ABSTRACT
Background
Airborne pollutants have collectively been classified as a known human carcinogen and, more broadly, affect the health of hundreds of millions of people worldwide. Benzene is a frequent component of air pollution, and strategies to protect individuals against unavoidable exposure to this and other airborne carcinogens could improve the public's health. Earlier clinical trials in Qidong, China, demonstrated efficacy in enhancing the detoxication of benzene using a broccoli sprout beverage.
Objectives
A randomized, placebo-controlled, multidose trial of a broccoli sprout beverage was designed to determine the lowest effective concentration that enhances benzene detoxication adjudged by enhanced excretion of the urinary biomarker, S-phenylmercapturic acid (SPMA).
Methods
Following informed consent, 170 subjects were randomly assigned in 5 blocks of 34 each to drink either a placebo beverage (n = 55) or 1 of 3 graded concentrations of a broccoli sprout beverage [full (n = 25), one-half (n = 35), and one-fifth (n = 55)] for 10 consecutive days. Concentrations of SPMA arising through induced benzene conjugation with glutathione were quantified by MS in sequential 12-h overnight urine collections during the intervention.
Results
MS was also used to quantify urinary sulforaphane metabolites in each dosing regimen that resulted in a median 24-h urinary output of 24.6, 10.3, and 4.3 µmol, respectively, confirming a dose-dependent de-escalation of the inducing principle within the beverage. A statistically significant increase in benzene mercapturic acids in urine was found for the high-dose group (+63.2%) during the 10-d period. The one-half dose (+11.3%) and one-fifth dose groups (−6.4%) were not significantly different from placebo controls.
Conclusions
An intervention with a broccoli sprout beverage enhanced the detoxication of benzene, an important airborne pollutant, when dosed at a concentration evoking a urinary elimination of ∼25 µmol sulforaphane metabolites per day, and it portends a practical and frugal population-based strategy to attenuate associated long-term health risks of air pollution. This trial was registered at clinicaltrials.gov as NCT02656420.
Keywords: broccoli, sulforaphane, glucoraphanin, air pollution, mercapturic acids, randomized clinical trial
Introduction
The WHO has provided analyses indicating that ∼7 million people die each year from exposures to air pollution (1). Furthermore, WHO concluded that globally, 40% of ischemic heart disease, 40% of stroke, 11% of chronic obstructive pulmonary disease, and 6% of lung cancer deaths are caused by outdoor air pollution (https://www.who.int/airpollution/en). Indeed, in 2013, the International Agency for Research on Cancer (IARC/WHO) classified outdoor air pollution, a complex mixture that often contains benzene, as a “Group 1 known human carcinogen” (2, 3). China is 1 of the world's largest emitters of anthropogenic air pollution, and levels of outdoor air pollution in China are among the highest in the world (4, 5). Our prior studies in Qidong, an area of rapidly growing economic development in the Yangtze River Basin, measured high concentrations of certain biomarkers of air pollutants, including benzene, aldehydes, and polycyclic aromatic hydrocarbons (6–8).
The diet is the major source of carbohydrates, proteins, fats, fiber, vitamins, and minerals (nutrients) that support intrinsic biochemical reactions essential for life, as well as the nonnutrient phytochemicals essential for enhancing health or quality of life. Certain nonnutrient dietary agents can enhance inherent detoxication pathways, such as the glutathione S-transferases (GSTs), and they have been utilized in a wide range of animal models to demonstrate efficacy in protecting against acute and chronic toxicities, including carcinogenesis, from environmental toxicants (9). Furthermore, the discovery of the role of the Keap1-Nrf2 signaling pathway has revealed a mechanistic roadmap for the identification of potentially effective dietary protective agents (10). A principal bioactive formed in broccoli, sulforaphane (SF) (11), is an effective anticarcinogen in animal models (12) and acts in part through inducing detoxication, antioxidant, and anti-inflammatory enzymes by activating the Keap1-Nrf2 signaling pathway (10). As a translation of these experimental studies, a series of placebo-controlled, randomized intervention trials with several formulations of broccoli sprout beverage have been conducted in China (3, 4). The safety of broccoli sprout beverage has been well established in prior phase I clinical trials (7, 10, 13, 14).
Urinary mercapturic acids are stable detoxication end products resulting from initial glutathione conjugation and subsequent metabolism of the parent air pollutants, and they can be formed from nonenzymatic or GST-catalyzed reactions (15, 16). These biomarkers can play multiple, seemingly paradoxical, roles in studies on human health. Commonly, they have been used as indices of internal dose and as such are physiologically integrated measures of either ambient or occupational exposures linked to adverse health effects. Dose–response relations between workplace air measures of benzene and urinary excretion of their mercapturic acid derivatives [e.g., S-phenylmercapturic acid (SPMA)] have been reported (17). These biomarkers have also served as measures of pharmacodynamic action to assess the impact of interventions to enhance carcinogen detoxication in the settings of crossover or other randomized clinical trials in which enhanced benzene mercapturic acid excretion was measured and associated with protection (6, 7, 18). The current study sought to define a minimally effective dose for the enhanced detoxication of air pollutants to serve as a guidepost in dose selection for broccoli-based interventions in an array of disease prevention settings.
Methods
Study design and participants
Adults in good general health without a history of major chronic illnesses were randomly assigned into a placebo-controlled trial for assessing the dose–response pharmacokinetics and pharmacodynamics of a beverage enriched with glucoraphanin (GR) and SF prepared from 3-day-old broccoli sprouts. Study participants were recruited from the villages of Qing Jia and Ji Zi in the rural farming community of He He Township, Qidong, Jiangsu Province, China. A total of 714 individuals were screened at local clinics during 4 d in December 2015 (Table 1, Figure 1). Written consent was obtained from all participants. The protocol was approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health and the Qidong Liver Cancer Institute and registered with clinicaltrials.gov (NCT02656420). A medical history, physical examination, and routine renal and hepatic function tests were used to screen the individuals, aged 21–65 y, by methods identical to those described for our previous interventions in this region (6, 8). Less than half (261 people; Figure 1) of the individuals from the screened group were eligible, of which the initial 170 were randomized. The randomization protocol was done in 5 blocks of 34 individuals to ensure distribution of 170 participants into each dose group as calculated by the power analysis (placebo, n = 55; full dose, n = 25; half dose, n = 35; and one-fifth dose, n = 55). All of these selected individuals returned to the clinics on the first day of the study and were given their identification code. Overall, there were 43 men (19%) and 127 women (81%), with a median age of 58 y (range: 24–65 y). None of the women in the study were current smokers. Although this was a tightly controlled dietary intervention, participants were under no dietary restrictions throughout the trial.
TABLE 1.
