Abstract
Using prospective data from the Early Determinants of Mammographic Density study (United States, 1959–2008, n = 1121), we examined the associations between maternal body size, birth size, and infant and early childhood growth during 3 time periods (0–4 months, 4–12 months, and 1–4 years) and benign breast disease (BBD) using multivariable logistic regression with generalized estimating equations. A total of 197 women (17.6%) reported receiving a diagnosis of BBD by a physician. Higher body mass index at age 7 years was inversely associated with BBD risk. Rapid weight gain from age 1 year to 4 years, defined as an increase of least 2 major percentiles (e.g., 5th, 10th, 25th, 50th, 75th, and 95th) relative to stable growth, defined as remaining within 2 percentiles, was also inversely associated with BBD (odds ratio (OR) = 0.51, 95% confidence interval (CI): 0.23, 1.15). In contrast, rapid weight gain in infancy was positively associated with BBD relative to stable growth (from 0 to 4 months, OR = 1.65, 95% CI: 1.04, 2.62; from 4 to 12 months, 1.85, 95% CI: 0.89, 3.85), independent of birth weight, which was not associated with BBD. Our results suggest that patterns of early-life weight gain are important to BBD risk. Thus, susceptibility to BBD, like susceptibility to breast cancer, might start in early life.
Keywords: benign breast disease, birth weight, BMI, body size, infant growth, maternal size
Body size, often assessed by weight or body mass index (BMI), across the life course has been consistently associated with breast cancer (BC) risk (1, 2), with adult body size positively associated with postmenopausal BC (3–6) and adolescent body size inversely associated with pre- and postmenopausal BC (7, 8). Adolescent body size might also be inversely associated with the risk of benign breast disease (BBD) (9, 10), a well-established BC risk factor (11).
Little is known about whether early infant and childhood growth is associated with BBD and BC risk. A recent study found that maternal prepregnancy BMI and gestational weight gain were inversely associated with BBD risk (12). Birth weight, which is positively associated with maternal body size (13) and premenopausal BC (14, 15), was not associated with BBD (10, 12, 16). Longitudinal studies of growth patterns starting at birth are essential to understanding when the positive association between weight and BC risk observed at birth reverses direction and becomes an inverse relationship, as is consistently observed in adolescence. Prior studies lacked information on body size between birth and adolescence to investigate this question.
We addressed these gaps in the literature by investigating the relationships between maternal body size, body size at birth, and postnatal changes in height and weight through age 4 years with risk of BBD, using data from an adult follow-up study of 2 US birth cohorts from the 1960s that prospectively collected early-life anthropometric measures.
METHODS
Study participants
The Early Determinants of Mammographic Density (EDMD) study is a follow-up study of 2 US birth cohorts—the Boston, Massachusetts, and Providence, Rhode Island, sites of the National Collaborative Perinatal Project (NCPP) and the California Child Health and Development Studies—that enrolled pregnant women between 1959 and 1966 (with deliveries extending into 1967) (17–19). We used prospective data from pregnant mothers and growth data on their children through age 7 years. The Early Determinants of Mammographic Density study enrolled 1,134 female participants when they were ages 39–49 years (details and eligibility criteria reported elsewhere (19, 20)). This analysis includes 1,121 (98.9%) of the 1,134 participants who reported their BBD status at an adult interview, including 286 sibling sets. This study was approved by the institutional review boards at Columbia University Medical Center, Kaiser Permanente, Brigham and Women’s Hospital, and Brown University.
Maternal and childhood data collection
Mothers reported their current smoking status, prepregnancy weight, and educational level at antenatal clinic visits. Study staff measured maternal height and weight during pregnancy. We calculated gestational weight gain by subtracting the self-reported prepregnancy weight from the measured weight at the end of pregnancy (17, 18, 20). We calculated gestational age as the date of delivery minus the date of the last menstrual period. Trained personnel weighed infants to the nearest gram shortly after birth and measured length to the nearest centimeter from crown to heel. In the National Collaborative Perinatal Project, trained clinical staff measured weight and height at follow-up visits through early childhood, including at 4 months, 12 months, and 4 years of age (17). Height and weight data through at least age 5 years were abstracted from medical records for Child Health and Development Studies participants (18). Height and weight at age 7 years were available for the majority of the cohort (78.6%).
We examined postnatal growth using the within-cohort percentile rank change in weight and height from 0 to 4 months, 4 to 12 months, and 1 to 4 years as continuous variables. We applied individual cubic splines to interpolate measures at these exact times (21). We also examined patterns of height and weight gain during these time periods based on changes in the within-cohort percentiles, using similar cutoffs to the Centers for Disease Control and Prevention growth chart reference percentiles. We defined rapid and slow growth patterns, respectively, for height and weight as an increase or decrease of at least 2 major percentiles (5th, 10th, 25th, 50th, 75th, and 95th) during the time period. We compared girls having these patterns with girls who had stable growth, defined as those whose rank remained within 2 major percentiles. We also examined the impact of smaller changes in height and weight gain on BBD risk, distinguishing between crossing 1 major percentile and 2 or more major percentiles compared with staying within 1 major percentile. There is a direct mapping between percentile rank and z scores (21). For example, an increase from the 50th to the 75th percentile is equivalent to a 0.67 increase in z score, a cutoff that has been used previously to define rapid infant weight gain (22).
