ABSTRACT
Background
The preferred macronutrient dietary composition, and the health consequences of dietary fat reduction specifically, have been debated for decades. Here we provide a comprehensive overview of long-term health outcomes in the Women's Health Initiative Dietary Modification (DM) trial.
Objective
The DM trial aimed to examine whether a low-fat dietary pattern would reduce the risk of invasive breast cancer, colorectal cancer, and, secondarily, coronary heart disease (CHD), with various other health outcomes also considered.
Methods
The DM trial is a randomized controlled trial conducted at 40 centers in the US, among 48,835 postmenopausal women aged 50–79 y with baseline intake of ≥32% energy from fat. Participants were randomly assigned to a low-fat dietary pattern intervention group or to a usual-diet comparison group, during 1993–1998. Intervention goals were to reduce fat intake from ∼35% to 20% of total energy, in conjunction with increasing vegetables and fruit to 5 servings/d and grains to 6 servings/d.
Results
Over an 8.5-y (median) intervention period, intervention and comparison group differences included lower fat by 8–10%, and higher carbohydrate by 8–10%, of total energy, in conjunction with higher consumption of vegetables, fruit, and grains. Time-to-outcome analyses did not show significant differences between intervention and comparison groups for invasive breast cancer, colorectal cancer, or CHD, either over the intervention period or over longer-term cumulative follow-up. Additional analyses showed significant intervention group benefits related to breast cancer, CHD, and diabetes, without adverse effects. Over a 19.6-y (median) follow-up period, HRs (95% CIs) were 0.84 (0.74, 0.96) for breast cancer followed by death, and 0.87 (0.77, 0.98) for diabetes requiring insulin.
Conclusions
Reduction in dietary fat with corresponding increase in vegetables, fruit, and grains led to benefits related to breast cancer, CHD, and diabetes, without adverse effects, among healthy postmenopausal US women.
This trial was registered at clinicaltrials.gov as NCT00000611.
Keywords: cancer, carbohydrate, cardiovascular disease, diabetes, health benefits and risks, low-fat dietary pattern, nutritional behavioral intervention, randomized controlled trial
Introduction
The Women's Health Initiative (WHI) dietary modification (DM) trial was substantially motivated by rodent feeding experiments showing benefits of a low-fat diet for reduction in mammary and colorectal tumors, and by concerns about the reliability of related human observational studies that rely on self-reported diet. The DM trial enrolled 48,835 postmenopausal US women during 1993–1998. Forty percent of participants were randomly assigned to a low-fat dietary pattern intervention that included goals of augmenting intake of vegetables, fruits, and grains, whereas the remaining 60% were assigned to a usual-diet comparison group. After a median 8.5 y of intervention through 31 March, 2005, intervention and comparison contrasts between randomized groups included HRs (95% CI) of 0.91 (0.83, 1.01) for invasive breast cancer (1) and 1.08 (0.90, 1.29) for colorectal cancer (2), the dual primary trial outcomes—and 0.97 (0.88, 1.08) for coronary heart disease (CHD) (3), the designated secondary trial outcome. The breast cancer HR differed (P = 0.04) by tumor receptor status, with an HR (95% CI) of 0.64 (0.49, 0.84) for estrogen receptor positive, progesterone receptor negative tumor incidence (1). Also, a global index, defined as the time to the earliest of these outcomes and death from any other cause, was used in trial monitoring (4), as was total mortality, with respective HRs (95% CIs) of 0.96 (0.91, 1.02) and 0.98 (0.91, 1.07) during the intervention period (1).
Eighty-three percent of participants in each group consented to additional nonintervention follow-up through 30 September, 2010, and 86% of participants subsequently consented to a further open-ended follow-up. All randomly assigned women were followed for mortality, in part through periodic matches to the National Death Index (NDI).
Analyses over the intervention period and the cumulative follow-up identified nominally significant intervention benefits related to breast cancer (5, 6), CHD (7), and diabetes (8). Here we update trial results for these and other important clinical outcomes, over the longest follow-up period for which complete outcome data are available, which for most outcomes entails a median follow-up period of 19.6 y.
The CHD analyses (7) provided evidence of postrandomization confounding by statin use among participants with prior cardiovascular disease (CVD) or hypertension at enrollment. Accordingly, we also present analyses, for all clinical outcomes considered, that stratify on baseline hypertension status among participants without prior CVD. For completeness, additional exploratory analyses are provided that give results for each outcome stratified by BMI, and by waist circumference, at enrollment.
Methods
Ethics
The WHI is funded by the National Heart, Lung, and Blood Institute. The study protocol was reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, Seattle, WA, where the Clinical Coordinating Center is located, and by the Institutional Review Boards at each of the 40 participating clinical centers. Participating women provided written informed consent for their DM trial participation over the trial intervention phase (ended 31 March, 2005) and had the opportunity to reconsent for nonintervention follow-up through 30 September, 2010, and again for open-ended subsequent follow-up.
Trial design
Details of the DM trial design, procedures, and implementation have been published (1, 4, 9). Briefly, 48,835 postmenopausal women aged 50–79 y with no prior history of breast or colorectal cancer, and with dietary fat intake estimated using an FFQ to be ≥32% of total energy intake, were recruited during 1993–1998 at 40 US clinical centers. Forty percent of participants were randomly assigned to a low-fat dietary pattern intervention, whereas the remaining 60% were assigned to a usual-diet comparison group. Intervention group goals included a reduction in fat from ∼35% of energy at baseline, to 20% of energy, in conjunction with increases in vegetables and fruit to 5 servings/d, and grains to 6 servings/d. Energy restriction, weight loss, or unsaturated fat substitution for saturated fat were not intervention goals. Comparison group participants received written health-related materials only.
