Supplemental Digital Content is available in the text
Keywords: Alzheimer's disease, meta-analysis, risk factor, vitamin D
Abstract
Background:
Considerable controversy exists on the association between serum vitamin D concentrations and Alzheimer disease (AD) risk. This study aimed to synthesize the association of serum vitamin D concentrations with AD in adults.
Methods:
PubMed, Embase, and Cochrane library databases were searched for prospective cohort studies with data on serum vitamin D concentrations and AD risk.
Result:
The studies that reported the adjusted relative risks (RRs) with 95% confidence intervals (CIs) of AD associated with serum vitamin D concentrations were included and subjected to subgroup analyses. Six prospective cohort studies with 1607 AD cases and 21,692 individuals were included in the meta-analysis. In 4 cohort studies with information about serum vitamin D concentrations <25 and 25 to 50 nmol/L, the random effects summary estimate did not show an increased risk of AD after adjustment for the established risk factors, while 3 cohort studies reported the RRs for incident AD per standard deviation (SD) decrease in serum vitamin D concentration and the random effects summary estimate did not show an increased risk of AD after adjustment for the established risk factors.
Conclusions:
The current meta-analysis indicated that serum vitamin D deficiency (<25 nmol/L) or insufficiency (25–50 nmol/L) was not statistically significant and associated with the risk of AD.
1. Introduction
Alzheimer disease (AD) is the most common cause of dementia, and up to 75% of all the cases of dementia are attributable to AD.[1] The average lifespan is expected to increase by an additional 10 years by 2050, and no definitive cure for AD has yet been found.[2] The accumulation of dysfunctional proteins, such as amyloid beta (Aβ) and tau protein derivatives in the brain, followed by oxidative damage and inflammation, leading to deranged energy metabolism, localized synaptic failure, and neuronal loss are speculated as the pathogenic hallmarks of AD.[3]
As the population continues to age rapidly, identifying the modifiable risk factors for AD with respect to lifestyle and diet is essential. In addition to the potentially modifiable risk factors of AD such as obesity, diabetes, hypertension, and smoking, a potential prognostic role for vitamin D deficiency has been proposed.[4]
Vitamin D has been implicated to be crucial in maintaining the cognitive function in old age.[5] Vitamin D receptors are present in the brain regions responsible for memory development and cognitive functions and may also be involved in plaque clearance.[6,7] Furthermore, the cutoffs for vitamin D deficiency and the optimum value for physical and mental health have not yet reached a global consensus.[8–10]
A meta-analysis of studies comparing the patients with and without dementia has indicated that those with AD have low levels of vitamin D.[11] Similarly, a meta-analysis of cross-sectional studies has identified that low serum vitamin D concentrations are associated with prevalent AD.[12,13]
The serum vitamin D concentrations independently increase the incidence of AD or whether this correlation is confounded by the population's prevalent modifiable risk factors in the primary prevention of AD is demonstrated in the recently published meta-analysis of 8 cohort studies. These studies showed that high levels of serum vitamin D status were associated with a low risk of AD.[14] However, the meta-analysis found insufficient evidence in the subgroup meta-analysis by adjusting for baseline cardiovascular disease (CVD) and cancer (yes vs no), adjusting for physical activity (yes vs no), adjusting for serum cholesterol (yes vs no), and adjusting for alcohol (yes vs no).
To fill in these gaps, we systematically evaluated the association between serum vitamin D concentrations and incidence of AD by conducting an updated meta-analysis of prospective cohort studies.
2. Material and methods
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist[15] for reporting the results in this systematic review.
2.1. Literature search
A systematic search of PubMed, Embase, and the Cochrane library databases was conducted to retrieve the potential prospective cohort studies published before March 2018. The terms used for the PubMed search are provided in online-only Supplemental Data 1, and similar searches were conducted in the Embase and Cochrane library. The current search was restricted to studies conducted in humans, and no restriction was imposed with respect to the language of publications. We also screened the reference lists of eligible reviews and included the relevant articles in these lists. When the same or a similar patient cohort was included in these publications, only the most recent or complete report was selected for analysis.
2.2. Study selection
Studies that satisfied the following criteria were included in the current meta-analysis:
-
(1)
the study of adult patients had a prospective cohort design;
-
(2)
the exposure of interest was serum vitamin D at the baseline;
-
(3)
the outcome of interest was AD;
-
(4)
the reported risk estimates [relative risk (RR) or hazard ratio (HR)] and the corresponding 95% confidence intervals (CIs) of dementia or AD; and
-
(5)
a follow-up period >1 year.
