Abstract
Background
Minority adolescents are at highest risk for obesity and extreme obesity; yet, there are few clinical trials targeting African American adolescents with obesity.
Purpose
The purpose of the study was to develop an adaptive family-based behavioral obesity treatment for African American adolescents using a sequential multiple assignment randomized trial (SMART) design.
Methods
Fit Families was a SMART where 181 African American adolescents (67% female) aged 12–17 were first randomized to office-based versus home-based behavioral skills treatment delivered from a Motivational Interviewing foundation. After 3 months, nonresponders to first phase treatment were rerandomized to continued home-based behavioral skills treatment or contingency management with voucher-based reinforcement for adolescent weight loss and for caregiver adherence to the program. All interventions were delivered by community health workers. The primary outcome was treatment retention and percent overweight.
Results
All adolescents reduced percent overweight by −3.20%; there were no significant differences in percent overweight based on treatment sequence. Adolescents receiving home-based delivery in Phase 1 and contingency management in Phase 2 completed significantly more sessions than those receiving office-based treatment and continued skills without CM (M = 8.03, SD = 3.24 and M = 6.62, SD = 2.95, respectively). The effect of contingency management was strongest among older and those with lower baseline confidence. Younger adolescents experienced greater weight reductions when receiving continued skills (−4.90% compared with −.02%).
Conclusions
Behavioral skills training can be successfully delivered to African American adolescents with obesity and their caregivers by community health workers when using a home-based service model with incentives. More potent interventions are needed to increase reductions in percent overweight and may need to be developmentally tailored for younger and older adolescents.
Keywords: Minority, Adolescent, Obesity, Skills, Motivational interviewing, SMART
Contingency management and home-based delivery helps retain African American adolescents in family-based behavioral obesity treatment.
Introduction
Rates of obesity and extreme obesity continue to rise in adolescents in the USA and minority adolescents continue to be most at risk [1]. However, recent reviews suggest a dearth of behavioral clinical trials in African American adolescents with obesity [2, 3]. Of existing trials, only a few have shown that participants lost weight and weight loss was at best modest [4–8]. When African American adolescents have participated in behavioral intervention trials for obesity, they have been at high risk for drop out [9]. Many have suggested home-based service delivery to increase access to behavioral health services [10, 11], particularly when delivered by community health workers [12].
Motivation to engage in the difficult behaviors necessary for weight loss may be another factor that affects weight loss success or failure (e.g., controlling portion sizes, self-monitoring, environmental control, and managing hunger and cravings) [13]. Several studies have indicated low motivation on the part of African American adolescents to engage in these behaviors [5, 6, 14]. Contingency management (CM) is an evidence-based strategy for increasing extrinsic motivation for behavior change by offering competing behavioral incentives (e.g., money and prizes) to counteract the reinforcement inherent in unhealthy foods and sedentary activities. CM has been tested extensively in the adolescent substance abuse literature [15] and has been recommended for pediatric obesity [16, 17], though obesity trials with African American adolescents have not explicitly employed this strategy.
Delivering services in the home to increase access and incorporating CM to increase motivation may improve treatment response, but these interventions are costly and may not be necessary for all families. Optimal interventions would be those that (a) are initially tailored and individualized based on participants’ presenting characteristics and (b) can change based upon the participants’ success or failure in treatment [18, 19]. Sequential multiple assignment randomization trials (SMART) are part of the newest generation of improvements in clinical trial design and methodology, which allow the development of such adaptive treatments [20]. The advantages of a SMART design over separate experiments testing different treatment strategies at different critical decision points include the involvement of the same participants in all phases of intervention development, being able to understand how initial and subsequent stage treatments work with (synergistically) or against (antagonistically) each other, and the ability to generate hypotheses about moderators of sequenced treatments.
The goal of the present SMART was to develop a 6 month adaptive treatment for weight loss in African American adolescents with primary obesity delivered by community health workers. We first tested whether family-based behavioral treatment would have greater success when delivered in the home (HBT) than in the office (OBT). For families who did not respond after 3 months, we tested whether the addition of CM would enhance outcomes compared with continued family-based behavioral treatment. Based on the literature recommending home-based services for high-risk minority populations [10–12], we hypothesized that HBT would result in greater session completion and more weight loss after 3 months compared with OBT. Second, we hypothesized that the addition of 3 months CM would result in greater session completion and more weight loss compared with continued behavioral skills (CS).
A secondary aim was to test moderators of weight loss. We hypothesized that African American adolescents with obesity with lower initial motivation, as defined by perceived importance and confidence, at baseline would require home-based and CM treatments to achieve significant reductions in percent overweight. We also explored age as a moderator to determine whether younger adolescents responded differently from older adolescent to the intervention sequences.
