Abstract
Antibiotics during infancy, delivery, and breastfeeding affect the intestinal microbiota in early life and is associated with allergic disease. Gastroenteritis (GE) during infancy also affects intestinal microbiota in early life, however, its relationship to allergic disease has not been investigated.
Data of 45,499 males and 49,430 females, from birth to 5 years of age, were collected from a national database in Taiwan. Subjects were categorized into early GE (GE within 0–6 months) and non-early GE group (no GE within 0–6 months). The rates of asthma (AS), allergic rhinitis (AR), and atopic dermatitis (AD) over 5 years were evaluated and compared between the groups. In patients with AS, AR, and AD, the number of clinical visits and drug prescriptions for the allergic disease was also evaluated to assess the effect of early GE on allergic disease.
After adjusting for the effect of GE in later life and other factors, the rates of AS [OR (odds ratio) 1.54, 95% confidence interval (CI) 1.48–1.60], AR [OR 1.49, 95% CI 1.45–1.54], and AD [OR 1.40, 95% CI 1.33–1.47] were higher in the early GE group than in the non-early GE group. The magnitude of the increase was higher in females than in males. In those with AS, AR, and AD, the number of clinical visits and drug prescriptions was not different between the early GE and non-early GE groups. In children with early GE, good control of GE in the following years lowered the rate of allergic disease.
Early-life GE was associated with increased rates of AS, AR, and AD in later life and this was trend more prominent in females.
Keywords: allergic disease, allergic rhinitis, asthma, atopic dermatitis, gastroenteritis, infant
1. Introduction
Asthma (AS), allergic rhinitis (AR), and atopic dermatitis (AD) are common chronic diseases, which have a negative impact on the quality of life and school performance.[1] These diseases can be controlled but they are not curable. Therefore, understanding the risk factors and preventing the development of allergic diseases is crucial. However, the causes and risk factors for allergic diseases are not fully understood.
Early-life events or diseases, such as perinatal circumstances or early allergen exposure are reported to increase the prevalence of allergic diseases.[2] Additionally, changes in the intestinal microbiota in early life have been reported to affect the development of allergic diseases in later life.[3,4] Early-life environmental factors, such as caesarian section delivery, infant feeding mode, early microbial exposure, and the use of antibiotics in infancy can alter the gut microflora and lead to allergic diseases in later life.[3,4] Moreover, acute gastroenteritis (GE) and intestinal infection have been reported to result in significant changes in the gut microbiota.[5] We speculate that early-life GE might change the gut microbiota in infants and could be associated with allergic diseases. However, evidence to support this hypothesis is scarce.
Using the National Health Insurance Research Database (NHIRD) in Taiwan, we performed a large, longitudinal, population-based research study to establish the relationship between early-life GE and allergic diseases. The effect of early-life GE on the development of allergic disease and subsequent frequencies of clinical visit and medical drug prescription for the allergic disease were evaluated.
2. Methods
2.1. Database
The NHIRD was created by the National Health Research Institute (NHRI) in Taiwan.[6,7] The NHRI randomly sampled a representative database of 1 million subjects in 2010 through systematic sampling and this served as our data source. The database provides information on patient identification, birth date, sex, diagnostic codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9), prescription drugs, medical care facilities, and other items. The institutional review board of Chung Shan Medical University Hospital, Taichung, Taiwan, approved this study (ethics approval number: CS13006).
2.2. Data collection and study flowchart
The study flowchart is presented in Figure 1. Subjects born between 2000 and 2008 were randomly selected from the NHIRD. Their claims data from birth to 5 years old (yo) were analyzed. We excluded children with malignant neoplasm, congenital anomalies of the digestive system, and anyone who had undergone surgery of the gastrointestinal system. The subjects were divided into the following groups: early-life GE (early GE) and non-early life GE (non-early GE). GE was diagnosed according to the ICD9 code including infectious or noninfectious etiology as follows: the diagnosis of noninfectious GE and colitis (ICD9 558.9), intestinal infectious diseases (ICD9 001–009), other intestinal helminthiases (ICD9 127), and unspecified intestinal parasitism (ICD9 129) before 6 months old (mo). Those with GE before 6 mo were included in the early-GE group, and those without were in the non-early GE group.
Figure 1.

Study flowchart.
2.3. Rate of allergic disease between early GE and non-early GE group
Rates of AS, AR, and AD between early GE and non-early GE groups were compared. The criteria for AS, AR, and AD were as follows: at least 2 diagnoses of AR (ICD9 477), AS (ICD9 493), or AD (ICD9 691.8) within 5 yo. Because AS, AR, and AD are chronic diseases, those with disease duration of <180 days were excluded. The mean number of clinical visits for GE from 6 mo to 5 yo were compared between the groups to investigate if early-life GE would increase the frequency of GE in later life or not.