Demographic characteristics of the screening population and trial participants, by treatment arm1
| Trial participants | |||||
|---|---|---|---|---|---|
| Characteristics | Screening population (n = 714) | Placebo (n = 55) | 1/5 dose (n = 55) | 1/2 dose (n = 35) | Full dose (n = 25) |
| Women, % (n) | 71.2 (508) | 69.1 (38) | 78.2 (43) | 80.0 (28) | 72.0 (18) |
| Age, y | 57.5 [52, 62] | 56 [53, 61] | 60 [53, 63] | 58 [50, 64] | 59 [56, 62] |
| BMI, kg/m2 | 24.9 [23.1, 27.3] | 25.1 [23.1, 26.7] | 24.7 [22.6, 26.8] | 23.9 [22.1, 26.6] | 23.5 [21.6, 25.8] |
| BMI among women, kg/m2 | 25.1 [23.1, 27.3] | 24.8 [23.1, 26.7] | 24.8 [22.6, 26.7] | 24.0 [22.1, 26.4] | 23.5 [21.6, 25.8] |
| BMI among men, kg/m2 | 24.6 [23.1, 27.4] | 25.4 [23.6, 27.4] | 24.2 [21.8, 28.1] | 23.2 [20.9, 26.7] | 22.2 [21.3, 27.9] |
| Current smokers, % (n) | 12.5 (89) | 5.5 (3) | 7.3 (4) | 8.6 (3) | 20.0 (5) |
| Current smokers among women, % (n) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Current smokers among men, % (n) | 43.2 (89) | 17.7 (3) | 33.3 (4) | 42.9 (3) | 71.4 (5) |
Values are median [25th percentile, 75th percentile] unless otherwise noted.
FIGURE 1.
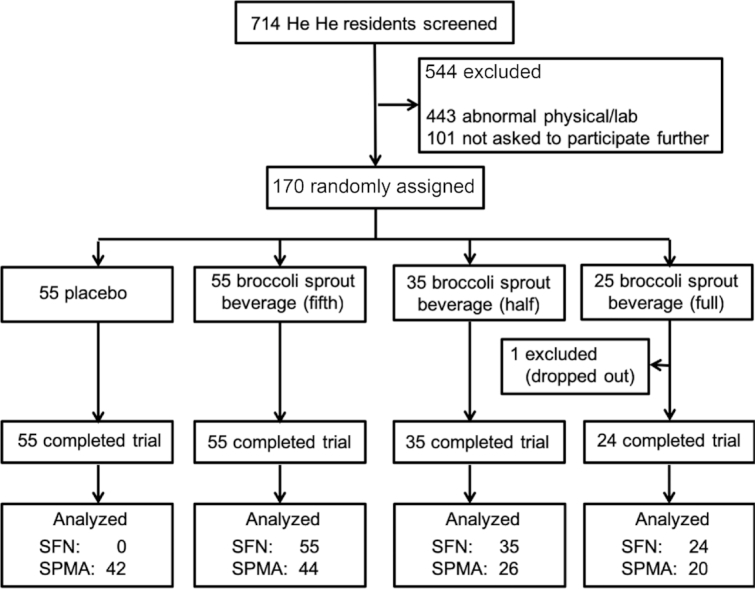
CONSORT plot of the intervention trial. Of the 170 participants, 169 completed the entire trial. SF metabolites were analyzed in all samples from the 3 broccoli sprout beverage dose groups but not those from participants receiving placebo beverages. The smaller number of SPMA analyses reflects the catastrophic failure of the MS near the completion of the fourth out of 5 randomization blocks. CONSORT, Consolidated Standards of Reporting Trials; SF, sulforaphane; SPMA, S-phenylmercapturic acid.
The trial was conducted in mid-January 2016. Participants, who were blinded to the beverage allocation, consumed a placebo beverage or 1 of 3 doses of a broccoli sprout beverage for 10 consecutive days. Participants met local doctors and study investigators at 1 of 5 designated local sites between 1630 and 1730 each evening for distribution of the intervention beverages. Compliance was determined by visual observation of consumption of the assigned beverage. Placebo and broccoli sprout beverages were prepared fresh each afternoon from bulk powders and brought to He He daily for distribution. To control pH, ascorbic acid was added to urine collection containers shortly before distribution to participants, and complete overnight and daytime (∼12 h each) urine samples were collected following consumption of the beverages on each day of the study. Once collected, total urine volumes were measured, and aliquots were prepared and transported to the Qidong Liver Cancer Institute for immediate storage at −20°C. Blood samples were collected on the first and last day of the study. Blood chemistries were measured by the clinical laboratory of the Qidong People's Hospital. Aliquots of urine and serum from each sample were shipped frozen to Baltimore at the end of the study in accordance with the export and import regulations of the People's Republic of China and the United States, respectively.
Preparation of the broccoli sprout beverages
The study was conducted using rehydrated, previously lyophilized broccoli sprout powders rich in either GR or SF that were produced by the Cullman Chemoprotection Center at the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, under conditions suitable for clinical use as an Investigational New Drug. Powders were stored at −80°C until shipment to China. The preparation of the GR- and SF-rich powders has been described previously (19, 20). Powders were titered for GR and SF content shortly before beginning the study as described previously (21, 22). At the Qidong Liver Cancer Institute, powders were dissolved in sterile water to which an equal volume of pineapple juice (Dole) was added along with lime juice (Safeway brand) in a final ratio of 47:47:6 by volume. The full dose contained 600 μmol GR and 40 µmol SF, whereas the half dose contained 300 and 20 µmol GR and SF, respectively, and the one-fifth dose contained 125 and 8 µmol of GR and SF, respectively, all in 100 mL of beverage. The placebo beverage contained the same liquid components, to which 1% molasses vol:vol was added to provide color masking. This lot of broccoli sprout powder has been used previously in intervention studies (6).
GR and SF in urine
Measurement of SF metabolites in urine was performed by isotope dilution mass spectrometric assay as reported previously by Egner et al. (23). Positive LC electrospray ionization tandem MS (LC-ESI-MS/MS) was carried out using a Thermo-Finnigan TSQ Advantage triple quadrupole mass spectrometer coupled to a Thermo-Finnigan Accela ultraperformance LC and HTC PAL autoinjector. Chromatographic separation of analytes was achieved using a 1.9-µm 100 × 1 mm2 Thermo Hyper Gold column maintained at 40°C.
Analysis of SPMA in urine
Following the thawing of the urine sample, the urine was centrifuged for 10 min at 1000 × g at room temperature, and 2 mL was removed for analysis. The 2-mL sample was applied to an Agilent Technologies C18 500 mg clean-up column (catalog no. 52,102,052). The column was initially washed with 3 mL acetone followed by 3 mL H2O, and then 2-mL urine samples, each containing an SPMA-d5 internal standard (CDN Isotopes), were introduced. This sample was washed with 3 mL H2O, and the SPMA was eluted with 3 mL acetone. Following evaporation to dryness, the sample was redissolved in 1 mL of the initial mobile phase for analysis by LC-ESI-MS/MS-selected reaction monitoring (SRM).