Method
Participants
Inclusion criteria were (a) self-identifying as African American, (b) being between the ages of 12 years 0 months and 16 years 11 months, (c) having BMI ≥ 95th percentile for age and gender, (d) residing with the identified primary caregiver, (e) living within 30 miles of the urban children’s hospital affiliated with the university, (f) primary caregiver willing to participate in treatment, and (g) speaking English. Exclusion criteria were (a) obesity secondary to medication prescribed for another medical condition (e.g., steroids and antipsychotics) or secondary to a chronic condition (e.g., Down syndrome, Prader–Willi syndrome, and Cushing’s syndrome), (b) conditions causing potential daily fluid fluctuations (e.g., diabetes insipidus, congestive heart failure, and dialysis), (c) medical conditions that prevent participation in normal exercise, (d) pregnancy or another medical condition where weight loss is contraindicated, (e) thought disorder (e.g., schizophrenia or other psychosis), suicidal, or homicidal, or (f) serious cognitive impairment. Potential participants were required to have a medical provider give clearance prior to enrollment if the participant had (a) a diagnosis of asthma, diabetes, or hypertension; (b) initial blood pressure readings averaging above 140/90; or (c) problems after physical activity reported on the Physical Activity Readiness Questionnaire [21–23].
The university’s Institutional Review Board approved the study, and the study was registered in ClinicalTrials.gov (NCT01350531). Three recruitment methods were used. First, potential participants were recruited from primary care, endocrine, cardiology, and asthma clinics. They were approached by medical staff who provided a brief study overview. Interested caregivers completed a release of contact information form to allow information to be provided to study personnel. Second, electronic medical records identified potential participants based on BMI and age. A letter was sent to the participant’s caregiver describing the study with a contact number for opting out. Third, families recruited from community settings (e.g., health fairs) completed a release of contact information form. Study staff then contacted potential participants to complete eligibility screening. See Brogan Hartlieb et al. [24] for more information regarding recruitment strategies.
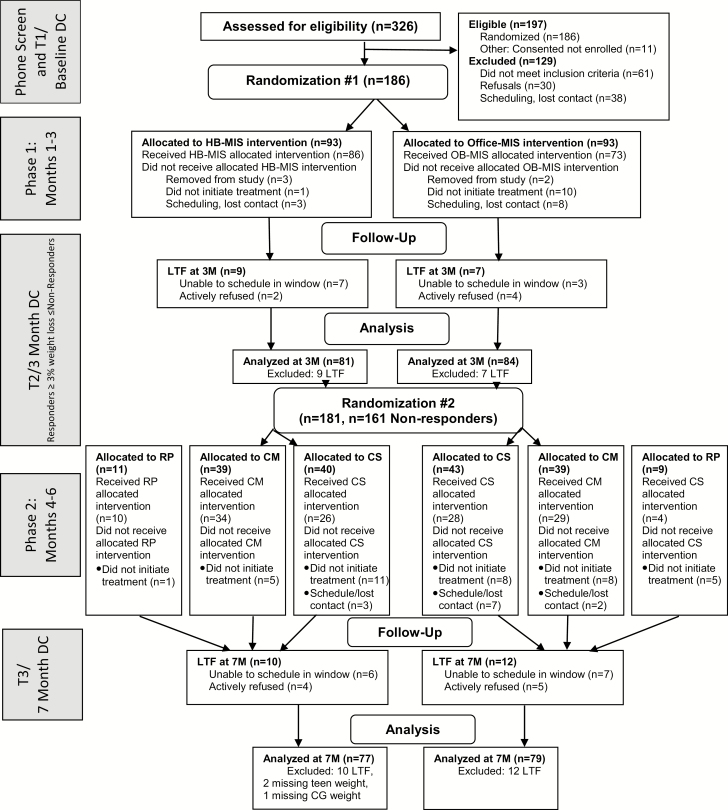
Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) diagram of participant flow throughout the study. Phone screening was conducted with 326 families. Sixty-one were ineligible, 30 refused, and 38 were lost before completing the consent process. One hundred eighty-six families enrolled. Five were excluded by the research team (two due to counselor error and three determined to be ineligible after the baseline visit), resulting in a sample of 181 adolescents and their caregiver. Table 1 demonstrates demographic characteristics at baseline.
Fig. 1.
Participant flow following Consolidated Standards of Reporting Trials (CONSORT) guidelines. CG caregiver; CM contingency management; CS continued skills; DC data collection; HB home-based; LTF lost to follow-up; MIS motivational interviewing and skills; OB office-based; RP relapse prevention.
Table 1.
Participant baseline characteristics (n = 181)
| M or % | SD or N | Range | Correlation with youth percent overweight | ||
|---|---|---|---|---|---|
| Teen weight, in pounds | 229.96 | 51.13 | 133.00 | 451.00 | .897*** |
| Teen BMI | 38.15 | 7.45 | 25.70 | 60.50 | .976*** |
| Teen percentage overweight | 96.81 | 37.59 | 35.38 | 218.47 | –- |
| Teen age | 14.26 | 1.44 | 12.04 | 17.01 | −.031 |
| Teen gender (reference = male) |
32.6% | 59 | −.043 | ||
| Caregiver weight, in pounds | 245.64 | 67.15 | 133.00 | 625.00 | .166* |
| Caregiver BMI | 41.28 | 11.179 | 22.40 | 103.99 | .213** |
| Caregiver percentage overweight | 89.68 | 51.76 | 3.13 | 378.86 | .214** |
| Caregiver parenting status (reference = single parent) |
63.5% | 115 | −.039 | ||
| Caregiver educational status (reference = high school or lower) |
38.7% | 70 | −.130† | ||
| Median family income | US$12,000–15,999 | US$5,000–11,999, US$25,000–34,999a | Less than US$5,000 | US$100,000 or more | −.095 |
| Treatment dose | 21.05 | 12.22 | .00 | 44.00 | .135† |
| Importance | 6.19 | 1.69 | .99 | 10.00 | .023 |
| Confidence | 6.88 | 1.71 | 1.94 | 10.00 | −.026 |
aInterquartile Range (IQR).