2.4. Clinical visits and drug prescription for allergic disease
Children with AS were divided into the following groups: AS early GE (AS patients with early-life GE) and AS non-early GE (AS patients without early-life GE). Children with AR and AD were also divided into similar groups. Throughout 5 years, the mean number of clinical visits for AS, AR, and AD were compared between the respective groups. Based on whether given prescription during the 5 years, the prescription rate for inhaled and nasal steroid as well as topical drugs tacrolimus/pimecrolimus in AS, AR, and AD subjects were compared between the various groups. These drugs are expensive in Taiwan and are only prescribed if patients have severe symptoms. Anatomical Therapeutic Chemical (ATC) code J R03AK and R03BA were used for inhaled steroids, R01AD was used for nasal steroids, and D11AX14 and D11AX15 were used for tacrolimus and pimecrolimus, respectively.
2.5. Selective groups
In early GE and non-early GE groups, if GE was well-controlled in later life (during 6 mo–5 yo), the subjects were further divided into group 1, early GE with good control, and group 2, non-early GE with good control. In the early GE and non-early GE groups, if there was poor GE control in later life (during 6 mo–5 yo), the subjects were further divided into group 3, early GE with poor control, and group 4, non-early GE with poor control. The criteria for good GE control was: 5 or fewer clinical visits for GE, without emergency room (ER) visit and admission related to GE between 6 mo and 5 yo. The criterion for poor GE control was 5to 15 clinical visits for GE with at least 2 ER visits or 1 admission for GE between 6 mo and 5 yo. AS, AR, and AD rate was compared between these groups. We compared the rates between group 1 and group 2, to assess whether good control of GE in later years decreases the rates of allergic disease in those with early GE.
2.6. Determining confounding factors
The following factors that affect allergic and gastrointestinal diseases were included in our analysis: preterm, low/high birth weight (ICD9 764–766), perinatal infection (infections specific to the perinatal period, ICD9 771), obesity (ICD9 278), and nutritional deficiencies (ICD9). Antibiotic use in infancy[8] and early-life infection[9] or respiratory disease[10] are often accompanied by gastrointestinal disease and are associated with the development of allergic diseases. We included the following diagnoses as possible confounders in our analysis: prescribed antibiotics for systemic use, infectious disease (infections other than intestinal infection, ICD9 010-136 or urinary tract infection, ICD9 599.0), and respiratory disease (ICD9 460–488) before 6 mo. ATC codes J01, J02, J04, and J05 were used for “antibiotics for systemic use”. Despite GE in early life, GE in later life might also affect the development of allergic diseases. The number of GE visits during 6 mo and 5 yo was used as a surrogate for GE conditions in later life.
The socioeconomic status was defined according to the parent's occupation which includes
-
(1)
teacher or public official,
-
(2)
company employee,
-
(3)
other jobs,
-
(4)
peasant or fisherman, and
-
(5)
low-/non-fixed income earner.
Based on Liu's report,[11] the urbanization levels were grouped into seven levels. Level 1 is the most urbanized and level 7 is the least urbanized. Due to low frequency, levels 5 to 7 were grouped together as level 5. All the factors mentioned above were considered as risk factors and were adjusted for.
2.7. Statistical analysis
All analyses were performed using SAS version 9.1 for Windows (SAS Inc., Cary, NC) and PASW Statistics 18 (IBM, Armonk, NY). The Chi-square test was used to compare the rate of AS, AR, and AD between groups. The t test was used to assess differences in the frequency of oral disease and the number of clinical visits between groups. Multivariate logistic regression and multivariate regression analyses were used to adjust for confounding factors (socioeconomic status, urbanization, perinatal infection, preterm, low/high birth weight, obesity, nutritional deficiencies, infections or respiratory disease before 6 mo, use antibiotics before 6 mo, and number of GE visits during 6 mo and 5 yo). A 2-sided P value of <.05 was defined as statistically significant.
3. Results
3.1. Study flowchart and demographic data
The study flowchart and demographic data are presented in Figure 1 and Table 1, respectively. In total, 94,929 subjects were enrolled. Of all subjects, 22,187 (23.4%) met the criteria for early GE. Of all subjects, 15,246 (16.1%) met the AS criteria, 23,731 (25.0%) met the AR criteria, and 8537 (9.0%) met the AD criteria. We found a significant difference in socioeconomic status, urbanization, perinatal infection, nutritional deficiencies, infections or respiratory disease before 6 mo, and use of antibiotics before 6 mo between early GE and non-early GE groups, for both sexes. High respiratory disease rate was noted due to low payment and convenient clinical visits in Taiwan.
