Abstract
This retrospective study shows the results of a 2 years application of a clinical pathway concerning the indications to NOM based on the patient's hemodynamic answer instead of on the injury grade of the lesions.
We conducted a retrospective study applied on a patient's cohort, admitted in “Azienda Ospedaliero-Universitaria Ospedali Riuniti of Ancona” and in the Digestive and Emergency Surgery Department of the Santa Maria of Terni hospital between September 2015 and December 2017, all affected by blunt abdominal trauma, involving liver, spleen or both of them managed conservatively. Patients were divided into 3 main groups according to their hemodynamic response to a fluid administration: stable (group A), transient responder (group B) and unstable (group C). Management of patients was performed according to specific institutional pathway, and only patients from category A and B were treated conservatively regardless of the injury grade of lesions.
From October 2015 to December 2017, a total amount of 111 trauma patients were treated with NOM. Each patient underwent CT scan at his admission. No contrast pooling was found in 50 pts. (45.04%). Contrast pooling was found in 61 patients (54.95%). The NOM overall outcome resulted in success in 107 patients (96.4%). NOM was successful in 100% of cases of liver trauma patients and was successful in 94.7% of splenic trauma patients (72/76). NOM failure occurred in 4 patients (5.3%) treated for spleen injuries. All these patients received splenectomy: in 1 case to treat pseudoaneurysm, (AAST, American Association for the Surgery of Trauma, grade of injury II), in 2 cases because of re-bleeding (AAST grade of injury IV) and in the remaining case was necessary to stop monitoring spleen because the patient should undergo to orthopedic procedure to treat pelvis fracture (AAST grade of injury II).
Non-operative management for blunt hepatic and splenic lesions in stable or stabilizable patients seems to be the choice of treatment regardless of the grade of lesions according to the AAST Organ Injury Scale.
Keywords: angiography, embolization, hemodynamic stability, liver trauma, non-operative management, splenic trauma, trauma
1. Introduction
Trauma is the major cause of mortality in population under 40 years; and abdominal trauma is the third common trauma with a high rate of morbidity and mortality.[1] Spleen represent the most commonly damaged organ during abdominal blunt trauma and is affected in about 33% of patients with traumatic abdominal injuries.[2–4] Liver is the solid organ with highest injury rate in abdominal injuries, and approximately 15% to 20% of these refer to hepatic trauma. Hepatic injury takes the third place in abdominal injury and 80% to 90% of hepatic injuries are blunt ones.[5,6] In 2013 1 study showed that the liver was the mostly affected organ and younger people were more vulnerable to hepatic and pancreatic injury.[7] The most important change in trauma patient's care over the last decades is represented by the switch to selective non-operative management (NOM).[8] It started with isolated pediatric splenic lesions but actually it is considered the gold standard treatment for trauma patients with specific parameters. In 2017 Ruscelli et al[9] presented the experience of the Cesena Trauma Center in which 732 patients were treated with NOM for blunt hepatic and splenic injuries reporting statistically significant positive results. Regardless the degree of hepatic or splenic lesions, the authors treated traumatized patients with NOM or embolization according to their hemodynamic response. In order to add data and reinforce the previous results, we present now our experience in the Ancona Trauma Center and in the Digestive and Emergency Surgery Department of the Santa Maria of Terni hospital, 2 different Institutions but similar results using the same pathway.
2. Materials and methods
According to the protocol in use at the Ancona Trauma Center, all blunt trauma patients were evaluated in order to assess after the primary survey their specific response to fluid challenge. According to the criteria of Advanced Trauma Life Support (ATLS), patients have been classified in 3 different categories: STABLE (A), TRANSIENT RESPONDER (B) or UNSTABLE (C). The considered parameters were: blood gas analysis, systolic and diastolic arterial pressure, heart rate and breathing rate. The ideal systolic blood pressure value was settled at 90 mmHg for isolate blunt abdominal trauma, and 110 mmHg when a cranial Trauma was associated. The STABLE (A) category included patients in which after the first 1500 cc bolus, the blood pressure rises and then is maintained on physiological levels with infusions at maintenance speed. The TRANSIENT RESPONDER (B) included patients that achieve the stability after a fluid challenge but they did not maintain it without them. The UNSTABLE (C) category includes patients without any response to fluid challenge that need an immediate surgical exploration.