Also, studies that reported results as per the unit increment or decrement in serum vitamin D were included. The studies were excluded if:
-
(1)
it had a cross-sectional or case-control design or clinical trial;
-
(2)
unadjusted or only age- or gender-adjusted RR was reported;
-
(3)
studies conducted among patients with specific diseases, such as hospitalized patients;
-
(4)
the study was duplicated;
-
(5)
the follow-up period of the study was <1 year.
2.3. Data abstraction
Two reviewers independently reviewed the full text of selected eligible studies and extracted the following information: the first author's name, publication year, study design, country of origin, number of participants, participants’ age, follow-up years, gender, assessment method of serum vitamin D and AD, number of AD cases, and adjusted covariates. Any discrepancy was resolved by discussion to reach a consensus between the 2 authors; the original authors were contacted in case supplementary information was required. According to the World Health Organization (WHO)[16] and the US Institute of Medicine,[8] a sufficient vitamin D status was defined as a deseasonalized serum vitamin D concentration >50 nmol/L, while vitamin D insufficiency was defined as a deseasonalized serum vitamin D concentration between 25 and 50 nmol/L, and deseasonalized vitamin D deficiency at <25 nmol/L (to convert to ng/mL, divide by 2.496).
2.4. Assessment of study quality
The quality was assessed in accordance with the Newcastle-Ottawa scale (NOS) for non-randomized studies.[17] A score of up to 9 stars was assigned to each study: participant selection (up to 4 stars), comparability of study groups (up to 2 stars), and assessment of outcome or exposure of the cohorts (up to 3 stars). A high score represented a superior methodological quality. The scores of 0 to 3, 4 to 6, and 7 to 9 indicated low, moderate, and high quality, respectively.
2.5. Statistical analysis
Multivariate-adjusted outcome data were used for analysis, and HRs and incidence rate ratio were considered as approximate RRs. These values in every study were converted using their natural logarithms and standard errors calculated based on the corresponding 95% CIs. The results were expressed as RRs with 95% CIs (a fixed-effect approach was used unless a significant heterogeneity was noted, in which case, a random-effects statistical model was applied).[18] The heterogeneity of the study was explored using tau2, and the amount of variance in the summary effect due to between-study heterogeneity was defined by I2. This was considered as statistically significant at the P < .10 level, as determined by the Cochran Q statistical test.[19] If heterogeneity was evident, subgroup synthesis and sensitivity analysis were employed to explicate the contribution to the heterogeneity. Subgroup analyses of serum vitamin D were conducted by adjusting for the baseline CVD and cancer (yes vs no), adjusting for physical activity (yes vs no), adjusting for serum cholesterol (yes vs no), and adjusting for the presence of alcohol (yes vs no). Publication bias was assessed by Egger test and the symmetry of the funnel plot.[20–22] In the case of publication bias, the “nonparametric trim-and-fill” method was used for computing the risk estimates corrected for this bias.[23] All statistical analyses were performed using the Review Manager version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK) and Stata version 11 (Stata Corporation, College Station, TX). Except otherwise noted, differences with a P value < .05 were considered as statistically significant.
