Abstract
Identification and management of patients at high bleeding risk undergoing percutaneous coronary intervention are of major importance, but a lack of standardization in defining this population limits trial design, data interpretation, and clinical decision-making. The Academic Research Consortium for High Bleeding Risk (ARC-HBR) is a collaboration among leading research organizations, regulatory authorities, and physician-scientists from the United States, Asia, and Europe focusing on percutaneous coronary intervention–related bleeding. Two meetings of the 31-member consortium were held in Washington, DC, in April 2018 and in Paris, France, in October 2018. These meetings were organized by the Cardiovascular European Research Center on behalf of the ARC-HBR group and included representatives of the US Food and Drug Administration and the Japanese Pharmaceuticals and Medical Devices Agency, as well as observers from the pharmaceutical and medical device industries. A consensus definition of patients at high bleeding risk was developed that was based on review of the available evidence. The definition is intended to provide consistency in defining this population for clinical trials and to complement clinical decision-making and regulatory review. The proposed ARC-HBR consensus document represents the first pragmatic approach to a consistent definition of high bleeding risk in clinical trials evaluating the safety and effectiveness of devices and drug regimens for patients undergoing percutaneous coronary intervention.
Keywords: clinical trial protocols as topic, hemorrhage, percutaneous coronary intervention
Abbreviations
- ACS
acute coronary syndrome
- AF
atrial fibrillation
- ARC
Academic Research Consortium
- BARC
Bleeding Academic Research Consortium
- bAVM
brain arteriovenous malformation
- BMS
bare metal stent
- CAD
coronary artery disease
- CKD
chronic kidney disease
- DAPT
dual antiplatelet therapy
- DES
drug-eluting stent
- eGFR
estimated glomerular filtration rate
- HBR
high bleeding risk
- HR
hazard ratio
- ICH
intracranial hemorrhage
- NIS
Nationwide Inpatient Sample
- NSAID
nonsteroidal anti-inflammatory drug
- OAC
oral anticoagulant
- OR
odds ratio
- PCI
percutaneous coronary intervention
- TIA
transient ischemic attack
- VKA
vitamin K antagonist
The evolution of percutaneous coronary intervention (PCI) over the last 40 years has facilitated treatment of increasingly complex patient populations. One such population comprises patients at high bleeding risk (HBR). In early trials of first-generation drug-eluting stents (DES), the protocol-recommended dual antiplatelet therapy (DAPT) duration was 3 to 6 months, but as a result of concerns about late thrombotic events, this was increased to 12 months in studies initiated after 2006.1 Coinciding with this shift, patients considered to be at HBR were either excluded from or underrepresented in clinical trials. The accepted practice in such patients was bare metal stent (BMS) implantation, given that 1 month of DAPT was considered sufficient at that time. Until recently, even more inclusive studies of contemporary DES continued to exclude patients for whom guideline-recommended DAPT was considered unsuitable.2,3
Recently, 3 randomized trials comparing DES and BMS with 1 month of DAPT in patients perceived to be at increased bleeding risk showed superior safety and efficacy with DES.4–6 These reports quickly generated global attention as an important public health concern given that, as recently as 2014, BMSs were used in 20% of coronary stenting procedures in patients ≥65 years of age in the United States, with 18.2% of BMS recipients having a predicted bleeding risk of ≥5%/y.7
The challenges in defining the optimal management of patients undergoing PCI at HBR include a paucity of relevant clinical data and the use of heterogeneous definitions of HBR that limit interpretation, generalization, and pooling of published data. In 2006, the first Academic Research Consortium (ARC) provided standardized definitions of ischemic end points for coronary stent trials, and in 2011, the Bleeding ARC (BARC) provided bleeding end point definitions, both of which have gained wide acceptance in clinical study design, demonstrating the value of consensus-based definitions in the PCI field.8,9
With this in mind, the aim of the ARC-HBR initiative is to define HBR in patients undergoing PCI on the basis of a literature review and clinical consensus with the primary goal of advancing the consistency and quality of data collection and reporting, thereby supporting organizations tasked with making recommendations for clinical practice or regulatory decisions.10 To this end, 2 meetings of the ARC-HBR group were organized by the Cardiovascular European Research Center (Massy, France) in Washington, DC, in April 2018 and Paris, France, in October 2018. International academic experts; representatives of the US Food and Drug Administration, the Japanese Pharmaceuticals and Medical Devices Agency, and a European Notified Body (DEKRA, Arnhem, the Netherlands); and observers from the device and pharmaceutical industries attended (participants are listed in the Appendix in the online-only Data Supplement).
Contemporary clinical trials of coronary stents and antiplatelet therapy: not generalizable to patients at HBR
Regulatory approval processes for medical devices differ between jurisdictions.11 In the United States, for example, completed pivotal randomized trials of investigational DES submitted for US Food and Drug Administration review have been prospective, multicenter studies with high internal validity, but enrollment has been limited to highly selected patients and lesions.12–18 Patients considered unsuitable for protocol-mandated DAPT duration have been excluded. Although more recent DES trials have had more liberal enrollment criteria, per protocol or de facto, they have continued to exclude patients with advanced renal impairment, prior bleeding, prior recent stroke, and hematologic abnormalities (Table I in the online-only Data Supplement).16–18
Many investigator-initiated “all-comer” randomized trials included some patients at increased bleeding risk.2,19–24 However, only a minority of screened patients tend to be enrolled, mean patient age is similar to that in earlier trials, patients unsuitable for long-term DAPT continue to be systematically excluded, and details on the proportion of patients taking oral anticoagulation (OAC) or with other bleeding risk factors are not consistently reported.2,19–24 Thus, despite broader inclusion criteria, subjects at HBR are still underrepresented in contemporary studies.
Clinical trials of DAPT strategies after stenting have also excluded patients at HBR, with reported major bleeding rates at 1 year varying between 0.3% and 2.8% (Table 1).25–34
Table 1.
One-year bleeding rates in trials of antiplatelet therapy after coronary stenting
| Trial (Year of Publication) | Patients, n | Type of Patients | Inclusion of Periprocedural Bleeding | Overall Bleeding, % | Bleeding Definition Used | Adjudication of Bleeding Events |
|---|---|---|---|---|---|---|
| RESET (2012)25 | 2117 | Selected low bleeding risk | Yes | 0.7 | TIMI major or minor | CEC adjudicated |
| EXCELLENT (2012)26 | 1443 | Selected low bleeding risk | Yes | 1 | TIMI major or minor | CEC adjudicated |
| ARCTIC (2012)27 | 2440 | All comers | Yes | 2.8 | STEEPLE major | CEC adjudicated |
| PRODIGY (2012)28 | 1970 | All comers | No (first 30 d excluded) | 2.0† | BARC 3 or 5 | CEC adjudicated |
| OPTIMIZE (2013)29 | 3119 | Selected low bleeding risk | Yes | 0.5 | Protocol-defined | CEC adjudicated |
| DAPT* (2014)30 | 22 866 | Selected low bleeding risk | Yes | 2.7 | GUSTO moderate or severe | CEC adjudicated |
| SECURITY (2014)31 | 1399 | Selected low bleeding risk | Yes | 0.9 | BARC 3 or 5 | CEC adjudicated |
| PRECISE-DAPT (2017)32 | 14 963 | Selected low bleeding risk | No (first 7 d excluded) | 1.5 | TIMI major or minor | CEC adjudicated |
| SMART-DATE (2018)34 | 2712 | ACS | Yes | 0.3† | BARC 3 or 5 | CEC adjudicated |
| GLOBAL LEADERS (2018)33 | 15 968 | All comers | Yes | 1.9† | BARC 3 or 5 | Site reported |
Bleeding definitions are shown in the Appendix in the online-only Data Supplement. ACS indicates acute coronary syndrome; ARCTIC, bedside monitoring to adjust antiplatelet therapy for coronary stenting; BARC, Bleeding Academic Research Consortium; CEC, Clinical Events Committee; DAPT, Dual Antiplatelet Therapy Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting; GLOBAL LEADERS, Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; OPTIMIZE, Optimized Duration of Clopidogrel Therapy Following Treatment With the Endeavor; PRECISE-DAPT, Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy; PRODIGY, Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study; RESET, Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation; SECURITY, Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month Dual-Antiplatelet Therapy; SMART-DATE, Safety of 6-Month Duration of Dual Antiplatelet Therapy After Acute Coronary Syndromes; STEEPLE, Safety and Efficacy of Enoxaparin in PCI Patients, an International Randomized Evaluation; and TIMI, Thrombolysis in Myocardial Infarction.
First year after enrollment, before randomization. †One-year bleeding rates were obtained as personal communications from the principal investigators of these 3 trials.
Contemporary clinical trials in patients at increased risk of bleeding
Three randomized trials investigating short DAPT durations have been completed in patients undergoing PCI perceived to be at increased bleeding risk,4–6 and many trials are currently ongoing (Table II in the online-only Data Supplement). Inclusion criteria in these trials largely reflect exclusion criteria in prior DES studies of patients not at HBR receiving different DAPT durations, but there is significant heterogeneity with respect to the patient populations included.
Among completed studies, the LEADERS FREE trial (Polymer-free drug-coated coronary stents in patients at high bleeding risk; n=2466) was the trial most broadly inclusive of patients at HBR to date, with a mean of 1.7 bleeding risk criteria per patient.4 The ZEUS trial (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates; n=1606) enrolled uncertain DES candidates on the basis of criteria for high thrombotic, restenotic, or bleeding risk,35 with a prespecified subgroup analysis of patients who met criteria for HBR (ZEUS-HBR; n=828).5 The SENIOR trial (Drug-eluting stents in elderly patients with coronary artery disease: a randomized single-blind trial; n=1200) included elderly patients with no other specified inclusion criteria associated with increased bleeding risk.6 The most frequently met criterion associated with increased bleeding risk in all 3 trials was advanced age (in 64%, 51%, and 100% of patients in LEADERS FREE, ZEUS-HBR, and SENIOR, respectively), although the lower cutoff for age differed between trials (>80 years in ZEUS-HBR versus ≥75 years in LEADERS FREE and SENIOR). The second most frequently met characteristic was indication for OAC in 36%, 38%, and 18% of patients, respectively. Although renal impairment was the third most commonly met criterion in LEADERS FREE (19%), it was not a prespecified criterion for HBR status in ZEUS-HBR. Early planned surgery was a bleeding risk inclusion criterion in LEADERS FREE (met in 16% of patients), but such patients were excluded in ZEUS-HBR and SENIOR. Prior hemorrhagic stroke was also an inclusion criterion in LEADERS FREE but was an exclusion criterion in SENIOR, and although it was not an exclusion criterion in ZEUS-HBR, no information on its prevalence is provided. Bleeding rates according to inclusion criteria in LEADERS FREE are shown in Table III in the online-only Data Supplement.
The differences in eligibility criteria and enrolled patient populations in completed trials are reflected in the differences in bleeding event rates. In LEADERS FREE and ZEUS-HBR, the 1-year rates of BARC 3 to 5 bleeding in patients treated with 1-month DAPT after PCI were 7.3% and 4.2%, respectively, and in the SENIOR trial, the 1-year BARC 3 to 5 bleeding rate in patients treated with 1 to 6 months of DAPT after PCI was ≈3.5%. Such differences highlight the need for a standardized definition of HBR.
Currently available bleeding risk scores
At least 6 scores have been developed that predict long-term bleeding risk in patients taking antiplatelet therapy.32,36–39 The 2017 European Society of Cardiology focused update on DAPT in coronary artery disease (CAD) recommended (Class IIb recommendation, Level of Evidence A) that the use of risk scores such as the PRECISE-DAPT (Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy) and DAPT scores may be considered to guide antiplatelet therapy after PCI.40
The main features of existing scores are summarized in Table 2, and the variables in each score are shown in Table IV in the online-only Data Supplement.32,36–39,41 Advanced age is the only variable common to all scores, but age cutoffs for increased bleeding risk and their relative weights vary between risk scores. In addition, although baseline anemia was found to be one of the strongest independent predictors of bleeding assessed in PARIS (patterns of non-adherence to anti-platelet regimens in stented patients), BleeMACS (Bleeding Complications in an Multicenter Registry of Patients Discharged With Diagnosis of Acute Coronary Syndrome), the Dutch aspirin score, and PRECISE-DAPT,32,36–38 it was not assessed in the development of the REACH (Reduction of Atherothrombosis for Continued Health Registry) or DAPT score.39,41 Moreover, definitions of anemia differed between studies.
Table 2.
Scores assessing long-term bleeding risk in patients taking antiplatelet therapy
| REACH39 | Dutch ASA Score37 | DAPT41* | PARIS38 | PRECISE-DAPT32 | BleeMACS36 | |
|---|---|---|---|---|---|---|
| Year of publication | 2010 | 2014 | 2016 | 2016 | 2017 | 2018 |
| Development data set | REACH registry | Dutch ASA registry | DAPT randomized trial | PARIS registry | Pooled analysis of 8 randomized trials | BleeMACS registry |
| Development data set, n | 56 616 | 235 531 | 11 648 | 4190 | 14 963 | 15 401 |
| Patient population | Risk of atherothrombosis† | New low-dose aspirin users | Stable and event-free patients 12 mo after PCI | Stable and unstable patients undergoing PCI | Stable and unstable patients undergoing PCI | Patients with ACS undergoing PCI |
| Bleeding outcome | Serious bleedingat 2 y | Upper GI bleeding at a median follow-up of 530 d | Major bleeding between 12 and 30 mo after PCI | Major bleedingat 2 y | Out-of-hospital bleeding at a median follow-up of 552 d | Serious spontaneous bleeding at 1 y |
| Bleeding definition used | Protocol-defined | First episode of upper GI bleeding | GUSTO moderate or severe | BARC 3 or 5 | TIMI major or minor | Protocol-defined |
| Proportion of patients at HBR | 25% (score >11) | 83.1% (score ≥1) | 23.4% (score −2 to 0) | 8% (score ≥8) | 25% (score ≥25) | 25% (score ≥26) |
| Rate of bleedingin the HBR subgroup | 2.76%(at 2 y) | 1%–35% for scores from 2 to 13 | 2.7%(between 13 and 30 mo) | 10.7%(at 2 y) | 1.8%–4.2%(at 1 y) | 8.03%(at 1 y) |
| Also evaluates thrombotic risk | No | No | Yes | Yes | No | No |
| Score range | 0 to 23 | 0 to 15 | –2 to 10 | 0 to 14 | 0 to 100 | 0 to 80 |
| Development discrimination | AUC 0.68 | AUC 0.64 | AUC 0.68 | AUC 0.72 | AUC 0.73 | AUC 0.71 (0.72 in internal validation) |
| Validating data set | CHARISMA | Dutch health insurance database | PROTECT | ADAPT-DES | PLATO andBern PCI registry | SWEDEHEART |
| Validating dataset, n | 15 603 | 32 613 | 8136 | 8130 | 8595 and 6172 | 96 239 (ACS+PCI);93,150 (ACS) |
| Validation discrimination | AUC 0.64 | AUC 0.63 | AUC 0.64 (bleeding) | AUC 0.64 | AUC 0.70 and 0.66 | AUC 0.65 (ACS+PCI);AUC 0.63 (ACS) |
Bleeding definitions are shown in the Appendix in the online-only Data Supplement. ACS indicates acute coronary syndrome; ADAPT-DES, Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents; ASA, aspirin; AUC, area under the curve; BARC, Bleeding Academic Research Consortium; BleeMACS, Bleeding Complications in a Multicenter Registry of Patients Discharged With Diagnosis of Acute Coronary Syndrome; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; DAPT, Dual Antiplatelet Therapy Trial; GI, gastrointestinal; GUSTO, Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries; HBR, high bleeding risk; PARIS, patterns of non-adherence to anti-platelet regimens in stented patients; PCI, percutaneous coronary intervention; PLATO, Platelet Inhibition and Patient Outcomes; PRECISE-DAPT, Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy; PROTECT, Patient Related Outcomes With Endeavor Versus Cypher Stenting Trial; REACH, Reduction of Atherothrombosis for Continued Health Registry; and TIMI, Thrombolysis in Myocardial Infarction.