Table 1.
Comparison of demographic data and characteristics between early-GE and non-early-GE group, in males and in females, respectively.

3.2. Rate of allergic diseases between early GE and non-early GE group
In total, the rate of AS was 20.8% in early GE and 14.6% in the non-early group, (OR 1.54, 95% CI 1.48–1.60, adjusted P value <.001). The rate of AR was 31.0% in early GE and 23.2% in non-early GE group (OR 1.49, 95% CI 1.45–1.54, adjust P value <.001). The rate of AD was 11.3% in early GE and 8.3% in non-early GE group (OR 1.40, 95% CI 1.33–1.47, adjust P value <.001). The results according to sex are presented in Table 2. The rates of AS, AR, and AD were all higher in the early GE group for both sexes. These results were all statistically significant, after adjusting the number of GE visits during 6 mo and 5 yo and other confounders. Compared to early GE males, females with early GE had higher rates of AS (OR: male 1.35; female 1.71), AR (OR: male 1.29; female 1.67), and AD (OR: male 1.25; female 1.56).
Table 2.
Comparison of the rate of AS, AR, and AD between early GE group vs non-early GE group, in males and females, respectively.

3.3. Frequencies of clinical visits and prescribed medication for each allergic disease
Next, we investigated the effect of early-life GE on the subsequent frequencies of clinical visit and medical drug prescription in the 5 years. The results are presented in Table 3. In AR females with early GE, there was a slight increase in clinic visits for AR compared to females with non-early GE (9.51 AR visit times in AR early GE group and 8.48 AR visit times in AR non-early GE group, P value .049). No difference in the number of drug prescriptions (inhaled, nasal steroid, and tacrolimus/pimecrolimus) and other visit times between groups.
Table 3.
Comparison of the mean clinical visits (over 5 years) for AS, AR, and AD; drugs prescription rates (inhaled steroid for AS, nasal steroid for AR and tacrolimus/pimecrolimus for AD) between early GE vs non-early GE, in males and females, respectively.

3.4. Rate of allergic diseases between selective groups
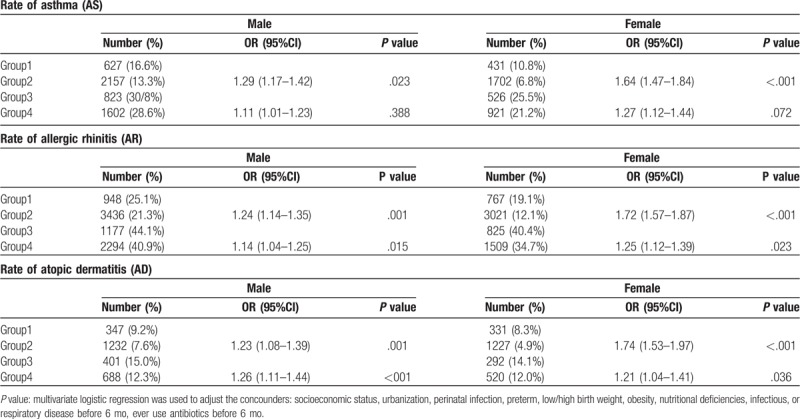
The results are presented in Table 4. Those subjects with poor GE control in later life (group 3 and 4) had dramatically higher rates of allergic disease compared to those with good control in later life (group 1 and 2), regardless of early or non-early GE. A difference was noted in both sexes. In those with good control in later life, children with early GE (group 1) still had higher rates of AS, AR, and AD compared to children without early GE (group 2). The magnitude of the increased rate was higher in females for the 3 allergic diseases (females had higher OR). In those with poor control in later life, children with early GE (group 3) had higher rates of AR and AD as compared to children without early GE (group 4).
Table 4.
Comparison of the rate of AS, AR, and AD between group 1 vs group 2 and group 3 vs group 4, in males and females, respectively.
4. Discussion
Our population-based longitudinal cohort study elucidated the association between early-life GE and increased rates of AS, AR, and AD in later life (5 yo). The magnitude of the increased rate was higher in females for the 3 allergic diseases. However, the association between early-life GE and the number of clinical visits and drug prescriptions for the allergic diseases was weak or absent. The results indicated that early-life GE is associated with the development of allergic disease but not with the subsequent frequencies of clinical visits and medical drug prescription.