3. Results
3.1. Literature search and characteristics
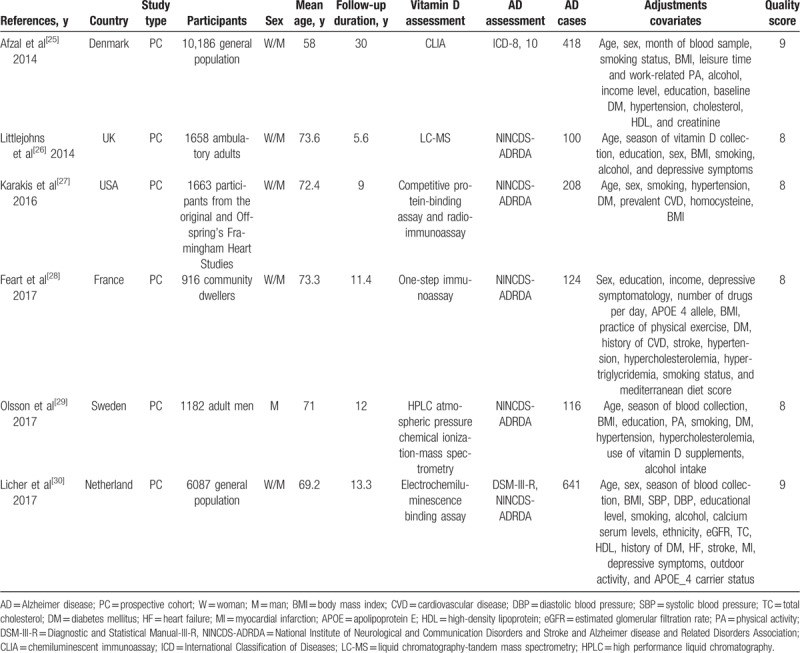
Figure 1 illustrates the study selection process and the results of our literature search, which retrieved a total of 706 studies. After excluding the duplicate records and the reports with findings of studies that did not meet the inclusion criteria, 19 potentially eligible studies were assessed by reviewing the full text. Consequently, 13 articles were further excluded due to the following reasons: the outcome was the absence of AD (n = 4), review or meta-analysis (n = 8), or lack of a cohort design (n = 1). Finally, the present meta-analysis included results from 6 independent prospective cohort studies.[24–29] Six studies on the relationship between serum vitamin D concentration and AD risk (1607 AD cases among 21,692 individuals) were published between 2014 and 2017 (Table 1).[24–29] The number of participants ranged from 916 (in the study conducted by Feart et al[27]) to 10,186 (in the study conducted by Afzal et al[24]). Furthermore, the follow-up duration ranged from 5.6 to 30 years,[24,25] with a median of 10.2 years. All articles were graded as high-quality according to the NOS (online-only Supplemental Table 1).
Figure 1.
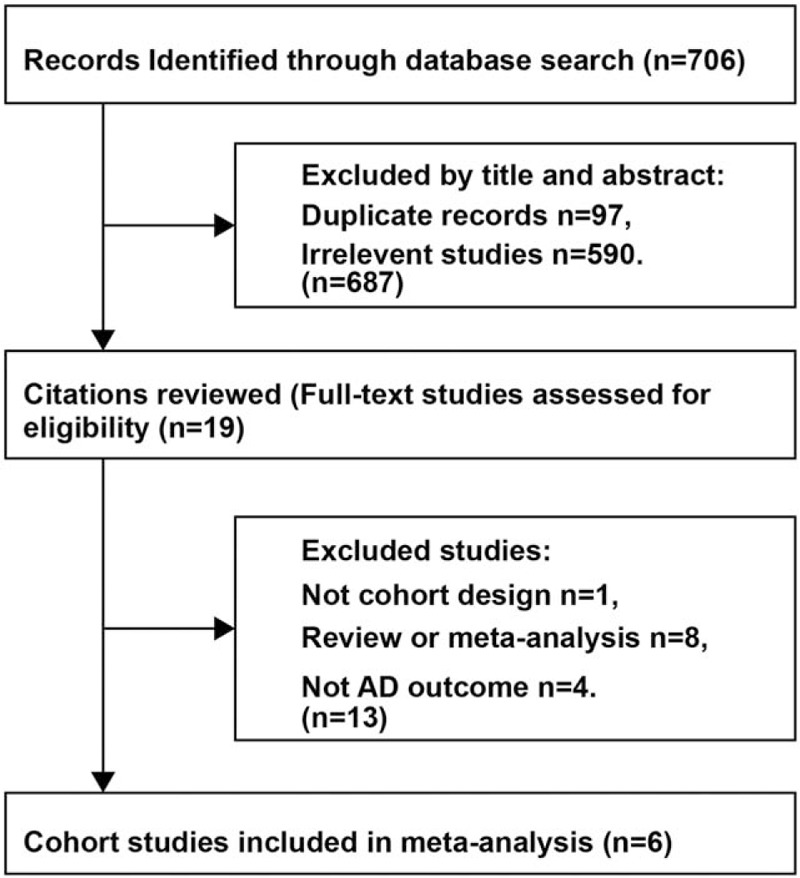
Process of literature search and study selection.
Table 1.
Study characteristics.
3.2. Vitamin D concentrations and risk of AD
In 4 cohort studies with information about the serum vitamin D concentrations <25 nmol/L, the random effects summary estimate did not show an increased risk of AD after adjustment for the established risk factors (RR 1.55, 95% CI: 0.97–2.49; P = .07) (Fig. 2) with significant heterogeneity across studies (I2 = 57%).[24–27] No significant publication bias was observed according to the funnel plot (online-only Supplemental Fig. 1) and Egger's test (P = .535).