The DAPT score is not purely a bleeding risk score; rather, it is a score to predict benefit versus harm of prolonged dual antiplatelet therapy (>1 year) in patients after percutaneous coronary intervention. Thus, it integrates covariates independently associated with bleeding (but not ischemic) risk and vice versa. †Risk of atherothrombosis in REACH was defined as cardiovascular disease, coronary artery disease, peripheral artery disease, or ≥3 cardiovascular risk factors.
Five variables (prior malignancy, congestive heart failure, body mass index <25 or ≥35 kg/m2, hypercholesterolemia, and elevated white cell count) are present in only 1 score. Furthermore, all scores omit certain important variables known to be associated with HBR because their prevalence is low in patients with CAD or those undergoing PCI (eg, severe liver disease, bleeding diatheses, or thrombocytopenia), because they were rarely recorded in the derivation data sets (eg, history of cancer or prior bleeding, use of nonsteroidal anti-inflammatory drugs [NSAIDs], or planned surgery), or because collinearity with other selected predictors may have overshadowed their significance.
Such differences in risk prediction scores reflect heterogeneity in the patient populations studied, the variables assessed (and their definitions), and the bleeding definitions used in the development cohorts. At best, these scores have moderate accuracy for predicting bleeding, with C statistics in the development cohorts ranging from 0.64 to 0.73 (Table 2). Moreover, none of these scores was validated in HBR patient populations, highlighting the need for standardized HBR criteria for evaluating such patients.
Defining HBR criteria
HBR is defined as a BARC 3 or 5 bleeding risk of ≥4% at 1 year or a risk of an intracranial hemorrhage (ICH) of ≥1% at 1 year. Thus, a major criterion for ARC-HBR is defined as any criterion that, in isolation, is considered to confer a BARC 3 or 5 bleeding risk of ≥4% at 1 year or any criterion considered to be associated with a risk of ICH of ≥1% at 1 year. A minor criterion for ARC-HBR is defined as any criterion that, in isolation, is considered to confer increased bleeding risk, with a BARC 3 or 5 bleeding rate of <4% at 1 year.
The cutoff value of 4% for BARC 3 or 5 bleeding was based on consensus of the participants, taking into account that 1-year major bleeding rates in trials of DAPT after PCI, which largely excluded patients at HBR, were <3% (Table 1) and that, in DES trials enrolling patients at HBR, 1-year BARC 3 to 5 bleeding rates were higher (7.2% in LEADERS FREE [with 1.7 HBR criteria per patient] and 4.2% in ZEUS-HBR despite only 1 month of DAPT after PCI) and 3.5% in the SENIOR trial (in which age ≥75 years was the sole inclusion criterion).
Proposed HBR definition
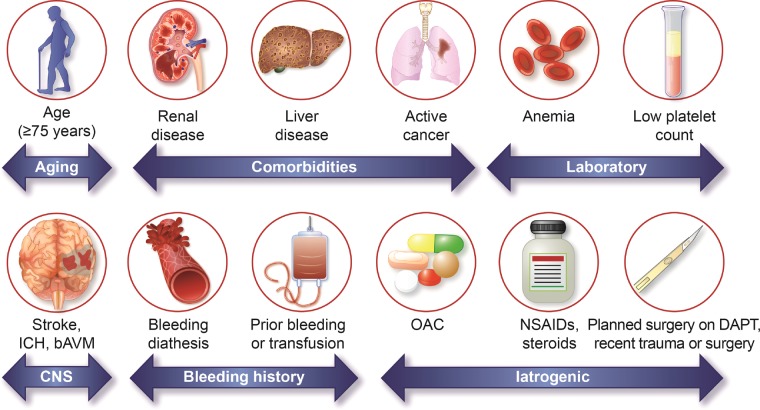
Twenty clinical criteria were identified as major or minor by consensus, supported by published evidence (Table 3 and Figure). Patients are considered to be at HBR if at least 1 major or 2 minor criteria are met. The definition is thus binary. Although it is recognized that the coexistence of increasing numbers of risk factors for bleeding is associated with a stepwise increase in risk of BARC 3 to 5 bleeding,5 sufficient data are not currently available to create a point-based score that would take into account the relative weight of each HBR criterion. Nonetheless, the presence of increasing numbers of major or minor criteria in any patient further increases bleeding risk, which may be considered in clinical decision-making and clinical trial analysis. The proposed consensus-based definition takes into account the available evidence for patients at HBR undergoing PCI and is pragmatic for application to clinical trials supporting clinical practice recommendations and regulatory review. The criteria making up the definition are discussed below. Associated major (preferably BARC 3 or 5) bleeding rates or rates of ICH at 1 year are provided when available. Factors that were considered but not deemed HBR criteria are also discussed.
Table 3.
Major and minor criteria for hbr at the time of PCI
| Major | Minor |
|---|---|
| Age ≥75 y | |
| Anticipated use of long-term oral anticoagulation* | |
| Severe or end-stage CKD (eGFR <30 mL/min) | Moderate CKD (eGFR 30–59 mL/min) |
| Hemoglobin <11 g/dL | Hemoglobin 11–12.9 g/dL for men and 11–11.9 g/dL for women |
| Spontaneous bleeding requiring hospitalization or transfusion in the past 6 mo or at any time, if recurrent | Spontaneous bleeding requiring hospitalization or transfusion within the past 12 mo not meeting the major criterion |
| Moderate or severe baseline thrombocytopenia† (platelet count <100 × 109/L) | |
| Chronic bleeding diathesis | |
| Liver cirrhosis with portal hypertension | |
| Long-term use of oral NSAIDs or steroids | |
| Active malignancy‡ (excluding nonmelanoma skin cancer) within the past 12 mo | |
| Previous spontaneous ICH (at any time)Previous traumatic ICH within the past 12 moPresence of a bAVMModerate or severe ischemic stroke§ within the past 6 mo | Any ischemic stroke at any time not meeting the major criterion |
| Nondeferrable major surgery on DAPT | |
| Recent major surgery or major trauma within 30 d before PCI |
bAVM indicates brain arteriovenous malformation; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; HBR, high bleeding risk; ICH, intracranial hemorrhage; NSAID, nonsteroidal anti-inflammatory drug; and PCI, percutaneous coronary intervention.
This excludes vascular protection doses.42†Baseline thrombocytopenia is defined as thrombocytopenia before PCI. ‡Active malignancy is defined as diagnosis within 12 months and/or ongoing requirement for treatment (including surgery, chemotherapy, or radiotherapy).
National Institutes of Health Stroke Scale score ≥5.
Figure.
Factors associated with an increased bleeding risk after percutaneous coronary intervention. bAVM indicates brain arteriovenous malformation; CNS, central nervous system; DAPT, dual antiplatelet treatment; ICH, intracranial hemorrhage; NSAID, nonsteroidal anti-inflammatory drug; and OAC, oral anticoagulation.
Age
Age ≥75 years is considered a minor ARC-HBR criterion (Table 3).
Although elderly patients represent the fastest-growing patient subgroup undergoing PCI,43,44 they tend to be underrepresented in randomized trials of DES and DAPT. In the SENIOR trial, which included patients ≥75 years of age (mean age, 81.4±4.2 years) treated with 1 or 6 months of DAPT after coronary stenting (DES versus BMS), the 1-year rate of BARC 3 to 5 bleeding was ≈3.5%. Indeed, elderly patients undergoing PCI tend to have more comorbidities and coexisting risk factors for bleeding compared with younger patients.45 A substudy of elderly patients (≥75 years) enrolled in the LEADERS FREE trial (n=1564) showed that patients who qualified for inclusion on the basis of age alone (n=562) had a lower rate of 1-year BARC 3 to 5 bleeding compared with the overall elderly population (3.2% versus 7.8%, respectively).46 Nonetheless, in the development cohorts of bleeding risk scores in patients undergoing PCI, advanced age generally persisted as an independent predictor of bleeding after adjustment for coexisting bleeding risk factors.32,38,41,47–51
In a patient-level meta-analysis of 6 randomized trials (n=11 473) comparing short (≤6 months) and longer (12 months) DAPT duration after PCI, short DAPT halved the rate of protocol-defined major bleeding at 1 year in patients ≥65 years of age (0.5% versus 1.1%; hazard ratio [HR], 0.46 [95% CI, 0.24–0.88]; P=0.02), without increasing ischemic events (2.4% versus 3.0%; HR, 0.84 [95% CI, 0.60–1.16]; P=0.2856). In contrast, in younger patients, short DAPT failed to reduce bleeding (0.3% versus 0.5%; HR, 0.59 [95% CI, 0.26–1.34]; P=0.21), but ischemic events were significantly increased (2.4% versus 1.4%; HR, 1.67 [95% CI, 1.14–2.44]; P=0.0082), suggesting differential bleeding–ischemic risk profiles in elderly versus younger patients after PCI.52
In summary, bleeding risk increases with age with some confounding resulting from comorbidities, which tend to accumulate in elderly patients. With this in mind, it must be acknowledged that biological age and chronological age may differ. Although the relationship between age and bleeding risk appears to be continuous, a pragmatic decision was made to use a binary variable in the current definition.
Oral anticoagulation
The anticipated use of long-term OAC (with a vitamin K antagonist [VKA] or non–vitamin K OAC) after PCI is considered a major ARC-HBR criterion (Table 3).
The most common indication for OAC in patients undergoing PCI is coexisting atrial fibrillation (AF). When treating such patients, physicians must balance the risk of thromboembolism with AF, the risk of stent thrombosis and myocardial infarction after PCI, and the risk of bleeding on combined antithrombotic therapy.53 Bleeding risk is magnified in the setting of triple antithrombotic therapy (OAC plus DAPT).54
In the WOEST trial (What Is the Optimal antiplatelet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting; n=573), 1-year rates of BARC 3 to 5 bleeding in patients on VKAs after PCI were 6.5% and 12.7% in the double (VKA plus clopidogrel) and triple (VKA plus aspirin and clopidogrel) therapy arms, respectively (HR, 0.49 [95% CI, 0.28–0.86]; P=0.011).55 In the ISAR-TRIPLE trial (Intracoronary Stenting and Antithrombotic Regimen-Testing of a 6-Week versus a 6-Month Clopidogrel Treatment Regimen in Patients with Concomitant Aspirin and Oral Anticoagulant Therapy Following Drug-Eluting Stenting; n=614), patients on a VKA undergoing PCI were randomized to treatment with triple therapy for 6 weeks versus 6 months, with continuation of VKA and aspirin thereafter.56 At 9 months, rates of BARC 3 to 5 bleeding were ≈11.1% and 10.4%, respectively, with comparable bleeding event rates between treatment groups.
In the PIONEER AF-PCI trial (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) and RE-DUAL PCI trial (Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting), patients with AF undergoing PCI were allocated to treatment with dual therapy consisting of a non–vitamin K OAC and a P2Y12 inhibitor or triple therapy consisting of a VKA, a P2Y12 inhibitor, and aspirin. Although bleeding rates were lower in patients on dual therapy, it is unclear to what extent this was attributable to the omission of aspirin as opposed to the use of a non–vitamin K OAC instead of a VKA.57,58 In PIONEER AF-PCI (n=2124), 1-year BARC 3 to 5 bleeding rates were 4.1% with dual therapy including low-dose rivaroxaban (15 mg daily), 4.4% with triple therapy including very-low-dose rivaroxaban (2.5 mg twice daily), and 7.9% with triple therapy including a VKA. In RE-DUAL PCI (n=2725), respective rates of TIMI (Thrombolysis in Myocardial Infarction) major/minor bleeding at 14 months were 3.0% versus 7.0% in patients treated with dual therapy with dabigatran 110 mg twice daily versus triple therapy with warfarin (HR, 0.41 [95% CI, 0.26–0.63]; P<0.001) and 3.5% versus 6.3% in those treated with dual therapy including dabigatran 150 mg twice daily versus triple therapy including warfarin (HR, 0.53 [95% CI, 0.33–0.85]; P=0.009). In both trials, bleeding rates in the groups treated with triple therapy with a VKA were markedly lower than those observed in WOEST and ISAR-TRIPLE, indicating an overall lower bleeding risk profile in the populations enrolled, possibly explained by the stricter patient selection criteria in PIONEER AF-PCI and RE-DUAL PCI.
Although bleeding risk may differ between VKAs and novel anticoagulants and between individual novel anticoagulants (Table V in the online-only Data Supplement) and different doses, exposure times and variations in renal function may confer differential bleeding risks. Weighting the relative bleeding risk with different OAC regimens is beyond the scope of this definition.
Chronic kidney disease
Severe or end-stage chronic kidney disease (CKD; estimated glomerular filtration rate [eGFR] <30 mL/min) is considered a major ARC-HBR criterion, and moderate CKD (eGFR, 30–59 mL/min) is considered a minor ARC-HBR criterion (Table 3).
