorp).
RESULTS
In total, 1040 individuals provided genital samples, of whom 402 tested positive for at least one HPV type (HPV-6/11/16/18/31/42/51) (Figure 1). In 492 dyads, genital samples were available from women and their first male sexual partners; of these, 225 dyads included at least one individual who was positive for ≥1 of the studied HPV types. None of the participants were HIV-infected or otherwise immunosuppressed. Cohort characteristics are displayed in Table 1. The median age at baseline was 20 years (range, 18–26 years) for women and 22 years (range, 18–45 years) for men. Approximately two-thirds of participants were born in Canada. Twelve percent of women had received ≥1 dose of the HPV vaccine at baseline. The median number of lifetime vaginal sex partners was 5 for both men and women. On average, couples were having vaginal sex for 4 months at the baseline visit, for a median of 60 encounters. Of all women and men, 37.9% and 39.3% had a positive genital sample for ≥1 HPV type during the first 2 visits, respectively. In dyads, 23.3% and 24.2% were concordant for ≥1 HPV type at visit 1 and 2, respectively. Approximately 10% of participants had an incident HPV infection at the second visit, while more than one-third of HPV-infected individuals had cleared their infection at the second visit.
Table 1.
Characteristics of Participants in the Human Papillomavirus Infection and Transmission Among Couples Through Heterosexual Activity (HITCH) Cohort Study
| Characteristic | Women | Men | Dyad |
|---|---|---|---|
| (n = 501) | (n = 539) | (n = 492) | |
| Age at baseline, y, median (range) | 20 (18–26) | 22 (18–45) | |
| Born in Canada | 67.9% | 63.9% | |
| Vaccinated against HPV at baseline | 12.0% | 0.4% | |
| Lifetime No. of vaginal sex partners at baseline, median (IQR) | 5 (2–9) | 5 (3–11) | |
| Days between first vaginal sex with HITCH partner and baseline visit, median (IQR) | … | … | 118 (78–155) |
| No. of vaginal sex encounters with HITCH partner at baseline, median (IQR) | … | … | 60 (33–102) |
| Individuals positive for any HPV (6/11/16/18/31/42/51) during the first 2 visits | 37.9% | 39.3% | … |
| HPV concordance at baseline, no./No. (%) | … | … | 107/460 (23.3%) |
| HPV concordance at the second visit, no./No. (%) | … | … | 88/364 (24.2%) |
| Individuals with ≥1 incident HPV infection at the second visit, no./No. (%) | 42/445 (9.4%) | 43/352 (12.2%) | … |
| Individuals who cleared ≥1 HPV infection at the second visit, no./No. (%) | 54/150 (36.0%) | 45/134 (33.6%) | … |
Dyads were only included if the woman and her first male sexual partner had both provided a genital sample.
Abbreviations: HITCH, HPV Infection and Transmission Among Couples Through Heterosexual Activity; HPV, human papillomavirus; IQR, interquartile range.
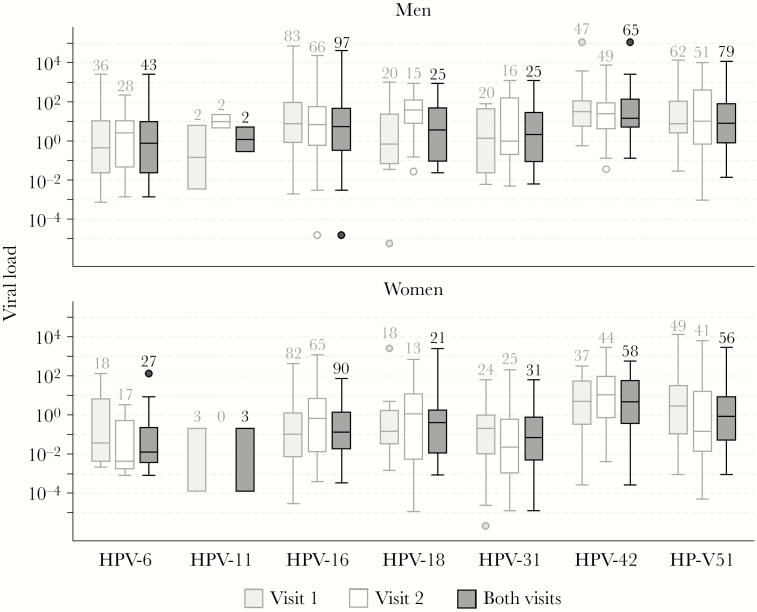
Type- and sex-specific HPV viral loads varied considerably (from <0.001 to 112 410 copies/cell), and tended to follow a log-normal distribution (Figure 2, Supplementary Table 2). Penile samples had on average a higher viral load than vaginal samples (P < .001). In both sexes, viral loads differed significantly between types, the median number of copies per cell ranging from 1.3 (HPV-31) to 26 (HPV-42) in penile samples, and from 0.01 (HPV-6) to 8 (HPV-42) in vaginal samples. There was no consistent change in the pattern of viral load distributions between the 2 visits.