A first intervention year included 18 dietary behavioral sessions in groups of size 8–15 and 1 individual session, led by centrally trained and certified nutritionists. Quarterly group sessions continued throughout the intervention period, which reached its planned completion on 31 March, 2005, after a median 8.5 y. Dietary intake was monitored by obtaining FFQs at baseline, 1 y, and approximately every 3 y thereafter throughout the intervention period, and by blood specimen analyses for certain nutritional variables in a random 5.8% subsample of participants (1). Four-day food records were also obtained at baseline from all participants and were analyzed routinely for nutrient content in this same 5.8% subsample.
Intervention and comparison group dietary differences included lower fat by 8–10% of total energy with similar fractional reductions for saturated and unsaturated fat; and higher carbohydrate by 8–10% of energy, with increases in vegetables, fruit, and grains. The intervention group had a significant 2.2-kg lower weight than did the comparison group at 1 y postrandomization, although some of this difference dissipated later in the intervention period (10). The dietary differences were somewhat larger at 1 y than later in the intervention period (3, 10), dropping to ∼8% of energy for both fat and carbohydrate toward the end of the intervention period, and were relatively small postintervention (11).
Outcomes
Cancer outcomes were coded centrally using the US National Cancer Institute's SEER system throughout the intervention and postintervention phases, whereas CVD outcomes were centrally adjudicated by expert committee only through 30 September, 2010. Cancer incidence and all mortality data, with periodic NDI matching, are included here through 30 September, 2016, the most recent time for which NDI data are complete. In addition to the primary trial outcomes, results are presented for total invasive cancer incidence and for a set of cancer sites that were recently designated as obesity-related (12). CHD was defined as nonfatal myocardial infarction plus CHD death. Total stroke was defined as ischemic plus hemorrhagic stroke. Total CVD was defined as CHD plus coronary artery bypass graft or percutaneous coronary intervention, plus total stroke. Diabetes was not a protocol-designated outcome, but treated diabetes information was documented semiannually during the intervention phase and annually during the extended follow-up, and these self-reported data were found to be consistent with medication inventories (13). The median cumulative follow-up duration is 13.4 y for adjudicated CVD outcomes and 19.6 y for other outcomes.
Statistics
Intervention and comparison group disease rate contrasts either include all 48,835 randomly assigned women, or a “baseline healthy” cohort of 47,179 participants who were without a history of CVD at enrollment. Prior history of invasive breast cancer or colorectal cancer were trial exclusionary criteria, as was any cancer except nonmelanoma skin cancer within the previous 10 y. All contrasts use intention-to-treat and time-to-event methods.
HRs contrasting the intervention and comparison groups are estimated using Cox regression with baseline rates stratified on age group, race/ethnicity, hysterectomy status, prior history of the outcome under analysis (if applicable), randomization status in the WHI hormone therapy trials (estrogen-alone, estrogen-alone placebo, estrogen plus progestin, estrogen plus progestin placebo, not randomized), and study phase (intervention and postintervention; time-dependent). For a specified clinical outcome the time to response is days from randomization to first relevant clinical event, whereas times for noncases were censored at the earliest of end of the study phase, loss to follow-up, or death. Cumulative results represent overall findings over the total aforementioned follow-up periods.
Monitored outcomes include the invasive breast cancer and colorectal primary outcomes, the secondary CHD outcome, a global index defined as the earliest of these outcomes or death from any other cause, and total mortality. Analyses for other outcomes can be considered as secondary.
All statistical tests are 2-sided and nominal P values ≤ 0.05 are regarded as significant. P values do not adjust for multiple outcomes, nor for sequential monitoring during the intervention phase. All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc.) and R software version 2.15 (R Foundation for Statistical Computing).
Results
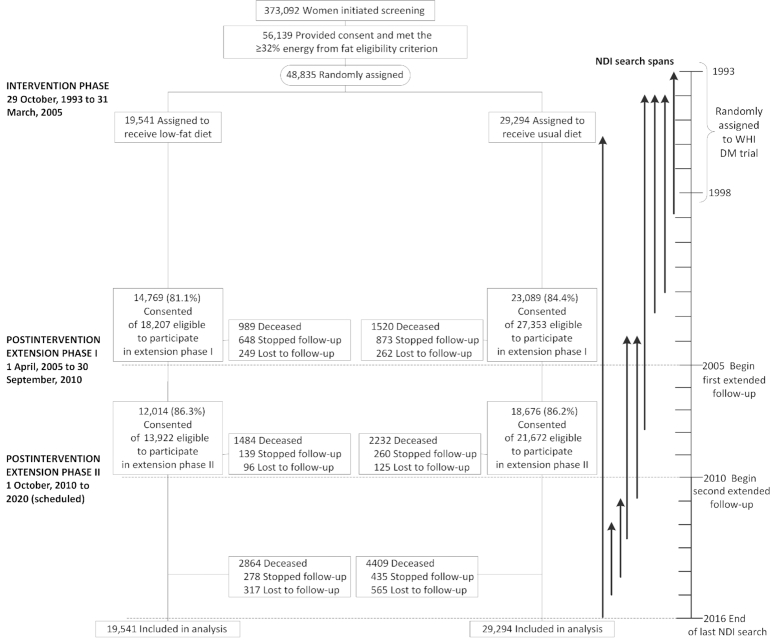
Figure 1 shows the number of participants by randomization group in the intervention and postintervention study phases. As shown in Table 1, baseline characteristics including demographic, medical history, and prerandomization diets were generally well-balanced between randomization groups. Table 2 shows dietary contrasts between the intervention and comparison groups at 1 y after randomization based on FFQ data. Fat intake was assessed as lower in the intervention group by ∼11% of total energy, with similar fractional reductions for saturated and unsaturated fat, whereas percentage of energy from carbohydrate was estimated to be higher among intervention women by ∼10%, and percentage of energy from protein by ∼1%. Servings of vegetables and fruit were higher by 1.2 servings to 5.1/d, and grains was higher by ∼0.7 servings to 5.4/d, in the intervention group.