Approximately 30% of patients undergoing PCI have an eGFR <60 mL/min,59 but patients with severe CKD have generally been excluded from randomized trials. Even mild CKD is an independent risk factor for bleeding after PCI,60,61 and the risk increases incrementally with worsening CKD (Table 4).60–64 One mechanism may be reduced clearance of certain antithrombotic medications. In the PRECISE-DAPT bleeding risk score,32 eGFR <30 mL/min in isolation places patients in the highest quartile for bleeding risk, whereas milder CKD is associated with a slightly to moderately increased bleeding risk.
The increased bleeding risk with CKD must be considered in the context of a proportionately increased risk of ischemic events (Table 4), making this balance more sensitive in patients with CKD compared with most other HBR criteria. In the DAPT score, a clinical decision tool to identify patients expected to derive benefit versus harm from prolonged DAPT after PCI, CKD is not a variable because the associated bleeding risk was balanced by an almost identical ischemic risk.41
Table 4.
Impact of CKD on clinical outcomes after PCI
| CrCl, mL/min | Major Bleeding |
Ischemic Events |
|||||
|---|---|---|---|---|---|---|---|
| End Point(s) and Duration of Follow-Up | Event Rate, % | P Value | End Point(s) and Duration of Follow-Up | Event Rate, % | P Value | ||
| EVENT registry62 (n=4791) | >75 (n=2827, (59%) | In-hospital TIMI major or minor bleeding, major vascular complications, or transfusion/TIMI major bleeding | 3.3/0.2 | <0.0001/0.56 | MI in hospital/at 1 y | 5.7/7.2 | <0.001/0.0007 |
| 50–75 (n=1253, 26%) | 5.0/0.3 | 7.3/9.2 | |||||
| 30–49 (n=571, 12%) | 8.8/1.2 | 8.2/10.7 | |||||
| <30 (n=140, 3% | 14.3/0.0 | 10.0/11.4 | |||||
| ACUITY trial60 (n=13 819) | ≥60 (n=11 350, 80.9) | ACUITY major bleeding at 30 d | 3.6 | <0.0001 | Death resulting from any cause, MI, or unplanned revascularization at ischemia) 30 d/1 y | 7.0/14.4 | <0.0001/0.001 |
| <60 (n=2469, 19.1%) | 9.2 | 10.8/21.6 | |||||
| HORIZON-AMI trial61 (n=3397) | ≥60 (n=2843, 83.7%) | ACUITY major bleeding at 30 d/1 y/2 y | 5.7/6.0/6.7 | <0.0001/<0.0001/ <0.0001 | Death, reinfarction, TVR, or stroke at 30 d/1 y/2 y | 4.3/10.1/19.8 | <0.0001/<0.0001/ <0.0001 |
| 30–60 (n=506, 14.9%) | 12.1/14.3/16.9 | 9.9/18.5/30.0 | |||||
| ≤30 (n=48, 1.4%) | 45.2/45.2/45.2 | 29.2/49.3/70.0 | |||||
| PARIS Registry63 (n=4584) | ≥60 (n=3745, 82.0%) | BARC 3 or 5 bleeding at 2 y | 3.04 | NR | Cardiac death, probable/definite ST, or clinically indicated TVR at 2 y | 10.20 | NR |
| <60 (n=839, 18.0%) | 8.94 | 16.81 | |||||
| ADAPT-DES64 (n=8410) | ≥60 (n=7043, 83.7%) | ACUITY major bleeding at 2 y | 7.5 | <0.001 | Cardiac death, MI, or ischemia-driven TLR at 2 y | 9.9 | <0.001 |
| <60 (n=1367 (16.3%) | 13.9 | 15.3 | |||||
Bleeding definitions are shown in the Appendix in the online-only Data Supplement. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; ADAPT-DES, Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents; BARC, Bleeding Academic Research Consortium; CKD, chronic kidney disease; CrCl, creatinine clearance; EVENT, Evaluation of Drug Eluting Stents and Ischemic Events; HORIZON-AMI, Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction; MI, myocardial infarction; NR, not reported; PARIS, patterns of non-adherence to anti-platelet regimens in stented patients; PCI, percutaneous coronary intervention; ST, stent thrombosis; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; and TVR, target vessel revascularization.
From the data presented, the consensus decision was to use CKD stages rather than eGFR as a continuous variable in the definition (Table 4).
Anemia
A hemoglobin level <11 g/dL is considered a major ARC-HBR criterion. A hemoglobin level of 11 to 12.9 g/dL for men and 11 to 11.9 g/dL for women is considered a minor ARC-HBR criterion (Table 3).
Anemia defined by World Health Organization criteria (hemoglobin <13 g/dL in men and <12 g/dL in women) is frequently encountered in patients undergoing PCI, with a reported prevalence of 21.6% in the Bern DES Registry.65 Anemia correlates with the risk of future bleeding in patients undergoing PCI. The 1-year risk of BARC 3 or 5 bleeding in patients with acute coronary syndrome (ACS) treated with PCI followed by prasugrel or ticagrelor in the RENAMI registry (Registry of New Antiplatelets in Patients With Myocardial Infarction; n=4424) was significantly higher in patients with World Health Organization–defined anemia compared with those without (5.4% versus 1.5%, respectively; P=0.001).66 In a meta-analysis of 44 studies including >230 000 patients undergoing PCI, anemia (defined by World Health Organization criteria in the majority of studies) was present in 16% of patients and was associated with a 2-fold risk of subsequent bleeding (as defined in individual studies; adjusted risk ratio, 2.31 [95% CI, 1.44–3.71]), as well as an increased risk of ischemic events and mortality.67 Furthermore, bleeding risk increased with increasing severity of anemia.
Baseline anemia was also found to be an important predictor of bleeding in the development cohorts of a number of bleeding risk scores. In PARIS, baseline anemia (hemoglobin <12 g/dL in men and <11 g/dL in women) was a strong predictor of 2-year BARC 3 or 5 bleeding (9.5% with versus 2.7% without anemia; adjusted HR, 2.72 [95% CI, 1.83–4.04]; P<0.0001).38 In BleeMACS, hemoglobin <11 g/dL was the strongest predictor of serious spontaneous bleeding (defined in the Appendix in the online-only Data Supplement) at 1 year (adjusted HR, 2.41 [95% CI, 1.29–4.50]; P<0.001), and hemoglobin of 11.0 to 13.9 g/dL was also associated with a significantly increased bleeding risk (adjusted HR, 1.59 [95% CI, 1.14–2.21]; P=0.006) compared with hemoglobin ≥14 g/dL.36 In the Dutch aspirin score, anemia (defined by diagnosis-related groups) was also found to be one of the most important predictors of a first upper gastrointestinal bleed on aspirin therapy (adjusted HR, 2.3 [95% CI, 1.9–2.8]; P<0.01).37 In PRECISE-DAPT, each 1-g/dL increase in hemoglobin between 10 and 12 g/dL was independently associated with a reduction in the risk of TIMI major/minor bleeding at 1 year (adjusted HR, 0.67 [95% CI, 0.53–0.84]; P=0.001).32
Prior bleeding and transfusion
Spontaneous (nonintracranial) bleeding requiring hospitalization or transfusion in the past 6 months (or at any time if recurrent) is considered a major ARC-HBR criterion, and a first spontaneous (nonintracranial) bleed requiring hospitalization or transfusion >6 and <12 months before PCI is considered a minor ARC-HBR criterion (Table 3).
Information on the risk of subsequent bleeding in patients with a prior bleeding event who undergo PCI is scarce. Nonetheless, in the PRECISE-DAPT score, prior spontaneous bleeding at any time was found to be an important predictor of future bleeding and, in isolation, places patients in the highest quartile for bleeding risk.32 In patients (n=320) presenting with peptic ulcer bleeding on aspirin monotherapy randomized to treatment with clopidogrel versus aspirin plus esomeprazole after confirmed ulcer healing, respective 1-year rates of recurrent ulcer bleeding (defined in the Appendix in the online-only Data Supplement) were 8.6% versus 0.7% (difference, 7.9% [95% CI, 3.4–12.4]; P=0.001).68 In another randomized trial in patients (n=153) with acute peptic ulcer bleeding on aspirin monotherapy, recurrent ulcer bleeding (defined in the Appendix in the online-only Data Supplement) at 30 days occurred in 10.3% versus 5.4% of patients allocated to aspirin plus pantoprazole versus aspirin discontinuation (HR, 1.9 [95% CI, 0.6–6.0]; P=0.25).69
Data on the association between previous blood transfusion and subsequent bleeding risk in patients undergoing PCI are scarce. In 1 randomized trial of transfusion strategies in patients without PCI with acute upper gastrointestinal bleeding, patients (n=921) were assigned to a restrictive (maintain hemoglobin >7 g/dL) or liberal (maintain hemoglobin >9 g/dL) transfusion strategy. The rate of further in-hospital bleeding (defined in the Appendix in the online-only Data Supplement) was significantly lower in patients allocated to the restrictive strategy (10% versus 16%; adjusted HR, 0.68 [95% CI, 0.47–0.98]; P=0.03).70 The highest rates of recurrent bleeding occurred in the setting of acute blood transfusion, suggesting that the timing of transfusion appears to an important determinant of bleeding risk. Bleeding rates at 1 year were not reported.
Thrombocytopenia
Moderate or severe baseline thrombocytopenia (platelet count <100×109/L) is considered a major ARC-HBR criterion (Table 3).
Baseline thrombocytopenia refers to thrombocytopenia that is present before PCI. This is distinct from acquired thrombocytopenia after PCI, which results from a postprocedural decline in platelet count in a patient without baseline thrombocytopenia. Thrombocytopenia is classified as mild (100–149×109/L), moderate (50–99×109/L), or severe (<50×109/L).71 The reported prevalence of baseline thrombocytopenia in patients undergoing PCI is ≈2.5% in the United States and 1.5% in Japan.72,73 Patients with thrombocytopenia are underrepresented in randomized trials of DES and DAPT, and those who are enrolled generally have no more than mild thrombocytopenia because a platelet count of <100×109/L is a common exclusion criterion.
Thrombocytopenia is a risk factor for both bleeding and ischemic complications. In an analysis from the US Nationwide Inpatient Sample (NIS) database, 32 565 patients with chronic thrombocytopenia at the time of PCI were propensity-matched with patients without thrombocytopenia.72 The risks of in-hospital postprocedural bleeding, defined by International Classification of Diseases codes for in-hospital complications (10.9% versus 4.9%; odds ratio [OR], 2.40 [95% CI, 2.05–2.72]; P<0.0001), and mortality (6.5% versus 2.9%; OR, 2.30 [95% CI, 1.90–2.70]; P<0.0001) were significantly higher in patients with thrombocytopenia.72 A post hoc analysis of patients with ST-segment–elevation myocardial infarction treated with PCI in the HORIZONS-AMI trial (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction; n=3476) showed a higher rate of 30-day ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy)-HORIZONS major bleeding (defined in the Appendix in the online-only Data Supplement) in 146 patients with baseline mild thrombocytopenia compared with those without thrombocytopenia (15.4% versus 9.1%; P=0.01).74
Bleeding risk appears to be proportional to the degree of thrombocytopenia. A pooled analysis of 3 Japanese studies including patients undergoing PCI (n=19 353) showed increased rates of GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) moderate/severe bleeding (defined in the Appendix in the online-only Data Supplement) at 3 years in patients with baseline mild thrombocytopenia (9.9% versus 6.9%; adjusted HR, 1.20 [95% CI, 1.03–1.40]; P=0.02) and moderate/severe thrombocytopenia (23.1% versus 6.9%; adjusted HR, 2.35 [95% CI, 1.80–3.08]; P<0.001) compared with patients without thrombocytopenia.73
Chronic bleeding diatheses
The presence of a clinically significant chronic bleeding diathesis is considered a major ARC-HBR criterion (Table 3).
Chronic bleeding diatheses include inherited or acquired conditions known to be associated with increased bleeding risk such as platelet dysfunction, von Willebrand disease (prevalence of 1%–2% in the general population), inherited or acquired clotting factor deficiencies (including factors VII, VIII [hemophilia A], IX [hemophilia B], and XI), or acquired antibodies to clotting factors, among others.75–77 For the purpose of the current HBR definition, thrombocytopenia is discussed separately.
Data on bleeding rates after PCI in patients with bleeding diatheses are scarce because such patients are generally excluded from DES and DAPT trials. In ZEUS-HBR, hematologic disorders or any known coagulopathy-associated bleeding diathesis (including prior or current thrombocytopenia, defined as platelet count <100×109/L) was a criterion conferring HBR status in 95 patients (11.5%).5
Among 796 patients with von Willebrand disease followed up for 1 year, 75 (9.4%) required clotting factor replacement therapy for 232 bleeding events.75 In a series of 54 patients with hemophilia A or B undergoing coronary angiography or PCI, major periprocedural bleeding occurred in 3 patients (6%), and 11 patients (20%) had a bleeding event (predominantly minor) within 1 year.78 The most important and reliable predictor of bleeding in patients with bleeding diatheses is a personal history of bleeding, which may be assessed with a bleeding questionnaire.79 However, given the lack of data and the low prevalence of such conditions in patients undergoing PCI, attempting to weight the differential bleeding risks with different bleeding diatheses and their levels of severity is beyond the scope of the current definition.
Cirrhosis with portal hypertension
The presence of cirrhosis with portal hypertension is considered a major ARC-HBR criterion (Table 3).