Figure 2.
Log10 distribution of viral loads by sex, human papillomavirus (HPV) type, and visit number. Boxes represent the 25th to 75th percentiles, the black horizontal line inside the boxes represents the median, and whiskers represent the full range of values up to 1.5 times the interquartile range. For values outside this range, the full range is marked by dots. Average viral loads between HPV types differed significantly, both in men and in women (P < .001, Kruskal–Wallis test).
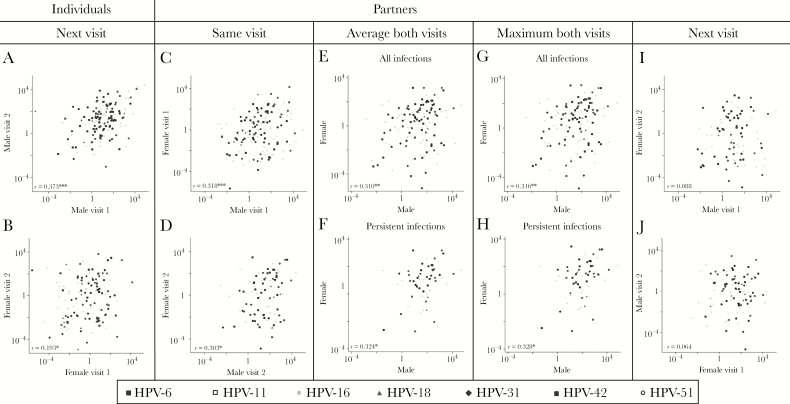
Correlations in type-specific viral loads were evaluated within HPV-positive individuals and sexually active couples (Figure 3, Supplementary Figures 1–5). For individuals with a persistent HPV infection, type-specific HPV viral loads at baseline were significantly correlated with viral loads at follow-up, particularly in men (correlation coefficient [r] = 0.38, P < .001; Figure 3A) but also in women (r = 0.19, P = .018; Figure 3B). Viral loads for partners who both tested positive for the same HPV type were significantly correlated with each other, both at the first and second visits (r = 0.318, P < .001 and r = 0.303, P = .040, respectively; Figure 3C and 3D). When comparing the average or maximum viral load at visits 1 and 2 in men and women who had a type-specific HPV infection during at least 1 visit (Figure 3E and 3G) or at both visits (Figure 3F and 3H), type-specific HPV viral loads significantly correlated between partners (r varied between 0.310 and 0.328, P < .05). Viral loads of men and women at visit 1 did not correlate with their sexual partner’s type-specific viral load at visit 2 (r = 0.088, P = .498 and r = 0.064, P = .605, respectively; Figure 3I and 3J, Supplementary Figure 5).
Figure 3.