† p < .075; *p < .05; **p < .01; ***p < .001.
Procedure
Data were collected in home at baseline, 3, and 7 months by research assistants blind to treatment condition. After baseline, the project manager randomized the family to 3 months of home- or office-based treatment (1:1 allocation). The project statistician developed a password-protected randomization spreadsheet before study launch using www.randomization.com and specified a permuted block algorithm with blocks of varying sizes (n = 2 and 4) with groups stratified based on adolescent percent overweight (high: at least 88.0% above the CDC’s median age- and gender-normed BMI; low: less than 88.0% above the median) and presence or absence of adolescent comorbidities (e.g., asthma and hypertension). The project manager notified the family, interventionist, and clinical supervisor of the Phase 1 assignment.
Half way through the end of treatment (3 months postbaseline), research assistants collected weight measurements to assess response to treatment. Participants achieving a weight loss of ≥3% of original body weight were identified by the project manager as responders and assigned to 3 months of relapse prevention (RP). This benchmark was selected based on National Heart Lung and Blood Institute recommendations of 1% weight loss per month.
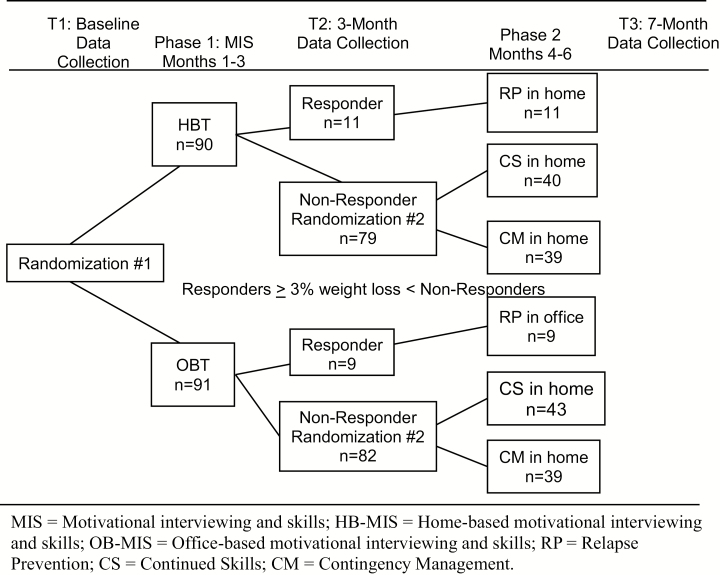
Using the same procedures at baseline, the project manager rerandomized the nonresponding participants into CM or CS, following the Phase 2 randomization spreadsheet (on a separate tab in the same password-protected file as for Phase 1 assignment, generated by the statistician) for nonresponders (1:1 allocation ratio). The interventionist contacted the family with their Phase 2 assignment. The 7 month data collection was completed within 1 month of the second phase ending. Figure 2 demonstrates the SMART randomization. Families were compensated for their time with US$50 each for the baseline and 7 month data collection, and US$10 for the 3 month data collection.
Fig. 2.
Sequential Multiple Assignment Randomization Trial (SMART) design and participant flow. MIS Motivational interviewing and skills; HB-MIS home-based motivational interviewing and skills; OB-MIS office-based motivational interviewing and skills; RP relapse prevention; CS continued skills; CM contingency management.
Treatments
All intervention components were delivered using Motivational Interviewing (MI) [25], a method of communication that was designed to increase intrinsic motivation and was recently adapted for adolescents in general [26] and African American adolescents in particular [27, 28].
Phase 1: Office-based behavioral skills treatment (OBT) and home-based behavioral skills treatment (HBT)
These treatments were designed to be very similar except for delivery setting. In both arms, a community health worker delivered weekly 1 hr face-to-face sessions to the adolescent and their primary caregiver for 3 months. In both cases, a community health worker (a paraprofessional counselor) delivered weekly 1 hr face-to-face sessions, with the first session focusing on engaging the family in treatment. The second two sessions were conjointly delivered with a registered dietitian to provide education in nutrition and physical activity and to develop a plan to either reduce their food intake by 500 kcal or to consume a maximum of 1,600–2,000 kcal per day. The remaining sessions focused on behavioral skills training integrated with MI. Content included self-monitoring food and physical activity levels, stimulus control of food and inactivity triggers both in and out of the home, managing hunger and food cravings, and parenting. A panel of experts in pediatric obesity and in cognitive-behavioral interventions for minority families chose the modules, which were piloted with families prior to the trial [17].