STABLE and TRANSIENT RESPONDER patients underwent computed tomography (CT) scan in order to perform a NOM while UNSTABLE patients were scheduled for immediate surgery (C) after the primary survey. According to our protocol only type A and B patients were considered eligible to NOM.
The only inclusion criteria for NOM was:
-
(1)
patients of categories A and B according to the ATLS.
The exclusion criteria for NOM were:
-
(1)
Need of more than 1000 cc of blood transfusions to maintain the stability, with an associated abdominal contamination source.
-
(2)
Presence of peritonitis at the time of admission.
-
(3)
Presence of other non-abdominal major lesions, such as thoracic, neurological, vascular, orthopedic, maxillofacial, urological, and requiring immediate surgical intervention.
-
(4)
Impossibility to receive an adequate follow-up (radiological or laboratory).
-
(5)
Impossibility to provide an immediate embolization or a surgical procedure in case of NOM failure.
Between September 2015 and December 2017, nr. 85 consecutive patients were admitted with blunt abdominal trauma, involving liver and/or spleen and/or kidney, at the Emergency Room of the “Azienda Ospedaliero-Universitaria Ospedali Riuniti of Ancona” Trauma Center. In the same period, 26 patients were admitted to the digestive and emergency surgery department of the Santa Maria of Terni hospital. For all patients, demographics, type of management, radiological, operative details, and postoperative outcomes were retrospectively collected and analyzed. Patients who died either at the scene or during transportation were excluded. The main evaluated outcomes are mortality, length of hospital stay, rate of treatment success, and complications. Patients management included a specific institutional developed protocol. In the two centers of the study the same protocol was adopted in treating these patients. First, hemodynamic stability was evaluated according to the patients’ response to fluid challenge, in all 3 categories A, B, and C. All patients in classes A and B performed CT scan while C class patients did not perform the CT scan. If a source of bleeding was detected at the CT scan, an immediate angiography was performed in order to control it and solve it. We did not perform any embolization in class C patients, and also in class A and B patients if there was the presence of an intraperitoneal contrast pooling; no embolization was also performed when CT scan was negative for contrast blush. Only patients with blunt abdominal trauma from category A and B were considered eligible for NOM.
Using the AUDIT methodology, our Trauma Service developed a clinical pathway to observe these patients during NOM. The NOM monitoring was initially built up according to the mortality frequencies observed. Then different protocols were established for splenic and hepatic trauma, distinguishing between major and minor lesions for each one. A major splenic and hepatic trauma was defined for patients with hemodynamic response type B and/or treated by embolization. All the remaining conditions have been considered as minor trauma and a less intensive monitoring has been carried on. The radiological monitoring was performed with Contrast Enhanced Ultrasound (CEUS) and a second CT scan was used only at 24 hours from the trauma event, when a first CT scan detected a contrast pooling that was not confirmed by angiography. Failure of NOM was considered in case of:
-
(1)
Onset of hypotension, tachycardia, and oligo-anuria during monitoring.
-
(2)
Decrease in hemoglobin associated with progressive increase of hemoperitoneum assessed by CEUS.
-
(3)
Need to infuse over 4 U.I. of blood in the first 24 hours to maintain and stable the parameters. In this case, after a new assessment of the hemodynamic response, the patient was immediately transferred to the operating room or angiography suite.
The research was undertaken according to the Italian Privacy Laws regarding the collection, storage, and analysis of private data. Formal approval by the Ethics Committee is not required as the study is non-interventional, anonymous, and retrospective. A signed consent for the processing and analysis of data for scientific purposes was obtained by all patients or their relatives at the time of admission or during hospitalization.