Correlation of human papillomavirus (HPV) viral loads within individuals (scatter plots, A and B) or between sexually active partners (scatter plots, C–J). To calculate correlation coefficients (r), multilevel mixed-effects linear regression was used, allowing for adjustment of repeated measures (ie, multiple HPV types were evaluated per individual/couple). A, HPV viral loads were strongly correlated between visit 1 and 2 in men with a persistent HPV infection (n = 152 in 114 men, r = 0.373, P < .001). B, HPV viral loads were significantly correlated between visit 1 and 2 in women with a persistent HPV infection (n = 145 in 117 women, r = 0.193, P = .018). C, HPV viral loads were strongly correlated between sexually active partners who were both positive for an identical HPV type at the baseline visit (n = 141 in 107 dyads, r = 0.318, P < .001). D, HPV viral loads were significantly correlated between sexually active partners who were both positive for an identical HPV type at the follow-up visit (n = 88 in 69 dyads, r = 0.303, P = .040). E, Mean HPV viral loads were strongly correlated in sexually active partners who were positive for an identical HPV type during at least one visit (n = 125 in 87 dyads, r = 0.310, P = .003). F, Mean HPV viral loads were significantly correlated in sexually active partners who both had a persistent infection of an identical HPV type (n = 59 in 49 dyads, r = 0.324, P = .028). G and H, Repeating the analysis in E and F, but using the maximum instead of mean viral load, provided similar results (all infections: r = 0.316, P = .003; persistent infections: r = 0.328, P = .027). I, HPV viral loads in male genital samples at visit 1 were not significantly correlated to type-specific viral loads in their female partner’s genital samples at visit 2 (n = 92 in 79 dyads, r = 0.088, P = .498). J, HPV viral loads in female genital samples at visit 1 were not significantly correlated to type-specific viral loads in their male partner’s genital samples at visit 2 (n = 98 in 76 dyads, r = 0.064, P = .605). *P < .05; **P < .01; ***P < .001.
Next, we evaluated whether HPV viral loads could predict HPV prevalence, incidence, and clearance within individuals and between partners. Table 2 shows the prevalence, incidence, and clearance of HPV infections based on type-specific viral loads in individuals and in their sexual partner. There were dose-dependent differences; for example, the prevalence of a type-specific HPV infection in women was 2.5% when men were HPV negative at the same visit, but respectively increased to 46.7%, 54.5%, and 75.2% when men had low, medium, or high type-specific viral loads. This viral load–dependent increase in HPV prevalence in sexual partners was observed during visits 1 and 2 (Supplementary Table 3).
Table 2.
Human Papillomavirus (HPV) Prevalence, Incidence, and Clearance Based on HPV Type-Specific Viral Loads in Men and Women
| HPV Prevalence | HPV Incidence | HPV Clearance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | HPV Status at a Given Visit | HPV Viral Load, Tertilesa | Individual | Partner | Partner | Individual | Partner | |||
| At Next Visit | At Same Visit | At Next Visit | At Same Visit | At Next Visit | At Next Visit | At Same Visit | At Next Visit | |||
| Men | Negative | 2.4% (55/2249) | 2.5% (119/4786) | 2.8% (71/2512) | 0.8% (14/1765) | 1.0% (21/2096) | NA | 46.8% (22/47) | 26.0% (13/50) | |
| Positive | Low | 54.1% (33/61) | 46.7% (57/122) | 46.8% (29/62) | 20.7% (6/29) | 18.5% (5/27) | 45.9% (28/61) | 25.0% (5/20) | 26.9% (7/26) | |
| Medium | 85.5% (59/69) | 54.5% (72/132) | 57.7% (41/71) | 16.7% (4/24) | 25.0% (5/20) | 14.5% (10/69) | 37.0% (10/27) | 26.8% (11/41) | ||
| High | 84.5% (60/71) | 75.2% (100/133) | 52.1% (37/71) | 63.6% (7/11) | 20.0% (3/15) | 17.1% (12/70) | 13.6% (6/44) | 35.7% (15/42) | ||
| Women | Negative | 1.6% (41/2533) | 3.3% (158/4825) | 3.3% (70/2153) | 1.3% (21/1656) | 1.3% (23/1747) | NA | 38.2% (29/76) | 34.5% (20/58) | |
| Positive | Low | 56.4% (31/55) | 58.8% (70/119) | 54.0% (27/50) | 21.1% (4/19) | 29.4% (5/17) | 31.6% (18/57) | 12.0% (3/25) | 20.0% (5/25) | |
| Medium | 81.5% (44/54) | 56.8% (63/111) | 53.3% (32/60) | 41.2% (7/17) | 31.8% (7/22) | 30.2% (19/63) | 19.0% (4/21) | 21.4% (6/28) | ||
| High | 69.8% (37/53) | 81.4% (96/118) | 81.5% (44/54) | 60.0% (9/15) | 50.0% (3/6) | 23.8% (15/63) | 3.8% (1/26) | 11.6% (5/43) | ||
Abbreviations: HPV, human papillomavirus; NA, not available.