All skills modules included modeling, caregiver and adolescent rehearsal, and feedback. Adolescents were also weighed weekly at the beginning of each session to assess and problem solve barriers and facilitators of weight loss. Counselors also attempted a second 15–45 min session per week on the home (HBT) or by phone (OBT) to discuss homework completion, address any barriers, and complete any missed components from the previous session (HBT). If the first session of the week was completely missed, the second session focused on the agenda of that session. To increase the likelihood of OBT attending treatment, families in OBT received a US$10 gift card per session for attendance, and parking vouchers or transportation via taxi was also provided for intervention sessions.
Phase 2: Continued skills (CS) and contingency management (CM)
The second phase of treatment was the same length and dose as the first phase. Both CS and CM were implemented in participants’ homes. In CS, the health worker first assessed the barriers to weight loss in the previous phase, and then together with the family selected which additional skills to focus on during 3 months of treatment (possible modules included reducing emotional eating, increasing planning and organizational skills, strengthening food refusal skills, and managing distorted cognitions). Families also had the option to repeat any session from the first phase.
The CM incentive structure was developed after extensive piloting (see Hartlieb et al. [17]). A voucher-based system was used to provide incentives to the teen for weight loss and to the caregiver for administering the CM program. Youth earned 20 points for losing at least one pound each week (calculated as an average between weight in session and a mid-week weigh-in). Youth earned 4 additional points each successive week they met their goal and 40 bonus points if they lost at least four pounds in one 4 week period. If youth missed more than one weekly goal in a 4 week period, the points available to earn was reset to 20. When caregivers missed any of their goals (delivering adolescent incentives, attending sessions, and ensuring that the youth recorded daily weights), their points were reset to 20, but they could still earn 40 bonus points every 4 weeks if they met their goals each week of that window. No points were deducted once earned. Each point was equivalent to US$1. Youth and parents could each earn up to US$624 in vouchers for products available from amazon.com (except dietary supplements, food, weapons, alcoholic beverages, and cigarettes). Rather than training in new skills, the health worker guided the caregiver in administering M and discussed barriers and facilitators of weight loss. If the caregiver did not administer the CM, the health worker did so that the youth would still receive points for success. Among families who completed at least one session, adolescents earned an average of US$184.98 (SD = 132.24) and caregivers earned an average of US$271.48 (SD = 186.93).
Phase 2: relapse prevention (RP)
Youth who responded to the first phase interventions (i.e., lost at least 3% of their original weight) were assigned to RP for the second phase. For 3 months of RP, the location of treatment remained the same as it was for Phase 1 (office-based or home-based), and session frequency was reduced to one face-to-face session per week. Treatment consisted of modules designed to explore values and commitment to treatment, managing slips, and reinforcing facilitators of weight loss from the previous 3 months.
Quality assurance procedures
First, counselors were hired based on a structured behavioral interview targeting knowledge, skills, and past performance and experience and performance in a video assessment of simulated encounters-revised [29] that assesses potential for MI competence. Once hired, health workers completed a total of 80 hr of didactic training with a psychologist and a dietitian who were both MI trainers. They also spent 50 hr role-playing and 170 hr in individual or interactive training activities. During the initial training period, role play sessions were coded based on the Motivational Interviewing Treatment Integrity (MITI 3.1) [30] and session checklists, and results were discussed during supervision. Health workers had to reach beginner competency on the MITI to be cleared to see participants.
Throughout the trial, health workers received 1 hr per week of individual supervision, 2 hr per week of group supervision, and a weekly phone meeting with an expert consultant to address difficult cases. All sessions were recorded, and the supervisor rated one session per week per counselor for fidelity to CBT skills training components and MI competence [31]. Monthly, each counselor had 2 hr case review sessions with the clinical supervisor and dietitian. The supervisor and dietitian also provided quarterly 3 hr booster trainings.
Measures
Primary outcomes
Consistent with previous research with adolescents with primary obesity, our primary outcome was percent overweight [14, 32–35] measured at baseline and at post-treatment (7 months) calculated as the percentage over median age- and gender-normed BMI. BMI was computed using Centers for Disease Control Epi Info software version 3.5.1. Weight was assessed with the Seca 869 scale and height was assessed using the Seca 213 Stadiometer. Adolescent weight was the average of two measurements collected between 1 and 9 days apart (Mdifference = 4.42, SD = 2.10). Participants’ change in percent overweight was calculated by subtracting their percent overweight at 7 months from the baseline value. Numbers of sessions completed were extracted from chart review matched with counselor logs.
Moderators
Rollnick’s Readiness Rulers [36] were adapted to assess adolescents’ self-efficacy for and perceived importance of making lifestyle changes necessary for weight loss, as these are two critical dimensions of motivation [37]. Adolescents rated 15 items assessing how important it was to them to makes changes in their eating, exercise, and sedentary behaviors and how confident they were that they could make these changes. Ratings were made on a 10-point ruler anchored at 1 = not important/not confident, 5 = “in the middle,” and 10 = very important/very confident. Both measures demonstrated adequate internal consistence (Importance Ruler: α = .88 and Confidence Ruler: α = .89) in the present sample. The perceived efficacy scale is referred to as confidence and the perceived importance scale is referred to as importance in the subsequent analyses.