3. Results
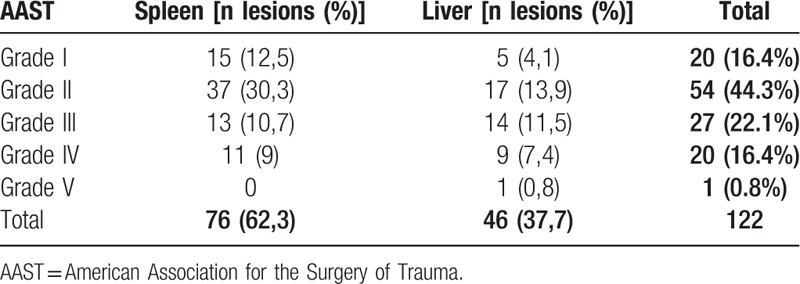
From October 2015 to December 2017 at the Emergency Room of the “Azienda Ospedaliero-Universitaria Ospedali Riuniti di Ancona” Trauma Center a total amount of 85 trauma patients were treated with NOM. In the same period 26 patients were treated with NOM in Terni at the Department of Digestive and Emergency Surgery. Average hospital stay was 12.4 days (7–65 days). The number of lesions for each organ involved, the grading of the lesions according to the American Association for the Surgery of Trauma (AAST) Organ Injury Scale and associated lesions are listed in Tables 1–3, respectively. At the time of hospitalization 39 patients (32%) had lesions also in other organs. In our study, 86 patients belonging to the stable category (77.47%) and 25 patients belonging to the transient responders’ category (22.52%) were included.
Table 1.
Number of lesions for each organ involved.
![]()
Table 3.
Associated lesions.
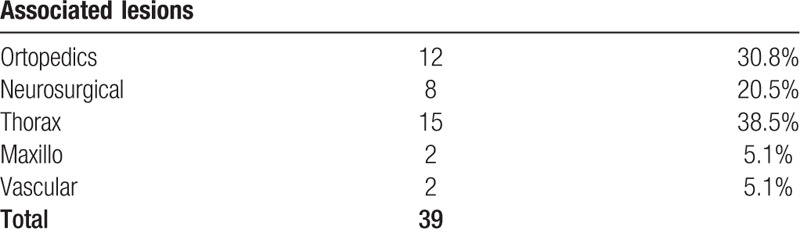
Table 2.
Grade of the lesions according to the AAST Organ Injury Scale.
During the NOM all these patients have been followed by the trauma surgeon. Each patient underwent CT scan at his admission. No contrast pooling was found in 50 pts. (45.04%). Contrast pooling was found in 61 patients (54.95%): in 47 cases (77.04%) it was arterial, in 3 cases (4.91%%) it was venous and in 11 patients(18.04%) it was doubtful. Of these last 11 patients, 6 (54.54%) underwent angiography and 2 (33.33%) underwent angioembolization. In the other 5 patients (45.45%) angiography was not performed. The 3 patients with venous blush detected at CT underwent to angiography and one of these (33.33%) had an embolization procedure. This means that 56 patients (91.80%) underwent angiography and 50 of them (89.28%) had embolization. (Fig. 1)
Figure 1.
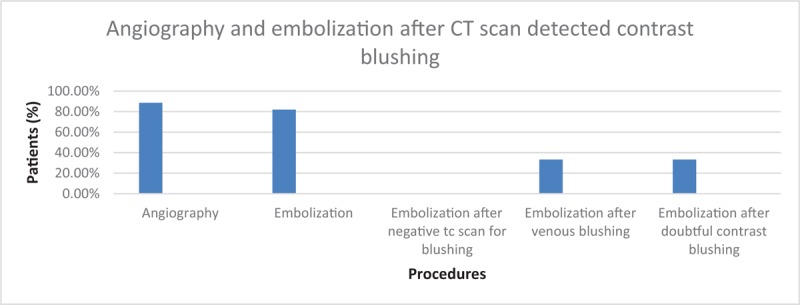
Angiography and embolization after TC scan detected contrast blushing.