aTertiles were defined per HPV type, by sex, and by visit; eg, a man with an HPV-16–positive genital sample at visit 1 was considered to have a medium viral load if his viral load was above the 33rd but below the 67th percentile of all detected viral loads for HPV-16 in male genital samples at the first visit.
Type-specific HPV prevalence was significantly higher in individuals who were HPV positive at the previous visit than in HPV-negative individuals, with ORs ranging from 47.9 to 209.7 depending on sex and viral load tertile (P < .001; Table 3). Similarly, HPV prevalence and incidence were significantly increased in individuals whose sexual partner had an HPV infection with a low viral load compared to when the sexual partner was HPV negative, with ORs ranging from 22.7 to 57.8 (P < .001). Furthermore, HPV clearance was associated with HPV positivity in the sexual partner, regardless of viral load; for example, the odds that a man had cleared an HPV infection was 0.2 (95% CI, .1–.9) when comparing male partners of HPV-positive women with a low type-specific viral load to male partners of HPV-negative women (Table 3).
Table 3.
Association of Human Papillomavirus (HPV) Positivity With HPV Prevalence, Incidence, and Clearance in Genital Samples of Individuals and Their Sexual Partners
| HPV Prevalence | HPV Incidence | HPV Clearance | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | HPV Status | Individual | Partner | Partner | Partner | |||
| At Next Visit | At Same Visit | At Next Visit | At Same Visit | At Next Visit | At Same Visit | At Next Visit | ||
| Men | Negative | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Positive | 125.5*** (73.8–213.4) | 85.1*** (59.2–122.2) | 40.6*** (27.0–61.2) | 51.8*** (19.6–136.5) | 33.4*** (13.0–85.6) | 0.3** (.1–.7) | 1.2 (.6–2.7) | |
| Low VLa | 47.9*** (26.0–88.5) | 49.3*** (30.1–80.8) | 31.9*** (17.7–57.7) | 32.5*** (11.5–92.0) | 30.5*** (8.2–114.4) | 0.3 (.1–1.3) | 1.1 (.4–3.1) | |
| Women | Negative | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Positive | 209.7*** (112.9–389.2) | 85.6*** (59.6–122.9) | 65.7*** (39.3–110.0) | 57.8*** (23.1–144.6) | 44.3*** (16.6–118.1) | 0.2*** (.1–.5) | 0.3* (.1–1.0) | |
| Low VLa | 195.7*** (86.7–441.7) | 57.8*** (35.2–95.0) | 42.4*** (21.0–85.5) | 22.7*** (6.2–83.1) | 37.1*** (9.7–141.4) | 0.2* (.1–.9) | 0.4 (.1–1.6) | |
Data are presented as odds ratios (95% confidence intervals), calculated using multilevel mixed-effects logistic regression, adjusting for repeated measurements. HPV-negative individuals were compared to all HPV-positive individuals (second row) or individuals with low VLs (lowest tertile, third row).
Abbreviations: HPV, human papillomavirus; VL, viral load.
aTertiles were defined per HPV type, by sex, and by visit; eg, a man with an HPV-16–positive genital sample at visit 1 was considered to have a low viral load if his viral load was below the 33rd percentile of all detected viral loads for HPV-16 in male genital samples at the first visit.
*P < .05.