Data Analysis Plan
We used generalized estimating equations (GEE) to test the study hypotheses according to the approach outlined by SMART developers Nahum-Shani and colleagues [19]. Robust (sandwich) errors were estimated to account for repeated measures. Phase 1 treatment, Phase 2 treatment, and the interaction of Phase 1 and 2 treatments were the primary predictor variables in all models. In brief, data from adolescents demonstrating a treatment response at the end of Phase 1 (i.e., ≥3% decrease in weight) were replicated and assigned to both Phase 2 arms from which their Phase 1 arm originated. Each participant’s data were then weighted as the inverse of their selection probability.
Adolescent and caregiver demographic characteristics and the total number of treatment sessions completed (dose) were correlated with youth baseline percent overweight at p < .075 (Table 1) and, thus, were included as covariates. We used SPSS, version 23, for these analyses. The alpha was set at p < .05 using a modified intent-to-treat sample.
Power
Power for the SMART adaptive treatment design was based on Murphy (2005) [38]. Because nonresponders are rerandomized in Phase 2, the planned sample size was based on the between-groups comparison of the Phase 2 treatment (CM vs. CS), a conservative approach. The two-sided, independent samples t-test with a Type-I error rate of 5% has 80% power to detect a between-groups difference in weight of 3.5% overweight within a sample size of 180.
Missing data
Twenty-two participants (12.2%) were missing weight outcome data. One additional participant was missing caregiver weight data (covariate) across all data collection points. Bivariate analyses revealed no primary outcome baseline differences between those with and without missing data. Under the assumption of missing at random, the missing values analysis expectation-maximization procedure was used to estimate missing data. The results of primary outcome analysis using these estimated data were no different from the analyses excluding missing data; thus, results reported reflect the analyses with missing data excluded.
Primary outcomes
Two multivariate models estimated to evaluate changes in the primary outcomes. The first evaluated change in youths’ percent overweight, which was calculated by subtracting their percent overweight at 7 months from the baseline value. The second assessed the effect of different treatment modalities on session completion.
Moderators of weight loss
Three multivariable models were estimated to explore potential tailoring variables. Youths’ perceptions of importance, self-efficacy/confidence, and adolescent age at study entry were evaluated as potential time invariant moderator variables. Factors were created from these variables using a median split to define categories (Mdnimp = 6.35; Mdnconf = 7.12; Mdnage = 14.09). Each moderator was tested in a separate model which included, in addition to the aforementioned predictors and covariates, the following moderator variables: Phase 1 treatment * moderator, Phase 2 treatment * moderator, and Phase 1 treatment * Phase 2 treatment * moderator. Estimated marginal means were generated to examine differences among predictor variables.
Results
Descriptive Analyses
Table 1 presents participants’ characteristics at study entry. Caregiver BMI, caregiver educational status, and dose of treatment received were associated at p < .075 with the primary outcome of adolescent percent overweight at baseline and were included as covariates in weight outcome analyses. There were no significant differences between groups in the percent overweight at entry to Phase 1 or Phase 2 treatment.
Primary Outcomes
Adjusted for covariates, adolescents reduced their percent overweight by −3.20% over the course of treatment regardless of treatment component received, and specific treatment components did not predict weight loss. On average, adolescents and their caregivers participated in 21.05 (SD = 12.22) treatment sessions over the course of the 6 months of treatment. Families randomized to home-based treatment in Phase 1 attended 8.03 more sessions (95% CI: 4.79, 11.27) than did families randomized to office-based treatment (26.89 vs. 18.85 sessions). Families randomized to CM in Phase 2 attended 6.62 more sessions (95% CI: 3.67, 9.57) than did families randomized to CS (26.19 vs. 18.56 sessions). Table 2 presents the results of the GEE model.
Table 2.
Primary outcomes
| Wald chi-square | df | P | |
|---|---|---|---|
| Change in percentage overweight | |||
| Intercept | 4.918 | 1 | .027 |
| Phase 1 treatment: HBT vs. OBT | 0.040 | 1 | .842 |
| Phase 2 treatment: CM vs. CS | 1.360 | 1 | .244 |
| Full treatment sequence: Phase 1 * Phase 2 | 0.053 | 1 | .818 |
| Caregiver educational status | 1.801 | 1 | .180 |
| Caregiver BMI | 1.232 | 1 | .267 |
| Dose of treatment | 1.125 | 1 | .289 |
| Dose of treatment | |||
| Intercept | 48.985 | 1 | .000 |
| Phase 1 Treatment: HBT vs. OBT | 23.575 | 1 | .000 |
| Phase 2 Treatment: CM vs. CS | 19.330 | 1 | .000 |
| Full treatment sequence: Phase 1 * Phase 2 | .683 | 1 | .408 |
| Caregiver educational status | .019 | 1 | .890 |
| Caregiver BMI | .190 | 1 | .663 |
Moderators of Percent Overweight
There were significant moderator effects for teen confidence (Table 3). Across all sequences, adolescents who entered treatment with higher confidence reduced their percentage overweight −3.88% more (95% CI: −7.40, −0.35, p = .031) than those with lower confidence (−5.52% compared with −1.64%). In addition, confidence significantly moderated Phase 2 treatment. When confidence was low, adolescents receiving CM were more likely to reduce percent overweight compared with adolescents receiving CS (−3.65% compared with .38%). When confidence was high, both CM and CS groups reduced percent overweight (−6.44% compared with −4.59%). Perceived importance was not a significant moderator of weight loss.