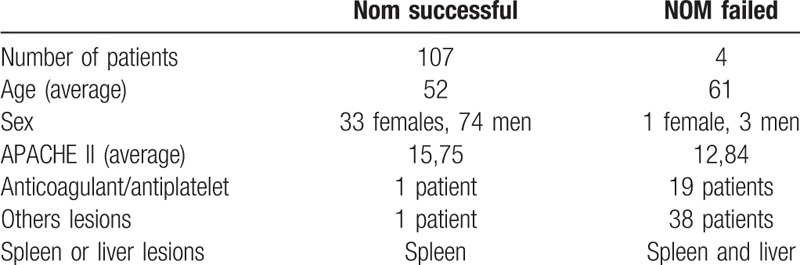
The NOM resulted successful in 107 patients (96.39%). NOM success rate resulted 100% for the liver group and 94.73% for the spleen group (72 of 76 patients). NOM failure occurred in 4 patients (5.26%). Of these, one patient was in the transient responder category and three patients in the stable category. Thus, NOM was successful in 96.52% of stable patients and in 96% of transient responder patients. The average age of the failed NOM group was 61 years and the patients were men and 1 woman, 1 patient was on antiplatelet therapy. In the NOM successful group, the average age was 52 years and there were 33 women and 74 males, 19 patients were on anticoagulants or antiplatelet therapy. The differences between the groups are summarized in the table. (Table 4)
Table 4.
Characteristics of the patients.
In the group of patients where NOM failed, all patients were treated for spleen injuries and all of them received splenectomy: in 1 case to treat pseudoaneurysm (AAST grade of injury II) in 2 cases because of re-bleeding (AAST grade of injury IV) after angioembolization and in the remaining case because the follow up of damaged spleen was stopped in order to operatively manage a concomitant pelvis fracture (AAST grade of injury II). (Table 5)
Table 5.
NOM failure in spleen trauma.

The patient with the pseudoaneurysm was male, 73 years old, on cardioaspirin therapy, he had no other traumas and the Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score was 17. Regarding to patients with re-bleeding: 1 was a 55-year-old male without comorbidity or other traumas and his APACHE II score was 14, while the other was a 63-year-old woman also without comorbidity or other traumas and the APACHE score II was 17. The last patient was a 53-year-old male, he had no co-morbidity but had pelvic fractures and the APACHE II score was 15.
During the monitoring with CEUS we found a blush recurrence after embolization in 3 cases (2.7%) within 30 hours and in 2 cases (1.8%) within 72 hours. The overall complications rate was 5.4% and occurred in 6 patients, 3 of the spleen group and 3 of the liver. Two patients with spleen injuries developed pseudoaneurysms: 1 of them underwent splenectomy during NOM while the other received angioembolization. Furthermore, a patient with splenic lesions due to new bleeding was subjected to splenectomy. Two patients with liver injuries had liver abscess and one 1 had biliary leakage all of them were treated non-operatively. Ninty-two (82.88%) patients completed the follow up period with a medium length of 21 days for the liver (13–32 days) and 36 days for the spleen (1–87). Eight patients were lost at follow up and we missed data for 11 patients. During the follow up period, each patient was controlled by a CEUS scan at 15, 30, and 60 days. If a scan resulted not completely clear, the radiologist could request a further study with CT scan or RMN. In our experience only 7 (7.6%) patients required a further TC/RMN scan.