LC-ESI-MS/MS-SRM analysis was performed on a TSQ Quantum Ultra instrument (Thermo). The HPLC instrument was equipped with a 250 × 4.6 mm Synergi C12, 4 µm, Max-RP, 80 Å pore size column (Phenomenex). For analysis of SPMA, elution was isocratic for 20 min with 55% 15 mM NH4OAc, pH 6.8, and 45% MeOH at a flow rate of 0.8 mL/min. The ESI ion source was operated in the negative ion mode; N2 sheath gas pressure was 30 pounds per square inch (psi), and N2 auxiliary gas pressure was 5 psi. The capillary temperature was 200°C with a voltage of −35 V. Other MS parameters were as follows: collision energy, 13 V; argon collision gas pressure, 1 mTorr; peak width: quartile 1 [full width at half maximum (FWHM)], 0.70; quartile 3 (FWHM), 0.70; scan width (m/z), 0.40; and scan time (s), 0.1. The limit of detection for SPMA was 0.07 pmol/mL urine. For analysis, samples below the limit of detection were imputed as 0.049 [= 0.07/(20.5)].
Analysis of urinary cotinine
Urinary cotinine levels were analyzed using a high-sensitivity quantitative ELISA (cotinine ELISA kits for human urine; Calbiotech). All samples were diluted 1- to 20-fold in MilliQ water (Millipore) to fit into the range of the standard curve. All samples were run in duplicate; standards and blanks were run every 40 samples.
Analysis of 8-iso-prostaglandin F2α in urine
The method was based on that described by Yan et al. (24, 25). 8-iso-prostaglandin F2α (8-iso-PGF2α) and 8-iso-PGF2α-d4 were procured from Cayman Chemical. For each sample, a 200-µL aliquot of urine was analyzed using an LC-ESI-MS/MS on a Vantage triple-quadrupole MS (Thermo Scientific) with a Dionex Ultimate 3000 rapid separation HPLC system (Thermo Scientific). The following transitions were monitored: 8-iso-PGF2α, m/z 353 → m/z 193 (quantifier) and m/z 353 → m/z 173 (qualifier); 8-iso-PGF2α-d4, m/z 357 → m/z 197 (quantifier) and 357 m/z → 177 m/z (qualifier). Fragmentation was achieved with a collision energy of 25 V and gas pressure of 1.2 mTorr. Argon was the collision gas. Quadrupole resolution was set at m/z 0.7 for both quartile 1 and quartile 3, with a scan width of m/z 0.1 and scan time of 0.25 s. The capillary temperature was set at 255°C, N2 sheath gas pressure at 40 psi, and N2 auxiliary gas pressure at 2 psi.
Analysis of PGE2 metabolites in urine
PGE2 metabolite (PGE-M) analysis was performed as described previously with modifications (25, 26). In brief, a 400-µL aliquot of urine was spiked with 120 pmol PGE-M-d6 (Cayman Chemical) and analyzed using an LC-MS/MS system for SRM analysis on a TSQ Quantum Discovery Max instrument (Thermo Fisher Scientific) operated in the negative ion atmospheric pressure chemical ionization mode. The following transitions were monitored: PGE-M-d6, m/z 315 → m/z 297 (quantifier) and m/z 315 → m/z 149 (qualifier); and PGE-M, m/z 309 → m/z 291 (quantifier) and m/z 309 → m/z 143 (qualifier). Other MS/MS parameters were as follows: collision energy for transitions: 15 V for m/z 315 → m/z 297, m/z 309 → m/z 291 and 23 V for m/z 315 → 149, m/z 309 → 143; quartile 1 = 0.7 FWHM and quartile 3 = 0.7 FWHM; scan width, m/z = 0.10; scan time, 0.25 s; vaporizer temperature, 80°C; capillary temperature, 270°C; N2 sheath gas pressure, 60 psi; and N2 auxiliary gas pressure, 5 psi. Argon was the collision gas.
Statistical analyses
Study design calculations were based on the results from our previous clinical trial (4) that identified a 61% increase in urinary SPMA excretion associated with 600 µmol of GR and 40 µmol of SF that was rapid and persistent over time. The linear mixed model used to estimate the treatment effect over time also provided the between-subject variability (variance = 0.3) and the within-subject variability (variance = 0.5) of SPMA in the log scale. From these variance parameters, and different predicted effect sizes of 60%, 50%, and 40% to account for decreased doses and an assumed drop-out of ∼10%, the study included a total of 170 participants, with the number of participants in each arm related to the expected increase in urinary SPMA excretion. Each arm provides at least 80% power to detect the predicted increases in urinary SPMA excretion.
To characterize the levels of SF metabolites by active treatment arm, graphical displays of individual data, daily medians, and overall summarized SF metabolites are presented. Overall SF metabolites were summarized by a log-linear mixed effects model with random intercepts within each treatment arm. Each day of the study was assessed as a potentially meaningful independent variable, but the overall SF metabolite concentrations did not systematically differ across time. The final model for each treatment arm included a main effect of intercept with random intercepts to account for repeated measurements within individuals, and this estimate was converted to the geometric mean with 95% CIs. Intraclass correlation coefficients were estimated from these models to summarize within-person correlation.
The primary comparison of interest was SPMA levels by treatment arm. Substantial within-individual variability and minimal within-individual correlation were expected (6) and observed. To summarize first 12-h SPMA concentrations over the study course by participant, the SPMA geometric mean (on the log scale) for each individual was calculated and used as the outcome. Treatment arms (placebo and one-fifth, one-half, and full doses) were independent (categorical) variables in a linear regression model, with placebo as the reference. Because the outcomes were log-transformed, the parameter estimates were converted to percentage differences from placebo for the active treatment arm for a meaningful interpretation and to be consistent with the similar previous trial (6). Last, to explore modified per-protocol effects, the within-individual average 12-h SPMA concentrations and the within-individual average 24-h SF concentrations during the course of the study were plotted.
Results
Compliance, data collection completeness, and tolerability
As indicated by the CONSORT (Consolidated Standards of Reporting Trials) diagram in Figure 1, 170 individuals were randomly assigned into 4 treatment arms with an unequal distribution, based on power calculations, of 55 in the placebo beverage arm and 55, 35, and 25 in the one-fifth, one-half, and full-dose broccoli sprout beverage arms, respectively. The intervention groups did not differ significantly (P > 0.05) by gender, age, or BMI (Table 1). The 15 current smokers were all men (approximately one-third of the male participants). One individual dropped out early in the study, for reasons not attributed to treatment, and had been in the full-dose beverage arm. All other participants completed the study and provided the requested urine and blood samples throughout. There were no abnormal clinical chemistry values for blood samples collected on the first or last days of the intervention.