Table 3.
Moderators of change in percentage overweight
| Wald chi-square | df | P | |
|---|---|---|---|
| Teen confidence | |||
| Intercept | 5.954 | 1 | .015 |
| Phase 1 treatment: HBT vs. OBT | 0.783 | 1 | .376 |
| Phase 2 treatment: CM vs. CS | 0.501 | 1 | .479 |
| Full treatment sequence: Phase 1 * Phase 2 | 0.629 | 1 | .428 |
| Teen confidence | 4.649 | 1 | .031 |
| Phase 1 Treatment * Teen Confidence | 0.049 | 1 | .825 |
| Phase 2 Treatment * Teen Confidence | 4.007 | 1 | .045 |
| Phase 1 Treatment * Phase 2 Treatment * Teen Confidence | 1.831 | 1 | .176 |
| Caregiver educational status | 2.938 | 1 | .087 |
| Caregiver BMI | 0.813 | 1 | .367 |
| Dose of treatment | 2.023 | 1 | .155 |
| Teen importance | |||
| Intercept | 5.264 | 1 | .022 |
| Phase 1 treatment: Home vs. Office | 0.055 | 1 | .815 |
| Phase 2 treatment: CM vs. CS | 1.402 | 1 | .236 |
| Full treatment sequence: Phase 1 * Phase 2 | 0.038 | 1 | .846 |
| Teen importance | 0.554 | 1 | .457 |
| Phase 1 Treatment * Teen Importance | 0.081 | 1 | .776 |
| Phase 2 Treatment * Teen Importance | 0.686 | 1 | .408 |
| Phase 1 Treatment * Phase 2 Treatment * Teen Importance | 0.105 | 1 | .746 |
| Caregiver educational status | 1.931 | 1 | .165 |
| Caregiver BMI | 1.164 | 1 | .281 |
| B | 1.451 | 1 | .228 |
| Adolescent age | |||
| 4.634 | 1 | .031 | |
| Phase 1 treatment: HBT vs. Office OBT | 0.081 | 1 | .776 |
| Phase 2 treatment: CM vs. CS | 1.359 | 1 | .244 |
| Full treatment sequence: Phase 1 * Phase 2 | 0.001 | 1 | .974 |
| Adolescent age | 0.307 | 1 | .580 |
| Phase 1 Treatment * Adolescent Age | 1.333 | 1 | .248 |
| Phase 2 Treatment * Adolescent Age | 8.031 | 1 | .005 |
| Phase 1 Treatment * Phase 2 Treatment * Adolescent Age | 2.205 | 1 | .138 |
| Caregiver educational status | 1.811 | 1 | .178 |
| Caregiver BMI | 0.860 | 1 | .354 |
| Dose of treatment | 0.999 | 1 | .318 |
Age was a significant moderator of adolescent weight loss during Phase 2 treatment (Table 3). Older adolescents assigned to CM reduced their percentage overweight more −5.64% more than their peers assigned to CS who essentially lost no weight (−5.72% compared with −0.08%). On the other hand, younger adolescents assigned to CS reduced their percentage overweight −4.88% more than their older peers in CS who lost essentially no weight (−4.90% compared with −.02%).
Discussion
A family-based intervention consisting of evidence-based behavioral weight loss skills training delivered from an MI foundation by front-line public health workers, with intensive training, resulted in high rates of obesity treatment retention and small amounts of weight loss in African American adolescents. The few obesity intervention trials with this population have shown poor retention with weight stabilization at best, or at worst, weight gain [2, 4]. The use of a SMART design allowed for testing of multiple treatment components (office vs. home-based and CM vs. continued skills) in order to identify which treatment components and sequences might be most effective in promoting weight loss in African American adolescents. In the present study, use of a home-based intervention delivery approach was associated with more sessions completed even when office-based intervention was incentivized and transportation provided. Among nonresponders, a voucher-based reinforcement program that included incentives for caregiver session completion in addition to adolescent weight loss both resulted in greater treatment retention.
However, although these strategies may have increased obesity intervention retention in African American adolescents who are at high risk for drop-out and ensured weekly session family-based behavioral skills treatment for 6 months, weight loss was only moderate. Thus, new and more potent treatments may still be necessary for increased treatment retention to result in greater weight loss for African American adolescents. Continued translation of basic behavioral and social science into innovative obesity interventions is warranted, and SMART and other innovative methods can help us to ensure that only interventions with the strongest potential and tailored for specific subpopulations are tested in full scale trials [39–41].
Moderator analyses suggested that adolescents who entered treatment with higher confidence reduced percent overweight by almost 6%. Perceived importance was not a significant moderator. The MI platform of intervention delivery may have targeted the importance of behavior change sufficiently and reduce the impact of this component of motivation on outcomes. MI training for weight loss providers may need to be adapted further to emphasize provider skill in MI strategies to improve confidence (as opposed to importance) before entering weight loss treatment such as provider’s affirming statements and provider’s open questions to elicit internal strengths and external supports [26].