**P < .01.
***P < .001.
Higher viral loads were associated with an increased HPV prevalence and incidence and a decreased clearance within couples (Table 4). HPV persistence was higher in men with a high compared to low type-specific viral load at the first visit (OR, 4.6 [95% CI, 2.0–10.5]). For participants with a high type-specific viral load, their sexual partners were more likely to be HPV positive at the same visit (ORs, 5.3 [95% CI, 2.5–11.2] for men and 5.9 [95% CI, 2.2–15.5] for women). The association between HPV viral loads and partner’s HPV prevalence at the same visit was independent of visit number (Supplementary Table 4). Moreover, when women had a high viral load, it increased the odds that their male sexual partner had a type-specific HPV infection at the next visit (OR, 4.7 [95% CI, 1.5–14.6]). Women were at an increased risk of an incident HPV infection when their partner had a high type-specific viral load at the same visit (OR, 6.7 [95% CI, 1.5–30.7]). Men were less likely to have cleared an infection if they had high viral loads at the previous visit (OR, 0.2 [95% CI, .1–.5]). For other comparisons, although the magnitude of the ORs also suggested that increased viral loads increased HPV prevalence and incidence, and decreased clearance, associations were not significant (Table 4).
Table 4.
Association of Human Papillomavirus (HPV) Viral Loads With Type-Specific HPV Prevalence and Clearance Within an Individual at the Next Visit, and With Type-Specific HPV Prevalence, Incidence, and Clearance in Genital Samples of Their Heterosexual Partners at the Same or Next Visit
| HPV Prevalence | HPV Incidence | HPV Clearance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | HPV Viral Load, Tertilesa | Individual | Partner | Partner | Individual | Partner | |||
| At Next Visit | At Same Visit | At Next Visit | At Same Visit | At Next Visit | At Next Visit | At Same Visit | At Next Visit | ||
| Men | Low | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Medium | 5.0*** (2.2–11.6) | 1.6 (.8–3.0) | 1.6 (.8–3.1) | 0.8 (.2–3.1) | 1.5 (.4–6.0) | 0.2*** (.1–0.5) | 2.2 (.3–18.5) | 1.0 (.3–3.0) | |
| High | 4.6*** (2.0–10.5) | 5.3*** (2.5–11.2) | 1.2 (.6–2.5) | 6.7* (1.5–30.7) | 1.1 (.2–5.4) | 0.2** (.1–.5) | 0.3 (.0–4.0) | 1.5 (.5–4.4) | |
| Women | Low | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Medium | 1.1 (.5–2.4) | 0.9 (.4–2.2) | 1.1 (.4–2.6) | 3.1 (.4–24.8) | 2.2 (.0–136.4) | 0.9 (.4–2.0) | 3.2 (.3–38.1) | 1.1 (.3–4.1) | |
| High | 1.2 (.5–2.7) | 5.9*** (2.2–15.5) | 4.7** (1.5–14.6) | 8.6 (.5–159.2) | 206.1 (.1–633 888) | 0.7 (.3–1.5) | 0.2 (.0–7.0) | 0.5 (.1–2.0) | |
Data are presented as odds ratios (95% confidence intervals), calculated using multilevel mixed-effects logistic regression, adjusting for repeated measurements.
Abbreviation: HPV, human papillomavirus.
aTertiles were defined per HPV type, by sex, and by visit; eg, a man with an HPV-16–positive genital sample at visit 1 was considered to have a medium viral load if his viral load was above the 33rd but below the 67th percentile of all detected viral loads for HPV-16 in male genital samples at the first visit.
*P < .05.
**P < .01.
***P < .001.
DISCUSSION
Prevention of cervical cancer is increasingly relying on the detection of HPV infections [29]. While HPV screening particularly increases the sensitivity for detection of CIN grade 3 or worse, false-positive results remain a problem, increasing the costs and burden for women with an HPV-positive test who do not have and will not develop cervical lesions, for example because of spontaneous clearance of infections [30, 31]. Furthermore, HPV-positive women may know that they are infected with a sexually transmitted infection, but cannot estimate the risk of infecting their sexual partner. Therefore, we need to improve our knowledge of HPV transmission and persistence. Intuitively, one would assume that increased HPV viral loads imply viral shedding and consequently an increased risk for HPV transmission and persistence. Indeed, various studies have found an association between viral loads and HPV-related genital lesions, suggesting that high viral loads facilitate HPV transmission [16–18, 32–34]. To obtain additional insights, we quantitated HPV viral loads and studied their effect on HPV transmission and persistence in young, recently formed sexually active, heterosexual couples, when most transmission is presumed to occur. Our data suggest that HPV viral loads are correlated in these couples and may predict to some extent HPV prevalence, incidence, and clearance within individuals and their sexual partners.