The reported prevalence of cirrhosis in patients undergoing PCI in the United States is 1.2%.80 The bleeding risk in chronic liver disease may be related to impaired hemostasis (resulting from coagulation factor deficiency, thrombocytopenia, platelet dysfunction, or increased fibrinolysis)81 or to esophageal varices in the presence of portal hypertension. Bleeding complications on antithrombotic therapy in such patients are potentially catastrophic.82
Patients with severe liver disease are generally excluded from DES and DAPT trials. In the LEADERS FREE trial, although severe chronic liver disease was an inclusion criterion for HBR, <1% of enrolled patients fulfilled this criterion.4 The finding of obstructive CAD during transplantation workup in patients with end-stage liver disease is an increasingly common scenario. A single-center study of patients (n=1221) who underwent orthotopic liver transplantation over a 10-year period in the United States reported that 38.6% of patients underwent coronary angiography and 4.7% underwent PCI before transplantation, with rates of both increasing over time.83
Data from the NIS registry (n=4 376 950) showed that liver disease was an independent predictor of in-hospital gastrointestinal bleeding in patients undergoing PCI (OR, 2.59 [95% CI, 2.22–3.02]; P<0.001).84 In another retrospective study of PCI procedures (n=1 051 252) in the NIS, 26.0% of patients with cirrhosis had a coagulopathy at baseline, 20.5% had anemia, and 3.9% had a hematologic or oncological malignancy.80 The in-hospital mortality rate over the study period (3.6%) was higher compared with historical studies of the NIS database (0.5%–1.1%), and the most common postprocedural complications were hemorrhage (6.6% of patients) and the need for transfusion (11.3% of patients). In a retrospective study of patients with cirrhosis and CAD (n=148) treated by either coronary stenting with DAPT or medical therapy with aspirin monotherapy, the rate of gastrointestinal bleeding at 1 year was 22% versus 5%, respectively (P=0.003).85 An observational study of patients with chronic hepatitis B virus (n=1674) showed significantly higher bleeding rates (defined as International Society on Thrombosis and Haemostasis major bleeding or clinically relevant nonmajor bleeding)86,87 in patients taking antiplatelet therapy compared with those without antiplatelet therapy (9.5% versus 1.8%; HR, 3.28 [95% CI, 1.98–5.42]; P< 0.001).88 Although Child-Pugh and Mayo End-Stage Liver Disease criteria are used as exclusion criteria in some DES and DAPT trials, such scores were validated for predicting mortality in end-stage liver disease but not for predicting bleeding risk.89–91
Cancer
Active malignancy (excluding nonmelanoma skin cancer) is considered a major ARC-HBR criterion (Table 3). Active malignancy is defined as diagnosis within the previous 12 months or ongoing active cancer treatment (surgery, radiotherapy, chemotherapy, or immunotherapy). Cancer that is considered to be in complete remission or requires only maintenance therapy (eg, tamoxifen for breast cancer) is not considered active.
The prevalence of current or previous cancer in patients undergoing PCI in the US NIS database increased from 6.3% in 2004 to 9.5% in 2014.92 Of 6 571 034 patients undergoing PCI, 1.8% had a current cancer diagnosis and 5.8% had previous cancer. Current cancer was associated with higher rates of in-hospital bleeding (defined by International Classification of Diseases, Ninth Revision, Clinical Modification codes, shown in the Appendix in the online-only Data Supplement) compared with previous cancer and no cancer history (9.7% versus 4.2% versus 3.1%; OR [current versus no cancer], 1.92 [95% CI, 1.82–2.04] and OR [historical versus no cancer], 1.08 [95% CI, 1.03–1.13]) and ranged between 4.9% and 21.2% according to the type, site, and spread of the malignancy.92 Bleeding in cancer patients may be caused by local invasion, by a secondary systemic process, or by cancer treatment (Table VI in the online-only Data Supplement).
The LEADERS FREE trial included 239 patients (9.7%) with nonskin cancer diagnosed or treated within 3 years before the index PCI,4 with 1-year BARC 3 to 5 bleeding in 9.6%. In an observational study of patients ≥65 years of age undergoing PCI (n=22 798), late bleeding (defined as hospitalization for bleeding ≤1 year after discharge) was reported in 5.0% of patients with a history of cancer, which was an independent predictor of late bleeding (HR, 1.80 [95% CI, 1.09–2.96]; P=0.02).93
In the TRILOGY ACS trial (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes; n=9240), cancer incidence and outcomes were prospectively assessed among patients treated with DAPT (including clopidogrel or prasugrel) after ACS.94 A new diagnosis of cancer was made in 170 patients (1.8%), of whom 53.5% permanently discontinued DAPT and 59% required surgery or chemotherapy. GUSTO severe/life-threatening, or moderate bleeding occurred substantially more frequently among those with cancer versus those without (11.2% versus 1.5%).
Previous ischemic stroke or ICH
The presence of a brain arteriovenous malformation (bAVM), previous ICH at any time, and moderate or severe ischemic stroke (National Institutes of Health Stroke Scale score ≥5 on presentation) within 6 months before PCI are all considered major ARC-HBR criteria. Ischemic stroke at any time not meeting the major criterion is considered a minor ARC-HBR criterion (Table 3).
In the SCAAR Registry (Swedish Coronary Angiography and Angioplasty Registry), 5% to 6% of patients undergoing PCI reported a prior stroke.95 In the NCDR (National Cardiovascular Data Registry) Cath-PCI, ≈12% of enrolled patients had a history of cerebrovascular disease (defined as prior stroke or carotid stenosis).96 Pivotal DES trials, however, excluded patients with a prior stroke within 6 months of enrollment (Table I in the online-only Data Supplement). In trials of DES in patients perceived to be at increased bleeding risk, the prevalence of prior stroke was low, and bleeding rates for this subgroup were not reported. In LEADERS FREE, 1.6% of patients had ischemic stroke within the prior 12 months, and 1.3% had prior ICH.4 In ZEUS-HBR, prior stroke or transient ischemic attack (TIA) was reported in 8% of patients.5 In the SENIOR trial, ≈8% of the enrolled population had previous ischemic stroke; prior ICH was an exclusion criterion.6
Trials of DAPT after ACS have also excluded patients with prior ICH but not prior ischemic stroke/TIA.97–99 In the TRITON (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel)–TIMI 38 trial, patients with prior TIA or stroke (>3 months before inclusion) who received aspirin and prasugrel had higher rates of ischemic and hemorrhagic stroke at 15 months compared with patients without prior TIA/stroke (any stroke occurred in 6.5% [2.3% ICH] and 0.9% [0.2% ICH], respectively), resulting in a contraindication for prasugrel use in such patients.99 In contrast, in patients treated with aspirin and clopidogrel, rates of subsequent stroke did not significantly differ between patients with and those without prior TIA/stroke (1.2% [0% ICH] and 1.0% [0.3% ICH], respectively). In the PLATO trial (Platelet Inhibition and Patient Outcomes; n=18 624), patients with prior TIA/stroke (n=1152, 6.2%) treated with DAPT (including ticagrelor or clopidogrel) after PCI had significantly higher 1-year rates of ICH compared with those without prior stroke or TIA (0.8% versus 0.2%; unadjusted HR, 3.95 [95% CI, 1.82–8.55]; P=0.0005), with no significant difference in ICH rates between treatment groups (0.9% for ticagrelor versus 0.7% for clopidogrel; HR, 1.00 [95% CI, 0.25–3.99]).100 In the TRA-2P [Trial to Assess the Effects of Vorapaxar (SCH 530348; MK-5348) in Preventing Heart Attack and Stroke in Patients With Atherosclerosis]–TIMI-50 trial (n=26 449), patients with prior stroke 2 weeks to 1 year before enrollment (n=5746 [21.7%]) had a significantly higher rate of ICH at 3 years with vorapaxar compared with placebo added to standard antiplatelet therapy (2.4% versus 0.9%; HR, 2.55 [95% CI, 1.52–4.28]; P<0.001).101 The rates of ICH in patients without prior stroke were markedly lower in both treatment groups (0.6% [vorapaxar arm] and 0.4% [placebo arm]; HR, 1.55 [95% CI, 1.00–2.41]; P=0.049).
Rates of non-ICH bleeding do not appear to differ significantly between patients undergoing PCI with and without previous stroke. In PRECISE-DAPT, patients with and without prior stroke had similar rates of TIMI major/minor bleeding (HR, 1.16 [95% CI, 0.54–2.48]; P=0.70).32 In PARIS, rates of BARC 3 or 5 bleeding in patients with and without previous stroke were also similar (4.1% and 3.5%, respectively; P=0.66).38
Six major randomized trials have investigated potent antiplatelet therapy for secondary stroke prevention (Table 5).102–107 Three trials enrolled patients with acute minor stroke or TIA (<12–24 hours; National Institutes of Health Stroke Scale score <3–5) and showed no significant difference in ICH rates between patients treated with either DAPT or ticagrelor and those treated with aspirin monotherapy for 90 days.102–104 MATCH (Management of Atherothrombosis With Clopidogrel in High-Risk Patients) and PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) enrolled patients with recent stroke (≤90–120 days). In both trials, overall rates of bleeding and primary ICH were higher with DAPT compared with clopidogrel monotherapy, without a significant reduction in ischemic events.105,106 The SPS3 trial (Secondary Prevention of Small Subcortical Strokes) patients also showed significantly higher major bleeding rates and no significant reduction in recurrent stroke with DAPT compared with aspirin monotherapy in patients with recent symptomatic lacunar infarcts (≤180 days).107 However, in contrast to MATCH and PRoFESS, rates of ICH were comparable between treatment groups, but mortality rates were significantly higher with DAPT. In line with these findings, American Stroke Association/American Heart Association guidelines recommend (Class IIa, Level of Evidence B-R) that DAPT (aspirin and clopidogrel) initiated within 24 hours can be beneficial for early secondary prevention for a period of up to 90 days,108 but it is not recommended (Class III, Level of Evidence A) for routine long-term secondary prevention after minor stroke or TIA.109
Table 5.
Major Randomized Trials of Antiplatelet Therapy in Recent or Acute Ischemic Stroke or TIA
| Trial (Year of Publication) | Patients, n | Indication | Experimental Arm | ControlArm | Duration of Treatment and Follow-Up | Ischemic (Efficacy) Outcomes | Bleeding (Safety) Outcomes |
|---|---|---|---|---|---|---|---|
| Trials of antiplatelet therapy in recent stroke or TIA | |||||||
| MATCH (2004)105 | 7599 | Recent ischemic stroke or TIA (<3 mo)+≥1 additional vascular risk factor (all patients were on clopidogrel monotherapy at baseline) | Aspirin 75 mg once daily plus clopidogrel 75 mg once daily | Clopidogrel 75 mg once daily | 18 mo | Composite of ischemic stroke, MI, readmission, or vascular death: 15.7% vs 16.7% (absolute risk reduction, 1% [95% CI, –0.6 to 2.7]; P=0.244)* Ischemic stroke: 8% vs 9% (absolute risk reduction, 0.62% [95% CI, –0.6 to 1.9]; P=0.353) | Life-threatening bleeding: 2.6% vs 1.3% (absolute risk increase, 1.3% [95% CI, 0.6 to 1.9]; P<0.0001) Primary ICH: 1% vs <1% (absolute risk increase, 0.40 [95% CI, 0.04 to 0.76]; P=0.029) |
| PRoFESS (2008)106 | 20 332 | Recent ischemic stroke (<90 d before randomization)+age ≥50 y or ischemic stroke 90–120 d before randomization+2 additional vascular risk factors | Aspirin 25 mg plus extended-release dipyridamole 200 mg twice daily | Clopidogrel 75 mg once daily‡ | 30 mo | Stroke (any): 9.0% vs 8.8% (HR, 1.01 [95% CI, 0.92 to 1.11])* Ischemic stroke: 7.7% vs 7.9% (HR, 0.97 [95% CI, 0.88 to 1.07]; P=NS) Composite of stroke, MI, or death from vascular causes: 13.1% in both groups (HR, 0.99 [95% CI, 0.92 to 1.07]) | Major bleeding: 4.1% vs 3.6% (HR, 1.15 [95% CI, 1.00 to 1.32]) ICH: 1.4% vs 1.0% (HR, 1.42 [95% CI, 1.11 to 1.83]; P=0.006) |
| SPS3 (2012)107 | 3020 | Recent symptomatic lacunar infarct (≤180 d before randomization) | Aspirin 325 mg once daily plus clopidogrel 75 mg once daily | Aspirin 325 mg once daily | Mean, 3.4 y (range, 0–8.2 y) | Stroke (any): 2.5%/y vs 2.7%/y (HR, 0.92 [95% CI, 0.72 to 1.16]; P=0.48)* Ischemic stroke: 2.0%/y vs 2.4%/y (HR, 0.82 [95% CI, 0.63 to 1.09]; P=0.13) Death: 2.1% vs 1.4% (HR, 1.52 [95% CI, 1.14 to 2.04]; P=0.004) | Major bleeding: 2.1%/y vs 1.1%/y (HR, 1.97 [95% CI, 1.41 to 2.71]; P<0.001) ICH: 0.42%/y vs 0.28%/y (HR, 1.52 [95% CI, 0.79 to 2.93]; P=0.21) |
| Trials of antiplatelet therapy in acute stroke or TIA | |||||||
| CHANCE (2014)102 | 5170 | Acute (≤24 h) minor ischemic stroke (NIHSS score ≤3) or high-risk TIA† | Clopidogrel 75 mg once daily+aspirin 75 mg once daily (for the first 21 d) | Aspirin 75 mg once daily | 90 d | Stroke (any): 8.2% vs 11.7% (HR, 0.68 [95% CI, 0.58 to 0.82]; P<0.001)* Ischemic stroke: 7.9% vs 11.4% (HR, 0.67 [95% CI, 0.56 to 0.81]; P<0.001) Composite of stroke, MI, or cardiac death: 8.4% vs 11.9% (HR, 0.68 [95% CI, 0.59 to 0.82]; P<0.001) | Moderate or severe bleeding (GUSTO): 0.3% in both arms (P=0.73) Hemorrhagic stroke: 0.3% in both arms (HR, 1.01 [95% CI, 0.38 to 2.70]; P=0.98) |
| SOCRATES (2016)103 | 13 199 | Acute (≤24 h) nonsevere ischemic stroke(NIHSS score ≤5) or high-risk TIA† or symptomatic intracranial orextracranial arterial stenosis | Ticagrelor 90 mg twice daily | Aspirin 100 mg once daily | 90 d | Stroke, MI, or death: 6.8% vs 7.5% (HR, 0.89 [95% CI, 0.78 to 1.01]; P=0.07)* Ischemic stroke: 5.9 vs 6.6% (HR, 0.87 [95% CI, 0.76 to 1.00]; P=0.05) | Major bleeding (PLATO): 0.5% vs 0.6% (HR, 0.83 [95% CI, 0.52 to 1.34]; P=0.45) ICH: 0.2% vs 0.3% (HR, 0.68 [95% CI, 0.33 to 1.41; P=0.30) |
| POINT (2018)104 | 4881 | Acute (≤12h) minor ischemic stroke (NIHSS score ≤3) or high-risk TIA† | Aspirin 50–325 mg once daily plus clopidogrel 75 mg once daily | Aspirin 50–325 mg once daily | 90 d | Composite of ischemic stroke, MI, or death resulting from an ischemic vascular event: 5.0% vs 6.5% (HR, 0.75 [95% CI, 0.59 to 0.95]; P=0.02)* Ischemic stroke: 4.6% vs 6.3% (HR, 0.72 [95% CI, 0.56–0.92]; P=0.01) | Major bleeding: 0.9% vs 0.4% (HR, 2.32 [95% CI 1.10 to 4.87]; P=0.02) Hemorrhagic stroke: 0.2% vs 0.1% (HR, 1.68 [95% CI, 0.40 to 7.03]; P=0.47) |
Bleeding definitions are shown in the Appendix in the online-only Data Supplement. CHANCE indicates Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; HR, hazard ratio; ICH, intracranial hemorrhage; MATCH, Management of Atherothrombosis With Clopidogrel in High-Risk Patients; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; NS, not significant; PLATO, Study of Platelet Inhibition and Patient Outcomes; POINT, Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke; PRoFESS, Prevention Regimen for Effectively Avoiding Second Strokes; SOCRATES, Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes; SPS3, Secondary Prevention of Small Subcortical Strokes; and TIA, transient ischemic attack.
