Abstract
Throughout life, mammary tissue is strongly influenced by hormones. Scientists have hypothesized that synthetic chemicals with hormonal activities could disrupt mammary gland development and contribute to breast diseases and dysfunction. Bisphenol S (BPS) is an estrogenic compound used in many consumer products. In this study, CD-1 mice were exposed to BPS (2 or 200 μg/kg/day) during pregnancy and lactation. Mice exposed to 0.01 or 1 μg/kg/day ethinyl estradiol (EE2), a pharmaceutical estrogen, were also evaluated. Mammary glands from female offspring were collected prior to the onset of puberty, during puberty, and in early adulthood. Growth parameters, histopathology, cell proliferation and expression of hormone receptors were quantified. Our evaluations revealed age- and dose-specific effects of BPS that were different from the effects of EE2, and distinct from the effects of BPA that have been reported previously. These assessments suggest that individual xenoestrogens may have unique effects on this sensitive tissue.
Keywords: endocrine disruptor, terminal end buds, whole mount, estrogen receptor, morphology, contraceptive
1. Introduction
In the mouse, the mammary gland begins to develop at embryonic day 10 as the epithelial anlagen assembles over an underlying embryonic mesenchyme [1, 2]. By birth, the mammary gland is comprised of a rudimentary ductal tree [3]. With the onset of ovarian function and the production of estrogen at puberty, terminal end buds (TEBs) appear at the ends of the mammary ducts, with highly proliferative cells that allow the ducts to progress through the fat pad [4]. As the mouse reaches the height of puberty (approximately PND32–35, after vaginal opening is observed), the ductal tree grows past the lymph node, more branching points are observed and a larger percentage of the fat pad is filled with epithelium. By adulthood, the mammary ductal tree contains more secondary and tertiary branching, and the TEBs regress and form terminal ends. The ductal tree begins to develop alveolar buds, hollow epithelial structures capable of secreting milk, in response to hormones produced throughout the estrus cycle [5]. During pregnancy, these alveolar buds undergo a massive phase of proliferation and differentiation in preparation for lactation, producing lobuloalveolar structures [6].
In embryonic development, the mesenchymal cells express estrogen receptor (ER), whereas epithelial cells begin to express ER around the time of birth [7]. Studies from ER knockout mice demonstrate that estrogen action is not required for mammary gland development until the onset of puberty [8–10]. Yet, numerous studies have shown that the gland is sensitive to estrogenic compounds when exposures occur during early development [11, 12]. For example, mice exposed to 17β-estradiol, estradiol benzoate, diethylstilbestrol, the phytoestrogen genistein, or the mycoestrogen zearalenone during the perinatal period develop mammary glands with abnormal morphologies including ducts with a ‘beaded’ appearance [13–17]. Consistent with the concept of the developmental origins of health and disease, the effects of early life xenoestrogen exposures often manifest after the period of exposure, with some outcomes that are observed only in later adulthood [18, 19].
One estrogenic compound that has been extensively evaluated for its effects on the mammary gland is bisphenol A (BPA) [19–21], a chemical found in everyday consumer products including plastic water bottles and food containers, epoxy resins lining metal food and beverage cans, dental sealants, thermal receipt paper, medical and sports equipment, and others [22, 23]. BPA mimics estrogen in both cultured cells and live animals, although it also acts via agonism or antagonism of other hormone receptors including thyroid hormone receptor, androgen receptor, estrogen related receptor-γ, the aryl hydrocarbon receptor, and others [24, 25]. Developmental exposures to BPA alter mammary gland morphology and function in both mice and rats, with alterations to proliferation, increased numbers and density of TEBs and terminal ends, advanced development of alveolar buds, pre-neoplastic and neoplastic lesions, enhanced responses to estrogen and progesterone, and increased sensitivity to chemical carcinogens [reviewed in [19–21, 26–28]].