In our data, viral loads differed between HPV types and were sex dependent, with higher viral loads observed in male genital samples. Methodological differences might have biased these results. Women self-collected vaginal samples, whereas penile samples were collected by experienced nurses who directly scraped the penile epithelium, potentially explaining the higher viral loads found in penile samples. A previous study that compared self-collected vaginal samples to clinician-collected cervical samples reported lower viral loads in self-collected vaginal samples [35]. On the other hand, Bleeker et al used direct cervical and penile scraping to obtain samples from 109 women with CIN and 57 of their male partners, but also reported differences in viral loads between male and female genital samples and between different HPV types [16]. Therefore, HPV viral loads should be studied separately by sex and HPV type.
Viral loads were correlated within individuals at the 2 visits, which were scheduled 4 months apart but could be up to 9 months apart. This suggested that viral loads are relatively constant over time within individuals with persistent HPV infections. The correlation was stronger in men than in women, which may either suggest relatively more fluctuations in viral loads in women, or measurement errors due to self-sampling among women. Viral loads were also correlated in couples if both partners had an identical HPV infection, suggesting that increased viral loads increase transmission and/or viral activity in the partner. Some shedding of HPV-infected cells from the partner may have occurred [36], but we expect such contamination to be limited, as couples had been asked to abstain from intercourse in the 24 hours before the clinical visit.
Higher HPV viral loads were also associated with an increased type-specific HPV prevalence and incidence, and a decreased HPV clearance within the individual and/or in the sexual partner. These findings are in line with previous reports [13, 14, 16, 18]. While these trends were relatively consistent in the overall analyses, we noticed large variations when analyzing each HPV type separately. Numbers were small, limiting the value of observed variation. Furthermore, other parameters may influence the association between viral loads and HPV prevalence/incidence/clearance, such as circumcision status or condom use [37].
Several factors need to be considered when interpreting our findings. First, couples were already sexually active for up to 6 months at the time of the baseline visit, which means that a considerable amount of transmission had already occurred, and that viral load levels were already influenced by the viral load status of the sexual partner. However, no large-scale prospective cohort study has been able to follow young, sexually active couples earlier in their relationship than HITCH; to do so would entail logistical quandaries. We limited viral load assessment to the first 2 visits only, to maximize the opportunity to study transmission events early in the couple’s relationship. However, this prevented us from observing viral load fluctuations over long periods of follow-up. We only tested type-specific viral loads in genital samples that tested HPV positive in Roche’s Linear Array. As such, we may have missed samples that were false negative in the Linear Array, for example due to viral loads that were too low to be detected in the assay. Being a type-specific amplification assay, it is possible that the real-time PCR may have a lower threshold for detection of low numbers of viral copies than the Linear Array assay. In general, the Linear Array assay is considered a well-validated genotyping HPV test for use in epidemiologic studies, with good reproducibility and high sensitivity and specificity [38, 39]. Finally, some variables were self-reported, such as relationship status. We attempted to reduce bias by comparing the respondents’ answers at multiple occasions and by comparing the answer of both partners to particular questions such as for relationship status.
In summary, we analyzed data from a large, longitudinal cohort study of recently formed, heterosexual couples. We found that viral loads vary largely but are correlated within individuals and between heterosexual partners. Measuring viral load may aid in predicting HPV infection outcomes within sexually active couples. Future studies are needed to identify determinants for high HPV viral loads, and to determine whether viral loads predict future clinical disease, to advance our understanding of the natural history of HPV infections and provide insights on preventive strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and the following present and former staff members of the HPV Infection and Transmission Among Couples Through Heterosexual Activity (HITCH) cohort study: Vicky D’Anjou-Pomerleau, Jennifer Selinger, Elizabeth Montpetit-Dubrule, Jessica Sammut, Emilie Lapointe, Johanna Bleecker, Shady Rahayel, Gail Kelsall, Suzanne Dumais, Natalia Morykon, Amelia Rocamora, Nathalie Slavtcheva, Veronika Moravan, Hélène Voyer, Véronique Legault, and Julie Guénoun.
