Abstract
Objectives:
To measure the prevalence of asymptomatic bacteriuria (ASB) in persons with spinal cord injury (SCI) at the time of their annual examination and to examine the effect on urine testing during the annual examination on subsequent antibiotic use.
Design:
Retrospective cohort study.
Setting:
A major SCI center.
Participants:
Veterans (N=393) with SCI seen for an outpatient annual evaluation in 2012 or 2013.
Interventions:
Not applicable.
Main Outcome Measures:
Antibiotic use for bacteriuria within 7 days of the annual evaluation encounter.
Results:
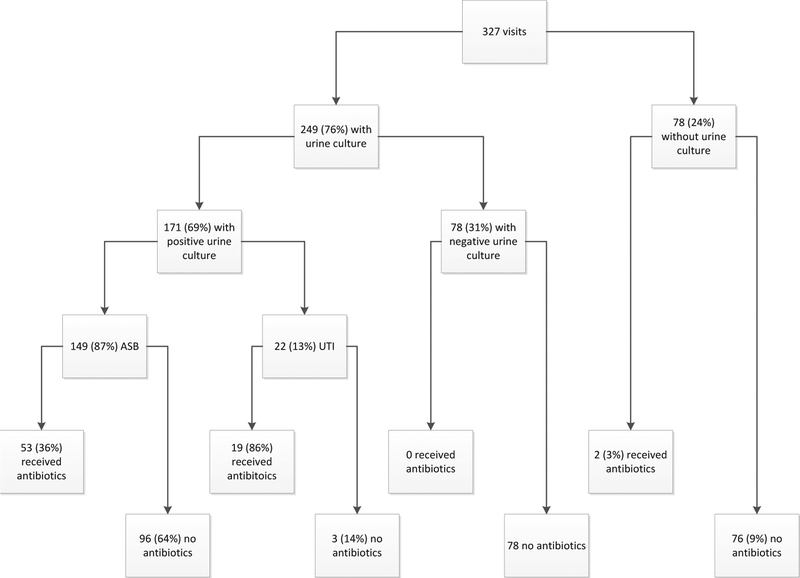
There were 327 clinic visits that met inclusion criteria; of these 327 veterans, 249 had a urine culture performed. A total of 171 urine cultures (69%) were positive for bacteria, of which 22 (13%) represented urinary tract infection (UTI) cases and 149 (87%) were ASB cases. More than a third of the ASB cases (n=53 [36%]) were treated with antibiotics. None of the 78 visits with negative urine cultures received antibiotics to treat the UTI; thus, a positive urine culture alone was associated with antibiotic use (P<.01). Factors predicting antibiotic use were higher age, nitrite presence on urinalysis, and urease-producing organism on culture media. When comparing bladder management strategies, indwelling catheterization was found to be associated with higher levels of pyuria and hematuria than did spontaneous voiding or intermittent catheterization (P<.01).
Conclusions:
Two-thirds of the urine cultures of persons with SCI presenting for their annual examination were positive. Most of the positive cultures represented ASB cases, and more than a third of these were treated with antibiotics. A better understanding of the mandate for urine testing at the annual examination and the outcomes of this practice is an important first step in developing antibiotic stewardship for UTI in persons with SCI.