Primary outcome. †High-risk TIA in CHANCE, SOCRATES, and POINT was defined as TIA with a moderate to high risk of stroke recurrence (defined as ABCD2 stroke risk score of ≥4; ABCD score assesses the risk of stroke based on age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes mellitus; scores range from 0–7, with higher scores indicating greater short-term risk of stroke). ‡Protocol amendment in PRoFESS: because of a concern with an increased risk of bleeding, after 2027 patients had been enrolled, the clopidogrel plus aspirin arm was modified, and the next 18 305 patients were randomized to either clopidogrel alone or the unmodified combination of low-dose aspirin and dipyridamole.
There is a lack of prospective data on DAPT and bleeding risk in patients with large strokes, prior ICH, and bAVMs. Patients with bAVMs have a high long-term risk of ICH.110 In a patient-level meta-analysis of 2525 patients with bAVM, the annual risk of first and recurrent ICH was 1.3% (95% CI, 1.0–1.7) and 4.8% (95% CI, 3.9–5.9), respectively.111 In a randomized study of unruptured bAVMs (n=223), the annual first ICH rate without interventional therapy was 2.0%.112 The incremental risk of ICH in patients with bAVM taking antiplatelet therapy is not known.
Planned major noncardiac surgery after PCI
Planned nondeferrable major surgery on DAPT after PCI is considered a major ARC-HBR criterion (Table 3).
After PCI, up to 17% of patients undergo an invasive diagnostic or therapeutic procedure within 1 year.113,114 The increased risk of bleeding in a patient on antiplatelet therapy undergoing major surgery must be balanced against the potential risks of discontinuing DAPT in the potentially prothrombotic perioperative setting.113,114 Important considerations include (1) the temporal relationship between PCI and surgery, (2) whether the surgery is deferrable, (3) the anticipated bleeding risk specific to the surgical procedure, and (4) the anticipated thrombotic risk as defined by patient, lesion, and procedural characteristics.
In the POISE-2 trial (Perioperative Ischemic Evaluation 2; n=10 010), 30-day major bleeding rates (defined in the Appendix in the online-only Data Supplement) after noncardiac surgery were higher with aspirin compared with placebo (4.6% versus 3.8%; HR, 1.23 [95% CI, 1.01–1.49]; P=0.04).115 Although clinical practice guidelines provide recommendations on perioperative management of antithrombotic therapy, they do not define the perioperative bleeding risk of different surgical procedures.116,117 To this end, a number of national multidisciplinary expert consensus documents have been published in an effort to standardize perioperative management of antithrombotic therapy based on balancing the predicted patient-specific ischemic risk with the anticipated procedure-specific bleeding risk.118–120
In summary, DAPT at the time of or shortly after surgery increases bleeding risk. Most elective surgery can be deferred beyond the proposed DAPT duration, and elective PCI is rarely necessary before elective major surgery. For urgent or nondeferrable surgery, the risk of stent thrombosis is much higher during the first month after PCI compared with subsequent months.121,122
PCI after recent major surgery or trauma
Major surgery or major trauma within 30 days before PCI is considered a major ARC-HBR criterion (Table 3).
The reported incidence of perioperative myocardial infarction after major noncardiac surgery is as high as 10%, depending on both patient and procedural characteristics.123 No data are available on bleeding rates when urgent PCI is required after recent major surgery or trauma. The bleeding risk of different types of surgery (including trauma surgery) has been reviewed recently.118
Long-term oral NSAID or steroid use
Long-term steroid or oral NSAID use (defined as planned daily intake for ≥4 d/wk) is considered a minor ARC-HBR criterion (Table 3).
NSAIDs represent the most widely used class of medications worldwide.124,125 Both oral NSAIDs and steroids are associated with increased gastrointestinal bleeding risk, which is dose-dependent and increases with long-term use.126,127 There is a paucity of data on bleeding risk in patients with long-term oral NSAID or steroid use after PCI because of underrepresentation or underreporting in randomized trials. Although long-term NSAID or steroid use was an inclusion criterion in both LEADERS FREE and ZEUS-HBR, this criterion was met in only 72 patients (2.8%) and 25 patients (3%), respectively.4,5 Moreover, their bleeding rates were not reported.
The risk of upper gastrointestinal bleeding is higher with NSAID monotherapy compared with aspirin monotherapy, and concomitant use of NSAIDs and aspirin substantially further increases the risk.37,128 In the CONCERN trial (n=514), patients with arthritis presenting with upper gastrointestinal bleeding on NSAIDs with a requirement for low-dose aspirin were randomized to celecoxib or naproxen (plus aspirin and esomeprazole) after confirmed ulcer healing. Recurrent upper gastrointestinal bleeding (defined in the Appendix in the online-only Data Supplement) rates were 5.6% and 12.3% at 18 months, respectively (HR, 0.44 [95% CI, 0.23–0.82]; P=0.008).129 In the CLASS study (Celecoxib Long-Term Arthritis Safety Study; n=8059), patients with arthritis were randomized to celecoxib or either ibuprofen or diclofenac. In the subgroup of patients taking aspirin, the rates of symptomatic upper gastrointestinal ulcers or complications (bleeding, perforation, and obstruction) at 6 months were 4.7% and 6.0%, respectively (P=0.49).130
Special considerations
Frailty
Frailty was not included as a criterion because of the paucity of data demonstrating a causative role in bleeding in patients undergoing PCI and the lack of a consensus on how frailty is best assessed.131 Bleeding risk may be increased in the setting of frailty as a result of more frequent falls, the inability to ambulate without assistance, or postural hypotension. When frailty was evaluated with a functional impairment score in the ACTION Registry (Acute Coronary Treatment and Intervention Outcomes Network), it was found to correlate with an increased risk of major in-hospital bleeding (defined in the Appendix in the online-only Data Supplement) in 112 000 elderly patients presenting with acute myocardial infarction undergoing cardiac catheterization. Major bleeding occurred in 6.4%, 10.3%, and 13.6% of patients with no, mild, and moderate to severe frailty, respectively (mild frailty–adjusted HR, 1.33 [95% CI, 1.23–1.44]; moderate to severe frailty–adjusted HR, 1.40 [95% CI, 1.24–1.58] compared with the group without frailty).132 The inclusion of advanced age and coexisting ARC-HBR criteria may account, to some degree, for frailty. Further studies on the impact of frailty on bleeding risk are encouraged.
Ethnicity
The role of ethnicity in post-PCI bleeding risk has not been fully elucidated. Nonetheless, lower doses of several antithrombotic regimens are recommended in Asian patients compared with patients in Europe or the United States because of greater bleeding concerns in Asians.133,134 Bleeding models developed in Western populations tend to underestimate bleeding risk in Asian populations.135 In a patient-level meta-analysis, which pooled 7 randomized trials (n=16 518; 8605 East Asians, 7913 non-Asians), major bleeding occurred more frequently in East Asians (0.6% versus 0.3%; P=0.001), whereas major adverse cardiac events occurred more frequently in non–East Asians (0.8% versus 1.8%; P<0.001),136 suggesting a differential ischemia/bleeding tradeoff in East Asians and non–East Asians. Further research is needed in this field.
Acute Coronary Syndromes
Compared with stable patients with CAD, patients with ACS are at increased thrombotic risk, warranting treatment with more potent, longer-duration antiplatelet therapy. However, such an approach inevitably increases bleeding risk. In a meta-analysis of 3 randomized trials of patients with ACS (n=17 393) undergoing PCI with bivalirudin or heparin plus a glycoprotein IIb/IIIa inhibitor, the rate of TIMI major/minor bleeding was 5.3% at 30 days.137 In selected patients with ST-segment–elevation myocardial infarction at low bleeding risk, respective 1-year rates of non–coronary artery bypass graft TIMI major/minor bleeding were 4.0% and 3.5% with ticagrelor and clopidogrel in the PLATO trial and 5.1% and 4.7% with prasugrel and clopidogrel in the TRITON-TIMI 38 trial.138,139 Other trials of patients with ACS with more stringent exclusion criteria have reported 2-year BARC 3 to 5 bleeding rates as low as 0.5% to 0.8%.34 Given that the increased bleeding risk in patients with ACS is attributable to the more aggressive antiplatelet therapy rather than the ACS per se, the consensus was not to consider ACS an HBR criterion.
DAPT Nonadherence
DAPT nonadherence after PCI is well described. In the PARIS study, at a time when guidelines recommended ≥12 months of DAPT for all patients after stenting, the rate of DAPT discontinuation was 2.6%, 11.8%, and 19.9% at 30 days, 6 months, and 12 months, respectively.140 In contrast, in trials investigating short DAPT regimens, nonadherence to recommended DAPT discontinuation may occur. For example, in the LEADERS FREE trial, despite a recommended 1-month DAPT duration, ≈9% remained on DAPT after 1 month.4 In the SENIOR trial, 20% of patients remained on DAPT at 12 months, well beyond the recommended 1 to 6 months.6 In the ZEUS trial, although all patients at HBR were prescribed DAPT for 30 days, 38% remained on DAPT at 2 months and 25% at 6 months.5,35 Although DAPT nonadherence may increase the risk of thrombotic complications, nonadherence with recommended discontinuation may increase bleeding complications.
Regulatory considerations
Studies of patients at HBR have intrinsic public health value and support the mission of regulatory bodies. Consensus definitions are necessary to improve the efficiency and predictability of study design and quality and can assist regulatory decision-making for safe and effective drugs and devices for patients at HBR in a timely fashion. Sex, nationality, and ethnic differences in bleeding risk may also be important considerations in trial design and the interpretation of study outcomes. This article reflects the consensus views of the ARC-HBR consortium and does not necessarily represent the practices, policies, requirements, or recommendations of the US Food and Drug Administration or the Japanese Pharmaceuticals and Medical Devices Agency. Furthermore, the recommendations in this document do not represent a regulatory requirement from either agency. Although regulators consider it acceptable to propose and justify alternative definitions and HBR criteria, they encourage investigators to discuss any proposed trial-specific definitions of HBR prospectively with the relevant regulatory bodies before study initiation.
Limitations
A number of important limitations of the proposed definition must be acknowledged. First, the chosen cutoff values for 1-year BARC 3 or 5 bleeding (4%) and ICH (1%) are arbitrary, according to the expert opinion of this group. Second, data on rates of BARC 3 or 5 bleeding or ICH at 1 year were not available for a number of criteria, in which case justification is based on consensus decision alone. Third, although the relationship between many criteria and bleeding is continuous, binary criteria have been used to simplify the definition and to facilitate its use in trial enrollment. In addition, the differential bleeding risks associated with the criteria have not been weighted beyond major and minor because of a lack of data to support such an approach. Finally, the definition has not been validated in an independent patient data set. To this end, as more data become available, we anticipate validation and recalibration of this initial set of HBR criteria.
Conclusions
In keeping with previous ARC initiatives, this ARC-HBR definition addresses an unmet need by providing a framework for evaluating treatment options for patients undergoing PCI at increased bleeding risk. It is expected that consistent use of the consensus definitions will improve our ability to tailor treatment to individual patient needs and to stimulate scientific progress, innovation, and quality control initiatives. We therefore encourage trialists and trial sponsors to consider using ARC-HBR definitions in clinical studies with reporting of BARC 3 or 5 bleeding rates to allow comprehensive and consistent assessment of patients at HBR.
The ARC-HBR group is cognizant that defining bleeding risk is the first step toward understanding the continuum of clinically meaningful risks and benefits in patients at HBR undergoing PCI. Evaluating and managing the risk of major bleeding must always be balanced by the assessment of the thrombotic risk. This balance will be addressed in a future phase of the ARC-HBR initiative.
Funding
The ARC-HBR group was entirely funded by multiple industry sponsors. Sponsors participated as observers in both meetings and were provided a copy of the document before submission. The contributing companies were Abbott Vascular, Alvimedica, Amgen, AstraZeneca, Biosensors, Biotronik, Boston Scientific, Celonova, Chiesi, Cordis, Daiichi Sankyo, Edwards Lifesciences, Janssen, Medinol, Medtronic, Orbusneich, Portola, Sanofi, Sinomed, Sahajanand Medical Technologies, and Terumo.