SF pharmacokinetics
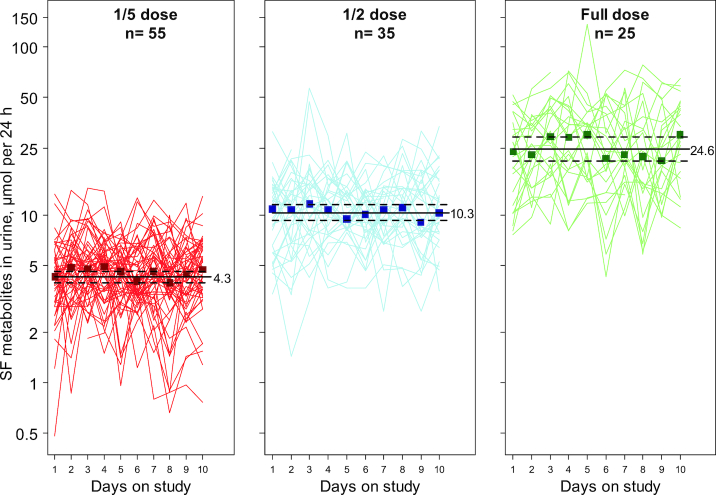
Isotope dilution MS was used to measure the concentrations of glutathione-derived conjugates of SF excreted in the urine during consecutive 12-h collections throughout the intervention period. SF-N-acetylcysteine, SF-cysteine, and free SF are the major urinary metabolites, accounting for 80–81%, 12–14%, and 5–7% of total SF and metabolites, respectively. The other glutathione-derived conjugates, SF-glutathione and SF-cysteinyl-glycine, accounted for <1% of total SF excretion, as described previously (6, 8). The distribution of individual urinary metabolites did not change during the course of the intervention, also as described previously (6, 8). Integrated across the 10-d study period, participants excreted a geometric average of 24.6, 10.3, and 4.3 µmol per 24 h total SF metabolites in urine following treatment with full, one-half, and one-fifth strength doses of beverage, respectively. The daily fluctuations in the excretion of the SF metabolites for each dose group are tracked in Figure 2. Thus, within the dose range used, bioavailability as a fraction of the administered dose remained constant. Moreover, this fraction remained constant across the 10 daily doses. Participants, irrespective of dose, consistently excreted the majority of each dose during the first (overnight) compared with the second 12-h post-dosing period: 71.1%, 71.8%, and 69.8% for full, one-half, and one-fifth strength doses, respectively (e.g., 15.2, 6.2, and 2.6 µmol).
FIGURE 2.
Urinary excretion of sulforaphane and its metabolites (SF-cysteine and SF-mercapturic acid) in micromoles per 24-h collections from participants randomly assigned to receive full, one-half, or one-fifth doses of the broccoli sprout beverage. Individual data points are represented by lines, with median concentrations by day represented by squares. The solid horizontal lines depict the estimated geometric mean from an intercept-only linear mixed model with random intercepts and corresponding 95% CIs displayed as dashed horizontal lines. SF, sulforaphane.
Excretion of SF metabolites exhibited relatively low reproducibility (i.e., minimal tracking) within individuals (Figure 2), with intraclass correlation coefficients of 0.41, 0.34, and 0.28 in the high to low dose groups, respectively, and this figure displays the relatively large intraindividual variability. Using the same full-dose formulation in a previous study, we reported an intraclass correlation coefficient for the repeated measures of SF urinary metabolites over 3 mo to be 0.35 (6, 8). All participants in the 3 broccoli beverage intervention arms excreted detectable amounts of SF metabolites in all samples, another metric indicative of exceptional compliance. Urine from participants drinking the placebo beverage was not measured for SF metabolites but is historically low in this region (3).
Air pollution concentrations
Data for the air quality index (AQI) in the Qidong region during the study period were provided by the Qidong Monitoring Station. AQI is calculated for 4 major air pollutants: particulate matter, ground-level ozone, carbon monoxide, and sulfur dioxide. Particulate matter ≤2.5 µ diameter typically drives the AQI in this region. An AQI of 100 generally corresponds to the national air quality standard for the pollutant. For purposes of public health messaging in Qidong, values of 0–50, 51–100, 101–150, 151–200, and 201–300 are termed good, moderate, lightly polluted, medially polluted, and heavily polluted, respectively. The daily AQI averaged 133 (lightly polluted) during the 10-d intervention period, with hourly excursions reaching a peak of 248 on day 9 and rarely dropping below 50 (Figure 3).
FIGURE 3.
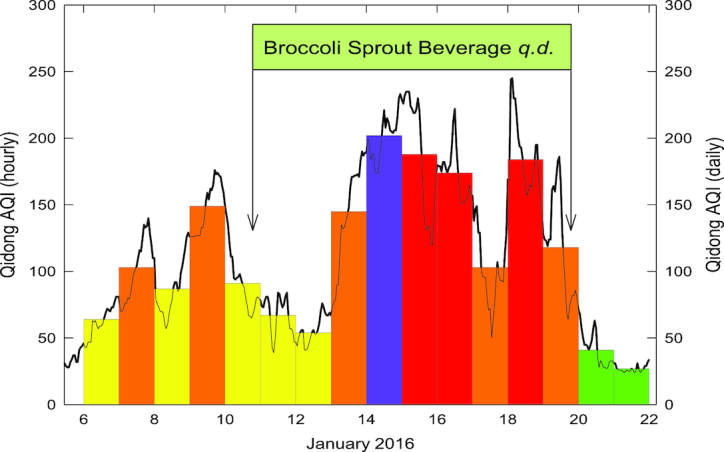
AQI in Qidong during the study period of January 2016. The solid line is the 1-h value, and bars are 24-h values. Values are means of measures at 2 monitors near the study site. Colors are those used to define China AQI categories: “good” (green), “moderate” (yellow), “lightly polluted” (orange), “medially polluted” (red), “heavily polluted” (purple). AQI, air quality index; q.d., daily.
As stated previously, none of the women enrolled in the study were self-reported smokers, whereas ∼35% of the enrolled men were smokers. Urinary cotinine concentrations measured from samples collected the day before the active intervention began were below the limit of detection (∼1 ng/mg creatinine) in 76 out of 127 women. For the 51 women who were positive, 28 had values <5 ng/mg creatinine. The remaining 23 women (18%) had concentrations that ranged from 5.4 to 30.2 ng/mg creatinine (median = 8.0 ng/mg creatinine), which are indicative of non-tobacco users with passive exposure to cigarette smoke. Fourteen men who completed the study, all self-reported smokers, had cotinine concentrations of 242–25,200 ng/mg creatinine (median = 1144 ng/mg creatinine). Median concentrations of cotinine among the assignment groups were similar to the 1 very high cotinine value in the placebo arm. Similar to the nonsmoking women, the 28 nonsmoking men had median concentrations below the limit of detection, with a highest value of 14.2 ng/mg creatinine. Thus, in this study group, self-reported smoking status was completely concordant with urinary cotinine measures; no women were smokers, and few had evidence of passive exposure to tobacco smoke.
Analyses of urinary PGE-M and iso-PGF2α
As a feasibility pilot study, urinary concentrations of PGE-M and iso-PGF2α were measured in the samples collected from participants enrolled in the placebo arm to assess whether these inflammation biomarkers tracked with changing levels of exposures to air pollutants. Using random effects ANOVA, there were no day-to-day differences in the distributions of values for either marker. The intraclass correlation coefficient was 0.50 for 8-iso-PGF2α and 0.57 for PGE-M. Thus, the within-individual variation was fairly high. Biomarker concentrations and the AQI measures taken at the time the samples were collected, and with 1- and 2-d lags between AQI value and biomarker, were examined. There was no association. Although these are useful markers in the context of chronic exposures, inflammation, and disease outcome (27, 28), they are not nimble enough to vary with day-to-day fluctuations in exposures (the dynamic range in AQI during the 10-d period was ∼5-fold).