After public concern was raised about the safety of BPA in consumer products, many manufacturers began removing BPA from products and labeling them “BPA-free”. Only recently has it become clear that many of these products are manufactured with other bisphenols including bisphenol S (BPS), which has a similar chemical structure and similar modes of action [29, 30]. In fact, BPS is now used in baby bottles, thermal receipt paper and other paper products; it has also been detected in canned foods [31–34]. Biomonitoring studies from the US and Asian countries indicate that human exposures to BPS are low but widespread, vary by location [35] and have increased over the last decade [36]. Although the effects of low dose BPS exposures are not yet well characterized, studies using human preadipocyte cell lines have shown that it increases the rate of adipocyte differentiation, induces lipid production in these cells, and increases the expression of adipogenic markers [37]. In zebrafish, BPS can induce the production of new neurons (via a process known as neurogenesis) in the hypothalamus [38]. In rats, low dose BPS exposures alter function of the heart at the whole organ, cellular, and protein levels of biological organization [39]. Finally, ongoing work in our lab has demonstrated that low dose exposures to BPS during pregnancy and lactation can disrupt mouse maternal behaviors, alter ERα expression in the maternal brain, and alter function of the lactating mammary gland [40–42]. Offspring exposed to BPS during perinatal development display abnormal social behaviors, altered body weight, abnormal responses of the female reproductive tract to estrogen, and disruptions to maternal care [41–44]. The effects of developmental exposure to BPS on mammary gland morphology, as well as mammary gland function and disease, remain unexplored to date.
In this study, we evaluated the effects of perinatal low dose BPS exposures on the developing mammary gland prior to puberty, during puberty, and in early adulthood. We also evaluated the same endpoints in mice developmentally exposed to 17α-ethinyl estradiol (EE2), a pharmaceutical estrogen commonly used as a positive control for estrogenicity [45, 46]. EE2 is the active estrogenic component found in oral contraceptives, which are used by more than 100 million women worldwide [47, 48]. Accidental use of oral contraceptives throughout the full duration of pregnancy is likely to be very rare, yet ~ 2 million women become pregnant each year while using oral contraceptives [49], with approximately 3% of pregnant women reporting that they took estrogenic pharmaceuticals during their first trimester [50]. Although EE2 has been used to study the effects of estrogens on sensitive organs at multiple stages of development [51], the effects of perinatal exposures on the developing mammary gland are poorly understood.
Using whole mount morphometric tools, histological and immunohistochemical analyses, we found significant effects of both BPS and EE2 on the developing mammary gland. Because of their similar modes of action, we hypothesized that the effects of BPS and EE2 would be comparable to previously published effects of low dose BPA exposures. Here, we report distinct effects of BPS and EE2 on the developing mammary gland, providing support that xenoestrogens promote compound-specific effects that also vary depending on the period of evaluation (pre-puberty, puberty, and adulthood).
2. Methods
2.1. Administration of Test Compounds
Pregnant female CD-1 mice (Charles River Laboratories, Stoneridge, NY) were individually housed (until parturition) in polysulfone cages with food (ProLab IsoDiet) and tap water (in glass bottles) provided ad libitum. The animals were maintained in temperature and light controlled (12h light, 12h dark, lights on at 0800 h) conditions at the University of Massachusetts, Amherst Central Animal Facility. All experimental procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
On pregnancy day eight, pregnant females were weighed and randomly allocated to treatment groups using software to normally distribute dams to groups based on body weight. From pregnancy day 9 to lactational day 20, dams were weighed daily and fed a small whole wheat wafer (Nabisco) treated with BPS, EE2 or vehicle alone (70% ethanol, allowed to dry prior to feeding). Wafers were dosed with solutions designed to deliver 2 or 200 μg BPS/kg/day or 0.01 or 1 μg EE2/kg/day. The high dose of BPS was selected based on two pilot studies showing that this dose disrupted maternal [40] and offspring behaviors [43]. The low dose was selected to better approximate human exposures; it is approximately 1–10x higher than typical human intake [32, 35]. The high dose of EE2 was selected because it has previously been shown to induce a uterotrophic response in prepubertal animals [52]; the low dose was selected because it has been shown to alter gene expression in the mouse mammary gland after prenatal exposures [7].
Dams delivered naturally with birth designated lactational day (LD) 0. Pregnancy loss was observed in all treatment groups, but there were no significant differences by treatment. These data were previously reported in [41, 53]. Litters were culled to 10 pups on LD1. Pups were weaned on postnatal day (PND) 21. One female from each litter was selected for necropsy at each age (PND24, PND32–35, week 9) as described below. Post-weaning mortality was rare. One female developed hydrocephaly and had to be euthanized; see description in [54].
2.2. Tissue Collections
At pre-pubertal (PND24), pubertal (PND32–35) and adult (9 week) periods of development, female mice were euthanized via CO2 inhalation. The left and right fourth inguinal mammary glands were isolated using standard dissection methods. One mammary gland was spread on a slide and fixed in neutral buffered formalin for later whole mount processing. The other mammary gland was fixed in neutral buffered formalin for paraffin embedding and histological analysis.
2.3. Whole Mount Processing and Evaluation
Whole mount mammary glands were fixed for 24–48 hours at room temperature, washed in phosphate buffered saline, and then processed through a series of alcohols and toluene to be defatted. The samples were rehydrated, stained overnight with carmine alum, dehydrated through a series of alcohols and xylene, and then sealed in k-pax heat bags with methyl salicylate.