Conflict of interest: Dr Colleran received financial compensation for her contribution to the preparation of this document. In accordance with the ARC charter, none of the other participants received fees or honoraria for their participation in the meetings or their contribution to this document. Dr Urban reports receiving speaker and consulting honoraria as an individual from Biosensors-Europe, Sinomed, and Terumo; participating in paid review activities (Clinical End Point Committee, Data Safety Monitoring Board) for Edward Lifesciences, Terumo, and Abbott Vascular; and serving as a medical codirector at the Cardiovascular European Research Center, a contract research organization based in Massy, France. Dr Mehran reports consultant fees to the institution from Abbott Laboratories and Spectranetics/Philips/Valcano Corp; consulting fees from Boston Scientific, Cardiovascular Systems Inc, Medscape, Siemens Medical Solutions, Regeneron Pharmaceuticals Inc, Roivant Sciences Inc, and Sanofi; being the spouse of a consultant for Abiomed and The Medicines Company; research funding to the institution from AstraZeneca, Bayer, Beth Israel Deaconess Hospital, BMS, CSL Behring, Eli Lilly and DSI, Medtronic, Novartis Pharmaceuticals, and Orbus Neich; scientific advisory board fees from PLx Opco Inc, dba PLx Pharma Inc; scientific advisory board fees to the institution from Bristol-Myers Squibb; executive committee fees from Janssen Pharmaceuticals and Osprey Medical; speaker engagements for Abbott Laboratories; equity from Claret Medical and Elixir Medical; and Data Safety Monitoring Board fees to the institution from Atermark Research Partners. Dr Angiolillo reports payments, consulting fees, or honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PLx Pharma Inc, Pfizer, Sanofi, and The Medicines Company; fees for participation in review activities from CeloNova and St. Jude Medical; institutional grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co, Merck, Novartis, Osprey Medical, and Renal Guard Solutions; and funding from the Scott R. MacKenzie Foundation and the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Award to the University of Florida UL1 TR000064 and National Institutes of Health/National Human Genome Research Institute U01 HG007269. Dr Byrne reports lecture fees from B. Braun Melsungen AG, Biotronik, Boston Scientific, and Micell Technologies and research funding to the institution from Boston Scientific and Celonova Biosciences. Dr Capodanno reports personal fees from Abbott Vascular, Bayer, Daiichi Sankyo, Pfizer, and AstraZeneca. Dr Cutlip reports receiving consultant fees from Celonova Biosciences and academic salary support from the Baim Institute for Clinical Research, an academic research organization in Boston, MA. Dr Eikelboom reports consulting fees and grant support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Pfizer, Janssen, and Sanofi-Aventis. Dr Gibson reports being chief executive officer of the Baim Institute for Clinical Research, in addition to receiving research grant funding from Angel Medical Corp, Bayer Corp, CSL Behring, Janssen Pharmaceuticals, Johnson & Johnson Corp, and Portola Pharmaceuticals; consultant fees from Amarin Pharama, Amgen, Bayer Corp, Boehringer Ingelheim, Boston Clinical Research Institute, Boston Scientific, Cardiovascular Research Foundation, CSL Behring, Chiesi, Duke Clinical Research Institute, Eli Lilly and Company, Gilead Sciences, Inc, Impact Bio LTD, Janssen Pharmaceuticals, Johnson & Johnson Corp, The Medicines Company, MedImmune, Medtelligence, Merck & Co, Inc, Microport, Novo Nordisk, PERT Consortium, Pharma Mar, Portola Pharmaceuticals, Sanofi, Somahlution, Vereseon Corporation, and Web MD; and royalties as a contributor from UpToDate in Cardiovascular Medicine. Dr Gregson reports personal fees from Edwards Lifesciences, MvRX, Amarin Corp, and BioSensors. Dr Haude reports grant support from Biotronik, Orbus Neich, AbboF, Medtronic, and Cardiac Dimensions; speaker’s bureau fees from Biotronik, Orbus Neich, AbboF, Medtronic, Lilly, Philips/ Volcano, and Cardiac Dimensions (Proctor); and consultant fees from Biotronik, Orbus Neich, and AbboF. Dr James reports institutional research grants from AstraZeneca, Bayer, Jansen, The Medicines Company, Abbot Vascular, and Boston Scientific, as well as honoraria from AstraZeneca, Bayer, and Medtronic. Dr Kimura reports consulting fees or honoraria from Abbott Vascular Japan Co, Ltd, Kowa Co, Ltd, Kowa Pharmaceutical Co Ltd, Sanofi K.K., Nippon Boehringer Ingelheim Co, Ltd, and Bristol-Myers Squibb K.K., as well as grant support from Otsuka Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Mitsubishi Tanabe Pharma Corp, Takeda Pharmaceutical Co Ltd, and Nippon Boehringer Ingelheim Co, Ltd. Dr Leon reports receiving research grants from Abbott Vascular, Boston Scientific, Medtronic, Biosensors, and SinomedEquity-Medinol. Dr Mylotte reports speaker fees from Biosensors and institutional research grants from Biosensors and Medtronic. Dr Pocock has served on steering committees or data monitoring committees for trials sponsored by AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Idorsia, Janssen, Medtronic, Novartis, Novo Nordisk, and Vifor, and has received grant funding from AstraZeneca and Merck. Dr Price reports receiving consulting honoraria from AstraZeneca, Chiesi USA, Medtronic, Boston Scientific, and Abbott Vascular/St Jude Medical; speaker’s bureau/fees from AstraZeneca, Chiesi USA, Medtronic, Boston Scientific, Abbott Vascular/St Jude Medical, and ACIST Medical; and institutional research grants from Daiichi-Sankyo. Dr Valgimigli reports grants and personal fees from Abbott, AstraZeneca, and Terumo; personal fees from Chiesi, Bayer, Daiichi Sankyo, Amgen, Alvimedica, Biosensors, and Idorsia; and grants from Medicure. Dr Varenne reports receiving consulting and research grants from Boston Scientific, Abbott Vascular, AstraZeneca, and Servier. Dr Yeh reports consulting and research grants from Abbott Vascular and Boston Scientific, research grants from Abiomed and AstraZeneca, and consulting fees from Biosense Webster, Medtronic, and Teleflex. Dr Krucoff reports receiving consulting and research grants from Abbott Vascular, Biosensors, Boston Scientific, Cook Medical, Medtronic, and OrbusNeich. Dr Morice is the chief executive officer of the Cardiovascular European Research Center, the contract research organization organizing the ARC-HBR initiative. The other authors report no conflicts.
Supplementary Material
This article has been copublished by Circulation.
References
- 1. King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW; 2005 WRITING COMMITTEE MEMBERS. 2007 Focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, writing on behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208 [DOI] [PubMed] [Google Scholar]
- 2. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, di Mario C, Davies S, van Geuns RJ, Eerdmans P, van Es GA, Meier B, Jüni P.. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163–1173. doi: 10.1016/S0140-6736(08)61244-1 [DOI] [PubMed] [Google Scholar]
- 3. Krucoff MW, Rutledge DR, Gruberg L, Jonnavithula L, Katopodis JN, Lombardi W, Mao VW, Sharma SK, Simonton CA, Tamboli HP, Wang J, Wilburn O, Zhao W, Sudhir K, Hermiller JB.. A new era of prospective real-world safety evaluation primary report of XIENCE V USA (XIENCE V Everolimus Eluting Coronary Stent System condition-of-approval post-market study). JACC Cardiovasc Interv. 2011;4:1298–1309. doi: 10.1016/j.jcin.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 4. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943 [DOI] [PubMed] [Google Scholar]
- 5. Ariotti S, Adamo M, Costa F, Patialiakas A, Briguori C, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Ferlini M, Vranckx P, Valgimigli M; ZEUS Investigators. Is bare-metal stent implantation still justifiable in high bleeding risk patients undergoing percutaneous coronary intervention? A pre-specified analysis from the ZEUS trial. JACC Cardiovasc Interv. 2016;9:426–436. doi: 10.1016/j.jcin.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 6. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, Helft G, Diaz Fernandez JF, Brugaletta S, Pinar-Bermudez E, Mauri Ferre J, Commeau P, Teiger E, Bogaerts K, Sabate M, Morice MC, Sinnaeve PR; SENIOR Investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7 [DOI] [PubMed] [Google Scholar]
- 7. Rymer JA, Harrison RW, Dai D, Roe MT, Messenger JC, Anderson HV, Peterson ED, Wang TY.. Trends in bare-metal stent use in the United States in patients aged >/=65 years (from the CathPCI Registry). Am J Cardiol. 2016;118:959–966. [DOI] [PubMed] [Google Scholar]
- 8. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 9. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H.. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 10. Krucoff MW, Mehran R, van Es GA, Boam AB, Cutlip DE.. The Academic Research Consortium governance charter. JACC Cardiovasc Interv. 2011;4:595–596. doi: 10.1016/j.jcin.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 11. Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Jüni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S.. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36:2608–2620. doi: 10.1093/eurheartj/ehv203 [DOI] [PubMed] [Google Scholar]
- 12. Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Münzel T, Popma JJ, Fitzgerald PJ, Bonan R, Kuntz RE; ENDEAVOR II Investigators. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206 [DOI] [PubMed] [Google Scholar]
- 13. Yeung AC, Leon MB, Jain A, Tolleson TR, Spriggs DJ, Mc Laurin BT, Popma JJ, Fitzgerald PJ, Cutlip DE, Massaro JM, Mauri L; RESOLUTE US Investigators. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011;57:1778–1783. doi: 10.1016/j.jacc.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 14. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Mahaffey KW, Cutlip DE, Fitzgerald PJ, Sood P, Su X, Lansky AJ; SPIRIT III Investigators. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903–1913. doi: 10.1001/jama.299.16.1903 [DOI] [PubMed] [Google Scholar]
- 15. Stone GW, Teirstein PS, Meredith IT, Farah B, Dubois CL, Feldman RL, Dens J, Hagiwara N, Allocco DJ, Dawkins KD; PLATINUM Trial Investigators. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57:1700–1708. doi: 10.1016/j.jacc.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 16. Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ, Feldman RL, Stein B, Dubois C, Grady T, Saito S, Kimura T, Christen T, Allocco DJ, Dawkins KD.. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Interv. 2015;8:e002372. [DOI] [PubMed] [Google Scholar]
- 17. Kandzari DE, Smits PC, Love MP, Ben-Yehuda O, Banai S, Robinson SD, Jonas M, Kornowski R, Bagur R, Iniguez A, Danenberg H, Feldman R, Jauhar R, Chandna H, Parikh M, Perlman GY, Balcells M, Markham P, Ozan MO, Genereux P, Edelman ER, Leon MB, Stone GW.. Randomized comparison of ridaforolimus- and zotarolimus-eluting coronary stents in patients with coronary artery disease: primary results from the BIONICS Trial (BioNIR Ridaforolimus-Eluting Coronary Stent System in Coronary Stenosis). Circulation. 2017;136:1304–1314. doi: 10.1161/CIRCULATIONAHA.117.028885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R; BIOFLOW V Investigators. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843–1852. doi: 10.1016/S0140-6736(17)32249-3 [DOI] [PubMed] [Google Scholar]
- 19. Byrne RA, Kastrati A, Kufner S, Massberg S, Birkmeier KA, Laugwitz KL, Schulz S, Pache J, Fusaro M, Seyfarth M, Schömig A, Mehilli J; Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Investigators. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30:2441–2449. doi: 10.1093/eurheartj/ehp352 [DOI] [PubMed] [Google Scholar]
- 20. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S.. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136–146. doi: 10.1056/NEJMoa1004130 [DOI] [PubMed] [Google Scholar]
- 21. Park KW, Kang SH, Kang HJ, Koo BK, Park BE, Cha KS, Rhew JY, Jeon HK, Shin ES, Oh JH, Jeong MH, Kim S, Hwang KK, Yoon JH, Lee SY, Park TH, Moon KW, Kwon HM, Hur SH, Ryu JK, Lee BR, Park YW, Chae IH, Kim HS; HOST–ASSURE Investigators. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based zotarolimus-eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-Safety & Effectiveness of Drug-Eluting Stents & antI-Platelet Regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63(pt A):2805–2816. doi: 10.1016/j.jacc.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen K, Maeng M, Kaltoft A, Thayssen P, Kelbaek H, Tilsted HH, Abildgaard U, Christiansen EH, Engstrøm T, Krusell LR, Ravkilde J, Hansen PR, Hansen KN, Abildstrøm SZ, Aarøe J, Jensen JS, Kristensen SD, Bøtker HE, Madsen M, Johnsen SP, Jensen LO, Sørensen HT, Thuesen L, Lassen JF; SORT OUT III Study Group. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet. 2010;375:1090–1099. doi: 10.1016/S0140-6736(10)60208-5 [DOI] [PubMed] [Google Scholar]
- 23. von Birgelen C, Kok MM, van der Heijden LC, Danse PW, Schotborgh CE, Scholte M, Gin RMTJ, Somi S, van Houwelingen KG, Stoel MG, de Man FHAF, Louwerenburg JHW, Hartmann M, Zocca P, Linssen GCM, van der Palen J, Doggen CJM, Löwik MM.. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388:2607–2617. doi: 10.1016/S0140-6736(16)31920-1 [DOI] [PubMed] [Google Scholar]
- 24. Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T, Bendz B, Stavnes S, Bjørnerheim R, Larsen AI, Slette M, Steigen T, Jakobsen OJ, Bleie Ø, Fossum E, Hanssen TA, Dahl-Eriksen Ø, Njølstad I, Rasmussen K, Wilsgaard T, Nordrehaug JE; NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. doi: 10.1056/NEJMoa1607991 [DOI] [PubMed] [Google Scholar]
- 25. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60:1340–1348. doi: 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 26. Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS.. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–513. doi: 10.1161/CIRCULATIONAHA.111.059022 [DOI] [PubMed] [Google Scholar]
- 27. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monségu J, Sabouret P, O’Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Barthélémy O, Beygui F, Silvain J, Vicaut E, Montalescot G; ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979 [DOI] [PubMed] [Google Scholar]
- 28. Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fucà G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589 [DOI] [PubMed] [Google Scholar]
- 29. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr, Nicolela EL Jr, Perin MA, Devito FS, Labrunie A, Salvadori D Jr, Gusmão M, Staico R, Costa JR Jr, de Castro JP, Abizaid AS, Bhatt DL; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–2522. doi: 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 30. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, Menozzi A, Pucci E, Mauri J, Cesana BM, Giustino G, Sardella G.. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64:2086–2097. doi: 10.1016/j.jacc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 32. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M; PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5 [DOI] [PubMed] [Google Scholar]
- 33. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Möllmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S; GLOBAL LEADERS Investigators. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940–949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 34. Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, Kim SH, Jeong JO, Bae JH, Kim BO, Cho JH, Suh IW, Kim DI, Park HK, Park JS, Choi WG, Lee WS, Kim J, Choi KH, Park TK, Lee JM, Yang JH, Choi JH, Choi SH, Gwon HC; SMART-DATE Investigators. 6-Month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391:1274–1284. doi: 10.1016/S0140-6736(18)30493-8 [DOI] [PubMed] [Google Scholar]
- 35. Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Airoldi F, Ferlini M, Liistro F, Dellavalle A, Vranckx P, Briguori C; ZEUS Investigators. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805–815. doi: 10.1016/j.jacc.2014.11.053 [DOI] [PubMed] [Google Scholar]
- 36. Raposeiras-Roubín S, Faxén J, Íñiguez-Romo A, Henriques JPS, D’Ascenzo F, Saucedo J, Szummer K, Jernberg T, James SK, Juanatey JRG, Wilton SB, Kikkert WJ, Nuñez-Gil I, Ariza-Sole A, Song X, Alexopoulos D, Liebetrau C, Kawaji T, Moretti C, Huczek Z, Nie SP, Fujii T, Correia L, Kawashiri MA, Caneiro-Queija B, Cobas-Paz R, Acuña JMG, Southern D, Alfonso E, Terol B, Garay A, Zhang D, Chen Y, Xanthopoulou I, Osman N, Möllmann H, Shiomi H, Giordana F, Gaita F, Kowara M, Filipiak K, Wang X, Yan Y, Fan JY, Ikari Y, Nakahayshi T, Sakata K, Yamagishi M, Kalpak O, Kedev S, Rivera-Asenjo D, Abu-Assi E.. Development and external validation of a post-discharge bleeding risk score in patients with acute coronary syndrome: the BleeMACS score. Int J Cardiol. 2018;254:10–15. doi: 10.1016/j.ijcard.2017.10.103 [DOI] [PubMed] [Google Scholar]