Financial support. The HITCH cohort study was supported by the Canadian Institutes of Health Research (CIHR; grant number MOP-68893, team grant number CRN-83320 to E. L. F.); and the US National Institutes of Health (grant number RO1AI073889 to E. L. F.). Supplementary and unconditional funding support was provided by Merck Frosst Canada Ltd and Merck & Co Ltd. Validation of viral load assays was supported by the Réseau sida et maladies infectieuses du Fonds de recherche du Québec–Santé. M. D. W. is funded by a CIHR Postdoctoral Fellowship Award.
Potential conflicts of interest. E. L. F. has served as an occasional advisor to companies involved with human papillomavirus (HPV) diagnostics (Roche, BD, Qiagen, Gen-Probe) and HPV vaccines (Merck, GSK). His institution has received unconditional grants from Merck in aid of investigator-initiated studies. F. C. has received grants through his institution from Merck, Becton Dickinson, and Roche, as well as honoraria from Merck and Roche for lectures on HPV. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Eurogin, Amsterdam, The Netherlands, 8–11 October 2017. Abstract P02-09.
References
- 1. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24(Suppl 1):S1–15. [DOI] [PubMed] [Google Scholar]
- 2. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016; 4:e609–16. [DOI] [PubMed] [Google Scholar]
- 3. Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis 2010; 10:862–74. [DOI] [PubMed] [Google Scholar]
- 4. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157:218–26. [DOI] [PubMed] [Google Scholar]
- 5. Muñoz N, Bosch FX, Castellsagué X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 2004; 111:278–85. [DOI] [PubMed] [Google Scholar]
- 6. Burchell AN, Richardson H, Mahmud SM, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol 2006; 163:534–43. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis 2008; 14:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burchell AN, Tellier PP, Hanley J, Coutlée F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology 2010; 21:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burchell AN, Coutlée F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis 2011; 204:1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nyitray AG, Menezes L, Lu B, et al. Genital human papillomavirus (HPV) concordance in heterosexual couples. J Infect Dis 2012; 206:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abalos AT, Harris RB, Nyitray AG, et al. Human papillomavirus type distribution among heterosexual couples. J Low Genit Tract Dis 2012; 16:10–5. [DOI] [PubMed] [Google Scholar]
- 12. Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013; 207:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marks M, Gravitt PE, Utaipat U, et al. Kinetics of DNA load predict HPV 16 viral clearance. J Clin Virol 2011; 51:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trevisan A, Schlecht NF, Ramanakumar AV, Villa LL, Franco EL; Ludwig-McGill Study Group Human papillomavirus type 16 viral load measurement as a predictor of infection clearance. J Gen Virol 2013; 94:1850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grabowski MK, Gray RH, Serwadda D, et al. High-risk human papillomavirus viral load and persistence among heterosexual HIV-negative and HIV-positive men. Sex Transm Infect 2014; 90:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bleeker MC, Hogewoning CJ, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis 2005; 41:612–20. [DOI] [PubMed] [Google Scholar]
- 17. Bleeker MC, Hogewoning CJ, Voorhorst FJ, et al. HPV-associated flat penile lesions in men of a non-STD hospital population: less frequent and smaller in size than in male sexual partners of women with CIN. Int J Cancer 2005; 113:36–41. [DOI] [PubMed] [Google Scholar]