FIGURE 1.
Participant flow diagram for the WHI trial of a low-fat dietary pattern through extended follow-up. Participants were randomly assigned to a low-fat dietary pattern intervention or usual-diet comparison group. All participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers. DM, dietary modification; NDI, National Death Index; WHI, Women's Health Initiative.
TABLE 1.
Baseline characteristics of Dietary Modification trial participants by low-fat dietary pattern intervention or usual-diet comparison group1
| Characteristic | Intervention (n = 19,541) | Comparison (n = 29,294) |
|---|---|---|
| Age at screening, y | 62.3 ± 6.9 | 62.3 ± 6.9 |
| Age group at screening, y | ||
| 50–59 | 7206 (36.9) | 10,792 (36.8) |
| 60–69 | 9083 (46.5) | 13,632 (46.5) |
| 70–79 | 3252 (16.6) | 4870 (16.6) |
| Race/ethnicity | ||
| White | 15,871 (81.2) | 23,891 (81.6) |
| Black | 2135 (10.9) | 3127 (10.7) |
| Hispanic | 751 (3.8) | 1094 (3.7) |
| American Indian | 88 (0.5) | 114 (0.4) |
| Asian/Pacific Islander | 431 (2.2) | 674 (2.3) |
| Unknown | 265 (1.4) | 394 (1.3) |
| High school diploma or GED | 15,158 (78.0) | 22,641 (77.8) |
| Family income ≥$50,000 | 7181 (39.0) | 10,612 (38.5) |
| Postmenopausal hormone use | ||
| Never | 8072 (41.3) | 12,102 (41.4) |
| Past | 2813 (14.4) | 4181 (14.3) |
| Current | 8639 (44.2) | 12,979 (44.4) |
| BMI, kg/m2 | 28.2 [24.8, 32.4] | 28.2 [24.9, 32.5] |
| Waist circumference, cm2 | 89.0 ± 13.9 | 89.0 ± 13.7 |
| Systolic BP, mm Hg | 127.5 ± 17.2 | 127.9 ± 17.2 |
| Diastolic BP, mm Hg | 75.9 ± 9.1 | 76.0 ± 9.1 |
| Physical activity, MET-h/wk | 6.3 [1.5, 15.0] | 6.3 [1.3, 14.7] |
| Smoking | ||
| Never | 9918 (51.4) | 15,029 (51.9) |
| Past | 8121 (42.1) | 11,979 (41.3) |
| Current | 1273 (6.6) | 1977 (6.8) |
| Hysterectomy | 8448 (43.2) | 12,755 (43.5) |
| Bilateral oophorectomy | 3884 (20.3) | 5997 (20.9) |
| Medical treatment received | ||
| Diabetes | 866 (4.4) | 1337 (4.6) |
| Hypertension or BP ≥140/90 mm Hg | 8382 (46.7) | 12,734 (47.4) |
| High cholesterol requiring medication | 2238 (11.5) | 3437 (11.7) |
| Statin use at baseline | 1207 (6.2) | 1836 (6.3) |
| Aspirin use ≥80 mg/d | 3437 (17.6) | 5400 (18.4) |
| Medical history | ||
| Myocardial infarction | 363 (1.9) | 549 (1.9) |
| CABG or PCI | 241 (1.2) | 321 (1.1) |
| Stroke | 205 (1.0) | 328 (1.1) |
| Family history of breast cancer2 | 3396 (18.3) | 4928 (17.8) |
| Menopausal hormone randomization group | ||
| CEE-alone | 615 (3.1) | 1039 (3.5) |
| CEE-alone placebo | 670 (3.4) | 1068 (3.6) |
| CEE + MPA | 972 (5.0) | 1457 (5.0) |
| CEE + MPA placebo | 925 (4.7) | 1304 (4.5) |
| Not randomized | 16,359 (83.7) | 24,426 (83.4) |
| CaD randomization group | ||
| CaD | 4767 (24.4) | 7827 (26.7) |
| CaD placebo | 4878 (25.0) | 7738 (26.4) |
| Not randomized | 9896 (50.6) | 13,729 (46.9) |
| Total energy, kcal/d3 | 1695.0 ± 451.8 | 1708.4 ± 462.7 |
| Percent energy from fat3 | 32.0 ± 6.6 | 32.5 ± 6.8 |
| Percent energy from protein3 | 16.9 ± 3.5 | 16.6 ± 3.5 |
| Percent energy from carbohydrates3 | 51.8 ± 7.9 | 51.6 ± 7.8 |
| Total dietary fiber, g/d3 | 17.3 ± 6.1 | 17.1 ± 6.0 |
Overall n = 48,835. Values are mean ± SD, n (%) of participants, or median [IQR]. Participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers. BP, blood pressure; CABG, coronary artery bypass graft; CaD, calcium and vitamin D; CEE, conjugated equine estrogens; GED, general education diploma; MET, metabolic equivalent unit; MPA, medroxyprogesterone acetate; PCI, percutaneous coronary intervention.
Indicates occurrence in participant's mother, sister, daughter, or grandmother.