Moderator analyses also suggested two tailoring variables that are relevant when considered future adaptive obesity treatments for African American adolescents. First, when confidence was low at baseline, CM had a clear advantage with a more than 4% difference between CM and CS in percent overweight reductions, whereas when confidence was high, both treatments were effective. Second, age was a significant moderator of treatment sequence. Older adolescents responded best to CM with a 6% reduction in percent overweight, whereas younger adolescents responded best to additional skills training with a 5% reduction in percent overweight. Because younger adolescents may have had less direct control over their own environments, continuing to focus on family-based skills may have been more successful than providing reinforcements. Alternatively, adapting CM procedures for young adolescents to focus on caregiver weight-related behaviors such as self-monitoring, environmental control, and modeling physical activity and nutritional changes may improve the effect of CM for younger adolescents [42].
In summary, the current SMART design was able to address multiple questions about intervention development and future directions in a single trial. CHWs were able to deliver comprehensive behavioral weight loss services when provided with intensive training and supervision and testing of more streamlined or technology-delivered training approaches may further promote replicability. Further analyses of other important secondary outcomes such as changes in physical activity and nutrition behaviors and biomarkers may reveal additional differences between treatment components to inform an adaptive treatment. In addition to testing other moderators related to possible obesity phenotypes such as food addiction and self-regulation [43, 44], the next step is to test an adaptive treatment that offers incentives for older youth and those with lower confidence while offering more typical skills training for younger adolescents and for adolescents with higher confidence and compare this adaptive treatment to standard treatment without tailoring. Strategies to increase confidence may result in improved weight loss for African American adolescents across phenotypes, and interventions delivered in the home and with incentives can increase intervention retention for high-risk African American families.
Funding
This work was funded by the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01HL097889).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Sylvie Naar, Deborah Ellis, April Idalski-Carcone, Angela J. Jacques-Tiura, Phillippe Cunningham, Thomas Templin, Kathryn Brogan Hartlieb, and K-L Cathy Jen declare that they have no conflict of interest.
Authors' Contributions S.N. oversaw all aspects. D.E. was involved in the development of research design and clinical manual. A.I.C. and T.T. were involved in the data analysis. A.J.J.-T. was involved in the project management and data analysis. P.C. was involved in the clinical supervision and fidelity monitoring. K.B.H. was involved in the training and project management. K.-L.C.J. was involved in the nutrition science expertise for design and delivery of clinical treatments.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Ogden CL, Carroll MD, Lawman HG, et al. . Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: A systematic review of randomized controlled trials. Obes Rev. 2013;14:265–278. [DOI] [PubMed] [Google Scholar]
- 3. Barr-Anderson DJ, Singleton C, Cotwright CJ, Floyd MF, Affuso O. Outside-of-school time obesity prevention and treatment interventions in African American youth. Obes Rev. 2014;15 (Suppl 4):26–45. [DOI] [PubMed] [Google Scholar]
- 4. Barr-Anderson DJ, Adams-Wynn AW, DiSantis KI, Kumanyika S. Family-focused physical activity, diet and obesity interventions in African-American girls: A systematic review. Obes Rev. 2013;14:29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Resnicow K, Taylor R, Baskin M, McCarty F. Results of go girls: A weight control program for overweight African-American adolescent females. Obes Res. 2005;13:1739–1748. [DOI] [PubMed] [Google Scholar]
- 6. Savoye M, Shaw M, Dziura J, et al. . Effects of a weight management program on body composition and metabolic parameters in overweight children: A randomized controlled trial. JAMA. 2007;297:2697–2704. [DOI] [PubMed] [Google Scholar]
- 7. Wadden TA, Stunkard AJ, Rich L, Rubin CJ, Sweidel G, McKinney S. Obesity in black adolescent girls: A controlled clinical trial of treatment by diet, behavior modification, and parental support. Pediatrics. 1990;85:345–352. [PubMed] [Google Scholar]
- 8. Williamson DA, Walden HM, White MA, et al. . Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity (Silver Spring). 2006;14:1231–1243. [DOI] [PubMed] [Google Scholar]
- 9. Jelalian E, Hart CN, Mehlenbeck RS, et al. . Predictors of attrition and weight loss in an adolescent weight control program. Obesity (Silver Spring). 2008;16:1318–1323. [DOI] [PubMed] [Google Scholar]
- 10. Snowden L, Masland M, Ma YF, Ciemens E. Strategies to improve minority access to public mental health services in California: Description and preliminary evaluation. J Community Psychol. 2006;34:225–235. [Google Scholar]
- 11. Gopalan G, Goldstein L, Klingenstein K, Sicher C, Blake C, McKay MM. Engaging families into child mental health treatment: Updates and special considerations. J Can Acad Child Adolesc Psychiatry. 2010;19:182–196. [PMC free article] [PubMed] [Google Scholar]