Whole mount mammary glands were viewed and imaged using a Zeiss Axio Imager dissection microscope and Zen Pro software. Quantification of epithelial structures in the mammary glands was completed using methods developed previously [55, 56]. In the pre-pubertal and pubertal glands, specific measurements included the area subtended by the ductal tree (ductal area), the growth of the longest duct from the center of the lymph node (ductal extension), the total number of terminal end buds (TEBs, defined as bulb-shaped structures ≥0.03 mm2), and area of TEBs. Average TEB size and TEB density were calculated from these values.
In the adult glands, images were collected at 35x magnification in the region just anterior to the central lymph node. A grid with 180 crosshairs was placed on each image, and the structure at each crosshair was counted. Volume fraction of epithelial structures (ducts, terminal ends, alveolar buds) was calculated from these measures (# of crosshairs with specific structure / total crosshairs on mammary tissue).
2.4. Embedding, Sectioning and Staining of Mammary Tissue
Tissues were fixed overnight at room temperature, washed in phosphate buffered saline, and then processed through a series of graded alcohols, followed by vacuum infiltration of paraffin and embedding. Paraffin-embedded tissues were sectioned with a Fisher rotary microtome at a thickness of 5μm and collected on positively charged glass slides. One section from each animal was stained with hematoxylin and eosin (H&E) and coverslipped with permanent mounting media using standard protocols. Samples were examined for basic histological features using a Zeiss inverted microscope and high resolution color camera.
2.5. Immunohistochemistry
5 μm sections were evaluated with immunohistochemistry for Ki67, a marker of proliferation, ERα and progesterone receptor (PR) using standard protocols [56]. Commercial antibodies were used including rabbit anti-ERα (EMD Millipore, Cat# 06–935, Temecula, CA); rabbit anti-Ki67 (Fisher Scientific, Cat# RM-9106-S1); rabbit anti-progesterone receptor (PR; Abcam, Cat# ab131486, Cambridge, MA); secondary antibody (goat anti-rabbit, Abcam, Cat# ab64256) followed by streptavidin peroxidase complex (Abcam, Cat# ab64269); and diaminobenzidene (DAB) chromogen (Abcam, Cat# ab64238) to visualize reactions. Samples were counterstained with hematoxylin and coverslipped with permanent mounting media. Images were collected using a Zeiss inverted microscope and a 40x objective, and expression of each marker was determined by counting a total of 200–1200 epithelial cells in each sample.
2.6. Statistics
SPSS v23 was used for all statistical analyses. All experiments (including dosing of animals and collecting of tissues at necropsy) were conducted by experimenters blind to treatment group. Further, all data were collected by experimenters blind to treatment group. For all continuous data, one-way ANOVA tests (with treatment as the independent variable) followed by Bonferroni posthoc analyses were used to compare treatment groups. Data were typically normally distributed. Reported in the text are posthoc comparisons between treated groups and the control group only. Chi Square analysis was used to compare categorical data (e.g., the presence or absence of TEB-like structures in whole mount mammary glands, the presence or absence of intraductal hyperplasias in histological sections). Because only one female was evaluated from each litter at each timepoint, statistical corrections for litter were not needed. Results were considered significant at p < 0.05 for all statistical tests. All data exhibited in the graphs represent mean ± SEM. Samples sizes are provided in each table and figure legend.
3. Results
3.1. BPS, but not EE2, alters mammary gland morphology at PND24
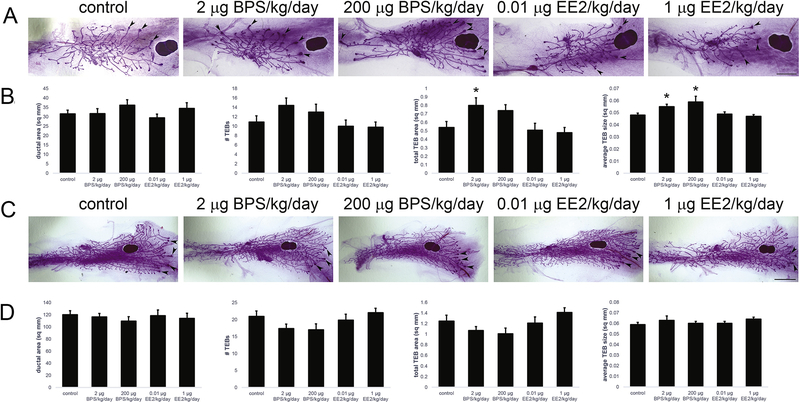
To quantify the morphology of the mammary gland at the pre-pubertal stage, growth parameters were evaluated in whole mount mammary glands at PND24 (Figure 1A). Parameters included ductal area, ductal extension, TEB number, total TEB area, average TEB size and TEB density. Quantification of these features revealed more developed mammary glands in both BPS-treated groups with significant increases in total TEB area and average TEB size (Figure 1B). EE2-treated females were not significantly different from controls for any growth parameter (Figure 1B).