Adult data collection
We conducted adult follow-up interviews using computer-assisted telephone interview methods. We asked whether a doctor had ever diagnosed participants with any form of BBD, which encompasses a variety of different phenotypes including cysts, lumps, and fibrocystic breasts. Participants who answered “yes” to that question were classified as having BBD. Participants also reported sociodemographic information, body size in adulthood, and medical and reproductive history.
Statistical analysis
We investigated differences according to BBD status in mean values of body size measures across the life course using t tests and frequencies of height and weight gain patterns using χ2 tests. We estimated odds ratios and their 95% confidence intervals relating body size across the life course to BBD status using logistic regression models with generalized estimating equations to account for correlations among siblings. We first examined the association between BBD status and recalled BMI during ages 20–29 years to replicate previous studies examining BMI in early adulthood and BBD (9, 10), and we then examined the association with BMI measured at ages 1, 4, and 7 years. To account for potential confounding, we first adjusted for age and site and then further adjusted for ponderal index at birth, gestational weight gain, maternal prepregnancy BMI, and other early-life factors. We also examined maternal prepregnancy BMI and gestational weight gain as categorical variables, defined by the Centers for Disease Control and Prevention thresholds for overweight and obesity. We tested for nonlinearity in the models assessing ponderal index at birth and BMI at ages 1, 4, and 7 years and during ages 20–29 years by adding their quadratic terms to the models, and we formally tested their significances using the Wald test. We present these exposures as continuous variables given that there was no evidence of nonlinearity (P > 0.05).
We then estimated the associations between growth during infancy and early childhood and BBD risk. We measured early-life growth paths by birth weight, birth length, and changes in weight and height from 0 to 4 months, 4 to 12 months, and 1 to 4 years, and we also categorized them as rapid, stable, and slow. All models adjusted for age at adult interview and site. To better understand how growth during specific time periods is associated with BBD, we ran a sequence of models with the base model including only birth weight and birth length as exposures, and sequentially expanding the model to include the later growth periods one at a time (i.e., weight and height gain from 0 to 4 months, then 4 to 12 months, and then 1 to 4 years). We adjusted for maternal and prenatal factors. We considered maternal age at registration, prepregnancy BMI, maternal height, maternal education, gestational weight gain, packs of cigarettes smoked per day during pregnancy, prematurity (birth prior to 37 weeks’ gestation), race/ethnicity, and BC family history as potential confounders. We retained a variable whose inclusion resulted in a change of ≥10% in the regression coefficients of any of the exposures of interest in future models. We considered BMI during ages 20–29 years as a potential mediator. We also restricted our sample to sibling sets only, and we used conditional logistic regression to estimate the associations between early-life growth and BBD risk at the family level.
We examined effect modification by site, birth weight, and BMI at age 7 years by adding cross-product terms to the models. To account for the regression-to-the-mean effect (i.e., catch-up and catch-down growth), we performed sensitivity analyses for rapid weight gain excluding infants born before 37 weeks (n = 42), infants with a birth weight below 2.5 kg (n = 45), and infants born small for gestational age (SGA) (n = 110). For slow weight gain, we excluded infants with a birth weight greater than 4 kg (n = 164) or infants born large for gestational age (n = 110). Being SGA and large for gestational age were defined as those infants below the within-cohort 10th and above the 90th percentiles of birth weight divided by gestational age, respectively. We also examined an interaction between SGA and rapid weight gain in either infant time period. We examined the distribution of early-life weight patterns according to BMI at age 7 years to investigate whether rapid weight gain in infancy and early childhood tracked to higher childhood BMI.
RESULTS
Of the 1,121 women, 197 (17.6%) reported a BBD diagnosis by a physician (Table 1). The average age at diagnosis for women with BBD was 30.8 years.
Table 1.