4. Discussion
The management of traumas of the spleen and of the liver has moved in recent years towards a conservative therapeutic choice defined NOM.[10,11] Indeed numerous scientific evidences have shown the importance of the immunological role played by the spleen and therefore the importance of preserving the spleen itself.[12] It has been demonstrated the importance of the mononuclear-phagocytes system to fight infections caused by encapsulated pathogens that determine the high mortality associated with post-splenectomy sepsis which is present approximately in up to 50% of cases.[13] Unlike liver lesions, splenic lesions can be fatal not only when the patient is hospitalized in the emergency department but also after some time due to the possible delayed rupture of sub-capsular hematomas or of pseudoaneurysms.[10]
The purpose of non-surgical management in trauma is to preserve the function of the spleen and of the liver, reducing the morbidity and mortality surgery related. It is associated with a lower rate of non-therapeutic laparotomies, a lower rate of blood transfusion, reduced overall morbidity and mortality rates and lower hospital costs.[14] In the proper selection of patients for nonoperative management the most important factor is the overall clinical condition of the patient.[10] In Olthof's article, it is emphasized that hemodynamic stability is fundamental for the success of the NOM in selected patients.[15] In the ninth edition of ATLS the patient is defined as “unstable” when: the blood pressure is <90 mmHg and the heart rate >120 bpm, with evidence of skin vasoconstriction (cold, slimy, decrease of the capillary refill), altered level of consciousness and / or shortness of breath.[16] Also the transient responder patients must be considered unstable patients.[10] Transient responder patients are those who show an initial response to the adequate infusion of fluids but then manifest the symptoms either of a persistent loss or of a perfusion deficit. International guidelines agree that patients with peritonitis or those who are hemodynamically unstable with evidence of intraperitoneal hemorrhage should undergo immediate exploratory laparotomy.[17,18]
NOM is nowadays considered the gold standard treatment for the hemodynamically stable patients with blunt splenic and hepatic traumas, in absence of peritonitis, pneumoperitoneum or associated lesions that requires laparotomy.[18–20] The guidelines of the Eastern Association for the Surgery of Trauma (EAST) do not contraindicate conservative treatment in patients with severe splenic injury diagnosed by CT, as long as they are hemodynamically stable.[17]
The classification of the AAST for hepatic and splenic traumas is universally used to express the severity of hepatic or splenic trauma. The single parameter of the severity of the injuries, however, cannot be used to establish whether a patient can be subjected to NOM or not. On the other hand, AAST scales could be very useful for angiography, in fact, according to many studies, patients with grade III splenic lesions should undergo angiography.[18]
Patient age, degree of splenic damage, presence/amount of hemoperitoneum, lesions concomitant to other organs, and presence of splenic vascular anomalies or pseudoaneurysms are all factors that influence the success of NOM and must therefore be considered in establishing the most suitable treatment in patients with traumas of the spleen and of the liver.[21–24]
Scientific literature has recently shown that a conservative treatment is not necessarily contraindicated in major splenic/hepatic traumas, in case of bleeding evidenced on CT, in expired neurological state, in patients over 55 years old, in the presence of associated lesions.[25–28]
Several studies confirm that the integration between CT in the early management of trauma and the NOM, in hemodynamically stable or responder patients, determine a better survival rate and should be the gold standard for both splenic and hepatic traumas.[29–32] It was demonstrated that NOM has many advantages compared to operative management (OM), such as reduction of complications, less need of blood components transfusion, lower mortality rate, and preservation of the immunologic spleen function.[9,17,32]
The present experience and the literature[33–35] prove that with some preexisting required conditions (surgeons experience, available hospital facilities including intensive care unit and 24 hours emergency operating room) the only criteria to choose to treat with NOM is the evaluation of the hemodynamic response of patients.
According to data reported in the literature, the non-surgical management of splenic lesions in adults is performed in about 85% of patients with blunt splenic lesions, with failure rates between 8% and 38%.[18,22,36,37] Analyzing the characteristics of the patients in whom the NOM failed, we did not show significant differences (Table 4).
The AAST Organ Injury Scale was assigned to all splenic and hepatic injuries based on CT scans. In our experience the AAST Organ Injury Scale was useless for the therapeutic decision-making process. We performed an immediate angiography only when the CT scan detect a source of bleeding, whether it is certain or doubtful, in order to control it and solve it. In our experience angioembolization was performed in 50 patients and was successful in 92% of cases. According to numerous studies, this technique applied to both spleen and liver traumas, has now become the most useful treatment for all the different grades of lesions. With an approximately 96% to 98% rate of success, it is also an additional factor on avoiding the failure of NOM.[38–44]
NOM is considered complete after a CEUS scan that confirms the resolution of the previous lesions.
The present study has limitations related to the fact that it is a retrospective study. The data are derived from observations on a group of patients treated in 2 years. Furthermore, although the same protocol was applied, the fact that the study was carried out in 2 different departments caused variations related to the differences in available instruments as well as different competences of health professionals.