Figure 2.
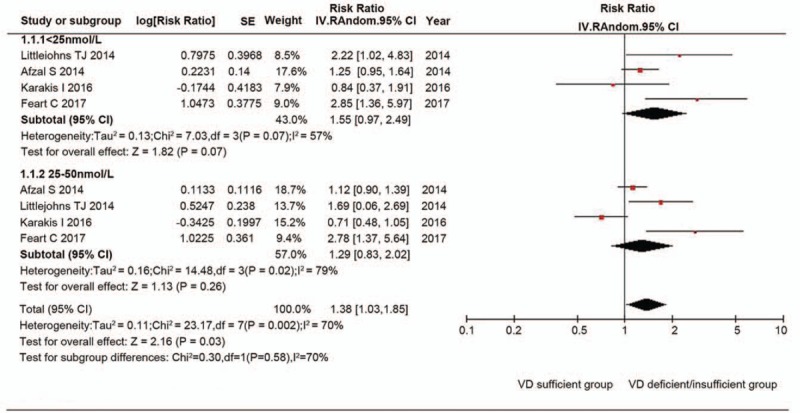
Random effects analysis of fully adjusted studies for the association between serum vitamin D concentrations and Alzheimer disease risk. The square box in the graph portrays the weight that each study contributed to the analysis. CI = confidence interval, IV = inverse variance, SE = standard error, VD = vitamin D.
In 4 cohort studies with information about serum vitamin D concentrations 25 to 50 nmol/L, the random effects summary estimate did not show an increased risk of AD after adjustment for the established risk factors (RR 1.29, 95% CI: 0.83–2.02; P = .26) (Fig. 2) with extreme heterogeneity across studies (I2 = 79%).[24–27] No significant publication bias was observed according to the funnel plot (online-only Supplemental Fig. 1) and Egger test (P = .507).
Furthermore, 3 cohort studies reported the RRs for the incidence of AD per SD decrease in serum vitamin D concentration. The random effects summary estimate did not show an increased risk of AD after adjustment for the established risk factors (RR 1.06, 95% CI: 0.96–1.18; P = .27) (online-only Supplemental Fig. 2) with significant heterogeneity across studies (I2 = 62%).[26,28,29] No significant publication bias was observed according to the funnel plot (online-only Supplemental Fig. 3) and Egger test (P = .918).
3.3. Subgroup analysis
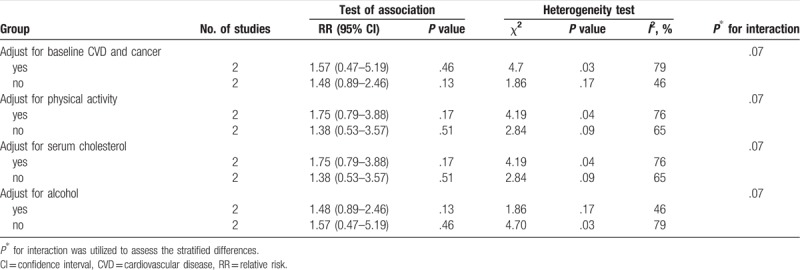
The serum vitamin D concentrations <25 nmol/L subgroup did not demonstrate an association of serum vitamin D concentration with increased risk of AD (Table 2). Additionally, in the serum vitamin D concentrations 25 to 50 nmol/L subgroup, similar associations were evident in several strata of the study characteristics (data not shown).
Table 2.
Stratified analyses of serum vitamin D concentrations <25 nmol/L and Alzheimer disease risk.
3.4. Sensitivity analysis
The robustness of our results was evaluated by sensitivity analysis. When studies included in the meta-analysis were excluded sequentially, the results of the meta-analysis remained largely unchanged. This indicated that the results of the present meta-analysis were stable (data not shown).
4. Discussion
4.1. Principal findings
The present meta-analysis provided additional evidence on the association of vitamin D concentration and the risk of AD. The current analysis indicated that serum vitamin D deficiency (<25 nmol/L) was not statistically significant and associated with the risk of AD (by 55%), and only marginally associated with the risk of AD in participants with serum vitamin D insufficiency (25–50 nmol/L) (by 29%). A 1 nmol/L decrement in serum vitamin D concentration was associated with a 6% high risk of AD, albeit not significantly.