Characteristics of Study Sample According to Benign Breast Disease Diagnosis (n = 1,121), Early Determinants of Mammographic Density Study, United States, 1959–2008
| Characteristic | Benign Breast Disease Diagnosis | |||||
|---|---|---|---|---|---|---|
| Yes (n = 197) | No (n = 924) | |||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Maternal | ||||||
| Prepregnancy BMIa | 185 | 22.8 (3.3) | 862 | 23.3 (3.9) | ||
| Weight gain during pregnancy, kg | 192 | 9.6 (3.5) | 895 | 9.3 (4.0) | ||
| Birth size | ||||||
| Birth weight, kg | 197 | 3.4 (0.6) | 924 | 3.5 (0.5) | ||
| Birth length, cm | 197 | 51.5 (2.7) | 917 | 51.2 (3.0) | ||
| Ponderal index at birthb | 197 | 25.0 (2.7) | 917 | 25.9 (6.5) | ||
| Early-life and childhood body size | ||||||
| Weight at 4 months, kg | 197 | 6.4 (0.8) | 922 | 6.4 (0.8) | ||
| Weight at 1 year, kg | 193 | 9.7 (1.3) | 914 | 9.7 (1.2) | ||
| Weight at 4 years, kg | 178 | 16.7 (2.3) | 887 | 16.7 (2.4) | ||
| Height at 4 months, cm | 195 | 62.2 (2.5) | 920 | 62.4 (2.9) | ||
| Height at 1 year, cm | 191 | 74.0 (3.1) | 912 | 73.8 (3.2) | ||
| Height at 4 years, cm | 176 | 101.2 (4.6) | 883 | 100.4 (4.8) | ||
| BMIa at 4 months | 195 | 16.6 (1.5) | 920 | 16.6 (1.9) | ||
| BMIa at 1 year | 191 | 17.7 (1.8) | 912 | 17.7 (1.7) | ||
| BMIa at 4 years | 176 | 16.2 (1.5) | 883 | 16.6 (1.8) | ||
| BMIa at 7 years | 139 | 16.2 (1.9) | 742 | 16.6 (2.2) | ||
| Early-life growth patternsc | ||||||
| Weight gain pattern, 0–4 monthsd | ||||||
| Rapid | 43 | 21.8 | 130 | 14.1 | ||
| Stable | 122 | 61.9 | 660 | 71.6 | ||
| Slow | 32 | 16.2 | 132 | 14.3 | ||
| Height gain pattern, 0–4 monthsd | ||||||
| Rapid | 19 | 9.7 | 137 | 14.9 | ||
| Stable | 139 | 71.3 | 639 | 69.5 | ||
| Slow | 37 | 19.0 | 144 | 15.7 | ||
| Weight gain pattern, 4–12 monthsd | ||||||
| Rapid | 16 | 8.3 | 54 | 5.9 | ||
| Stable | 168 | 87.0 | 813 | 88.9 | ||
| Slow | 9 | 4.7 | 47 | 5.1 | ||
| Height gain pattern, 4–12 monthsd | ||||||
| Rapid | 18 | 9.4 | 84 | 9.2 | ||
| Stable | 160 | 83.8 | 725 | 79.5 | ||
| Slow | 13 | 6.8 | 103 | 11.3 | ||
| Weight gain pattern, 1–4 yearsd | ||||||
| Rapid | 13 | 7.3 | 89 | 10.0 | ||
| Stable | 154 | 86.5 | 735 | 82.9 | ||
| Slow | 11 | 6.2 | 63 | 7.1 | ||
| Height gain pattern, 1–4 yearsd | ||||||
| Rapid | 20 | 11.3 | 101 | 11.5 | ||
| Stable | 139 | 79.0 | 696 | 78.8 | ||
| Slow | 17 | 9.7 | 86 | 9.7 | ||
| Adult | ||||||
| Age at interview, years | 197 | 44.1 (1.8) | 924 | 44.1 (1.9) | ||
| Age BBD first diagnosed by doctor, years | 196 | 30.8 (8.2) | ||||
| BMIa during ages 20–29 years | 197 | 21.7 (3.8) | 915 | 22.5 (4.4) | ||
| BMIa during ages 30–39 years | 197 | 23.8 (5.3) | 915 | 24.8 (5.9) | ||
| BMIa at interview | 194 | 26.6 (6.9) | 903 | 27.4 (6.5) | ||
Abbreviations: BMI, body mass index; cm, centimeters; SD, standard deviation.
a Weight(kg)/height(m)2.
b Weight(kg)/height(m)3.
c Rapid and slow patterns are defined as an increase or decrease, respectively, of ≥2 major percentiles (5th, 10th, 25th, 50th, 75th, and 95th) in weight or height relative to stable growth (staying within 2 major percentiles).
d Values do not sum to total due to missing data.
Body size (BMI)
We observed an inverse association between BMI during ages 20–29 years and BBD (per BMI unit, odds ratio (OR) = 0.96, 95% confidence interval (CI): 0.92, 1.01) (Table 2). This inverse association with BMI and BBD was seen at age 7 years as well, although it was not statistically significant in the multivariable-adjusted model (per BMI unit, OR = 0.93, 95% CI: 0.84, 1.03), but did not extend earlier in the life course. BMI at 4 years, BMI at 1 year, and ponderal index at birth were not independently associated with BBD risk in either the age- and site-adjusted or multivariable-adjusted model. Maternal prepregnancy BMI and gestational weight gain were also not associated with BBD risk (Web Table 1, available at https://academic.oup.com/aje).
Table 2.
Multivariable Models of Body Size Across the Life Course and Benign Breast Disease Diagnosis, Early Determinants of Mammographic Density Study, United States, 1959–2008
| Exposure | Age- and Site-Adjusted | Multivariable-Adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Model 1: maternal prepregnancy BMI, per unit increasea | 0.96 | 0.92, 1.00 | 0.97 | 0.93, 1.02 |
| Model 2: gestational weight gain, per 1-kg increaseb | 1.01 | 0.97, 1.04 | 1.00 | 0.96, 1.04 |
| Model 3: ponderal index at birth, per unit increasec | 0.96 | 0.92, 1.01 | 0.96 | 0.91, 1.02 |
| Model 4: BMI at age 1 year, per unit increased | 1.01 | 0.92, 1.11 | 1.05 | 0.95, 1.16 |
| Model 5: BMI at age 4 years, per unit increased | 0.93 | 0.85, 1.03 | 0.95 | 0.86, 1.06 |
| Model 6: BMI at age 7 years, per unit increased | 0.92 | 0.84, 1.00 | 0.93 | 0.84, 1.03 |
| Model 7: BMI during ages 20–29 years, per unit increased | 0.95 | 0.90, 0.99 | 0.96 | 0.92, 1.01 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
a BMI was calculated as weight (kg)/height (m)2. Multivariable model adjusted for site, age at interview, race/ethnicity, maternal age at registration, maternal education, maternal height, maternal cigarettes per day, and family history of breast cancer.
b Multivariable model adjusted for everything in model 1 plus maternal prepregnancy BMI and prematurity.
c Ponderal index was calculated as weight (kg)/height (m)3. Multivariable model adjusted for everything in model 2 plus gestational weight gain.
d BMI was calculated as weight (kg)/height (m)2. Multivariable model adjusted for everything in model 3 plus ponderal index at birth.