5. Conclusions
According to the present results and to our previous experience at Cesena Trauma Center the high success rate seems to prove that a NOM, both for liver and spleen, can be performed in stable or stabilizable patients, regardless the grade of organ lesions. In our protocol the hemodynamic response is the most important criterion to choose the NOM. Unstable patients should not undergo to NOM. Angiography should be performed also in case of doubt in the evaluation of the contrast pooling, in order to eliminate undetected bleeding, often cause of NOM failure.
Author contributions
Conceptualization: Paolo Ruscelli, Alessandro Gemini, Massimiliano Rimini, Claudio Renzi, Roberto Cirocchi, Vito D’Andrea, Amilcare Parisi.
Data curation: Alessandro Gemini, Massimiliano Rimini, Sergio Santella, Roberto Candelari, Enrico Paci, Vittorio Marconi, Claudio Renzi, Rita Commissari, Roberto Cirocchi, Vito D’Andrea, Amilcare Parisi.
Formal analysis: Paolo Ruscelli, Alessandro Gemini, Massimiliano Rimini, Roberto Candelari, Rita Commissari, Roberto Cirocchi, Alberto Santoro, Vito D’Andrea.
Investigation: Paolo Ruscelli, Massimiliano Rimini, Sergio Santella, Roberto Candelari, Marzia Rosati, Enrico Paci, Vittorio Marconi, Claudio Renzi, Rita Commissari, Roberto Cirocchi.
Methodology: Paolo Ruscelli, Alessandro Gemini, Marzia Rosati, Enrico Paci, Claudio Renzi, Roberto Cirocchi, Alberto Santoro, Vito D’Andrea, Amilcare Parisi.
Project administration: Paolo Ruscelli, Alessandro Gemini, Roberto Cirocchi, Vito D’Andrea, Amilcare Parisi.
Resources: Paolo Ruscelli, Claudio Renzi.
Software: Alessandro Gemini.
Supervision: Paolo Ruscelli, Marzia Rosati, Roberto Cirocchi, Alberto Santoro, Vito D’Andrea, Amilcare Parisi.
Validation: Paolo Ruscelli, Massimiliano Rimini, Sergio Santella, Roberto Candelari, Marzia Rosati, Vittorio Marconi, Claudio Renzi, Rita Commissari, Alberto Santoro, Vito D’Andrea, Amilcare Parisi.
Visualization: Alessandro Gemini, Claudio Renzi, Rita Commissari, Roberto Cirocchi.
Writing – original draft: Paolo Ruscelli, Alessandro Gemini, Claudio Renzi, Roberto Cirocchi.
Writing – review & editing: Paolo Ruscelli, Alessandro Gemini, Massimiliano Rimini, Sergio Santella, Roberto Candelari, Enrico Paci, Vittorio Marconi, Claudio Renzi, Roberto Cirocchi, Alberto Santoro, Vito D’Andrea.
Claudio Renzi orcid: 0000-0002-7022-3336.
Footnotes
Abbreviations: AAST = American Association for the Surgery of Trauma, APACHE II = Acute Physiologic Assessment and Chronic Health Evaluation, ATLS = Advanced Trauma Life Support, CEUS = Contrast Enhanced Ultrasound, CT = computed tomography, NOM = non-operative management, OM = operative management.
The authors have no funding and conflicts of interests to disclose.