Effects of broccoli sprout beverage on air pollution biomarkers
Isotope dilution MS was utilized to measure SPMA in urine that had been collected in 12-h overnight fractions during the course of the 10-d intervention trial. Due to catastrophic failure of the mass spectrometer, complete 10-d series of SPMA analyses were completed in only 132 of the 169 evaluable participants. Of the 1320 samples, >95% had detectable concentrations of SPMA. To directly compare results from this clinical trial with prior data reported in this population (6), the analyses reported in Figure 4 depict the individual subject's average concentrations of SPMA in total nanomoles per 12-h urine sample integrated across the 10-d intervention for each of the dose groups. The geometric mean for urinary SPMA excretion in the placebo group was 0.67 pmol/12 h. The 2011–2012 survey of the US adult population from NHANES could not determine a geometric mean for the distribution of SPMA because the proportion of results below the limit of detection was too high; however, the 50th percentile for SPMA was estimated to be ∼0.4 pmol/12 h in 884 smokers and <0.2 pmol/12 h in 1284 nonsmokers (29) (https://www.cdc.gov/exposurereport). Thus, exposures to benzene in the Qidong region of China are typically higher than those measured in the United States.
FIGURE 4.
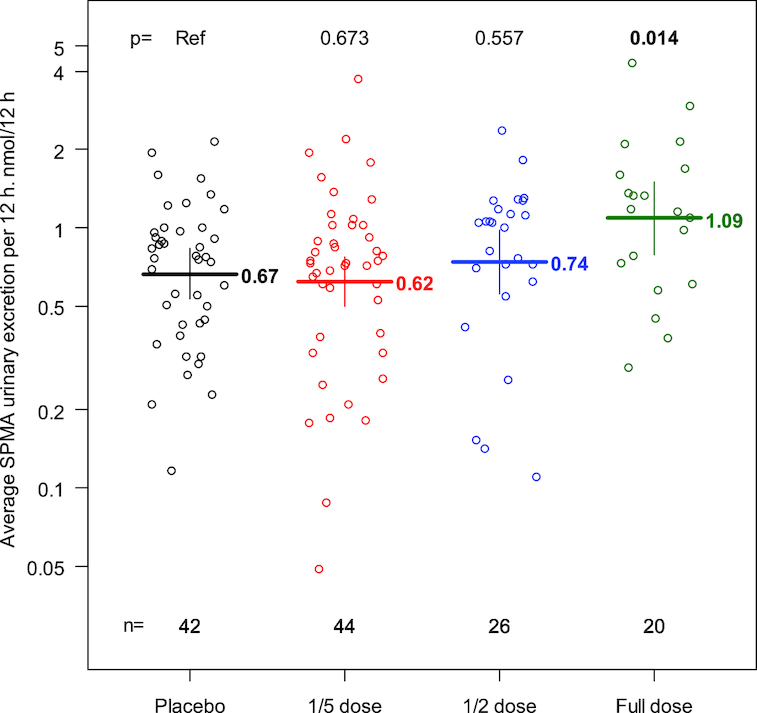
Individual subject average urinary excretion of SPMA per overnight 12 h (nanomoles per 12 h) in each dose group. Dashes depict geometric means within each dose group, with corresponding 95% CIs displayed as vertical bounds. P values are based on a linear regression model with placebo as the reference. SPMA, S-phenylmercapturic acid.
The data in Figure 4 demonstrate a significant increase in the excretion of SPMA in the full-dose group of 63.2% (95% CI: 10.6, 140.9). The increase in the one-half dose group of 11.3% (95% CI: −22.2, 59.1) and the decrease in the one-fifth dose group of −6.4% (95% CI: −31.3, 27.4) were not significant compared with placebo controls.
Figure 5 depicts the fractional difference compared with placebo for the excretion of SPMA in overnight urine compared with the average SF concentrations in micromoles in the same 12-h overnight urine. The 3 data points for 2016 represent the findings in this study, and the 2011 value represents data from our prior study for comparison purposes (6). Figure 6 provides further granularity for the data and explored individual subjects’ average overnight SPMA excretion compared with average SF metabolites, over 24 h. In this exploratory analysis, a nonparametric (Lowess) spline summarized this relation and suggests that >10 μmol SF per 24 h may represent the lowest effective dose of the broccoli sprout extract affecting the biomarker measurement. Thus, using the internal dose metric, excreted SF metabolite concentrations in urine will provide guidance for the design of future SF interventions regardless of formulation.
FIGURE 5.
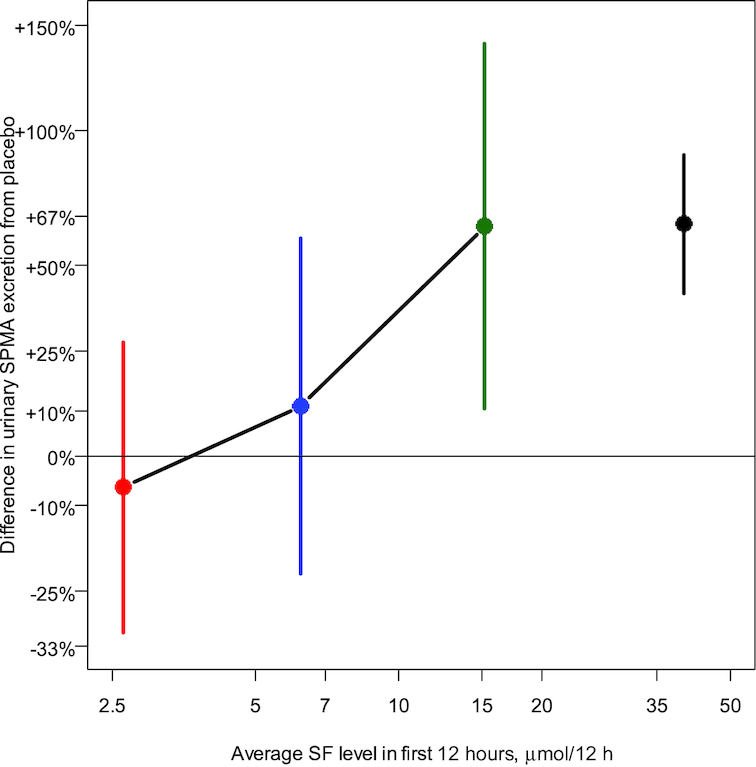
Relation between urinary excretion of SF and its metabolites and urinary SPMA. Percentage difference compared with placebo (one-fifth dose: red; one-half dose: blue; full dose: green) for the excretion of SPMA in overnight urine compared with the average SF concentrations in micromoles in the same 12-h overnight urine. The numbers of samples analyzed for SF metabolites/12 h were 55, 35, and 24 for one-fifth, one-half, and full doses, respectively, and 44, 26, and 20 samples were analyzed for determination of percentage differences in urinary SPMA excretion from placebo. The data point on the right (black) represents the findings from the prior study (full dose) for comparison (6). In that study, 137 participants received the full dose. SF, sulforaphane; SPMA, S-phenylmercapturic acid.