Keywords: Antimicrobial stewardship, Rehabilitation, Spinal cord injuries, Urinary tract infections
Urinary tract infections (UTIs) and other genitourinary complaints account for 30% of emergency department visits and hospitalizations among persons with spinal cord injury (SCI) in the United States and are the most common causes of health care utilization in this population.1 Bacteriuria, either asymptomatic (ASB) or symptomatic, is common after SCI. Many patients with SCI have a neurogenic bladder and need a bladder management strategy, either with indwelling (intraurethral or suprapubic) catheters or with intermittent catheterization programs. Adequate bladder drainage (often via chronic indwelling or intermittent catheterization) is vital to promote safe bladder pressures to protect kidney function, as well as to prevent bladder distention, but can predispose patients to infections.2 Sepsis arising from urinary tract organisms getting into the bloodstream is one of the leading causes of death in patients with SCI.3,4
Although the appropriate treatment of UTI in this population is clearly important, the potential for the overdiagnosis of UTI in patients with SCI is high because many patients with SCI have bladders colonized with urinary pathogens. It was estimated that 30% to 90% of patients with SCI had ASB, depending on the bladder management strategy used.5 Therefore, if urine is tested in a person with SCI, there is a high likelihood that it will yield a positive result. ASB does not require antibiotic treatment except in pregnancy and before urologic procedures.5,6 The propensity to treat a positive urinalysis or urine culture, even in persons without any symptoms or any UTI symptoms, is well documented and can lead to overdiagnosis of UTI and use of unnecessary antibiotics.7–9 Frequent courses of antibiotics put persons with SCI at risk of acquiring resistant pathogens, and it has been shown that the proportions of these pathogens are indeed high in populations with SCI.10–12
Evidence-based clinical practice guidelines published by the Infectious Diseases Society of America (IDSA) recommend against obtaining a urine for a urinalysis and/or urine culture in asymptomatic patients and recommend against treating ASB; these guidelines are endorsed by the U.S. Preventive Services Task Force.5,13 In the Veterans Health Administration (VHA) system, care for persons with SCI is driven by VHA directive 1176.01.14 A large component of this directive describes the components of the annual examination, which include a comprehensive medical evaluation, including laboratory and radiological testing, as well as functional and psychosocial evaluations. Currently, VHA directive 1176.01 recommends urine culture and urinalysis as part of this evaluation, regardless of the presence of UTI symptoms. This is essentially screening for ASB, which is contrary to other guidelines. Expert opinion that led to this recommendation in persons with SCI includes the common practice of screening for and preemptively treating ASB caused by stone-forming organisms such as Proteus species, but the outcomes of this practice are not known.
In addition to the above information, we need more information about how bacteriuria is tested and managed during the SCI annual examination as well as about the downstream effect of this management. Herein we describe in a major VHA SCI center the prevalence of ASB in veterans with SCI presenting for their annual examination as well as the rate of subsequent antibiotic treatment for ASB in this cohort. We also examined predictors of antibiotic use for ASB. We hypothesized that there would be no difference in the clinical outcomes between those treated for ASB and those who were not. Secondarily, we described how urinalysis and urine culture results obtained during the SCI annual evaluation varied by bladder management strategy.
Methods
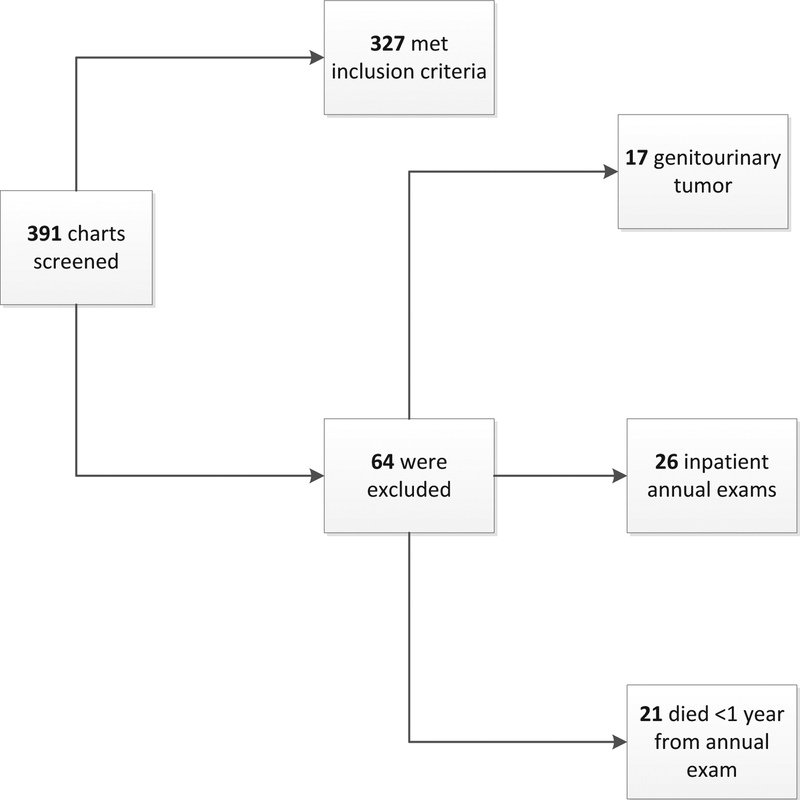
This was a retrospective cohort study design. Our cohort consisted of veterans seen for the SCI annual evaluation in 2012 or 2013 at the Michael E. DeBakey Veterans Affairs Medical Center as identified by the local Management of Information Outcomes Coordinator. Because many patients were seen multiple times per year, only the first qualifying visit for each patient was included. The electronic medical record (EMR) of each visit was screened for the following inclusion criteria: (1) annual evaluation was completed in the outpatient setting and (2) veterans survived for >1 year after the annual evaluation encounter. The visit was excluded if (1) their annual evaluation was completed in the inpatient setting (and therefore more likely to be associated with acute illness); (2) there was a history of genitourinary tract tumors; and (3) the veteran died <1 year after the annual evaluation encounter.