Early-life growth
Women who experienced rapid weight gain (an increase of ≥2 major percentiles) from 0 to 4 months were 1.65 times more likely to report a BBD diagnosis than women with stable growth (95% CI: 1.04, 2.62) (Table 3). The association between rapid weight gain between 4 and 12 months and BBD was also elevated (OR = 1.85, 95% CI: 0.89, 3.85). In contrast, rapid weight gain from 1 to 4 years was associated with a decreased risk of BBD (OR = 0.51, 95% CI: 0.23, 1.15). BMI during ages 20–29 years did not mediate associations between rapid weight gain and BBD (data not shown). There was no evidence of effect measure modification by birth weight, site, or BMI at age 7 years for any of the weight gain patterns from 0 to 4 years.
Table 3.
Multivariable Models of Birth Size and Patterns of Growth in Infancy and Early Childhood and Benign Breast Disease Diagnosis in the Overall Cohort and Within Families, Early Determinants of Mammographic Density Study, United States, 1959–2008
| Exposure | Overall Cohort | Sibling Sets | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a,b | Model 2a,c | Model 3a,d | Model 4a,e | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Birth weight, per 1-kg increase | 0.69 | 0.46, 1.04 | 0.71 | 0.46, 1.10 | 0.74 | 0.47, 1.15 | 0.51 | 0.17, 1.51 |
| Weight gain pattern, 0–4 months | ||||||||
| Rapidf | 1.61 | 1.03, 2.51 | 1.70 | 1.08, 2.68 | 1.65 | 1.04, 2.62 | 3.06 | 1.17, 7.98 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 1.36 | 0.85, 2.18 | 1.35 | 0.83, 2.19 | 1.40 | 0.86, 2.28 | 0.89 | 0.25, 3.15 |
| Weight gain pattern, 4–12 months | ||||||||
| Rapidf | 2.08 | 1.07, 4.06 | 2.04 | 1.00, 4.14 | 1.85 | 0.89, 3.85 | 1.09 | 0.25, 4.75 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 0.75 | 0.36, 1.55 | 0.65 | 0.30, 1.40 | 0.68 | 0.32, 1.48 | 0.87 | 0.12, 6.42 |
| Weight gain pattern, 1–4 years | ||||||||
| Rapidf | 0.55 | 0.26, 1.17 | 0.53 | 0.24, 1.13 | 0.51 | 0.23, 1.15 | 0.13 | 0.02, 0.78 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 0.69 | 0.34, 1.39 | 0.71 | 0.35, 1.46 | 0.72 | 0.36, 1.44 | 1.06 | 0.28, 4.01 |
| Birth length, per 1-cm increase | 1.04 | 0.97, 1.12 | 1.05 | 0.97, 1.15 | 1.05 | 0.96, 1.14 | 1.04 | 0.87, 1.24 |
| Height gain pattern, 0–4 months | ||||||||
| Rapidf | 0.78 | 0.45, 1.37 | 0.80 | 0.44, 1.43 | 0.76 | 0.42, 1.40 | 0.69 | 0.23, 2.05 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 1.05 | 0.66, 1.68 | 1.07 | 0.66, 1.74 | 1.04 | 0.63, 1.72 | 0.70 | 0.29, 1.71 |
| Height gain pattern, 4–12 months | ||||||||
| Rapidf | 0.86 | 0.47, 1.57 | 0.80 | 0.42, 1.52 | 0.92 | 0.48, 1.78 | 1.35 | 0.37, 4.94 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 0.89 | 0.48, 1.65 | 0.89 | 0.46, 1.72 | 0.82 | 0.42, 1.60 | 0.53 | 0.11, 2.48 |
| Height gain pattern, 1–4 years | ||||||||
| Rapidf | 0.87 | 0.48, 1.58 | 0.86 | 0.46, 1.61 | 0.87 | 0.46, 1.68 | 5.51 | 1.05, 28.99 |
| Stableg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Slowh | 1.52 | 0.84, 2.75 | 1.57 | 0.84, 2.94 | 1.51 | 0.80, 2.83 | 1.66 | 0.46, 5.98 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
a Estimates are adjusted only for growth measures in the same or earlier time periods.
b Model 1 included site, age at interview, birth weight, birth length, and changes in weight and height from 0 to 4 months, 4 to 12 months, and 1 to 4 years.
c Model 2 included all variables from model 1 plus maternal age at registration, maternal prepregnancy BMI, maternal height, and maternal education.
d Model 3 included all variables from model 1 plus maternal prepregnancy BMI, maternal height, maternal cigarettes per day, maternal weight gain during pregnancy, and prematurity.
e Model 4 was fitted using conditional logistic regression and adjusted for age at interview and growth measures in the same or earlier time periods only.
f Rapid growth is defined as an increase of ≥2 major percentiles (5th, 10th, 25th, 50th, 75th, and 95th).
g Stable growth is defined as staying within 2 major percentiles.
h Slow growth is defined as a decrease of ≥2 major percentiles (5th, 10th, 25th, 50th, 75th, and 95th).