FIGURE 6.
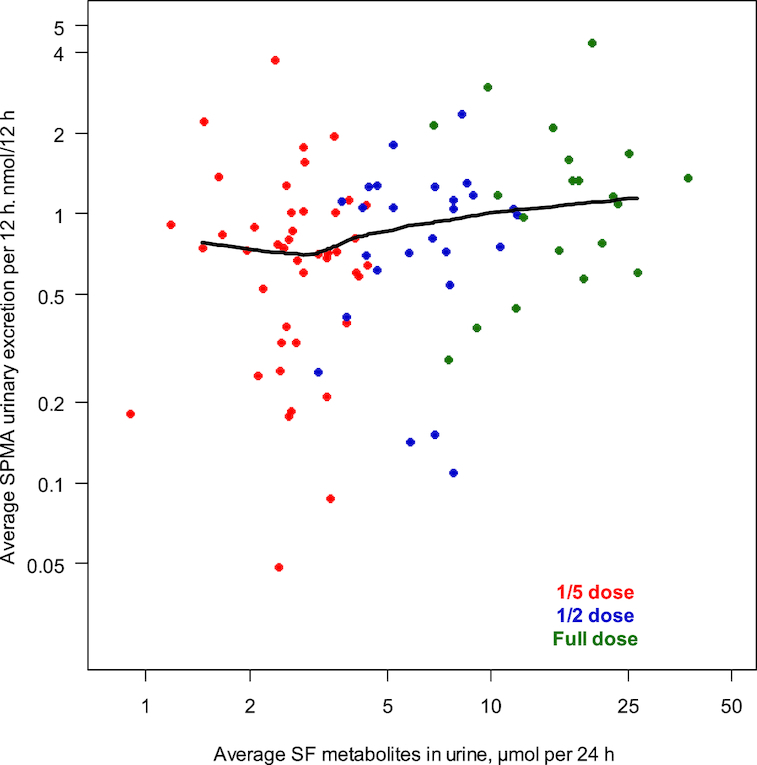
Relation between urinary excretion of SF and its metabolites and urinary SPMA. Individual subjects’ average SPMA compared with their average SF metabolites during 24 h with a Lowess regression. The numbers of samples analyzed for SF metabolites/12 h were 55, 35, and 24 for one-fifth, one-half, and full doses, respectively, and 44, 26, and 20 samples were analyzed for determination of percentage differences in urinary SPMA excretion from placebo. SF, sulforaphane; SPMA, S-phenylmercapturic acid.
Discussion
The IARC has classified outdoor air pollution as a Group 1 carcinogen (carcinogenic to humans) (2). The precise chemical and physical features of ambient air pollution, which comprises a myriad of individual chemical constituents, vary throughout the world due to differences in the sources of pollution, climate, and meteorology, but the mixtures of ambient air pollution invariably contain specific chemicals known to be carcinogenic to humans, including benzene (3). Environmental carcinogens, such as benzene, typically undergo metabolic activation in target cells to form reactive intermediates that damage DNA or other biomolecules (30). In populations suffering from unavoidable exposure to these pollutants, chemoprevention offers reasonable prospects for hazard risk reduction using strategies based on the mechanistic principles of carcinogenesis. Several completed clinical trials from our group have attempted to reduce the burden of damage imparted by environmental exposures (6–8, 31, 32). The end points for these trials were short-term modulations of biomarkers of carcinogen metabolism and/or DNA adducts and other forms of damage. Modulation of these biomarkers is presumptive evidence for a cancer risk reduction, a concept well validated in animal models. One means to alter the balance between bioactivation and detoxication is to induce the expression of GSTs, a multigene family of enzymes that facilitate the nucleophilic addition of glutathione to electrophilic centers in carcinogens, among many substrates (10, 33). The importance of this detoxication pathway is highlighted by studies in cell culture in which overexpression of GSTs renders them resistant to the DNA-damaging actions of carcinogens, whereas disruption of GST expression in cells or mice renders them more sensitive. Pharmacological induction of GST expression leads to reduced DNA damage and a substantive reduction in tumor incidence in animal models (34, 35).
Several key challenges in the design of clinical chemoprevention trials, especially food-based trials, are the selection of the dose, formulation, and dose schedule of the intervention material. Dose schedule is typically delineated by the convenience of a daily schedule and knowledge of the short biological half-life of SF. The other factors are less circumscribed. Selection of dose is complicated by the very different bioavailability of SF when administered in the precursor form of GR and when given as SF. In accordance with other reports in the literature, we have observed recoveries of excreted SF metabolites (principally SF-N-acetylcysteine) to typically range from 2% to 15% when GR was administered (8, 19, 36) and from 70% to 90% when SF was administered in broccoli preparations (8, 37). However, there is in fact enormous interindividual range following administration of GR-rich broccoli beverages, with extremes from 1% to 45%. Thus, bioavailability changes dramatically as a function of the formulation, which in turn reflects how broccoli sprout extracts are prepared (e.g., with or without exogenous myrosinase-catalyzed hydrolysis of GR). Therefore, reporting of administered dose of GR and/or SF may be a poor index of bioavailable/bioactive dose of SF. Given that SF has a short biological half-life (following an oral dose of 201 µmol of SF, the plasma peak at 1.25 h is 1.9 µM, t1/2 is 1.8 h, ∼60% of the dose is excreted within 8 h, and excretion is almost complete by 24 h) (38), we propose that the excreted amount of SF metabolites (SF + SF-cysteinyl-glycine + SF-cysteine + SF-N-acetylcysteine) in urine, which is a measure of internal dose, provides a more revealing and likely consistent view of the delivery of SF to study participants. In the current study, ∼25, ∼10, and ∼5 µmol of SF metabolites were excreted in 24 h with the full, half, and one-fifth doses of broccoli sprout powder, respectively. The lack of effect on SPMA excretion by the one-fifth and one-half doses and robust elevations in SPMA excretion with the full dose clearly delineate the one-fifth dose (120 µmol GR and 8 µmol SF) as a no-effect level and suggest a limited, if any, efficacy for the half dose. It is unlikely that any external dose, regardless of formulation, that does not achieve an initial 12-h excretion of >10 µmol SF metabolites will be effective in disease prevention.