From baseline 4-d food records on a 5.8% random subsample. Baseline FFQ data are not used here, owing to distortions related to the use of baseline FFQs for eligibility screening.
TABLE 2.
FFQ dietary intake at 1 y after randomization in the Dietary Modification trial in the low-fat dietary pattern intervention and usual-diet comparison groups1
| Intervention (n = 17,643) | Comparison (n = 26,116) | ||
|---|---|---|---|
| Postrandomization dietary characteristic | Mean ± SD | Mean ± SD | P value |
| Calories from fat, % | 24.3 ± 7.4 | 35.1 ± 6.9 | <0.001 |
| Calories from saturated fat, % | 8.0 ± 2.8 | 11.8 ± 2.9 | <0.001 |
| Calories from trans fat, % | 1.6 ± 0.8 | 2.5 ± 1.1 | <0.001 |
| Calories from polyunsaturated fat, % | 5.2 ± 1.8 | 7.2 ± 2.1 | <0.001 |
| Calories from monounsaturated fat, % | 8.9 ± 3.1 | 13.3 ± 2.9 | <0.001 |
| Calories from carbohydrates, % | 58.4 ± 8.9 | 47.9 ± 7.9 | <0.001 |
| Calories from sugar, % | 28.4 ± 7.4 | 23.2 ± 6.8 | <0.001 |
| Calories from other carbohydrates, % | 29.9 ± 6.3 | 24.7 ± 5.3 | <0.001 |
| Calories from protein, % | 17.7 ± 3.1 | 16.8 ± 3.1 | <0.001 |
| Dietary energy, kcal/d | 1520.3 ± 515.2 | 1612.3 ± 603.3 | <0.001 |
| Vegetable and fruit consumption, servings/d | 5.1 ± 2.3 | 3.9 ± 2.0 | <0.001 |
| Vegetable consumption, servings/d | 2.6 ± 1.4 | 2.1 ± 1.2 | <0.001 |
| Fruit consumption, servings/d | 2.5 ± 1.4 | 1.8 ± 1.1 | <0.001 |
| Total grain consumption, servings/d | 5.4 ± 2.6 | 4.7 ± 2.4 | <0.001 |
| Whole-grain consumption, servings/d | 1.4 ± 1.1 | 1.1 ± 0.9 | <0.001 |
| Other grain consumption, servings/d | 4.0 ± 2.1 | 3.6 ± 2.0 | <0.001 |
| Dietary fiber, g/d | 18.3 ± 7.3 | 15.1 ± 6.3 | <0.001 |
| Total carotenoids,2 mg/d | 12.4 ± 6.5 | 10.3 ± 5.6 | <0.001 |
Overall n = 43,759. n = 413 (2.3%) compared with 625 (2.3%) participants with implausible FFQ energy intake (<600 or >5000 kcal/d) were excluded from the intervention and the comparison group, respectively; there was no evidence of differential exclusions by randomization group (P = 0.73). Participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers.
Defined as the sum of α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein plus zeaxanthin.
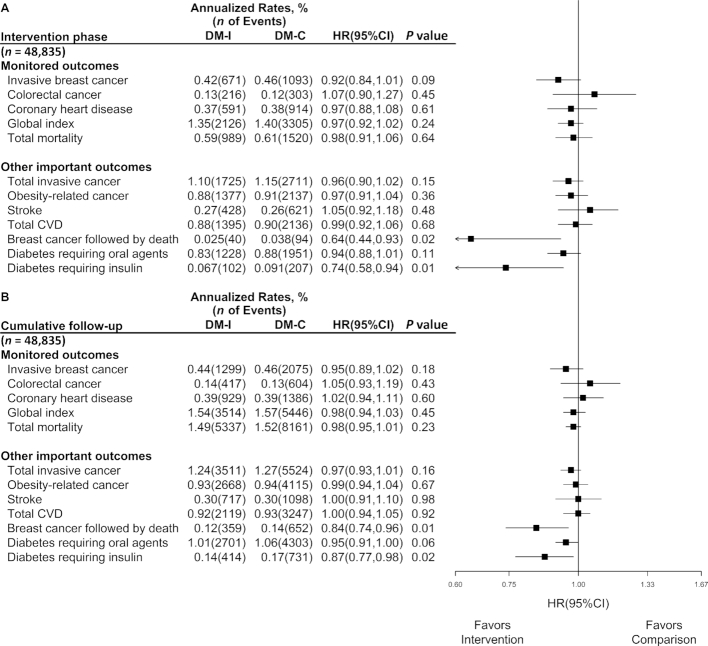
Figure 2 shows outcome comparisons between randomized groups during the intervention phase, and over the cumulative intervention and postintervention phases. Results during the intervention phase may differ slightly from those previously published as a result of database updates. This is also the case postintervention, for outcomes (CHD, global index, stroke, total CVD) that use adjudicated CVD incidence data. For all other listed adjudicated outcomes, results over the cumulative follow-up include some years (minimum of 2 y; 2.7, 3.0, and 6.0 y, respectively, for breast cancer, total mortality, and colorectal and other cancers) of follow-up beyond data used in prior publications. HR estimates in Figure 2 are mostly <1. Those for colorectal cancer (HR: 1.07; 95% CI: 0.90, 1.27) and stroke (HR: 1.05; 95% CI: 0.92, 1.18) are not, but are far from significant (P = 0.45 and 0.48, respectively). As during the intervention phase, there is a significant reduction in the composite outcome of breast cancer followed by death from any cause (HR: 0.84; 95% CI: 0.74, 0.96), and for diabetes requiring insulin (HR: 0.87; 95% CI: 0.77, 0.98), over the cumulative follow-up. Also, although not shown in Figure 2, there was a significant reduction in estrogen receptor positive, progesterone receptor negative breast cancer incidence over the long-term follow-up, with an HR (95% CI) of 0.77 (0.64, 0.94). The total mortality rate is precisely estimated, with 13,498 deaths, and yields an intervention compared with comparison group HR of 0.98 (95% CI: 0.95, 1.01), over the cumulative follow-up period. Also over the cumulative follow-up, in exploratory analyses (not shown in Figure 2), HRs (95% CIs) are 0.90 (0.82, 1.00) for diabetes requiring oral agents followed by death, with 610 and 1039 deaths in the intervention and comparison groups; and 0.65 (0.52, 0.82) for diabetes requiring insulin followed by death, with 106 and 248 deaths in the intervention and comparison groups.