Each eligible visit was then evaluated by chart review by a postdoctoral research fellow. Basic demographic information was extracted, including age, neurological level of injury using the American Spinal Injury Association Impairment Scale (AIS) classification, sex, ethnicity, etiology of injury, whether the patient resided in the community (which includes private residencies with or without home health assistance, personal care homes, or assisted living facilities), and bladder management strategy. With regard to neurological level of injury, the “not classified” group consisted of mostly chronic myelopathy cases that were not given an AIS classification. With regard to bladder management strategy, categories included spontaneous voiding, indwelling (intraurethral and suprapubic) catheters, intermittent catheterization, and other. The “other” category is a heterogeneous group that includes external catheter use and bladder augmentation cases.
Each visit was then classified as having ASB, UTI, neither, or indeterminate using a validated algorithm developed by our team.8 The EMR was examined for signs and symptoms of UTI as delineated by the IDSA clinical practice guidelines. Urinalysis and urine culture results were also retrieved from laboratory data available in the EMR. The first decision point was whether a patient had signs and symptoms of UTI. The IDSA guidelines have different thresholds for positivity for ASB and UTI. If no symptoms were present, the urine culture results were evaluated. A “positive” culture for ASB is defined as the presence of at least 105 organisms.5 A “positive” culture for UTI is defined as the presence of >102 organisms.2 For our study, if the patient had signs and symptoms of a UTI, a positive culture was defined as the presence of at least 102 organisms, and if the patient was asymptomatic, the threshold for positivity was 105 organisms. The type of each organism grown on urine culture was also recorded to examine the incidence of urease-producing organisms, such as Proteus species.
The primary outcome was documentation of antibiotics prescribed for the UTI within 7 days of the annual evaluation encounter on the basis of pharmacy data in the EMR and review of clinical notes. Health care utilization within 60 days after the annual evaluation was also recorded. These included number of emergency department visits, hospitalizations, subsequent urine cultures obtained, subsequent diagnosis of UTI (using the same method as described above), subsequent diagnosis of Clostridium difficile infection (based on laboratory data), and/or diagnoses of urologic complications (which included catheter malfunction, genitourinary stones, and hematuria).
To test for differences in outcomes between groups, the chi-square test was used for nominal variables and the Kruskal-Wallis test was used for continuous variables. Univariate and multivariate logistic regression models were used to identify predictors of antibiotic use in cases classified as ASB cases. A P value of <.05 was defined as statistically significant. This retrospective chart review research protocol was approved by the Baylor College of Medicine Institutional Review Board under a waiver of consent.
Results
Figure 1 shows participants in the study on the basis of inclusion and exclusion criteria. A total of 391 outpatient annual examinations occurred between January 1, 2012 and December 31, 2013; 327 were included and 64 were excluded on the basis of the criteria described above.
Fig 1.
Visual diagram of the inclusion and exclusion of patients.
Table 1 summarizes participant demographic characteristics grouped by neurological level of injury. Participants most commonly had AIS grade D SCI, were men, and were white. Those with tetraplegia and AIS grades A to C were significantly younger than the remainder of the cohort (P<.01). Most participants lived in the community. There was a significant difference in etiology across the groups, with the highest percentage of motor vehicle collision(41.5%) in patients with paraplegia (P =.02). There were no statistically significant differences in fall etiology between the groups (P =.24). Patients with tetraplegia were more likely to have indwelling catheters than were those with other levels of injury (P<.01).