This view is predicated on the assumption that activation of the Nrf2 stress response pathway, which in turn leads to elevated SPMA excretion in humans, occurs at lower or equivalent doses/concentrations of SF than does activation or inhibition of many other cellular processes that could contribute to maintenance of health. Given that induction of detoxication enzymes is reported to occur at sub-micromolar concentrations in cell culture (11), whereas some other putative targets require low to mid-micromolar concentrations for response (39), this assumption is plausible (10). However, few informative dose–response studies of SF attempting to link modulation of molecular pathways to extent of disease prevention have been conducted in vivo. Kong et al. (40) described inhibition of collagen-induced arthritis by graded intraperitoneal doses of SF (14.5–360 µmol/kg). Remarkable peak plasma concentrations of 210 µM at 1 h from the high dose of SF were associated with suppression of arthritis through modulation of autoimmune and cytokine responses and increased synoviocyte apoptosis. More modest peak plasma concentrations of SF (∼1 µM) have been associated with induction of Nrf2 signaling, enhanced apoptosis, and diminished Akt activity along with suppression of prostate tumor growth in a murine transgenic adenocarcinoma of mouse prostate model following feeding with broccoli sprout powder (41).
A practical question then lies in an estimate of the equivalency of broccoli sprout preparations used in clinical trials and what a consumer might expect to find in market-stage broccoli. Many factors make such an estimate fraught with uncertainty. Parameters to consider include plant genotypes affecting concentrations and speciation of glucosinolates, field environment, time from planting to harvest and harvest to consumer, as well as eating or cooking preferences and extent of conversion of GR to SF by plant myrosinase or by β-thioglucosidases present in the host's microbiome but not in host (human) tissues.
GR is by far the most abundant thioalkyl glucosinolate present in harvest heads of cultured broccoli, with estimates of 60–90% of total glucosinolate content and >95% of cytoprotective enzyme inducer activity by bioassay of fractionated plants (7, 42). Nearly all broccoli consumed in the United States is harvested from hybrid production fields. Kushad et al. (43) examined 14 F1 hybrid and open-pollenated cultivars in a single environment and observed a range in GR content of 1.5 to 21.7 µmol g−1 dry head weight. Similar scales of diversity have been reported by Fahey et al. (20). Thus, there is significant variation in the germplasm of broccoli plants. Median concentrations of ∼1 to 2 µmol g−1 are reported across these studies; however, various studies have grown cultivars of broccoli in several environments and found significant effects of both genotype and environment on GR content of the mature heads (42, 44, 45). Conaway et al. (46) examined the 24-h excretion of isothiocyanates following ingestion of 200 g of either fresh or steamed broccoli and reported estimated recoveries of 32.2% and 10.3% of the plant GR content, respectively. Similarly, Vermeulen et al. (47) reported on a small, randomized, free-living crossover feeding trial of raw and cooked broccoli. Peak plasma concentrations (∼0.1 µM) were achieved at 1.6 and 6 h for raw and cooked broccoli, respectively. Elimination half-lives of the SF N-acetylcysteine were comparable (∼2.5 h), indicating that the rate-limiting step for whole-body elimination of GR was likely the initial hydrolysis step. The investigators noted a better bioavailability when subjects were fed raw broccoli (37% of the administered dose) compared with cooked broccoli (3.4%). An earlier study by Shapiro et al. (48) reported urinary recovery of 21% of isothiocyanates following ingestion of broccoli containing 2 µmol GR g−1 fresh weight. Taken together, and assuming 2 µmol g−1 of GR in market-stage broccoli for the typical consumer and a conversion/excretion yield of 20%, a crude estimate for bioequivalence between the full dose of sprout beverage in the current study (∼25 µmol SF metabolites excreted/24 h) and market broccoli is 60 g (∼1 cup). Although this amount is a substantial serving size, the amount of SF that a typical serving would yield has a wide and opaque range for the consumer due to the factors discussed previously. Meta-analyses have found an inverse association between FFQ estimates of cruciferous vegetable intake and lung cancer risk, with intake of crucifers in the highest quartile in line with the estimated serving reported here (49). Other members of the Brassica family of vegetables, such as kale, cauliflower, Brussel sprouts, kohlrabi, radishes, watercress, horseradish, and cabbage, are all sources of other closely related isothiocyanates, but none are as potent inducers of Nrf2/Keap1 signaling as SF.
Several efforts have been undertaken to reduce this uncertainty. High-GR broccoli has been developed by introduction of the allele for the Myb28 transcription factor that modulates the expression of genes associated with sulfate assimilation and glucosinolate biosynthesis (36). Greater than 4-fold increases in 24-h urinary excretion of SF metabolites are observed when this MybBv/v genotype is fed as a broccoli soup compared with soup from standard broccoli. Thus, very moderate amounts of mature broccoli could deliver effective internal doses of SF. As an alternative approach to reduce this variability, the dietary supplement industry has introduced tablets formulated from milled broccoli seeds to provide GR and freeze-dried broccoli to provide myrosinase. Conversion of GR to SF in the gastrointestinal tract is enhanced by this strategy (21). Although some supplements are in current use in clinical trials, concern should be raised by the limited degree of quality control imparted into the manufacturing of such products. Pharmaceutical-grade formulations of stabilized SF are also currently in clinical trials (50, 51) and may further obviate concerns regarding interplant and interindividual variations, at least in interventions targeting higher risk individuals in predisease states. Nonetheless, regardless of formulations, dose selections should be guided by pharmacokinetic measures of excreted dose (over either 12 or 24 h) of SF metabolites. Reliable biomarkers of pharmacodynamic action could also be used but remain to be established.
Acknowledgments
The authors’ responsibilities were as follows—J-GC, DN, JZ, J-BW, SH, LJ, AM, JF, TK, and JG: designed the research; J-GC, JJ, PE, JZ, J-BW, X-FX, YS, Y-HZ, L-LL, Y-SC, YW, Y-RZ, SC, LJ, KK, AR, TK, and JG: conducted the research; PE, SC, SH, and JF: provided essential reagents/materials; PE, DN, JZ, LJ, and AM: analyzed data or performed statistical analyses; TK and JG: wrote the paper; J-GC, DN, TK, and JG: had primary responsibility for final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This work was supported in part by NIH grants R01 CA190610 and R35 CA197222.
J-GC, JJ, PE, and DN contributed equally to this work.
Abbreviations used: AQI, air quality index; ESI, electrospray ionization; FWHM, full width at half maximum; GR, glucoraphanin; GST, glutathione S-transferase; IARC, International Agency for Research on Cancer; PGE-M, PGE2 metabolite; psi, pounds per square inch; SF, sulforaphane; SPMA, S-phenylmercapturic acid; SRM, selected reaction monitoring; 8-iso-PGF2α, 8-iso-prostaglandin F2α.