Over the past decades, there has been growing evidence supporting the crucial role of intestinal microbiota in the maturation of the immune system in early life and its association with allergic diseases.[3] Diversity[12] and composition[13] of neonate intestinal microbiota were reported to be associated with allergy sensitization, eczema, and AS. From exposure to the mother's vaginal, fecal, or even in utero microbiota, intestinal microbial colonization begins at birth.[14] Early-life environmental factors, such as caesarian section delivery,[15] infant feeding mode,[16] early microbial exposure,[9] and antibiotic use in infancy,[8] have been reported to induce dysbiosis of the intestinal microbial. These environmental factors have also been reported to be associated with allergic disease.[8,9,17,18]
Bacterial,[5] viral,[19] and parasitic[20] gastrointestinal infections may result in significant differences in intestinal microbiota diversity. Hand et al reported that during gastrointestinal infection, tolerance to commensal microbiota is lost and the immunological profile changes as well.[21] Moreover, the non-infectious gastrointestinal disease may also influence the composition of intestinal bacteria.[22] These studies suggest that GE in early life may induce changes in the intestinal microbiota that can contribute to early-life environmental risk factors for the development of the allergic disease. However, there is little evidence supporting the association between early GE and allergic disease. In our present study, we report an association between early-life GE and allergic disease.
We speculated that those with early-life GE may have poor control of GE in later life. Our study found that during later life (6 mo–5 yo), mean clinical visits for GE was higher in the early GE group (10.38 visits) than that of the non-early GE group (4.79 visits). The gastrointestinal disease has been reported to be associated with higher rates of allergic disease in both children and adults.[23,24] Thus, the question posed is whether the increased rate of allergic disease is due to the early effects of GE or poor control of GE in later life. After adjusting the number of GE visits during 6 mo to 5 yo, we could exclude the interference of “later life GE” in allergic disease formation. In selective groups, both group 1 and group 2 had good GE control during 6 mo to 5 yo. Consequently, the effect of increased GE related-suffering in later life could be excluded. We compared the rate of allergic disease between group 1 (early GE) and group 2 (non-early GE). We found the rates of AS, AR, and AD were higher in group 1. Therefore, we concluded that early-life GE could affect the rate of allergic disease in later life, despite the condition of GE in later years.
To the best of our knowledge, only Ahn et al have observed a weak association between acute GE during the first year of life and childhood AS,[25] but the possibility of recall bias limits the conclusion in this cross-sectional study. On the other hand, the condition of GE in later life and its role in the development of AS were not discussed in the study.[25]
Our study has several strengths. First, our study was a retrospective cohort including large sample size and follow-up for 5 years. This large national survey is also representative of the general population in Taiwan. Second, previous studies focused on the prevalence of allergic diseases in relation to different environmental factors. We, additionally, evaluate the influence of the subsequent frequencies of clinical visit and medical drug prescription for allergic disease. Third, antibiotic use in infancy,[8] early-life infectious disease,[9] or early-life respiratory disease[10] is often accompanied by gastrointestinal disease and is associated with allergic disease. These important confounding factors were adjusted in our study, but are seldom adjusted in other studies. Lastly, by the control of GE in later life (comparing the rate of allergic disease between group 1 and group 2), or by adjusting GE visit times in later life, we exclude the effect of late GE on the development of allergic diseases. Thus, we could focus on the effect of early-life GE on the development of allergic diseases.
Our study has some limitations. First, family history, birth history, breastfeeding, and other environmental factors, which are associated with the allergic disease were not evaluated in this study. Second, there was no clinical evaluation or investigation of biological samples, such as intestinal microbiota, to support our hypotheses. Third, the percentages of infectious or non-infectious subjects in early-GE groups cannot be counted accurately in the database study. A case-control study in the future is necessary to find the association between early-infectious-GE, early-noninfectious-GE, and allergic disease.
5. Conclusions
Our results demonstrate that GE in early-life was associated with increased risk of subsequent development of AS, AR, and AD. This increased rate was higher in females, compared to males. In children with early-life GE, good control of GE in later life is associated with decreased development of AS, AR, and AD, compared to those with poor control. However, the development of allergic disease was still higher than those without early-life GE and good control of GE in later life.
Preventing the development of allergic disease before their development is essential. These results suggest that preventing GE in infancy might be a novel method to prevent the development of allergic diseases. Prevention of antibiotic abuse when a pediatrician treats GE would be a detailed plan to reduce the development of allergic diseases. However, further biological research, clinical data, and case-control studies are necessary to fully understand the association.