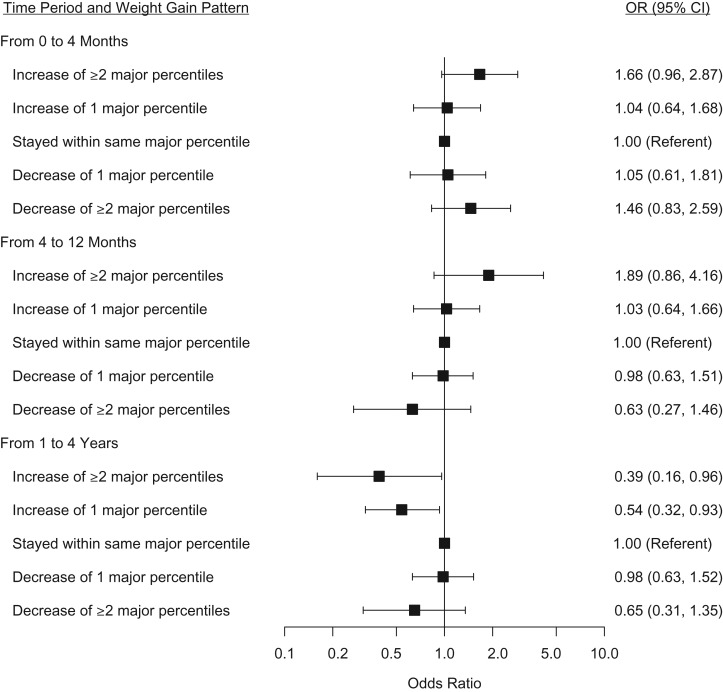
When we examined the association between changes of 1 and ≥2 major percentiles with BBD compared with those that stayed within 1 major percentile, the positive association between rapid infant weight gain and BBD was limited to those whose weight increased by at least 2 major percentiles from 0 to 4 and 4 to 12 months (Figure 1). An increase of 1 major percentile or more from 1 to 4 years was inversely associated with BBD. Slow weight gain and patterns of height gain in these time periods were not associated with BBD. Percentile rank changes in weight and height measured continuously from 0 to 4 months, 4 to 12 months, and 1 to 4 years were also not associated with BBD risk (Web Table 2), suggesting that the associations between weight gain and BBD risk are not linear and are limited to those who experience very fast weight gain.
Figure 1.
The association between early-life weight gain (using expanded definitions of rapid and slow weight gain) and benign breast disease diagnosis, Early Determinants of Mammographic Density Study, United States, 1959–2008. Models adjusted for site, age at interview, birth weight, birth length, height gain pattern in the same time period, height and weight gain pattern in previous time periods, maternal prepregnancy body mass index, maternal height, maternal cigarettes per day, maternal weight gain during pregnancy, and prematurity. CI, confidence interval; OR, odds ratio.
Sibling analysis
We observed similar associations between rapid weight gain and BBD within siblings, suggesting that family-level confounders do not explain the associations observed in the full cohort (Table 3). Within sibling sets, rapid weight gain from 0 to 4 months was associated with a more than 3-fold increased risk of BBD (OR = 3.06, 95% CI: 1.17, 7.98). Rapid weight gain from 1 to 4 years was associated with a decreased risk of BBD (OR = 0.13, 95% CI: 0.02, 0.78).
Rapid weight gain and size at birth
Birth weight and birth length were not associated with BBD (Table 3). We adjusted for weight and length at birth in all infant growth models. The inference was similar when we examined the ratio of birth weight/gestational age, a measure of fetal growth rate, instead of birth weight (data not shown). The inferences regarding infant weight gain and BBD risk were similar when we excluded preterm, low birth weight, or SGA infants (Web Figure 1, rapid weight gain) or high birth weight or large for gestational age infants (Web Figure 2, slow weight gain), suggesting that these associations hold in the majority of births and are not driven by the extremes of birth size. Further, we examined the association specifically with rapid weight gain in either infant time period and found that the association between rapid infant weight gain and BBD was apparent in SGA and non-SGA infants (for rapid weight gain and SGA, OR = 2.10, 95% CI: 1.03, 4.28; for rapid weight gain and non-SGA, OR = 1.66, 95% CI: 1.08, 2.54) compared with reference group of non-SGA infants without rapid weight gain (P for interaction = 0.53).
Rapid weight gain and body size in childhood
Because BMI at age 7 years was inversely associated with BBD risk, we examined whether rapid infant and early childhood weight gain tracked to higher childhood BMI. Girls who experienced rapid infant weight gain had a mean BMI at age 7 years that was similar to that of girls with stable growth (Web Figure 3). Rapid infant weight gain was associated with an increased risk of BBD regardless of BMI at age 7 years (P for interaction > 0.05 for infant weight patterns and BMI at age 7 years).