References
- [1].Miniño AM, Heron MP, Murphy SL, et al. Deaths: final data for 2004. Natl Vital Stat Rep 2007;55:1–19. [PubMed] [Google Scholar]
- [2].Smith J, Caldwell E, D’Amours S, et al. Abdominal trauma: a disease in evolution. ANZ J Surg 2005;75:790–4. [DOI] [PubMed] [Google Scholar]
- [3].Hancock GE, Farquharson AL. Management of splenic injury. JR Army Med Corps 2012;158:288–98. [DOI] [PubMed] [Google Scholar]
- [4].Dupuy DE, Raptopoulos V, Fink MP. Current concepts in splenic trauma. J Intensive Care Med 1995;10:76–90. [DOI] [PubMed] [Google Scholar]
- [5].Leenen LP. Abdominal trauma: from operative to nonoperative management. Injury 2009;40Suppl 4:S62–8. [DOI] [PubMed] [Google Scholar]
- [6].Hommes M, Navsaria PH, Schipper IB, et al. Management of blunt liver trauma in 134 severely injured patients. Injury 2015;46:837e842. [DOI] [PubMed] [Google Scholar]
- [7].Ara R, Khan N, Chakraborty RK, et al. Ultrasound evaluation of traumatic patient in a tertiary level hospital. Mymensingh Med J 2013;22:255e260. [PubMed] [Google Scholar]
- [8].Beuran M, Negoi I, Paun S, et al. – Selective nonoperative management of solid abdominal visceral lesions. Chirurgia (Bucur) 2010;105:317–26. [PubMed] [Google Scholar]
- [9].Ruscelli P, Buccoliero F, Mazzocato S, et al. Blunt hepatic and splenic trauma. A single Center experience using a multidisciplinary protocol. Ann Ital Chir 2017;88: pii: S0003469X17026483. [PubMed] [Google Scholar]
- [10].Coccolini F, Montori G, catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg 2017;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sartorelli KH, Frumiento C, Rogers FB, et al. Non-operative management of hepatic, splenic, and renal injuries in adults with multiple injuries. J Trauma 2000;49:56Y61. [DOI] [PubMed] [Google Scholar]
- [12].Zarzaur Bl, Rozycki GS. An update on nonoperative management of the spleen in adults. Trauma Surg Acute Care Open 2017;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Pathol 2001;54:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Petrone P, Anduaga MFP, Staffolani MJS, et al. Evolution of the treatment of splenic injuries: from surgery to non-operative management. Cir Esp 2017;95:420–7. [DOI] [PubMed] [Google Scholar]
- [15].Olthof DC, Van Der Vlies CH, Joosse P, et al. PYTHIA Collaboration Group. Consensus strategies for the nonoperative management of patients with blunt splenic injury: a Delphi study. J Trauma Acute Care Surg 2013;74:1567–74. [DOI] [PubMed] [Google Scholar]
- [16].American College of Surgeon's Commitee on Trauma. Advanced Trauma Life Support® (ATLS®) Student Manual 9th ed.ed., American College of Surgeon, Chicago; 2012. [Google Scholar]
- [17].Cathey KL, Brady WJ, Jr, Butler K. Blunt splenic trauma: characteristics of patients requiring urgent laparotomy. Am Surg 1998;65:450Y454. [PubMed] [Google Scholar]
- [18].Stassen NA, Bhullar I, Chen JD, et al. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;735 Suppl. 4:S294–300. [DOI] [PubMed] [Google Scholar]
- [19].Marconi FT, Escocia AD, Montego BTP, et al. Nonoperative management of splenic injury grade IV is safe using rigid protocol. Rev Col Bras Cir 2013;40:323–8. [DOI] [PubMed] [Google Scholar]
- [20].Bünyami Ö, Abdullah K, Bülent A, et al. Non-operative management (NOM) of blunt hepatic trauma: 80 cases. Ulus Travma Acil Cerr Derg, March 2014;20:97. [DOI] [PubMed] [Google Scholar]
- [21].Fonseca-Neto OC, Ehrhardt R, Miranda AL. Morbimortality in patients with hepatic trauma. Arq Bras Cir Dig 2013;26:129–32. [DOI] [PubMed] [Google Scholar]
- [22].Harbrecht BG, Ko SH, Watson GA, et al. Angiography for blunt splenic trauma does not improve the success rate of nonoperative management. J Trauma 2007;63:44–9. [DOI] [PubMed] [Google Scholar]
- [23].Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: multi- institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma 2000;49:177–89. [DOI] [PubMed] [Google Scholar]