FIGURE 2.
Monitored and other important outcomes in the Women's Health Initiative Dietary Modification trial (n = 48,835) during the 8.5-y (median) intervention phase, and over cumulative follow-up of 13.4 y for adjudicated CVD outcomes and 19.6 y for other outcomes. Summary statistics and forest plots are shown for randomly assigned groups, during the (A) intervention period and (B) cumulative follow-up. HRs (95% CIs) and P values are from Cox regression models with baseline hazard stratified on age at random assignment (50–54, 55–59, 60–69, and 70–79 y), ethnicity (white, black, and other), hysterectomy status (yes or no), prior disease (if applicable), randomization status in the hormone therapy trials (CEE, CEE-placebo, CEE + MPA, CEE + MPA placebo, and not randomized), and study phase (intervention phase, extension phase I, and extension phase II; time-dependent). Time to event is measured from date of randomization. P value is for the overall influence of random assignment on outcomes based on a score (log-rank) test. Analyses for diabetes outcomes was among participants without prevalent diabetes at baseline (n = 45,595). All participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers. CEE, conjugated equine estrogens; CVD, cardiovascular disease; DM-C, dietary modification comparison group; DM-I, dietary modification intervention group; MPA, medroxyprogesterone acetate.
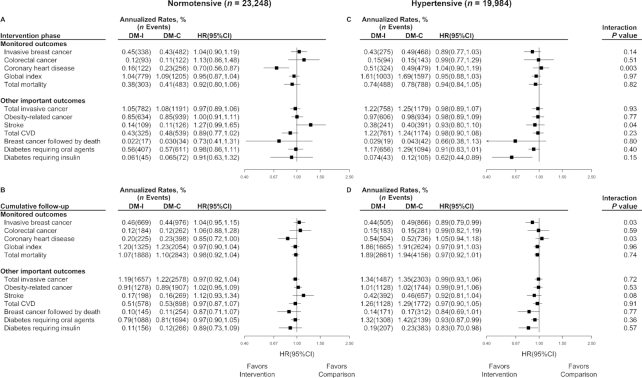
Supplemental Figure 1 shows analyses, like Figure 2, after excluding the 3.4% of women having prior CVD. This restriction to a baseline healthy cohort yielded HR results in the intervention period and during the cumulative follow-up that differed only a little from those in Figure 2. Figure 3 shows results for these same outcomes, stratified by whether participants without prior CVD were normotensive (54%) or hypertensive (46%) at enrollment. A significant reduction in CHD incidence (HR: 0.70; 95% CI: 0.56, 0.87) occurred among baseline normotensive participants where evidence of postrandomization confounding by statin use was absent, whereas there was no evidence for an intervention effect among baseline hypertensive participants, with a highly significant contrast between the 2 (P = 0.003). This interaction remains evident over the cumulative follow-up (P = 0.03).
FIGURE 3.
Monitored outcomes and other important outcomes in the Women's Health Initiative Dietary Modification trial among participants (n = 43,232) without prior CVD (3947 participants excluded based on missing hypertension status at baseline) during the 8.5-y (median) intervention phase (A, C), and over cumulative follow-up (B, D) of 13.4 y for adjudicated CVD outcomes and 19.6 y for other outcomes, stratified by baseline hypertension status (A, B: normotensive, compared with C, D: hypertensive). Summary statistics and forest plots are shown for randomly assigned groups without prior history of CVD during the intervention period (A, C), by hypertension status; likewise for cumulative follow-up (B, D). For each panel, regression models were expanded by stratifying on hypertension status (normotensive compared with hypertensive) and including an interaction term. P value is for the interaction between randomization group and hypertension status based on a score test. All participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers. CVD, cardiovascular disease; DM-C, dietary modification comparison group; DM-I, dietary modification intervention group.
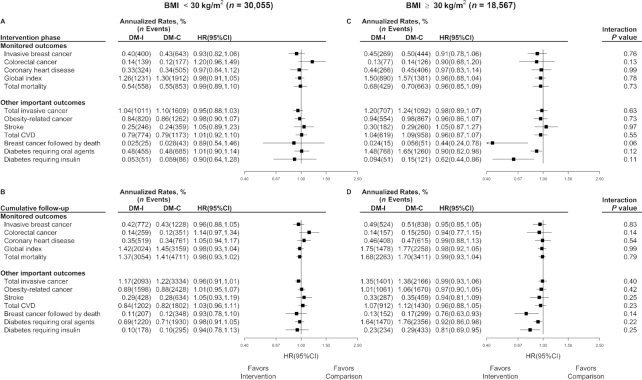
Figure 4 shows that there is little suggestion of intervention effects on any of the outcomes considered among women having baseline BMI (in kg/m2) <30, whereas the observed HR reductions in the overall trial cohort are more apparent among the 38.2% of women who were classified as obese (BMI ≥30) at enrollment. Very similar outcome patterns emerge for analyses of women having baseline waist circumference <88 cm compared with ≥88 cm (Supplemental Figure 2). Note, however, that HR interaction tests between strata in these exploratory analyses were mostly nonsignificant.