DISCUSSION
This study supports an inverse association between childhood and adult BMI and risk of BBD, and it further extends the literature to suggest that weight gain as early as infancy also influences BBD susceptibility, independent of birth weight and childhood body size. Although we observed associations between rapid weight gain in infancy and early childhood and BBD risk, BMI at ages 1 and 4 years were not associated with BBD. This finding is consistent with a previous study using the 1946 British birth cohort that did not find an association between BMI at ages 2 and 4 years and BC risk (23). Similar to the present study, this prior study reported an inverse association between BMI velocity from 2 to 4 years and BC risk. However, they did not have measurements between birth and 2 years and could not assess infant weight gain in relation to BC risk. A Swedish study of 405 BC cases and 1,081 controls found that neonates with an initial weight loss after birth of ≥200 g and/or rapid neonatal weight gain (≥25 g per day) after initial weight loss had an approximately 50% higher risk of BC compared with neonates who gained <25 g per day after an initial loss of <200 g. This association was roughly 2-fold in women aged less than 50 years (24). Together, this suggests that the rate of weight gain prior to childhood plays an important role in shaping the risk of BBD and BC in adulthood.
Infancy is associated with an activation of the hypothalamic-pituitary-gonadal axis, termed “minipuberty,” when breast tissue is present and might be stimulated by increased levels of reproductive hormones (25–27), which peak at 1–3 months of age and then gradually decline (28). The long-term effects of minipuberty are not well understood (29). Increases in height and faster peak height velocity during puberty, a critical period for breast development when growth and reproductive hormone levels are rapidly increasing (30), are associated with increased risk of BBD (9) and BC (7, 31). Some studies have also observed associations between increases in BMI during adolescence and decreased risks of BBD (16) and BC (7). Because the window of minipuberty during infancy might also be a biologically relevant time period for breast development (27), growth during this time period might plausibly be associated with later breast disease. Our results suggest that during infancy, rapid weight gain, not height gain, is associated with increased risk of BBD, which could reflect a mechanism different from the pathways that link pubertal growth and BBD risk.
Several mechanisms could underlie the observed association between rapid infant weight gain and increased risk of BBD and BC generally. Although infants who grow rapidly are at increased risk of obesity starting in childhood (21, 22, 32), the positive association between rapid infant weight gain and BBD in this study was not mediated by later body size. Infant weight gain is influenced by nutrition, with formula-fed infants more likely to have rapid weight gain than their breastfed peers (33, 34). Migrant and animal studies support the hypothesis that an energy-rich diet in early life might affect mammary gland development and BC risk (35–39), but previous studies have not observed an association between infant feeding and BBD (10, 12) or BC (40). Trichopoulos et al. (41) proposed an early-life etiological model that hypothesizes that BC risk depends on the number of mammary tissue–specific stem cells, which is determined in the perinatal period. Under this model, infant growth could influence BC risk through an effect on the number or replication rate of mammary tissue–specific stem cells, which is affected by postnatal levels of growth-enhancing hormones (41). Rapid weight gain in the first 2–3 years of life is associated with higher levels of insulin-like growth factor-I and growth hormone–binding protein in childhood (42), and higher levels of insulin-like growth factor-I in adulthood are associated with increased risk of premenopausal BC (43). Infant growth might also be associated with epigenetic changes, such as changes in DNA methylation of imprinted genes (44), which is associated with genomic instability and cancer risk in adulthood (45).
Our results suggest that weight gain during infancy influences BBD risk, but consistent with previous studies (10, 12), we did not observe an association between birth weight and BBD risk. We did not observe any consistent patterns relating maternal prepregnancy BMI and gestational weight gain to BBD risk, similar to prior studies examining these factors and BC risk (46, 47). However, maternal prepregnancy BMI and gestational weight gain are both higher in contemporary cohorts than in this cohort of pregnancies from the 1960s (48). Thus, we cannot rule out that higher maternal BMI and gestational weight gain are associated with BBD risk, and in fact, in the Growing Up Today Study of girls born in the 1980s, maternal prepregnancy BMI and gestational weight gain were inversely associated with BBD (12).
The prospective assessment of body size at birth and through early childhood by trained personnel limits the likelihood of exposure misclassification due to measurement error and is a major strength of this study. Many early-life exposures, including growth, are socially patterned (49, 50), making it difficult to rule out residual confounding by socioeconomic status as an explanation for early-life findings (51, 52). The consistency of the associations between rapid weight gain in infancy and early childhood and BBD risk in both the overall cohort and sibling subset, which controlled for family-level confounders such as socioeconomic status by design (53, 54), suggests that our findings are unlikely to be explained by social patterning.
Although BBD was self-reported, the prevalence of BBD in our study (17.6%) was similar to the prevalence of self-reported BBD in other cohorts (55, 56). A validation study in the Nurses’ Health Study II cohort found self-reports of biopsy-confirmed BBD to be accurate; in 621 women with self-reported BBD and pathology material available, 95% were confirmed by histologic review (57). Our results on childhood BMI and BBD risk were similar to those observed in previous studies that used reports of biopsy-confirmed cases (9, 10). We were unable to assess heterogeneity by BBD subtype without pathology reports. Because the increased risk of BC varies by BBD subtype, with a modest increase in BC risk, if any, for women with fibrocystic disease and the highest increase in risk for women with atypical hyperplasia (11, 58, 59), stronger effects in higher-risk BBD subtypes might be masked. In addition, given the relatively small number of BBD cases in each rapid weight gain group, the replication of results in larger studies is warranted.