- 18. Grabowski MK, Kong X, Gray RH, et al. Partner human papillomavirus viral load and incident human papillomavirus detection in heterosexual couples. J Infect Dis 2016; 213:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burchell AN, Tellier PP, Hanley J, Coutlée F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis 2010; 37:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Legault V, Burchell A, Goggin P, et al. Generic microtiter plate assay for triaging clinical specimens prior to genotyping of human papillomavirus DNA via consensus PCR. J Clin Microbiol 2011; 49:3977–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dahlstrom KR, Burchell AN, Ramanakumar AV, et al. Sexual transmission of oral human papillomavirus infection among men. Cancer Epidemiol Biomarkers Prev 2014; 23:2959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burchell AN, Rodrigues A, Moravan V, et al. Determinants of prevalent human papillomavirus in recently formed heterosexual partnerships: a dyadic-level analysis. J Infect Dis 2014; 210:846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tota JE, Ramanakumar AV, Villa LL, et al. Evaluation of human papillomavirus type replacement postvaccination must account for diagnostic artifacts: masking of HPV52 by HPV16 in anogenital specimens. Cancer Epidemiol Biomarkers Prev 2015; 24:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Zein M, Coutlée F, Tellier PP, Roger M, Franco EL, Burchell AN; HITCH Study Group Human papillomavirus infection and transmission among couples through heterosexual activity (HITCH) cohort study: protocol describing design, methods, and research goals. JMIR Res Protoc 2019; 8:e11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coutlée F, Rouleau D, Petignat P, et al. Canadian Women’s HIV study Group Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear Array HPV genotyping test. J Clin Microbiol 2006; 44:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez J, de Pokomandy A, Rouleau D, et al. HIPVIRG Study Group Episomal and integrated human papillomavirus type 16 loads and anal intraepithelial neoplasia in HIV-seropositive men. AIDS 2010; 24:2355–63. [DOI] [PubMed] [Google Scholar]
- 27. Azizi N, Brazete J, Hankins C, et al. Canadian Women’s HIV Study Group Influence of human papillomavirus type 16 (HPV-16) E2 polymorphism on quantification of HPV-16 episomal and integrated DNA in cervicovaginal lavages from women with cervical intraepithelial neoplasia. J Gen Virol 2008; 89:1716–28. [DOI] [PubMed] [Google Scholar]
- 28. Frigge M, Hoaglin DC, Iglewicz B. Some implementations of the boxplot. Am Stat 1989; 43:50–4. [Google Scholar]
- 29. Jeronimo J, Castle PE, Temin S, Shastri SS. Secondary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J Oncol Pract 2017; 13:129–33. [DOI] [PubMed] [Google Scholar]
- 30. Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method. J Natl Cancer Inst 2012; 104:178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sroczynski G, Schnell-Inderst P, Mühlberger N, et al. Cost-effectiveness of primary HPV screening for cervical cancer in Germany—a decision analysis. Eur J Cancer 2011; 47:1633–46. [DOI] [PubMed] [Google Scholar]
- 32. Ylitalo N, Sørensen P, Josefsson AM, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet 2000; 355:2194–8. [DOI] [PubMed] [Google Scholar]
- 33. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–14. [DOI] [PubMed] [Google Scholar]
- 34. Schlecht NF, Trevisan A, Duarte-Franco E, et al. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer 2003; 103:519–24. [DOI] [PubMed] [Google Scholar]
- 35. Labani S, Asthana S. Human papillomavirus viral load on careHPV testing of self-collected vaginal samples vs. clinician-collected cervical samples. Eur J Obstet Gynecol Reprod Biol 2014; 181:233–9. [DOI] [PubMed] [Google Scholar]
- 36. Malagón T, Burchell AN, El-Zein M, et al. HITCH Study Group Estimating HPV DNA deposition between sexual partners using HPV concordance, Y chromosome DNA detection, and self-reported sexual behaviors. J Infect Dis 2017; 216:1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis MA, Gray RH, Grabowski MK, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: a randomized trial in Rakai, Uganda. Int J Cancer 2013; 133:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinau M, Swan DC, Unger ER. Type-specific reproducibility of the Roche linear array HPV genotyping test. J Clin Virol 2008; 42:412–4. [DOI] [PubMed] [Google Scholar]
- 39. Xu L, Zhang H, Yue H, et al. Gas stunning with CO2 affected meat color, lipid peroxidation, oxidative stress, and gene expression of mitogen-activated protein kinases, glutathione S-transferases, and Cu/Zn-superoxide dismutase in the skeletal muscles of broilers. J Anim Sci Biotechnol 2018; 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.