FIGURE 4.
Monitored and other important outcomes in the Women's Health Initiative Dietary Modification trial (n = 48,622, 213 participants excluded owing to missing BMI data at baseline) during the 8.5-y intervention (median) phase (A, C), and over cumulative follow-up (B, D) of 13.4 y for adjudicated CVD outcomes and 19.6 y for other outcomes, stratified by baseline BMI group (A, B, <30; compared with C, D, ≥30). Summary statistics and forest plots are shown for randomly assigned groups during the intervention period by BMI group; likewise for cumulative follow-up. For each panel, regression models as used for Figure 2 were expanded by stratifying on BMI group (<30 compared with ≥30) and included an interaction term between randomization assignment and BMI group, with the corresponding interaction P value based on a score test. All participants were postmenopausal and in the age range 50–79 y when enrolled during 1993–1998 at 40 US clinical centers. BMI in kg/m2. CVD, cardiovascular disease; DM-C, dietary modification comparison group; DM-I, dietary modification intervention group.
Discussion
Summary of DM trial results during the intervention period and over the long-term follow-up
This report updates the health implications of a noteworthy dietary change from a relatively high-fat to a higher-carbohydrate diet in an ethnically and socioeconomically diverse cohort of postmenopausal US women, over a follow-up period of nearly 20 y. The reduction in total fat was accompanied by increased vegetables, fruits, and grains, with resulting increases in fiber and total carotenoid intakes.
Importantly, intervention and comparison groups did not differ significantly for the designated primary breast cancer or colorectal cancer outcomes, or for the secondary CHD outcome. Further data analyses that include long-term nonintervention follow-up show significant benefits related to breast cancer, but not other cancers (see also 14, 15), and benefits related to CHD and diabetes, with no observed adverse effects. Previously reported benefits continue to be evident over the longer cumulative follow-up period considered here.
Although overall mortality rates did not differ significantly between randomization groups overall, significant mortality reductions in conjunction with breast cancer and diabetes requiring insulin were observed over a 19.6-y (median) cumulative follow-up with HRs (95% CIs) of 0.84 (0.74, 0.96) and 0.87 (0.77, 0.98), respectively.
Concerning potential mediating variables that may help to explain clinical outcome findings, intervention group participants experienced a reduction in plasma estradiol and an increase in sex hormone binding globulin (1), as has also been observed in other intervention trials (16, 17), which could be relevant to breast cancer results. Also observed were small but favorable changes in blood pressure, LDL cholesterol, insulin, glucose, HOMA-IR, and metabolic syndrome score (8, 18, 19) that could be relevant to CHD and diabetes findings. Note that these changes are not supportive of the carbohydrate–insulin resistance hypothesis underpinning the Paleo diet (20). Also, the OmniCarb trial (21) showed little relation between carbohydrate glycemic index and influences on CVD and diabetes risk factors, suggesting that clinical findings here may have some robustness to the choice of carbohydrate that accompanies the fat reduction. Although neither energy reduction nor weight loss were intervention goals, intervention group women did lose some weight, especially early in the intervention period (10).
In exploratory analyses, favorable intervention effects were observed primarily in the 38.2% of women who were classified as obese (BMI ≥30) at baseline. Additional analyses (11) show that long-term difference in percentage of energy from fat between the intervention and comparison groups, as estimated by 24-h dietary recall, did not differ (P = 0.98) between the BMI <30 and BMI ≥30 strata so that differential intervention influences, rather than differential intervention adherence, likely underlie any outcome differences between obese and nonobese participants. One can ask also whether the small weight loss experienced by intervention participants provides an explanation for intervention benefit. The Figure 2 analyses were repeated with baseline weight, and weight change from baseline as a time-dependent variate, added to the Cox regression model. These analyses gave almost identical HRs to those shown in Figure 2, so that weight loss is unlikely to be an important mediator of estimated HR reductions among intervention group women.
There is an extensive epidemiologic literature on associations between dietary factors and chronic disease incidence and mortality. The cancer literature has recently been assembled by expert committee (22), without a conclusion concerning the role of dietary fat, or concerning the substitution of carbohydrate for fat. Likewise, reviews of the CVD epidemiology literature, although supporting the replacement of saturated by unsaturated fat, have no consensus concerning the replacement of saturated fat by carbohydrate, with concerns about substitution of refined carbohydrate (23, 24).
Recent observational literature projecting total mortality implications of replacing fat by carbohydrate is particularly interesting. Investigators for the Prospective Urban Rural Epidemiology study in 18 countries having disparate dietary and socioeconomic conditions proposed that global dietary guidelines should be re-examined in view of their observation of elevated (total) mortality among persons consuming low-fat, high-carbohydrate diets (25). Their analyses, which combine analytic and ecologic data sources, contrasted a highest quartile (77% of energy from carbohydrate) to a lowest quartile (46.4% of energy from carbohydrate). However, a recent cohort study in the United States reported elevated mortality risks with high-carbohydrate diets (>70% of energy) and even more so with low-carbohydrate diets (<40% of energy), with lowest mortality among persons having 50–55% of energy from carbohydrate (26). The authors presented additional analysis suggesting improved cardiovascular and noncardiovascular mortality if plant-based, but not animal-based, fat and protein replaced carbohydrate. Similarly, a 2018 review article (27), although not attempting a “premature consensus,” offers the perspective that chronic disease benefits may be able to be achieved with a broad range of ratios of dietary carbohydrate to fat, with carbohydrate and fat quality likely to be highly influential concerning health benefits and risks across such a range.