- 12. Balcazar H, Rosenthal EL, Brownstein JN, Rush CH, Matos S, Hernandez L. Community health workers can be a public health force for change in the United States: Three actions for a new paradigm. Am J Public Health. 2011;101:2199–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dietz WH, Robinson TN. Overweight children and adolescents - reply. N Engl J Med. 2005:353:1070–1071. [Google Scholar]
- 14. MacDonell K, Ellis D, Naar-King S, Cunningham P. Predictors of home-based obesity treatment efficacy for African American youth. Childrens Health Care. 2010:39:1–14. [Google Scholar]
- 15. Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80:276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barlow SE; Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120 (Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 17. Hartlieb KB, Naar S, Ledgerwood DM, et al. . Contingency management adapted for African-American adolescents with obesity enhances youth weight loss with caregiver participation: A multiple baseline pilot study. Int J Adolesc Med Health. 2015;29:3, pii. [DOI] [PubMed] [Google Scholar]
- 18. Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat Med. 2012;31:1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nahum-Shani I, Qian M, Almirall D, et al. . Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods. 2012;17:457–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Transl Behav Med. 2014;4:260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arraiz GA, Wigle DT, Mao Y. Risk assessment of physical activity and physical fitness in the Canada Health Survey mortality follow-up study. J Clin Epidemiol. 1992;45:419–428. [DOI] [PubMed] [Google Scholar]
- 22. Mottola M, Wolfe L. Active living and pregnancy. In: Quinney HA, Gauvin L, Wall AE, eds. Toward Active Living. Proceedings of the International Conference on Physical Activity, Fitness & Health Champaign, IL: Human Kinetics Publishers; 1994. [Google Scholar]
- 23. Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 24. Hartlieb KB, Jacques-Tiura AJ, Naar-King S, et al. . Recruitment strategies and the retention of obese urban racial/ethnic minority adolescents in clinical trials: The FIT families project, Michigan, 2010–2014. Prev Chronic Dis. 2015;12:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller W, Rollnick S.. Motivational Interviewing: Helping People Change. 3rd ed New York: Guilford Press; 2013. [Google Scholar]
- 26. Naar-King S, Suarez M.. Motivational Interviewing with Adolescents and Young Adults. New York: Guilford Press; 2011. [Google Scholar]
- 27. Macdonell K, Brogan K, Naar-King S, Ellis D, Marshall S. A pilot study of motivational interviewing targeting weight-related behaviors in overweight or obese African American adolescents. J Adolesc Health. 2012;50:201–203. [DOI] [PubMed] [Google Scholar]
- 28. Bean MK, Powell P, Quinoy A, Ingersoll K, Wickham EP III, Mazzeo SE. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: Results from the MI Values randomized controlled trial. Pediatr Obes. 2015;10:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosengren DB, Hartzler B, Baer JS, Wells EA, Dunn CW. The video assessment of simulated encounters-revised (VASE-R): Reliability and validity of a revised measure of motivational interviewing skills. Drug Alcohol Depend. 2008;97:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moyers T, Martin T, Manuel J, Miller W, Ernst D.. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1.1 (MITI 3.1.1). Unpublished manuscript, Albuquerque, NM: University of New Mexico; 2010. [Google Scholar]
- 31. Naar S, Safren S.. Motivational Interviewing and CBT: Combining Strategies for Maximum Effectiveness. New York: Guildford Press; 2017. [Google Scholar]
- 32. Ellis DA, Janisse H, Naar-King S, et al. . The effects of multisystemic therapy on family support for weight loss among obese African-American adolescents: Findings from a randomized controlled trial. J Dev Behav Pediatr. 2010;31:461–468. [DOI] [PubMed] [Google Scholar]
- 33. Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13:373–383. [DOI] [PubMed] [Google Scholar]
- 34. Naar-King S, Ellis D, Kolmodin K, et al. . A randomized pilot study of multisystemic therapy targeting obesity in African-American adolescents. J Adolesc Health. 2009;45:417–419. [DOI] [PubMed] [Google Scholar]
- 35. Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87:743–754. [DOI] [PubMed] [Google Scholar]
- 37. Miller WR, Rollnick S.. Motivational Interviewing: Helping People Change. New York:Guilford Press; 2012. [Google Scholar]
- 38. Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–1481. [DOI] [PubMed] [Google Scholar]
- 39. Czajkowski SM, Powell LH, Adler N, et al. . From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naar S, Czajkowski SM, Spring B. Innovative study designs and methods for optimizing and implementing behavioral interventions to improve health. Health Psychol. 2018;37:1081–1091. [DOI] [PubMed] [Google Scholar]
- 41. Carcone A. Analyzing patient-provider communication in clinical contexts to identify novel behavior change targets. Presented at: Workshop on Innovative Study Designs and Methods for Developing, Testing and Implementing Behavioral Interventions to Improve Health Bethesda, MD; 2014. [Google Scholar]
- 42. Seo DC, Sa J. A meta-analysis of obesity interventions among U.S. minority children. J Adolesc Health. 2010;46:309–323. [DOI] [PubMed] [Google Scholar]
- 43. Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57:711–717. [DOI] [PubMed] [Google Scholar]
- 44. Ziauddeen H, Alonso-Alonso M, Hill JO, Kelley M, Khan NA. Obesity and the neurocognitive basis of food reward and the control of intake. Adv Nutr. 2015;6:474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]