Table 1.
Patient demographic characteristics (N = 327)
| Characteristic | Tetraplegia and AIS Grades A–C (n = 71) |
Paraplegia and AIS Grades A–C (n = 118) |
All AIS Grade D (n = 123) |
Not Classified (n = 15) |
P* |
|---|---|---|---|---|---|
| Age (y) | 54 (21–77) | 60 (26–81) | 62 (30–84) | 62 (40–76) | <.01 |
| Sex: male | 69 (97.2) | 110 (93.2) | 117 (95.9) | 14 (93.3) | .61 |
| Ethnicity | .96 | ||||
| White | 42 (59.2) | 75 (63.6) | 71 (57.7) | 9(60) | |
| Non-Latino black | 26 (36.6) | 37 (31.4) | 47 (38.2) | 5 (33.3) | |
| Other | 3 (4.2) | 6 (5.1) | 5 (4.1) | 1 (6.7) | |
| Etiology of injury | <.01 | ||||
| Motor vehicle collision | 28 (39.4) | 49 (41.5) | 31 (25.2) | 1 (6.7) | |
| Fall | 17 (23.9) | 16 (13.6) | 27 (22.0) | 1 (6.7) | |
| Other | 26 (36.3) | 53 (44.9) | 65 (52.8) | 13 (86.7) | |
| Community dweller | 63 (88.7) | 116 (98.3) | 120 (97.6) | 15 (100) | <.01 |
| Bladder management | <.01 | ||||
| Spontaneous void | 6 (8.5) | 6 (5.1) | 61 (49.6) | 12 (80.0) | |
| Indwelling catheter | 29 (40.8) | 20 (16.9) | 7 (5.7) | 1 (6.7) | |
| Intermittent catheter | 22 (31.0) | 72 (61.0) | 44 (35.8) | 2 (13.3) | |
| Other | 14 (19.7) | 20 (16.9) | 11 (8.9) | 0(0) |
NOTE. Values are median (interquartile range) or n (%).
The P value refers to a χ2 comparison of the 4 categories of SCI classification, such that a significant P value means that at least 1 category differed significantly from the others.
Classification of cases, predictors of antibiotic use, and subsequent health care utilization
Figure 2 shows the classifications of 327 cases. Urine cultures were performed in 249 total cases (76%). Furthermore, 149 of 171 positive cultures (87%) were subsequently classified as representing ASB, and 53 of those cultures representing ASB (36%) were treated with antibiotics. None of the cases with negative urine cultures were treated with antibiotics; thus, a positive urine culture is significantly associated with antibiotic use, regardless of whether signs and symptoms of infection are present (P<.01).
Fig 2.
Visual diagram of the classification of cases and subsequent antibiotic use.
Table 2 presents the results of univariate and multivariate logistic regression analyses identifying predictors of antibiotic use for ASB within 7 days of the annual examination. Age, leukocyte esterase presence on urinalysis, nitrite presence on urinalysis, and urease-producing organism on culture were all significant in univariate analyses, and so they were included in the multivariate regression model. Age, nitrite presence on urinalysis, and urease-producing organism on culture all remained significant predictors of receiving antibiotics for ASB in multivariate analyses (P<.01 for all).
Table 2.
Predictors of antibiotic use for ASB
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Age | 1.06 (1.02–1.09) | <.01* | 1.05 (1.01–1.09) | <.01† |
| Sex | 1.09 (0.25–4.76) | .91 | NT | |
| Ethnicity | 0.78 (0.39–1.57) | .41 | NT | |
| AIS classification | 1.14 (0.50–2.58) | .76 | NT | |
| Bladder management | 2.31 (0.72–7.42) | .27 | NT | |
| Urine WBCs | 1.00 (1.00–1.01) | .10 | NT | |
| LE positive | 8.71 (1.11–68.22) | .04* | 4.88 (0.57–41.83) | .15 |
| Nitrite positive | 2.44 (1.22–4.88) | .01* | 2.78 (1.31–5.90) | <.01† |
| UPO on culture | 3.42 (1.69–6.89) | <.01* | 2.92 (1.38–6.18) | <.01† |
Abbreviations: CI, confidence interval; LE, leukocyte esterase; NT, not tested; OR, odds ratio; UPO, urease-producing organism (such as Proteus species).