In interpreting the observational literature on these topics, it is important to remember that observational study associations rely directly on self-reported dietary data for their principal exposure assessment, whereas our DM trial reports use dietary self-report data only for adherence assessment and make no use of such data for clinical outcome randomization group comparisons (e.g., Figures 2 –4). The measurement properties of self-reported fat and carbohydrate consumption, either absolute intake or as a fraction of calories, are largely unknown. For total energy intake, which is thought to correlate strongly with the fat content of the diet, estimated chronic disease associations are highly dependent on measurement error correction. For example, when a doubly labeled water biomarker is used to correct self-reported energy consumption for measurement error, strong positive associations of energy consumption with prominent cancers, CVDs, and especially diabetes are estimated (28). These associations were mostly not evident without measurement error correction.
Against this background, the DM randomized controlled trial provides valuable insight into chronic disease benefits and risks of a noteworthy replacement of fat by carbohydrate in a well-nourished population of postmenopausal US women. At 1 y after randomization, the intervention group reported ∼24% of energy from fat compared with ∼35% in the comparison group, ∼58% of energy from carbohydrate compared with ∼48% in the comparison group, and ∼18% compared with 17% of energy from protein. Saturated and unsaturated fats were reduced by similar fractions, and intervention group dietary changes included increases in vegetables, fruit, grains, micronutrients, and fiber, but also some increase in sugars (Table 2). These changes evidently resulted in health benefits related to breast cancer, CHD, and diabetes, without corresponding observed chronic disease risks. Overall, the intervention program resulted in a range of dietary changes that are mostly consistent with current concepts for a healthful diet, and maintenance of the dietary change proved to be practical for a large group of postmenopausal US women, over an 8.5-y (median) intervention period.
Strengths and limitations
Strengths of this large trial include its randomized controlled intervention design, and long-term follow-up with large numbers of carefully ascertained health-related outcomes. Intervention trials that are powered for chronic disease outcomes, and that include the type of nutritional behavioral intervention implemented in the WHI DM trial, are uncommon in the nutritional research area.
Trial limitations include the fact that only ∼70% of the targeted difference in percentage of energy from fat between the intervention and comparison groups was achieved, and grains servings also fell somewhat short of the targeted value (1). Also, these adherence assessments, even though bolstered to some degree by biomarkers (1), rely primarily on self-reported (FFQ) dietary data. Furthermore, the trial took place during a time of rapid increase in the use of statins. The magnitude of the 30% reduction in CHD benefit in the healthy, normotensive participant subset (7) could be influenced by subgroup multiple testing issues. Multiple testing issues for the range of clinical outcomes presented is a trial limitation more generally, especially for outcomes that were not designated as a part of the trial monitoring plan. Also, the trial intervention focused on total fat reduction, rather than targeting differential reduction for saturated and unsaturated fat, and the recommended increase in grains did not target whole grains, leaving many important nutrition and chronic disease questions unexamined. Finally, it is a limitation of an unblinded participant intervention trial with a follow-up period of nearly 20 y that differences other than the targeted dietary differences could emerge between randomization groups. Related to this, we have studied medication use based on periodic medication inventories among trial participants. Although medication patterns have changed in substantial ways over this lengthy time period, the only major difference we have observed in changes between randomized groups is that previously mentioned, of statin use patterns. We have also examined physical activity patterns, especially those related to leisure activity, but have not observed major differences in self-reported physical activity changes between randomization groups.
Conclusions
In summary, reduction in dietary fat with a commensurate increase in carbohydrate, with vegetable, fruit, and grain increases, did not show significant benefits for primary breast or colorectal cancer incidence or for secondary CHD incidence overall, but evidently led to some important health benefits during the intervention period and over the longer-term cumulative follow-up, without observed adverse health consequences. The observed benefits include reduction in breast cancer followed by death of ∼35% during the intervention and 15% over the cumulative follow-up; reduction in CHD incidence by ∼30% during the intervention and 15% over the cumulative follow-up among healthy normotensive women; and reduction in insulin-requiring diabetes by ∼25% during the intervention and 13% over the cumulative follow-up.
Supplementary Material
Acknowledgments
We thank the following WHI program investigators—Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD): Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center (Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Women's Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC). A list of all the investigators who have contributed to WHI science is available from: https://www.whi.org/researchers/Documents%20%Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. The DM trial protocol can be accessed at www.whi.org. The authors’ responsibilities were as follows—RLP, BVH, RTC, LFT, JEM, GLA, LEK, and JER: designed the research; RLP and AKA: analyzed the data and drafted the manuscript; and all authors: provided input to manuscript content, provided critical review of manuscript drafts, contributed actively to the final manuscript development, and approved the final manuscript.
Notes
Supported by NIH National Heart, Lung, and Blood Institute contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Author disclosures: RLP, AKA, BVH, CAT, LVH, LFT, JEM, GLA, LEK, MLN, KCJ, LS, and JER, no conflicts of interest. RTC is a consultant to AstraZeneca, Novaartis, Amgen, Genetech, Immunomedics, and Pfizer.
Decisions concerning study design, data collection and analysis, interpretation of results, the preparation of the manuscript, and the decision to submit the manuscript for publication resided with committees composed of Women's Health Initiative investigators that included National Heart, Lung, and Blood Institute representatives. The contents of the article are solely the responsibility of the authors and do not necessarily reflect the views of the US Department of Health and Human Services/National Heart, Lung, and Blood Institute.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; DM, Dietary Modification; NDI, National Death Index; WHI, Women's Health Initiative.