- 37. de Groot NL, Hagenaars MP, Smeets HM, Steyerberg EW, Siersema PD, van Oijen MG.. Primary non-variceal upper gastrointestinal bleeding in NSAID and low-dose aspirin users: development and validation of risk scores for either medication in two large Dutch cohorts. J Gastroenterol. 2014;49:245–253. doi: 10.1007/s00535-013-0817-y [DOI] [PubMed] [Google Scholar]
- 38. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S.. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. doi: 10.1016/j.jacc.2016.02.064 [DOI] [PubMed] [Google Scholar]
- 39. Ducrocq G, Wallace JS, Baron G, Ravaud P, Alberts MJ, Wilson PW, Ohman EM, Brennan DM, D’Agostino RB, Bhatt DL, Steg PG; REACH Investigators. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J. 2010;31:1257–1265. doi: 10.1093/eurheartj/ehq021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 41. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. doi: 10.1001/jama.2016.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 43. Gerber Y, Rihal CS, Sundt TM 3rd, Killian JM, Weston SA, Therneau TM, Roger VL.. Coronary revascularization in the community: a population-based study, 1990 to 2004. J Am Coll Cardiol. 2007;50:1223–1229. doi: 10.1016/j.jacc.2007.06.022 [DOI] [PubMed] [Google Scholar]
- 44. Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JWM, Moussa I, Oetgen WJ, Varosy PD, Vincent RN, Wei J, Curtis JP, Roe MT, Spertus JA.. Trends in U.S. cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. 2017;69:1427–1450. doi: 10.1016/j.jacc.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 45. Feldman DN, Gade CL, Slotwiner AJ, Parikh M, Bergman G, Wong SC, Minutello RM; New York State Angioplasty Registry. Comparison of outcomes of percutaneous coronary interventions in patients of three age groups (<60, 60 to 80, and >80 years) (from the New York State Angioplasty Registry). Am J Cardiol. 2006;98:1334–1339. doi: 10.1016/j.amjcard.2006.06.026 [DOI] [PubMed] [Google Scholar]
- 46. Morice MC, Talwar S, Gaemperli O, Richardt G, Eberli F, Meredith I, Zaman A, Fajadet J, Copt S, Greene S, Urban P.. Drug-coated versus bare-metal stents for elderly patients: a predefined sub-study of the LEADERS FREE trial. Int J Cardiol. 2017;243:110–115. doi: 10.1016/j.ijcard.2017.04.079 [DOI] [PubMed] [Google Scholar]
- 47. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP.. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW.. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076 [DOI] [PubMed] [Google Scholar]
- 49. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP.. In-hospital major bleeding during ST-elevation and non-ST-elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry(R)-GWTG. Am J Cardiol. 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 50. Rao SV, McCoy LA, Spertus JA, Krone RJ, Singh M, Fitzgerald S, Peterson ED.. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6:897–904. [DOI] [PubMed] [Google Scholar]
- 51. Pasea L, Chung SC, Pujades-Rodriguez M, Moayyeri A, Denaxas S, Fox KAA, Wallentin L, Pocock SJ, Timmis A, Banerjee A, Patel R, Hemingway H.. Personalising the decision for prolonged dual antiplatelet therapy: development, validation and potential impact of prognostic models for cardiovascular events and bleeding in myocardial infarction survivors. Eur Heart J. 2017;38:1048–1055. doi: 10.1093/eurheartj/ehw683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee SY, Hong MK, Palmerini T, Kim HS, Valgimigli M, Feres F, Colombo A, Gilard M, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Stone GW.. Short-term versus long-term dual antiplatelet therapy after drug-eluting stent implantation in elderly patients: a meta-analysis of individual participant data from 6 randomized trials. JACC Cardiovasc Interv. 2018;11:435–443. doi: 10.1016/j.jcin.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 53. Capodanno D, Angiolillo DJ.. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ Cardiovasc Interv. 2014;7:113–124. doi: 10.1161/CIRCINTERVENTIONS.113.001150 [DOI] [PubMed] [Google Scholar]
- 54. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP.. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138:527–536. doi: 10.1161/CIRCULATIONAHA.118.034722 [DOI] [PubMed] [Google Scholar]
- 55. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ‘t Hof AW, ten Berg JM; WOEST Study Investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 56. Fiedler KA, Maeng M, Mehilli J, Schulz-Schüpke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N.. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE Trial. J Am Coll Cardiol. 2015;65:1619–1629. doi: 10.1016/j.jacc.2015.02.050 [DOI] [PubMed] [Google Scholar]
- 57. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA.. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 58. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH; RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 59. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, Messenger JC, Rumsfeld JS, Spertus JA.. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3:e001380. doi: 10.1161/JAHA.114.001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mehran R, Nikolsky E, Lansky AJ, Kirtane AJ, Kim YH, Feit F, Manoukian S, Moses JW, Ebrahimi R, Ohman EM, White HD, Pocock SJ, Dangas GD, Stone GW.. Impact of chronic kidney disease on early (30-day) and late (1-year) outcomes of patients with acute coronary syndromes treated with alternative antithrombotic treatment strategies: an ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) substudy. JACC Cardiovasc Interv. 2009;2:748–757. doi: 10.1016/j.jcin.2009.05.018 [DOI] [PubMed] [Google Scholar]
- 61. Saltzman AJ, Stone GW, Claessen BE, Narula A, Leon-Reyes S, Weisz G, Brodie B, Witzenbichler B, Guagliumi G, Kornowski R, Dudek D, Metzger DC, Lansky AJ, Nikolsky E, Dangas GD, Mehran R.. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:1011–1019. doi: 10.1016/j.jcin.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 62. Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ, Saucedo JF; EVENT Investigators. In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: a report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc Interv. 2009;2:37–45. doi: 10.1016/j.jcin.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 63. Baber U, Li SX, Pinnelas R, Pocock SJ, Krucoff MW, Ariti C, Gibson CM, Steg PG, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Iakovou I, Dangas G, Aquino MB, Sartori S, Chieffo A, Moliterno DJ, Colombo A, Mehran R.. Incidence, patterns, and impact of dual antiplatelet therapy cessation among patients with and without chronic kidney disease undergoing percutaneous coronary intervention: results from the PARIS Registry (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients). Circ Cardiovasc Interv. 2018;11:e006144. doi: 10.1161/CIRCINTERVENTIONS.117.006144 [DOI] [PubMed] [Google Scholar]
- 64. Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Xu K, Parise H, Brodie BR, Stuckey TD, Stone GW.. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents registry. Circ Cardiovasc Interv. 2015;8:e001683. doi: 10.1161/CIRCINTERVENTIONS.115.001683 [DOI] [PubMed] [Google Scholar]
- 65. Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, Gloekler S, Binder RK, Wenaweser P, Moschovitis A, Khattab AA, Buellesfeld L, Zwahlen M, Meier B, Jüni P, Windecker S.. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interv. 2012;5:202–210. doi: 10.1161/CIRCINTERVENTIONS.111.965749 [DOI] [PubMed] [Google Scholar]
- 66. Guerrero C, Garay A, Ariza-Solé A, Formiga F, Raposeiras-Roubín S, Abu-Assi E, D’Ascenzo F, Kinnaird T, Manzano-Fernández S, Alegre O, Sánchez-Salado JC, Lorente V, Templin C, Velicki L, Xanthopoulou I, Cerrato E, Rognoni A, Boccuzzi G, Omedè P, Montabone A, Taha S, Durante A, Gili S, Magnani G, Conrotto F, Bertaina M, Autelli M, Grosso A, Blanco PF, Quadri G, Varbella F, Tomassini F, Queija BC, Paz RC, Fernández MC, Pousa IM, Gallo D, Morbiducci U, Dominguez-Rodriguez A, Valdés M, Alexopoulos D, Iñiguez-Romo A, Gaita F, Cequier Á.. Anemia in patients with acute coronary syndromes treated with prasugrel or ticagrelor: insights from the RENAMI registry. Thromb Res. 2018;167:142–148. doi: 10.1016/j.thromres.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 67. Kwok CS, Tiong D, Pradhan A, Andreou AY, Nolan J, Bertrand OF, Curzen N, Urban P, Myint PK, Zaman AG, Loke YK, Mamas MA.. Meta-analysis of the prognostic impact of anemia in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2016;118:610–620. doi: 10.1016/j.amjcard.2016.05.059 [DOI] [PubMed] [Google Scholar]
- 68. Chan FK, Ching JY, Hung LC, Wong VW, Leung VK, Kung NN, Hui AJ, Wu JC, Leung WK, Lee VW, Lee KK, Lee YT, Lau JY, To KF, Chan HL, Chung SC, Sung JJ.. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352:238–244. doi: 10.1056/NEJMoa042087 [DOI] [PubMed] [Google Scholar]
- 69. Sung JJ, Lau JY, Ching JY, Wu JC, Lee YT, Chiu PW, Leung VK, Wong VW, Chan FK.. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1–9. doi: 10.7326/0003-4819-152-1-201001050-00179 [DOI] [PubMed] [Google Scholar]
- 70. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C.. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801 [DOI] [PubMed] [Google Scholar]
- 71. McCarthy CP, Steg G, Bhatt DL.. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38:3488–3492. doi: 10.1093/eurheartj/ehx531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ayoub K, Marji M, Ogunbayo G, Masri A, Abdel-Latif A, Ziada K, Vallurupalli S.. Impact of chronic thrombocytopenia on in-hospital outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:1862–1868. doi: 10.1016/j.jcin.2018.05.033 [DOI] [PubMed] [Google Scholar]
- 73. Ito S, Watanabe H, Morimoto T, Yoshikawa Y, Shiomi H, Shizuta S, Ono K, Yamaji K, Soga Y, Hyodo M, Shirai S, Ando K, Horiuchi H, Kimura T.. Impact of baseline thrombocytopenia on bleeding and mortality after percutaneous coronary intervention. Am J Cardiol. 2018;121:1304–1314. doi: 10.1016/j.amjcard.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 74. Hakim DA, Dangas GD, Caixeta A, Nikolsky E, Lansky AJ, Moses JW, Claessen B, Sanidas E, White HD, Ohman EM, Manoukian SV, Fahy M, Mehran R, Stone GW.. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am Heart J. 2011;161:391–396. doi: 10.1016/j.ahj.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 75. de Raucourt E, Roussel-Robert V, Zetterberg E.. Prevention and treatment of atherosclerosis in haemophilia: how to balance risk of bleeding with risk of ischaemic events. Eur J Haematol. 2015;94(suppl 77):23–29. doi: 10.1111/ejh.12498 [DOI] [PubMed] [Google Scholar]
- 76. Sharma R, Flood VH.. Advances in the diagnosis and treatment of von Willebrand disease. Blood. 2017;130:2386–2391. doi: 10.1182/blood-2017-05-782029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Franchini M, Coppola A.. Atherothrombosis in von Willebrand disease: an analysis of the literature and implications for clinical management. Semin Thromb Hemost. 2012;38:185–199. doi: 10.1055/s-0032-1301416 [DOI] [PubMed] [Google Scholar]
- 78. Boehnel C, Rickli H, Graf L, Maeder MT.. Coronary angiography with or without percutaneous coronary intervention in patients with hemophilia: systematic review. Catheter Cardiovasc Interv. 2018;92:1–15. doi: 10.1002/ccd.27255 [DOI] [PubMed] [Google Scholar]
- 79. Federici AB, Bucciarelli P, Castaman G, Mazzucconi MG, Morfini M, Rocino A, Schiavoni M, Peyvandi F, Rodeghiero F, Mannucci PM.. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014;123:4037–4044. doi: 10.1182/blood-2014-02-557264 [DOI] [PubMed] [Google Scholar]
- 80. Singh V, Patel NJ, Rodriguez AP, Shantha G, Arora S, Deshmukh A, Cohen MG, Grines C, De Marchena E, Badheka A, Ghatak A.. Percutaneous coronary intervention in patients with end-stage liver disease. Am J Cardiol. 2016;117:1729–1734. doi: 10.1016/j.amjcard.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 81. Mitchell O, Feldman DM, Diakow M, Sigal SH.. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50. doi: 10.2147/HMER.S74612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Russo MW, Pierson J, Narang T, Montegudo A, Eskind L, Gulati S.. Coronary artery stents and antiplatelet therapy in patients with cirrhosis. J Clin Gastroenterol. 2012;46:339–344. doi: 10.1097/MCG.0b013e3182371258 [DOI] [PubMed] [Google Scholar]
- 83. Maddur H, Bourdillon PD, Liangpunsakul S, Joseph Tector A, Fridell JA, Ghabril M, Lacerda MA, Bourdillon C, Shen C, Kwo PY.. Role of cardiac catheterization and percutaneous coronary intervention in the preoperative assessment and management of patients before orthotopic liver transplantation. Liver Transpl. 2014;20:664–672. doi: 10.1002/lt.23873 [DOI] [PubMed] [Google Scholar]
- 84. Patel NJ, Pau D, Nalluri N, Bhatt P, Thakkar B, Kanotra R, Agnihotri K, Ainani N, Patel N, Patel N, Shah S, Kadavath S, Arora S, Sheikh A, Badheka AO, Lafferty J, Alfonso C, Cohen M.. Temporal trends, predictors, and outcomes of in-hospital gastrointestinal bleeding associated with percutaneous coronary intervention. Am J Cardiol. 2016;118:1150–1157. doi: 10.1016/j.amjcard.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 85. Krill T, Brown G, Weideman RA, Cipher DJ, Spechler SJ, Brilakis E, Feagins LA.. Patients with cirrhosis who have coronary artery disease treated with cardiac stents have high rates of gastrointestinal bleeding, but no increased mortality. Aliment Pharmacol Ther. 2017;46:183–192. doi: 10.1111/apt.14121 [DOI] [PubMed] [Google Scholar]
- 86. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 87. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. [DOI] [PubMed] [Google Scholar]
- 88. Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, Cho H, Ahn H, Cho YY, Yoo JJ, Cho Y, Lee DH, Cho EJ, Yu SJ, Lee DH, Lee JM, Kim YJ, Yoon JH.. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556–1569. doi: 10.1002/hep.29318 [DOI] [PubMed] [Google Scholar]
- 89. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR.. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172 [DOI] [PubMed] [Google Scholar]
- 90. Child CG, Turcotte JG.. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 91. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R.. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 92. Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, De Belder MA, Kwok CS, Rashid M, Fischman DL, Mamas MA.. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States [published online November 30, 2018]. Eur Heart J. doi: 10.1093/eurheartj/ehy769. https://academic.oup.com/eurheartj/advance-article-abstract/doi/10.1093/eurheartj/ehy769/5221009?redirectedFrom=fulltext. [DOI] [PubMed] [Google Scholar]
- 93. Ko DT, Yun L, Wijeysundera HC, Jackevicius CA, Rao SV, Austin PC, Marquis JF, Tu JV.. Incidence, predictors, and prognostic implications of hospitalization for late bleeding after percutaneous coronary intervention for patients older than 65 years. Circ Cardiovasc Interv. 2010;3:140–147. doi: 10.1161/CIRCINTERVENTIONS.109.928721 [DOI] [PubMed] [Google Scholar]
- 94. Roe MT, Cyr DD, Eckart D, Schulte PJ, Morse MA, Blackwell KL, Ready NE, Zafar SY, Beaven AW, Strickler JH, Onken JE, Winters KJ, Houterloot L, Zamoryakhin D, Wiviott SD, White HD, Prabhakaran D, Fox KA, Armstrong PW, Ohman EM; TRILOGY ACS Investigators. Ascertainment, classification, and impact of neoplasm detection during prolonged treatment with dual antiplatelet therapy with prasugrel vs. clopidogrel following acute coronary syndrome. Eur Heart J. 2016;37:412–422. doi: 10.1093/eurheartj/ehv611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fokkema ML, James SK, Albertsson P, Akerblom A, Calais F, Eriksson P, Jensen J, Nilsson T, de Smet BJ, Sjögren I, Thorvinger B, Lagerqvist B.. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2013;61:1222–1230. doi: 10.1016/j.jacc.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 96. Acharya T, Salisbury AC, Spertus JA, Kennedy KF, Bhullar A, Reddy HKK, Joshi BK, Ambrose JA.. In-hospital outcomes of percutaneous coronary intervention in America’s safety net: insights from the NCDR Cath-PCI Registry. JACC Cardiovasc Interv. 2017;10:1475–1485. doi: 10.1016/j.jcin.2017.05.042 [DOI] [PubMed] [Google Scholar]
- 97. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4 [DOI] [PubMed] [Google Scholar]
- 98. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsén M; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 99.European Medicines Agency. Efient public assessment report: product information. 2009. https://www.ema.europa.eu/documents/product-information/efient-epar-product-information_en.pdf. Accessed 25 October 2018.