Significant in univariate analysis.
Significant in multivariate analysis.
With regard to health care utilization 60 days after the annual evaluation, incidences were low overall, with no cases of C difficile infection captured in our cohort. There were no statistically significant differences in the number of emergency visits, hospitalizations, subsequent urine cultures obtained, subsequent diagnosis of UTI, or diagnoses of urologic complications between those who received antibiotics for ASB and those who did not.
Urinalysis and urine culture results grouped by bladder management strategy used and case classification
Tables 3 and 4 present urinalysis and urine culture results grouped by type of bladder management strategy used. There was a significant difference in the presence of pyuria, microhematuria, and nitrite on urinalysis between all 4 groups. Bladder management strategy was associated with the risk of having a urine culture obtained, with the odds ratio of having an indwelling catheter compared with spontaneous voiding being 4.8 (95% confidence interval, 1.74−13.5). In a more granular analysis, there was a significant difference in leukocyte esterase results between those who had indwelling catheters and those who performed intermittent catheterization, with nearly all those with indwelling catheters having a positive result (P<.01). Those with indwelling catheters also had higher levels of microhematuria than did those who performed intermittent catheterization (P<.01). However, with regard to nitrite levels, there was no significant difference between those who had indwelling catheters and those who performed intermittent catheterization; (P =.07). Urine cultures collected from indwelling catheters were more likely to be classified as positive cultures than 51/52 cultures (98%, P<0.01). There was no difference in the incidence of urease-producing organisms on culture between the groups.
Table 3.
Urinalysis results grouped by bladder management strategy used
| Variable | Spontaneous Void (n = 85) |
Intermittent Catheter (n = 140) |
Indwelling Catheter (n = 57) |
Other (n=45) | P |
|---|---|---|---|---|---|
| Urinalysis obtained | 67/85 (78.8) | 113/140 (80.7) | 54/57 (94.7) | 37/45 (82.2) | .69 |
| WBCs | 0 (0–2) | 11 (2–45) | 48 (10–122) | 20 (2–77) | <.01* |
| RBCs | 1 (0–2) | 1 (0–4) | 6 (1–22) | 1 (0–10) | <.01* |
| Nitrite positive | 8/67 (11.9) | 38/113 (33.6) | 26/54 (48.1) | 13/37 (35.1) | <.01* |
| LE positive | 22/67 (32.8) | 87/113 (77.0) | 53/54 (98.1) | 32/37 (86.5) | <.01* |
NOTE. Values are median (interquartile range) or n (%).
Abbreviations: LE, leukocyte esterase; RBC, red blood cell; WBC, white blood cell.
The P value refers to a χ2 comparison of the 4 categories of SCI classification, such that a significant P value means that at least 1 category differed significantly from the others.
Table 4.
Urine culture results grouped by bladder management strategy used
| Variable | Spontaneous Void (n=85) |
Intermittent Catheter (n=140) |
Indwelling Catheter (n = 57) |
Other (n =45) | P |
|---|---|---|---|---|---|
| Cultures obtained | 58/85 (68.2) | 103/140 (73.6) | 52/57 (91.2) | 36/45 (80.0) | .01* |
| Positive cultures | 21/58 (36.2) | 70/103 (68.0) | 51/52 (98.1) | 29/36 (80.6) | <.01* |
| UPO on culture | 9/21 (42.9) | 26/70 (37.1) | 28/51 (54.9) | 13/29 (44.8) | .28 |
NOTE. Values are n/total n (%).
Abbreviation: UPO, urease-producing organism (such as Proteus species).
The P value refers to a χ2 comparison of the 4 categories of SCI classification, such that a significant P value means that at least 1 category differed significantly from the others.
Table 5 presents urinalysis and culture results grouped by case classification (ASB or UTI). The median levels of pyuria and microhematuria were significantly different between the groups; however, the remainder of the urinalysis components and the incidence of urease-producing organisms on culture were not significantly different between ASB and UTI cases.
Table 5.