4.2. Strengths and limitations of the study
A major strength of our meta-analysis was the prospective design of the included cohort studies with adjusted RRs from general populations, which might have greatly reduced the potential of selection bias. In addition, we used NOS score and found that all studies had a high-quality. As the sample size of the single primary study was relatively small, our meta-analysis increased the statistical power to detect the possible association between serum vitamin D concentration and AD risk in order to determine the accurate risk estimation. Nevertheless, the potential limitations of the study should be considered. First, we could not exclude the potential bias due to the different methods used for assessing the vitamin D concentration. Second, primary studies only assessed the baseline serum vitamin D and did not perform repeated measurements over the follow-up period. Thus, without considering these factors, we might obtain a biased conclusion. Third, despite the major confounders seemed unlikely to alter the role of vitamin D concentration on the increment of AD risk based on the subgroup analyses of adjustments, other factors (such as unknown confounders and imprecise adjustment) potentially accounted for the observed association, which could not be ruled out. For example, information with respect to genetic factors was not available in most studies. Fourth, men and women have different overall AD incidence, while sufficient data was not available to perform gender subgroup analysis for exploring the effect of gender on the association between vitamin D deficiency and risk of developing AD.[30,31] Finally, only published cohort data was included in the current meta-analysis, which increased the risk of publication bias via the exclusion of unpublished studies. However, after carefully examining all the relevant articles, including several meta-analyses[12,13,32,33] and reviews,[34,35] any relevant unpublished studies were not retrieved.
4.3. Comparison with other studies
Several previous meta-analyses have returned consistent results on vitamin D concentration and dementia.[12,13,32,33] Similarly, another published meta-analysis that included 8 cohort studies including 28,354 participants reported that high levels of serum vitamin D were associated with a low risk of dementia and AD.[14] However, the meta-analysis did not find sufficient evidence in the subgroup meta-analysis by adjusting for baseline CVD, cancer, physical activity, serum cholesterol, and alcohol.
In recent decades, evidence indicated that vitamin D deficiency or insufficiency exerts its effects indirectly by increasing the levels of CVD risk factors and the risk of endothelial dysfunction—the 2 risk factors for the development of AD.[36,37] Shen et al included 5 study populations from 3 prospective cohort studies and found that vitamin D deficiency was associated with an increased risk of AD occurrence as compared to the subjects with serum vitamin D level >50 nmol/L.[33] Similarly, the large sample size in our meta-analysis allowed further analysis of the risk of different subgroups. Additionally, in a large prospective population-based cohort, Licher et al found that low serum vitamin D concentrations were associated with a high incidence of dementia.[29] Conversely,[29] Olsson et al did not find any no evidence supporting that baseline vitamin D concentration was an independent risk factor for dementia or cognitive impairment in adult males.[28] Furthermore, Olsson et al did not analyze the risk of serum vitamin D deficiency (<25 nmol/L) according to the guidelines of the National Academy of Medicine (NAM) in the USA.[28]
4.4. Potential mechanisms
Although the pathophysiological etiology of AD is not fully understood, different mechanisms of neurodegeneration have emerged, including deposition of amyloid plaques, inflammatory processes, neurofibrillary degeneration, glutamatergic excitotoxicity, excessive intraneuronal calcium influx, and oxidative stress.[3] Vitamin D is hypothesized to exert its effects via different neural pathways.[38,39] Although the exact mechanisms are unclear, evidence suggests that vitamin D might protect against cognitive dysfunction via its effect on neuroprotection, neurotransmission, synaptic plasticity, immune modulation, neuronal calcium regulation, and enhanced nerve conduction,[40,41] with secondary protective effects on vascular systems and modulation of vascular risk factors.[42] Additionally, the in vitro analysis showed that vitamin D treatment inhibits the production TNF-α and IL 6, suggesting an anti-inflammatory role.[43] In vitamin D-deficient mice, specific calcium channels are also shown to be unregulated leading to an increase in Ca2+ level and in vitro evidence has shown that vitamin D can downregulate these calcium channels.[44] Finally, vitamin D might partially prevent the AD-related defects in acetylcholine since vitamin D supplementation in rats caused an increase in choline acetyltransferase activity in several brain areas.[45] This experimentally proved the involvement of vitamin D in brain pathways together with the present finding that serum vitamin D concentrations are overall lower in AD as compared to controls provides new insight on the putative involvement of vitamin D in the course of AD.