- [24].Pachter HL, Guth AA, Hofstetter SR, et al. Changing patterns in the management of splenic trauma. Ann Surg 1998;227:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hashemzadeh SH, Hashemzadeh KH, Dehdilani M, et al. Non-operative management of blunt trauma in abdominal solid organ injuries: a prospective study to evaluate the success rate and predictive factors of failure. J Trauma 2010;65:267–74. [PubMed] [Google Scholar]
- [26].Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma 1989;29:1664–6. [PubMed] [Google Scholar]
- [27].Falimirski ME, Provost D. Nonsurgical management of solid abdominal organ injury in patients over 55 years of age. Am Surg 2000;66:631–5. [PubMed] [Google Scholar]
- [28].Sharma OP, Oswanski MF, Singer D, et al. Assessment of non-operative management of blunt spleen and liver trauma. Am Surg 2005;71:379–86. [PubMed] [Google Scholar]
- [29].Nix JA, Costanza M, Daley BJ, et al. Outcomes of the current management of splenic injuries. J Trauma 2001;50:835Y842. [DOI] [PubMed] [Google Scholar]
- [30].Petrowsky H, Raeder S, Zuercher L, et al. A quarter century experience in liver trauma: a plea for early computed tomography and conservative management for all hemodynamically stable patients. World J Surg 2012;36:247–54. [DOI] [PubMed] [Google Scholar]
- [31].Van der Wilden GM, Velmahos GC, Emhoff T, et al. Successful nonoperative management of the most severe blunt liver injuries: a multicenter study of the research consortium of new England centers for trauma. Arch Surg 2012;147:423–8. [DOI] [PubMed] [Google Scholar]
- [32].Beuran M, Gheju I, Venter MD, et al. Non-operative management of splenic trauma. J Med Life 2012;5:47–58. [PMC free article] [PubMed] [Google Scholar]
- [33].Koca B, Topgül K, Yürüker SS, et al. Non-operative treatment approach for blunt splenic injury: is grade the unique criterion? Ulus Travma Acil Cerrahi Derg 2013;19:337–42. [DOI] [PubMed] [Google Scholar]
- [34].Buccoliero F, Ruscelli P. Current trends in polytrauma management. Diagnostic and therapeutic algorithms operational in the Trauma Center of Cesena, Italy. Ann Ital Chir 2010;81:81–93. [PubMed] [Google Scholar]
- [35].Ruscelli P, Buccoliero F. Outcomes in polytrauma: comparison between the results achieved in the Cesena trauma center and in the regional registry of major trauma (RRGT) of Emilia Romagna. Ann Ital Chir 2014;85:6–15. [PubMed] [Google Scholar]
- [36].Velmahos GC, Zacharias N, Emhoff TA, et al. Management of the most severely injured spleen. Arch Surg 2010;145:456Y460. [DOI] [PubMed] [Google Scholar]
- [37].Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care 2013;17:R185doi: 10.1186/cc12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gaspar B, Negoi I, et al. Selective nonoperative management of abdominal injuries in polytrauma patients: a protocol only for experienced trauma centers. Medic J Clin Med 2014;9:168–72. [PMC free article] [PubMed] [Google Scholar]
- [39].Miller P, Chang M, Hoth JJ, et al. Prospective trial of angiography and embolization for all grade III to V blunt splenic injuries: nonoperative management success rate is significantly improved. J Am Coll Surg 2014;218:644–8. [DOI] [PubMed] [Google Scholar]
- [40].Foley PT, Kavnoudias H, Cameron PU. Proximal versus distal splenic artery embolisation for blunt splenic trauma: what is the impact on splenic immune function? Cardiovasc Intervent Radiol 2015;38:1143–51. [DOI] [PubMed] [Google Scholar]
- [41].Jing-Jing R, Liu D, Qing-Hua W. The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: a systematic review and meta-analysis. Mil Med Res 2017;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bhullar IS, Frykberg ER, Siragusa D, et al. Selective angiographic embolization of blunt splenic traumatic injuries in adults decreases failure rate of nonoperative management. J Trauma Acute Care Surg 2012;72:1127–34. [DOI] [PubMed] [Google Scholar]
- [43].Sosada K, Wiewióra M, Piecuch J. Literature review of non-operative management of patients with blunt splenic injury: impact of splenic artery embolization. Wideochir Inne Tech Maloinwazyjne 2014;9:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sivrikoz E, Teixeira PG, Resnick S, et al. Angiointervention: an independent predictor of survival in high-grade blunt liver injuries. Am J Surg 2015;209:742–6. [DOI] [PubMed] [Google Scholar]