References
- 1. Tian L, Sun S. Comparison of health impact of air pollution between China and other countries. Adv Exp Med Biol. 2017;1017:215–32. [DOI] [PubMed] [Google Scholar]
- 2. Loomis D, Grosse Y, Lauby-Secretan B, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–3. [DOI] [PubMed] [Google Scholar]
- 3. Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Vilahur N, Mattock H, Straif K et al.. Carcinogenicity of benzene. Lancet Oncol. 2017;18(12):1574–5. [DOI] [PubMed] [Google Scholar]
- 4. Chen B, Hong C, Kan H. Exposures and health outcomes from outdoor air pollutants in China. Toxicology. 2004;198(1–3):291–300. [DOI] [PubMed] [Google Scholar]
- 5. Lin J, Pan D, Davis SJ, Zhang Q, He K, Wang C, Streets DG, Wuebbles DJ, Guan D. China's international trade and air pollution in the United States. Proc Natl Acad Sci USA. 2014;111(5):1736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD et al.. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014;7(8):813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y et al.. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2605–13. [DOI] [PubMed] [Google Scholar]
- 8. Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, Zhu J, Zhang YH, Chen YS, Friesen MD et al.. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila). 2011;4(3):384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palliyaguru DL, Yuan JM, Kensler TW, Fahey JW. Isothiocyanates: translating the power of plants to people. Mol Nutr Food Res. 2018;62(18):e1700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, Talalay P. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91(8):3147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–100. [PubMed] [Google Scholar]
- 14. Chartoumpekis D, Ziros P, Chen J, Groopman J, Kensler T, Sykiotis G. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: results of a 12-week randomized trial. Food Chem Toxicol. 2019;126:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berhane K, Widersten M, Engstrom A, Kozarich JW, Mannervik B. Detoxication of base propenals and other α,β-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci USA. 1994;91(4):1480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pal A, Hu X, Zimniak P, Singh SV. Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of α,β-unsaturated aldehydes. Cancer Lett. 2000;154(1):39–43. [DOI] [PubMed] [Google Scholar]
- 17. Qu Q, Shore R, Li G, Su L, Jin X, Melikian AA, Roy N, Chen LC, Wirgin I, Cohen B et al.. Biomarkers of benzene: urinary metabolites in relation to individual genotype and personal exposure. Chem Biol Interact. 2005;153–154:85–95. [DOI] [PubMed] [Google Scholar]
- 18. Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Muñoz A, Egner PA, Chen JG, Qian GS, Chen TY et al.. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33(1):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila). 2012;5(4):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94(19):10367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fahey JW, Wade KL, Wehage SL, Holtzclaw WD, Liu H, Talalay P, Fuchs E, Stephenson KK. Stabilized sulforaphane for clinical use: phytochemical delivery efficiency. Mol Nutr Food Res. 2017;61(4). [DOI] [PubMed] [Google Scholar]
- 22. Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P. Sulforaphane bioavailability from glucoraphanin-rich broccoli: control by active endogenous myrosinase. PLoS One. 2015;10(11):e0140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egner PA, Kensler TW, Chen JG, Gange SJ, Groopman JD, Friesen MD. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol. 2008;21(10):1991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J Lipid Res. 2007;48(7):1607–17. [DOI] [PubMed] [Google Scholar]
- 25. Chen M, Carmella SG, Sipe C, Jensen J, Luo X, Le CT, Murphy SE, Benowitz NL, McClernon FJ, Vandrey R et al.. Longitudinal stability in cigarette smokers of urinary biomarkers of exposure to the toxicants acrylonitrile and acrolein. PLoS One. 2019;14(1):e0210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neale JR, Dean BJ. Liquid chromatography–tandem mass spectrometric quantification of the dehydration product of tetranor PGE-M, the major urinary metabolite of prostaglandin E(2) in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871(1):72–7. [DOI] [PubMed] [Google Scholar]
- 27. Yuan JM, Grouls M, Carmella SG, Wang R, Heskin A, Jiang Y, Tan YT, Adams-Haduch J, Gao YT, Hecht SS. Prediagnostic levels of urinary 8-epi-prostaglandin F2α and prostaglandin E2 metabolite, biomarkers of oxidative damage and inflammation, and risk of hepatocellular carcinoma. Carcinogenesis. 2019; [Epub ahead of print]. doi:10.1093/carcin/bgy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan JM, Carmella SG, Wang R, Tan YT, Adams-Haduch J, Gao YT, Hecht SS. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2α to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis. 2018;39(7):948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta (GA): CDC; 2019. [PubMed] [Google Scholar]
- 30. Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Smith MT, Zhang L, Li G, Shen M, Yin S et al.. Modeling human metabolism of benzene following occupational and environmental exposures. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2246–52. [DOI] [PubMed] [Google Scholar]
- 31. Wang JS,Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA et al.. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91(4):347–54. [DOI] [PubMed] [Google Scholar]
- 32. Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJ, Jacobson LP et al.. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci USA. 2001;98(25):14601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): predictive role for short-term molecular dosimetry. Cancer Res. 1991;51(20):5501–6. [PubMed] [Google Scholar]
- 35. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. [DOI] [PubMed] [Google Scholar]
- 36. Sivapalan T, Melchini A, Saha S, Needs PW, Traka MH, Tapp H, Dainty JR, Mithen RF. Bioavailability of glucoraphanin and sulforaphane from high-glucoraphanin broccoli. Mol Nutr Food Res. 2018;62(18):e1700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82(6):1283–91. [DOI] [PubMed] [Google Scholar]
- 38. Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316(1-2):43–53. [DOI] [PubMed] [Google Scholar]
- 39. Jiang X, Liu Y, Ma L, Ji R, Qu Y, Xin Y, Lv G. Chemopreventive activity of sulforaphane. Drug Des Devel Ther. 2018;12:2905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kong JS, Yoo SA, Kim HS, Kim HA, Yea K, Ryu SH, Chung YJ, Cho CS, Kim WU. Inhibition of synovial hyperplasia, rheumatoid T cell activation, and experimental arthritis in mice by sulforaphane, a naturally occurring isothiocyanate. Arthritis Rheum. 2010;62(1):159–70. [DOI] [PubMed] [Google Scholar]
- 41. Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, Li W, Kong AN. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26(10):2324–31. [DOI] [PubMed] [Google Scholar]
- 42. Farnham MW, Stephenson K, Fahey J. Capacity of broccoli to induce a mammalian chemoprotective enzyme varies among inbred lines. J Am Soc Hort Sci. 2000;125:482–8. [Google Scholar]
- 43. Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47(4):1541–8. [DOI] [PubMed] [Google Scholar]
- 44. Farnham MW, Stephenson KK, Fahey JW. Glucoraphanin level in broccoli seed is largely determined by genotype. HortSci. 2005;40:50–3. [Google Scholar]
- 45. Farnham MW, Wilson PE, Stephenson KK, Fahey JW. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breeding. 2004;123:60–5. [Google Scholar]
- 46. Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38(2):168–78. [DOI] [PubMed] [Google Scholar]
- 47. Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56(22):10505–9. [DOI] [PubMed] [Google Scholar]
- 48. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–8. [PubMed] [Google Scholar]
- 49. Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, Ji BT, Gao YT, Shu XO, Xiang YB. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol. 2013;24(7):1918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, Le Scodan R, Azzouzi AR, Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila). 2015;8(8):712–9. [DOI] [PubMed] [Google Scholar]
- 51. Cuadrado A, Rojo AI,Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW et al.. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18(4):295–317. [DOI] [PubMed] [Google Scholar]