In conclusion, the current meta-analysis indicated that serum vitamin D deficiency (<25 nmol/L) was neither statistically significant nor associated with the risk of AD; however, it was marginally associated with the risk of AD in participants with serum vitamin D insufficiency (25–50 nmol/L). Nowadays, lifestyle modification is the primary management of individuals with vitamin D deficiency or insufficiency. Thus, randomized clinical trials will be essential to address the issue of causality and determine whether vitamin D supplementation is effective for the prevention or treatment of AD.
Author contributions
Conceptualization: Kui Yang.
Data curation: Yongning Zhou.
Formal analysis: Yongning Zhou.
Investigation: Kui Yang, Xiaoguang Li.
Methodology: Jun Chen.
Project administration: Kui Yang.
Software: Jun Chen, Yongning Zhou.
Validation: Jun Chen, Xiaoguang Li.
Writing – original draft: Kui Yang.
Writing – review & editing: Kui Yang, Yongning Zhou.
Supplementary Material
Footnotes
Abbreviations: Aβ = amyloid beta, AD = Alzheimer's disease, CIs = confidence intervals, CVD = cardiovascular disease, HR = hazard ratio, RRs = relative risks, SD = standard deviation.
The manuscript does not contain clinical studies or patient data.
The authors have no conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 2009;11:111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- [3].Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329–44. [DOI] [PubMed] [Google Scholar]
- [4].Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res 2012;43:600–8. [DOI] [PubMed] [Google Scholar]
- [5].Perez-Lopez FR, Chedraui P, Fernandez-Alonso AM. Vitamin D and aging: beyond calcium and bone metabolism. Maturitas 2011;69:27–36. [DOI] [PubMed] [Google Scholar]
- [6].Annweiler C, Schott AM, Berrut G, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology 2010;62:139–50. [DOI] [PubMed] [Google Scholar]
- [7].Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer's disease: vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 2011;23:207–19. [DOI] [PubMed] [Google Scholar]
- [8].Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [10].Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull 2014;39:322–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao Y, Sun Y, Ji HF, et al. Vitamin D levels in Alzheimer's and Parkinson's diseases: a meta-analysis. Nutrition 2013;29:828–32. [DOI] [PubMed] [Google Scholar]
- [12].Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 2012;79:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Annweiler C, Montero-Odasso M, Llewellyn DJ, et al. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis 2013;37:147–71. [DOI] [PubMed] [Google Scholar]
- [14].Jayedi A, Rashidy-Pour A, Shab-Bidar S. Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response. Nutr Neurosci 2018;1–0. [DOI] [PubMed] [Google Scholar]
- [15].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [16].Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 2003;921:1–64. back cover. [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [23].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [24].Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement 2014;10:296–302. [DOI] [PubMed] [Google Scholar]
- [25].Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014;83:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham heart study. J Alzheimers Dis 2016;51:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feart C, Helmer C, Merle B, et al. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement 2017;13:1207–16. [DOI] [PubMed] [Google Scholar]
- [28].Olsson E, Byberg L, Karlstrom B, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr 2017;105:936–43. [DOI] [PubMed] [Google Scholar]
- [29].Licher S, de Bruijn R, Wolters FJ, et al. Vitamin D and the risk of dementia: the Rotterdam study. J Alzheimers Dis 2017;60:989–97. [DOI] [PubMed] [Google Scholar]
- [30].Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997;49:1498–504. [DOI] [PubMed] [Google Scholar]
- [31].Miech RA, Breitner JC, Zandi PP, et al. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology 2002;58:209–18. [DOI] [PubMed] [Google Scholar]
- [32].Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 2013;33:659–74. [DOI] [PubMed] [Google Scholar]
- [33].Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J 2015;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aspell N, Lawlor B, O'Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc Nutr Soc 2018;77:124–34. [DOI] [PubMed] [Google Scholar]
- [35].Keeney JT, Butterfield DA. Vitamin D deficiency and Alzheimer disease: common links. Neurobiol Dis 2015;84:84–98. [DOI] [PubMed] [Google Scholar]
- [36].de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 2004;3:184–90. [DOI] [PubMed] [Google Scholar]
- [37].O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4. [DOI] [PubMed] [Google Scholar]
- [38].Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009;34Suppl. 1:S265–77. [DOI] [PubMed] [Google Scholar]
- [39].Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med 2008;29:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100–5. [DOI] [PubMed] [Google Scholar]
- [42].Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lefebvre d’Hellencourt C, Montero-Menei CN, Bernard R, et al. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res 2003;71:575–82. [DOI] [PubMed] [Google Scholar]
- [44].Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001;21:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sonnenberg J, Luine VN, Krey LC, et al. 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology 1986;118:1433–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