Author contributions
Conceptualization: Min-Sho Ku, Hai-Lun Sun.
Data curation: Min-Sho Ku, Hui-Hsien Pan.
Investigation: Min-Sho Ku, Hui-Hsien Pan.
Methodology: Min-Sho Ku, Hui-Hsien Pan, Hai-Lun Sun.
Software: Min-Sho Ku, Hui-Hsien Pan, Hai-Lun Sun.
Supervision: Min-Sho Ku, Ko-Huang Lue.
Validation: Ko-Huang Lue.
Visualization: Ko-Huang Lue.
Writing – original draft: Hui-Hsien Pan.
Writing – review & editing: Min-Sho Ku, Ko-Huang Lue.
Footnotes
Abbreviations: AD = atopic dermatitis, AR = allergic rhinitis, AS = asthma, ATC = anatomical therapeutic chemical, CI = confidence interval, GE = gastroenteritis, ICD9 = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, OR = odds ratio.
The authors have no conflicts of interests to disclose.
References
- [1].Mario Sánchez-Borges, Bryan L, Martin, et al. The importance of allergic disease in public health: an iCAALL statement. World Allergy Organ J 2018;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bernsen RM, van der Wouden JC, Nagelkerke NJ, et al. Early life circumstances and atopic disorders in childhood. Clin Exp Allergy 2006;36:858–65. [DOI] [PubMed] [Google Scholar]
- [3].Zimmermann P, Messina N, Mohn WW, et al. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol 2019;143:467–85. [DOI] [PubMed] [Google Scholar]
- [4].Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol 2017;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mathew S, Smatti MK, Al Ansari K, et al. Mixed viral-bacterial infections and their effects on gut microbiota and clinical illnesses in children. Sci Rep 2019;9:86529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Department of health, Executive Yuan. National Health Insurance Evaluation and Projections. Taiwan: DoH, 1998: 1. [Google Scholar]
- [7].TM C. Taiwan's new national health insurance program: genesis and experience so far. Health Aff 2003;22:61–76. [DOI] [PubMed] [Google Scholar]
- [8].Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J 2011;38:295–302. [DOI] [PubMed] [Google Scholar]
- [9].Hill DA, Siracusa MC, Abt MC, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nature Med 2012;18:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol 2013;13:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large-scale health interview survey. J Health Manage 2006;4:1–22. [Google Scholar]
- [12].Abrahamsson TR, Jakobsson HE, Andersson AF, et al. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 2012;129:434–40. [DOI] [PubMed] [Google Scholar]
- [13].van Nimwegen FA, Penders J, Stobberingh EE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol 2011;128:948–55. [DOI] [PubMed] [Google Scholar]
- [14].Schaub B, Liu J, Höppler S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009;123:774–5. [DOI] [PubMed] [Google Scholar]
- [15].Stokholm J, Thorsen J, Chawes BL, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol 2016;138:881–9. [DOI] [PubMed] [Google Scholar]
- [16].Arrieta MC, Stiemsma LT, Amenyogbe N, et al. The intestinal microbiome in early life: health and disease. Front Immunol Front 2014;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Renz-Polster H, David MR, Buist AS, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy 2006;35:1466–72. [DOI] [PubMed] [Google Scholar]
- [18].van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003;58:833–43. [DOI] [PubMed] [Google Scholar]
- [19].Chen SY, Tsai CN, Lee YS, et al. Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Sci Rep 2017;11:46130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toro-Londono MA, Bedoya-Urrego K, Garcia-Montoya GM, et al. Intestinal parasitic infection alters bacterial gut microbiota in children. Peer J 2019;7:e6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hand TW, Dos Santos LM, Bouladoux N, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 2012;337:1553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spyros Vrakas, Konstantinos C, Mountzouris, et al. Intestinal bacteria composition and translocation of bacteria in inflammatory bowel disease. PLoS One 2017;12:e0170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ho SW, Lin CP, Ku MS. The impact of allergic rhinitis on gastrointestinal disorders among young adults. J Eval Clin Pract 2019;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [24].Powell N, Huntley B, Beech T, et al. Increased prevalence of gastrointestinal symptoms in patients with allergic disease. Postgrad Med J 2007;83:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahn KM, Lee MS, Hong SJ, et al. Fever, use of antibiotics, and acute gastroenteritis during infancy as risk factors for the development of asthma in Korean school-age children. J Asthma 2005;42:745–50. [DOI] [PubMed] [Google Scholar]