In conclusion, our results suggest that patterns of weight gain in infancy are important to BBD risk. Thus, susceptibility to BBD, like susceptibility to breast cancer, might start in early life. Future research should examine growth trajectories during sensitive windows, which might have independent effects on later disease risk. Windows should be defined narrowly based on developmental biology given that we found heterogeneity of associations of growth rate with BBD even within the short window of birth through age 4 years.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Mandy Goldberg, Lauren C. Houghton, Julie D. Flom, Mary Beth Terry); Child Health and Development Studies, Public Health Institute, Berkeley, California (Barbara A. Cohn, Piera Cirillo); Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York (Ying Wei); Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (Karin B. Michels); Institute for Prevention and Cancer Epidemiology, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany (Karin B. Michels); Herbert Irving Comprehensive Cancer Center, Irving Medical Center, Columbia University, New York, New York (Mary Beth Terry); and Imprints Center for Genetic and Environmental Lifecourse Studies, Mailman School of Public Health, Columbia University, New York, New York (Mary Beth Terry).
This work received funding from the National Cancer Institute (grants R01CA104842-03, K07CA90685, and 5T32CA09529), Eunice Kennedy Shriver National Institute of Child Health and Development (grant P01AG023028-01), and the National Institute for Environmental Health Sciences (grant ES009089).
Conflict of interest: none declared.
Abbreviations
- BBD
benign breast disease
- BC
breast cancer
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- SGA
small for gestational age
REFERENCES
- 1. Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10(1):15–32. [DOI] [PubMed] [Google Scholar]
- 2. Ruder EH, Dorgan JF, Kranz S, et al. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. 2008;8(4):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. [DOI] [PubMed] [Google Scholar]
- 4. White AJ, Nichols HB, Bradshaw PT, et al. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121(20):3700–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2). [DOI] [PubMed] [Google Scholar]
- 6. Gathirua-Mwangi WG, Zollinger TW, Murage MJ, et al. Adult BMI change and risk of breast cancer: National Health and Nutrition Examination Survey (NHANES) 2005–2010. Breast Cancer. 2015;22(6):648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351(16):1619–1626. [DOI] [PubMed] [Google Scholar]
- 8. Baer HJ, Tworoger SS, Hankinson SE, et al. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171(11):1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berkey CS, Willett WC, Frazier AL, et al. Prospective study of growth and development in older girls and risk of benign breast disease in young women. Cancer. 2011;117(8):1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baer HJ, Schnitt SJ, Connolly JL, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2889–2897. [DOI] [PubMed] [Google Scholar]
- 11. Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149(3):569–575. [DOI] [PubMed] [Google Scholar]
- 12. Berkey CS, Rosner B, Willett WC, et al. Prenatal factors and infant feeding in relation to risk of benign breast disease in young women. Breast Cancer Res Treat. 2015;154(3):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frederick IO, Williams MA, Sales AE, et al. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J. 2008;12(5):557–567. [DOI] [PubMed] [Google Scholar]
- 14. Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007–2025. [DOI] [PubMed] [Google Scholar]
- 15. Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8(12):1088–1100. [DOI] [PubMed] [Google Scholar]
- 16. Berkey CS, Rosner B, Tamimi RM, et al. Body size from birth through adolescence in relation to risk of benign breast disease in young women. Breast Cancer Res Treat. 2017;162(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Broman S. The collaborative perinatal project: an overview In: Mednick SA, Harway M, Finello KM, eds. Handbook of Longitudinal Research. New York, NY: Praeger Publishers; 1984:185–227. [Google Scholar]
- 18. Berg van den BJ. The California Child Health and Development Studies In: Mednick SA, Harway M, Finello KM, eds. Handbook of Longitudinal Research. New York, NY: Praeger Publishers; 1984:166–179. [Google Scholar]
- 19. Terry MB, Schaefer CA, Flom JD, et al. Prenatal smoke exposure and mammographic density in mid-life. J Dev Orig Health Dis. 2011;2(6):340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Susser E, Buka S, Schaefer CA, et al. The early determinants of adult health study. J Dev Orig Health Dis. 2011;2(06):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terry MB, Wei Y, Esserman D, et al. Pre- and postnatal determinants of childhood body size: cohort and sibling analyses. J Dev Orig Health Dis. 2011;2(2):99–111. [DOI] [PubMed] [Google Scholar]
- 22. Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–908. [DOI] [PubMed] [Google Scholar]
- 23. De Stavola BL, dos Santos Silva I, McCormack V, et al. Childhood growth and breast cancer. Am J Epidemiol. 2004;159(7):671–682. [DOI] [PubMed] [Google Scholar]
- 24. Lagiou P, Hsieh CC, Trichopoulos D, et al. Neonatal growth and breast cancer risk in adulthood. Br J Cancer. 2008;99(9):1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayasinghe Y, Cha R, Horn-Ommen J, et al. Establishment of normative data for the amount of breast tissue present in healthy children up to two years of age. J Pediatr Adolesc Gynecol. 2010;23(5):305–311. [DOI] [PubMed] [Google Scholar]