- 100. James SK, Storey RF, Khurmi NS, Husted S, Keltai M, Mahaffey KW, Maya J, Morais J, Lopes RD, Nicolau JC, Pais P, Raev D, Lopez-Sendon JL, Stevens SR, Becker RC; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attack. Circulation. 2012;125:2914–2921. doi: 10.1161/CIRCULATIONAHA.111.082727 [DOI] [PubMed] [Google Scholar]
- 101. Morrow DA, Alberts MJ, Mohr JP, Ameriso SF, Bonaca MP, Goto S, Hankey GJ, Murphy SA, Scirica BM, Braunwald E, Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events TSC and Investigators. Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke. 2013;44:691–698. [DOI] [PubMed] [Google Scholar]
- 102. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 103. Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KS; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35–43. doi: 10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]
- 104. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 106. Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA.. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 109. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 110. Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, Torner JC; American Heart Association Stroke Council. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e200–e224. doi: 10.1161/STR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 111. Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL; MARS Coinvestigators. Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology. 2014;83:590–597. doi: 10.1212/WNL.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ; International ARUBA Investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Capodanno D, Angiolillo DJ.. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785–2798. doi: 10.1161/CIRCULATIONAHA.113.003675 [DOI] [PubMed] [Google Scholar]
- 114. Banerjee S, Angiolillo DJ, Boden WE, Murphy JG, Khalili H, Hasan AA, Harrington RA, Rao SV.. Use of antiplatelet therapy/DAPT for post-PCI patients undergoing noncardiac surgery. J Am Coll Cardiol. 2017;69:1861–1870. doi: 10.1016/j.jacc.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 115. Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, Villar JC, Sigamani A, Biccard BM, Meyhoff CS, Parlow JL, Guyatt G, Robinson A, Garg AX, Rodseth RN, Botto F, Lurati Buse G, Xavier D, Chan MT, Tiboni M, Cook D, Kumar PA, Forget P, Malaga G, Fleischmann E, Amir M, Eikelboom J, Mizera R, Torres D, Wang CY, VanHelder T, Paniagua P, Berwanger O, Srinathan S, Graham M, Pasin L, Le Manach Y, Gao P, Pogue J, Whitlock R, Lamy A, Kearon C, Baigent C, Chow C, Pettit S, Chrolavicius S, Yusuf S; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105 [DOI] [PubMed] [Google Scholar]
- 116. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Lüscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C; Task Force Members. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282 [DOI] [PubMed] [Google Scholar]
- 117. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513 [DOI] [PubMed] [Google Scholar]
- 118. Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, Capodanno D, Leonardi S, Lettino M, Limbruno U, Menozzi A, Marchese UOA, Saia F, Valgimigli M, Ageno W, Falanga A, Corcione A, Locatelli A, Montorsi M, Piazza D, Stella A, Bozzani A, Parolari A, Carone R, Angiolillo DJ; Italian Society of Interventional Cardiology (SICI-GISE); Italian Society for the Study of Haemostasis and Thrombosis (SISET); Italian Society of Anesthesia and Intensive Care Medicine (SIAARTI); Italian Society of Surgery (SIC); Italian Society for Cardiac Surgery (SICCH); Italian Society of Vascular and Endovascular Surgery (SICVE); Italian Society of Urology (SIU); Italian Orthopaedic Society (SIOT); Italian Society of Thoracic Surgeons (SICT); Italian Federation of Scientific Societies of Digestive System Diseases (FISMAD); Italian Society of Digestive Endoscopy (SIED); Italian Association of Hospital Gastroenterology and Digestive Endoscopy (AIGO); Italian Association of Gastroenterology and Digestive Endoscopy (SIGE); Italian Society of Maxillofacial Surgery (SICMF); Italian Society of Reconstructive Plastic Surgery and Aesthetics (SICPRE); Italian Society of Gynecology and Obstetrics (SIGO); Italian Society of Neurosurgery (SINch); Italian Association of Hospital Pulmonologist (AIPO); Italian Society of Periodontology (SIdP); Italian Society of Ophthalmology (SOI); Italian Association of Hospital Otorhinolaryngologist (AOOI); Italian Association of Hospital Surgeons (ACOI); Association of Obstetricians Gynecologists Italian Hospital (AOGOI). A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery: surgery after stenting 2. JACC Cardiovasc Interv. 2018;11:417–434. doi: 10.1016/j.jcin.2017.10.05129519377 [Google Scholar]
- 119. Godier A, Fontana P, Motte S, Steib A, Bonhomme F, Schlumberger S, Lecompte T, Rosencher N, Susen S, Vincentelli A, Gruel Y, Albaladejo P, Collet JP; French Working Group on Perioperative Hemostasis (GIHP). Management of antiplatelet therapy in patients undergoing elective invasive procedures: proposals from the French Working Group on Perioperative Hemostasis (GIHP) and the French Study Group on Thrombosis and Hemostasis (GFHT): in collaboration with the French Society for Anesthesia and Intensive Care (SFAR). Arch Cardiovasc Dis. 2018;111:210–223. doi: 10.1016/j.acvd.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 120. Vivas D, Roldan I, Ferrandis R, Marin F, Roldan V, Tello-Montoliu A, Ruiz-Nodar JM, Gomez-Doblas JJ, Martin A, Llau JV, Ramos-Gallo MJ, Munoz R, Arcelus JI, Leyva F, Alberca F, Oliva R, Gomez AM, Montero C, Arikan F, Ley L, Santos-Bueso E, Figuero E, Bujaldon A, Urbano J, Otero R, Hermida JF, Egocheaga I, Llisterri JL, Lobos JM, Serrano A, Madridano O, Ferreiro JL.. Perioperative and periprocedural management of antithrombotic therapy: consensus document of SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT and AEU. Rev Esp Cardiol (Engl Ed). 2018;71:553–564. [DOI] [PubMed] [Google Scholar]
- 121. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW.. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6 [DOI] [PubMed] [Google Scholar]
- 122. Kimura T, Morimoto T, Nakagawa Y, Tamura T, Kadota K, Yasumoto H, Nishikawa H, Hiasa Y, Muramatsu T, Meguro T, Inoue N, Honda H, Hayashi Y, Miyazaki S, Oshima S, Honda T, Shiode N, Namura M, Sone T, Nobuyoshi M, Kita T, Mitsudo K; j-Cypher Registry Investigators. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009;119:987–995. doi: 10.1161/CIRCULATIONAHA.108.808311 [DOI] [PubMed] [Google Scholar]
- 123. Steely AM, Callas PW, Neal D, Scali ST, Goodney PP, Schanzer A, Cronenwett JL, Bertges DJ; Vascular Quality Initiative. Regional variation in postoperative myocardial infarction in patients undergoing vascular surgery in the United States. Ann Vasc Surg. 2017;40:63–73. doi: 10.1016/j.avsg.2016.07.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. White WB, Kloner RA, Angiolillo DJ, Davidson MH.. Cardiorenal safety of OTC analgesics. J Cardiovasc Pharmacol Ther. 2018;23:103–118. doi: 10.1177/1074248417751070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Singh G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: a view from the ARAMIS database: Arthritis, Rheumatism, and Aging Medical Information System. Am J Ther. 2000;7:115–121. [DOI] [PubMed] [Google Scholar]
- 126. Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A.. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. 2018;154:500–514. doi: 10.1053/j.gastro.2017.10.049 [DOI] [PubMed] [Google Scholar]
- 127. Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115 [DOI] [PubMed] [Google Scholar]
- 128. Masclee GM, Valkhoff VE, Coloma PM, de Ridder M, Romio S, Schuemie MJ, Herings R, Gini R, Mazzaglia G, Picelli G, Scotti L, Pedersen L, Kuipers EJ, van der Lei J, Sturkenboom MC.. Risk of upper gastrointestinal bleeding from different drug combinations. Gastroenterology. 2014;147:784–792.e9; quiz e13. doi: 10.1053/j.gastro.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 129. Chan FKL, Ching JYL, Tse YK, Lam K, Wong GLH, Ng SC, Lee V, Au KWL, Cheong PK, Suen BY, Chan H, Kee KM, Lo A, Wong VWS, Wu JCY, Kyaw MH.. Gastrointestinal safety of celecoxib versus naproxen in patients with cardiothrombotic diseases and arthritis after upper gastrointestinal bleeding (CONCERN): an industry-independent, double-blind, double-dummy, randomised trial. Lancet. 2017;389:2375–2382. doi: 10.1016/S0140-6736(17)30981-9 [DOI] [PubMed] [Google Scholar]
- 130. Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS.. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial: Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. [DOI] [PubMed] [Google Scholar]
- 131. Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, Malisoux L, Guillaume M, Witte DR.. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186:420–434. doi: 10.1093/aje/kwx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dodson JA, Hochman JS, Roe MT, Chen AY, Chaudhry SI, Katz S, Zhong H, Radford MJ, Udell JA, Bagai A, Fonarow GC, Gulati M, Enriquez JR, Garratt KN, Alexander KP.. The association of frailty with in-hospital bleeding among older adults with acute myocardial infarction: insights from the ACTION Registry. JACC Cardiovasc Interv. 2018;11:2287–2296. doi: 10.1016/j.jcin.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Numasawa Y, Kohsaka S, Ueda I, Miyata H, Sawano M, Kawamura A, Noma S, Suzuki M, Nakagawa S, Momiyama Y, Fukuda K.. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol. 2017;69:272–279. doi: 10.1016/j.jjcc.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 134. Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Nakamura M.. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–1692. [DOI] [PubMed] [Google Scholar]
- 135. Kohsaka S, Miyata H, Ueda I, Masoudi FA, Peterson ED, Maekawa Y, Kawamura A, Fukuda K, Roe MT, Rumsfeld JS, JCD-KiCS and NCDR. An international comparison of patients undergoing percutaneous coronary intervention: a collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database-Keio interhospital Cardiovascular Studies (JCD-KiCS). Am Heart J. 2015;170:1077–1085. [DOI] [PubMed] [Google Scholar]
- 136. Kang J, Park KW, Palmerini T, Stone GW, Lee MS, Colombo A, Chieffo A, Feres F, Abizaid A, Bhatt DL, Valgimigli M, Hong MK, Jang Y, Gilard M, Morice MC, Park DW, Park SJ, Jeong YH, Park J, Koo BK, Kim HS.. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545 [DOI] [PubMed] [Google Scholar]
- 137. Verheugt FW, Steinhubl SR, Hamon M, Darius H, Steg PG, Valgimigli M, Marso SP, Rao SV, Gershlick AH, Lincoff AM, Mehran R, Stone GW.. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:191–197. doi: 10.1016/j.jcin.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 138. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L; PLATO Study Group. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–2141. doi: 10.1161/CIRCULATIONAHA.109.927582 [DOI] [PubMed] [Google Scholar]
- 139. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM; TRITON-TIMI 38 Investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4 [DOI] [PubMed] [Google Scholar]
- 140. Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Berger PB, Iakovou I, Dangas G, Waksman R, Antoniucci D, Sartori S, Krucoff MW, Hermiller JB, Shawl F, Gibson CM, Chieffo A, Alu M, Moliterno DJ, Colombo A, Pocock S.. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.