Urinalysis and urine culture results in ASB and UTI cases
| Variable | ASB Cases (n=149) |
UTI Cases (n = 22) |
P |
|---|---|---|---|
| WBCs | 25 (5–70) | 61 (27–128) | <.01* |
| RBCs | 2 (0–7) | 6 (2–27) | .01* |
| Nitrite positive | 70 (47) | 10 (46) | .89 |
| LE positive | 133 (90) | 21 (96) | .36 |
| UPO on culture | 67 (45) | 9(41) | .72 |
NOTE. Values are median (interquartile range) or n (%).
Abbreviations: LE, leukocyte esterase; RBC, red blood cell; UPO, urease-producing organism (such as Proteus species); WBC, white blood cell.
The P value refers to a χ2 comparison of the 4 categories of SCI classification, such that a significant P value means that at least 1 category differed significantly from the others.
Discussion
Our results suggest that most positive urine cultures obtained during the SCI annual evaluation represent ASB cases and that more than a third of these ASB cases received antibiotics, contrary to the IDSA guidelines. Factors predicting antibiotic use were higher age, nitrite presence on urinalysis, and urease-producing organism on culture. With regard to urinalysis results, there were significant differences in the level of pyuria, level of micro-hematuria, and positive nitrate and leukocyte esterase results, depending on the type of bladder management strategy used, with patients with indwelling catheters having the highest proportion of these results. However, the ranges of pyuria and hematuria levels overlap between the ASB and UTI groups such that a threshold cannot be used to distinguish these 2 clinical conditions. Because a positive nitrate result on urinalysis was an independent predictor of antibiotic use for ASB, it suggests that providers are using urinalysis as a guide to initiate antibiotics before culture results.
One rationale for treating ASB in persons with SCI is to detect and prophylactically treat urease-producing organisms such as Proteus species.15 These organisms promote genitourinary stone formation, which is a major source of morbidity in this population.15 Our results suggest that the detection of urease-producing organisms was indeed a strong driving force of antibiotic use for ASB after the SCI annual examination. However, there is convincing SCI-specific evidence against screening for or treating ASB in persons with SCI. When ASB was treated in a cohort of catheter-free, primarily male, patients with SCI, 93% of patients were bacteriuric again within 30 days of a 7- to 14-day course of antibiotics, with the reinfecting strains showing increased antibiotic resistance.16 Lewis et al17 prospectively observed 52 persons with SCI for 4 to 26 weeks with weekly urine cultures; 78% of the cultures were positive, but only 6 episodes of symptomatic UTI were recorded. A small randomized placebo-controlled trial6 found the incidences of symptomatic UTI and recurrence of bacteriuria to be similar in those receiving prophylactic antibiotic treatment and those receiving the placebo. A prospective randomized trial18 of antibiotic treatment or no treatment of ASB enrolled 50 patients who performed intermittent catheterization and reported a similar incidence of symptomatic UTI during a mean follow-up period of 50 days, irrespective of whether prophylactic antibiotics were given.
Those with indwelling catheters were more likely to have a urine culture sent than were those who voided spontaneously or performed intermittent catheterization; this is likely a practical matter, in that urine from an indwelling catheter is much more readily obtained by clinic staff rather than relying on the patient to produce a sample on their own.
Study limitations
Our study has several limitations. First, it is a retrospective chart review study; identification of signs and symptoms of UTI were limited to the documentation available in the EMR. Second, our data are several years old and treatment patterns may have changed over this time. Third, this study describes the practice pattern of a single site, albeit one of the largest SCI centers in the United States. It is possible that some instances of UTI and health care outcomes were missed, because all patients were not followed up locally. This type of study cannot provide insight into the beliefs and attitudes of providers or patients surrounding urine testing and treatment at the annual examination.
Conclusions
This study shows that unnecessary antibiotic treatment of ASB is common in veterans presenting for their SCI annual evaluation. Antibiotic stewardship for bacteriuria in persons with SCI presents unique challenges but is vitally important to protect the health of this population. Antibiotic stewardship has become a national and even global priority.19–21 A better understanding of the mandate for urine testing at the annual examination and the outcomes of this practice is an important first step in developing antibiotic stewardship for UTI in persons with SCI.
Acknowledgments
Supported in part by the Department of Veterans Affairs, Veterans Health Administration Office of Research and Development, and the Center for Innovations in Quality, Effectiveness and Safety (grant no. CIN 13–413). Also supported in part by the National Institutes of Health (grant no. 5G12MD007605).