References
- 1. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM et al.. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative randomized controlled Dietary Modification trial. JAMA. 2006;295:629–42. [DOI] [PubMed] [Google Scholar]
- 2. Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, Anderson GL, Assaf AR, Bassford T, Bowen D et al.. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative randomized controlled Dietary Modification trial. JAMA. 2006;295:643–54. [DOI] [PubMed] [Google Scholar]
- 3. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL et al.. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative randomized controlled Dietary Modification trial. JAMA. 2006;295:655–66. [DOI] [PubMed] [Google Scholar]
- 4. Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. [DOI] [PubMed] [Google Scholar]
- 5. Chlebowski RT, Aragaki AK, Anderson GL, Thomson CA, Manson JE, Simon MS, Howard BV, Rohan TE, Snetselar L, Lane D et al.. Low-fat dietary pattern and breast cancer mortality in the Women's Health Initiative randomized controlled trial. J Clin Oncol. 2017;35:2919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chlebowski RT, Aragaki AK, Anderson GL, Simon MS, Manson JE, Neuhouser ML, Pan K, Stefanic ML, Rohan TE, Lane D et al.. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the Women's Health Initiative randomized clinical trial. JAMA Oncol. 2018;4:e181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prentice RL, Aragaki AK, Van Horn L, Thomson CA, Beresford SA, Robinson J, Snetselaar L, Anderson GL, Manson JE, Allison MA et al.. Low-fat dietary pattern and cardiovascular disease: results from the Women's Health Initiative randomized controlled trial. Am J Clin Nutr. 2017;106:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard BV, Aragaki AK, Tinker LF, Allison M, Hingle MD, Johnson KC, Manson JE, Shadyab AH, Shikany JM, Snetselaar LG et al.. A low-fat dietary pattern and diabetes: a secondary analysis from the Women's Health Initiative dietary modification trial. Diabetes Care. 2018;41:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 10. Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, Rodabough RJ, Snetselaar L, Thomson C, Tinker L et al.. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative dietary modification trial. JAMA. 2006;295:39–49. [DOI] [PubMed] [Google Scholar]
- 11. Thomson CA, Van Horn L, Caan BJ, Aragaki AK, Chlebowski RT, Manson JE, Rohan TE, Tinker LF, Kuller LH, Hou L et al.. Cancer incidence and mortality during the intervention and postintervention periods of the Women's Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2014;23:2924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer – viewpoint of the IARC working group. N Engl J Med. 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Margolis KL, Qi L, Brzyski R, Bonds DE, Howard BV, Kempainen S, Liu S, Robinson JG, Safford MM, Tinker LT et al.. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, Pettinger M, Lane DS, Lessin L, Yasmeen S et al.. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative dietary modification trial. J Natl Cancer Inst. 2007;99:1534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chlebowski RT, Anderson GL, Manson JE, Prentice RL, Aragaki AK, Snetselaar L, Beresford SAA, Kuller LH, Johnson K, Lane D et al.. Low-fat dietary pattern and cancer mortality in the Women's Health Initiative (WHI) randomized controlled trial. JNCI Cancer Spectrum. 2018;2:pky065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prentice RL, Thompson DJ, Clifford C, Gorbach S, Goldin B, Byar D. Dietary fat reduction and plasma estradiol concentration among healthy postmenopausal women. J Natl Cancer Inst. 1990;82:129–34. [DOI] [PubMed] [Google Scholar]
- 17. Rock CL, Flatt SW, Pakiz B, Quintana EL, Heath DD, Rana BK, Natarajan L. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism. 2016;65(11):1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allison MA, Aragaki AK, Ray RM, Margolis KL, Beresford SA, Kuller L, O'Sullivan M, Wassertheil-Smoller S, Van Horn L. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: tertiary analysis of the WHI dietary modification trial. Am J Hypertension. 2015;29(8):959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howard BV, Curb JD, Eaton CB, Kooperberg C, Ockene J, Kostis JB, Pettinger M, Rajkovic A, Robinson JG, Rossouw J et al.. Low-fat dietary pattern and lipoprotein risk factors: the Women's Health Initiative Dietary Modification trial. Am J Clin Nutr. 2010;91:860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borkman M, Campbell LV, Chrisholm DJ, Storlein LH. Comparison of the effects on insulin sensitivity of high carbohydrate and high fat diets in normal subjects. J Clin Endocrinol Metab. 1991;72:432–7. [DOI] [PubMed] [Google Scholar]
- 21. Sacks FM, Carey VJ, Anderson CA, Miller ER 3rd, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J et al.. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA. 2014;312:2531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. [Internet]. Continuous Update Project Expert Report 2017; London: WCRF International; [cited Mar 2019]. Available from: www.dietandcancerreport.org. [Google Scholar]
- 23. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Astrup A, Dyerberg J, Edwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, Krauss RM, Lecerf JM, LeGrand P et al.. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010?. Am J Clin Nutr. 2011;93:684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A et al.. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390:2050–62. [DOI] [PubMed] [Google Scholar]
- 26. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, Solomon SD. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. 2018;3:e419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ludwig DS, Willett WC, Volek JS, Neuhouser ML. Dietary fat: from foe to friend. Science. 2018;362:764–70. [DOI] [PubMed] [Google Scholar]
- 28. Zheng C, Beresford SA, Van Horn L, Tinker LF, Thomson CA, Neuhouser ML, Di C, Manson JE, Mossavar-Rahmani Y, Seguin R et al.. Simultaneous association of total energy consumption and activity-related energy expenditure with cardiovascular disease, cancer, and diabetes risk among postmenopausal women. Am J Epidemiol. 2014;180:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.