- 26. Chellakooty M, Schmidt IM, Haavisto AM, et al. Inhibin A, inhibin B, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone–binding globulin levels in 473 healthy infant girls. J Clin Endocrinol Metab. 2003;88(8):3515–3520. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt IM, Chellakooty M, Haavisto AM, et al. Gender difference in breast tissue size in infancy: correlation with serum estradiol. Pediatr Res. 2002;52(5):682–686. [DOI] [PubMed] [Google Scholar]
- 28. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 29. Schooling CM, Houghton LC, Terry MB. Potential intervention targets in utero and early life for prevention of hormone related cancers. Pediatrics. 2016;138(suppl 1):S22–S33. [DOI] [PubMed] [Google Scholar]
- 30. Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57(2):112–137. [PubMed] [Google Scholar]
- 31. Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400–2409. [DOI] [PubMed] [Google Scholar]
- 32. Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klag EA, McNamara K, Geraghty SR, et al. Associations between breast milk feeding, introduction of solid foods, and weight gain in the first 12 months of life. Clin Pediatr (Phila). 2015;54(11):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Griffiths LJ, Smeeth L, Hawkins SS, et al. Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child. 2009;94(8):577–582. [DOI] [PubMed] [Google Scholar]
- 35. John EM, Phipps AI, Davis A, et al. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2905–2913. [DOI] [PubMed] [Google Scholar]
- 36. Parkin D, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer. 1996;32(5):761–771. [DOI] [PubMed] [Google Scholar]
- 37. Lagiou P, Adami HO, Trichopoulos D. Early life diet and the risk for adult breast cancer. Nutr Cancer. 2006;56(2):158–161. [DOI] [PubMed] [Google Scholar]
- 38. Hilakivi-Clarke L, Onojafe I, Raygada M, et al. Breast cancer risk in rats fed a diet high in n-6 polyunsaturated fatty acids during pregnancy. J Natl Cancer Inst. 1996;88(24):1821–1827. [DOI] [PubMed] [Google Scholar]
- 39. Hilakivi-Clarke L, Clarke R, Onojafe I, et al. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci U S A. 1997;94(17):9372–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wise LA, Titus-Ernstoff L, Newcomb PA, et al. Exposure to breast milk in infancy and risk of breast cancer. Cancer Causes Control. 2009;20(7):1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trichopoulos D, Adami HO, Ekbom A, et al. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008;122(3):481–485. [DOI] [PubMed] [Google Scholar]
- 42. Ong KK, Elmlinger M, Jones R, et al. Growth hormone binding protein levels in children are associated with birth weight, postnatal weight gain, and insulin secretion. Metabolism. 2007;56(10):1412–1417. [DOI] [PubMed] [Google Scholar]
- 43. Allen NE, Roddam AW, Allen DS, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92(7):1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Temple IK, Clayton-Smith J, Mackay DJG. Chapter 9. Imprinting disorders of early childhood In: Michels KB, ed. Epigenetic Epidemiology. Berlin, Germany: Springer Science & Business Media; 2012. [Google Scholar]
- 45. Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. [DOI] [PubMed] [Google Scholar]
- 46. Sanderson M, Williams MA, Daling JR, et al. Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12(4):397–407. [DOI] [PubMed] [Google Scholar]
- 47. Wilson KM, Willett WC, Michels KB. Mothers’ pre-pregnancy BMI and weight gain during pregnancy and risk of breast cancer in daughters. Breast Cancer Res Treat. 2011;130(1):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wijlaars LP, Johnson L, van Jaarsveld CH, et al. Socioeconomic status and weight gain in early infancy. Int J Obes (Lond). 2011;35(7):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Den Berg G, Van Eijsden M, Galindo-Garre F, et al. Low maternal education is associated with increased growth velocity in the first year of life and in early childhood: the ABCD study. Eur J Pediatr. 2013;172(11):1451–1457. [DOI] [PubMed] [Google Scholar]
- 51. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81–82:21–37. [DOI] [PubMed] [Google Scholar]
- 52. Smith GD. Reflections on the limitations to epidemiology. J Clin Epidemiol. 2001;54(4):325–331. [DOI] [PubMed] [Google Scholar]
- 53. Susser E, Eide MG, Begg M. Invited commentary: the use of sibship studies to detect familial confounding. Am J Epidemiol. 2010;172(5):537–539. [DOI] [PubMed] [Google Scholar]
- 54. Richmond RC, Al-Amin A, Smith GD, et al. Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Hum Dev. 2014;90(11):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Horn-Ross PL, Canchola AJ, West DW, et al. Patterns of alcohol consumption and breast cancer risk in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(3):405–411. [PubMed] [Google Scholar]
- 56. Ishitani K, Lin J, Manson JE, et al. Caffeine consumption and the risk of breast cancer in a large prospective cohort of women. Arch Intern Med. 2008;168(18):2022–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Su X, Colditz GA, Willett WC, et al. Genetic variation and circulating levels of IGF-I and IGFBP-3 in relation to risk of proliferative benign breast disease. Int J Cancer. 2010;126(1):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carter CL, Corle DK, Micozzi MS, et al. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128(3):467–477. [DOI] [PubMed] [Google Scholar]
- 59. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.