Disclosures: L. Grigoryan receives funding support from Zambon Pharmaceuticals. The other authors have nothing to disclose.
List of abbreviations:
- AIS
American Spinal Injury Association Impairment Scale
- ASB
asymptomatic bacteriuria
- EMR
electronic medical record
- IDSA
Infectious Diseases Society of America
- SCI
spinal cord injury
- UTI
urinary tract infection
- VHA
Veterans Health Administration
Footnotes
Presented in part at the 2016 Society for Hospital Epidemiology of America Conference, May 18–21st, 2016, Atlanta, GA, and 2016 American Academy of Physical Medicine and Rehabilitation Annual Assembly, October 20–23, 2016, New Orleans, LA.
References
- 1.Skelton F, Hoffman JM, Reyes M, Burns SP. Examining health-care utilization in the first year following spinal cord injury. J Spinal Cord Med 2015;38:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50: 625–63. [DOI] [PubMed] [Google Scholar]
- 3.NSCISC National Spinal Cord Injury Statistical Center, The University of Alabama at Birmingham. 2012 Annual Statistical Report − Public Version. Available at: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf. Accessed January 8, 2018.
- 4.Rabadi MH, Mayanna SK, Vincent AS. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord 2013;51:784–8. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–54. [DOI] [PubMed] [Google Scholar]
- 6.Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987;138:336–40. [DOI] [PubMed] [Google Scholar]
- 7.Naik AD, Skelton F, Amspoker AB, Glasgow RA, Trautner BW. A fast and frugal algorithm to strengthen diagnosis and treatment decisions for catheter-associated bacteriuria. PLoS One 2017;12:e0174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trautner BW, Bhimani RD, Amspoker AB, et al. Development and validation of an algorithm to recalibrate mental models and reduce diagnostic errors associated with catheter-associated bacteriuria. BMC Med Inform Decis Mak 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: identifying provider barriers to evidence-based care. Am J Infect Control 2014;42: 653–8. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick MA, Suda KJ, Safdar N, et al. Unique risks and clinical outcomes associated with extended-spectrum b-lactamase enterobacteriaceae in veterans with spinal cord injury or disorder: a case-case-control study. Infect Control Hosp Epidemiol 2016;37:768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick MA, Suda KJ, Safdar N, et al. Changes in bacterial epidemiology and antibiotic resistance among veterans with spinal cord injury/disorder over the past 9 years. J Spinal Cord Med 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suda KJ, Patel UC, Sabzwari R, et al. Bacterial susceptibility patterns in patients with spinal cord injury and disorder (SCI/D): an opportunity for customized stewardship tools. Spinal Cord 2016;54:1001–9. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: reaffirmation recommendation statement. Am Fam Physician 2010;81:505. [PubMed] [Google Scholar]
- 14.U.S. Department of Veterans Affairs. VHA handbook 1176.01: spinal cord injury and disorders (SCI/D) system of care. Available at: http://www.va.gov/vhapublications/ViewPublication.asp?pub_IDZ2365. Accessed January 8, 2018.
- 15.Hung EW, Darouiche RO, Trautner BW. Proteus bacteriuria is associated withsignificantmorbidityinspinalcordinjury.SpinalCord 2007;45:616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waites KB, Canupp KC, DeVivo MJ. Eradication of urinary tract infection following spinal cord injury. Paraplegia 1993;31:645–52. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RI, Carrion HM, Lockhart JL, Politano VA. Significance of asymptomatic bacteriuria in neurogenic bladder disease. Urology 1984;23:343–7. [DOI] [PubMed] [Google Scholar]
- 18.Maynard FM, Diokno AC. Urinary infection and complications during clean intermittent catheterization following spinal cord injury. J Urol 1984;132:943–6. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Vital signs Making health care safer: stop spread of antibiotic resistance. Atlanta: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 20.The White House Office Washington. National action plan for combating antibiotic-resistant bacteria. Available at: https://www.hsdl.org/?view&did=763992. Accessed December 8, 2016.
- 21.High Level Meeting on Antimicrobial Resistance. Available at: https://www.un.org/pga/71/wp-content/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108E.pdf. Accessed January 8, 2018.