Figure 1: Perinatal exposures to BPS alter mouse mammary gland morphology prior to, but not during, puberty.
A) Whole mount mammary glands collected from females at PND24, prior to the onset of puberty [as measured by vaginal opening]. Arrowheads indicate TEBs. Scale bar = 4mm. B) Quantification of growth parameters reveals significant effects of perinatal BPS exposure on TEB parameters. EE2 treatment had no significant effects on any parameter. C) Whole mount mammary glands collected from females during puberty. Arrowheads indicate TEBs. Scale bar = 2mm. D) Neither BPS nor EE2 altered growth parameters in whole mount mammary glands at puberty. In all panels, * p < 0.05, Bonferroni posthoc after significant ANOVA. Sample sizes: control = 20; 2 μg BPS/kg/day = 14; 200 μg BPS/kg/day = 10; 0.01 μg EE2/kg/day = 15; 1 μg EE2/kg/day = 12.
3.2. Neither BPS or EE2 disrupts mammary gland morphology at puberty
To evaluate the effects of BPS and EE2 on the pubertal mammary gland, the same endpoints evaluated at PND24 were examined in whole mount mammary glands collected from animals at PND32–35, after vaginal opening (Figure 1C). No significant effects of either BPS or EE2 were observed for any morphological parameters (Figure 1D).
3.3. Developmental exposures to BPS and EE2 alter morphology of the mammary gland in adulthood
To examine the effects of developmental exposures to BPS and EE2 on the adult mammary gland, images were collected from whole mounts in the region just anterior to the lymph node (Figure 2A). A 180-point grid and unbiased stereological methods were used to quantify structures in the mammary gland including ducts, terminal ends and alveolar buds. Females exposed to 200 μg BPS/kg/day and either dose of EE2 had significantly more alveolar buds compared to controls (Figure 2B). Females exposed to 2 μg BPS/kg/day also had significantly more terminal ends compared to controls (Figure 2C). Finally, all treated groups had more total epithelium in the mammary gland compared to controls, although only the females exposed to 1 μg EE2/kg/day were significantly different from controls (p<0.01, Figure 2D).
Figure 2: Developmental BPS and EE2 exposures alter adult mammary gland morphology including the retention of TEBs.
A) Whole mount mammary gland collected from females in early adulthood (9 weeks of age). Arrows indicate terminal ends, arrowheads indicate alveolar buds. Scale bar = 0.5mm. B) Quantification of alveolar buds in adult mammary glands. C) Quantification of terminal ends. D) Quantification of all epithelium. E) Examples of normal terminal ends (arrowheads) and TEB-like structures (arrows) in adult mammary glands. In all panels, * p < 0.05, Bonferroni posthoc after significant ANOVA. Sample sizes: control = 23; 2 μg BPS/kg/day = 16; 200 μg BPS/kg/day = 13; 0.01 μg EE2/kg/day = 16; 1 μg EE2/kg/day = 11.
Additional evaluations of whole mount morphology revealed the presence of TEBs at the leading edge of several mammary glands (Figure 2E). These TEBs had a similar appearance to TEBs observed in pubertal mammary glands. Importantly, these TEB-like structures were only observed in xenoestrogen treated females, with the majority observed in BPS-exposed animals (Table 1).
Table 1.
TEB-like structures are observed in BPS-treated females in adulthood
| Treatment group | Number of females evaluated | Incidence of TEB- like structures (%) | p-value, Chi Square compared to controls |
|---|---|---|---|
| Controls | 23 | 0/23 (0%) | |
| 2 μg BPS/kg/day | 16 | 10/16 (63%) | p<0.01 |
| 200 μg BPS/kg/day | 13 | 7/13 (54%) | p<0.01 |
| 0.01 μg EE2/kg/day | 16 | 2/16 (13%) | n.s. |
| 1 μg EE2/kg/day | 11 | 0/11 (0%) | n.s. |
3.4. Developmental BPS exposure decreases proliferation in the mammary epithelium at PND24 and increases proliferation in adulthood
To evaluate the effects of both xenoestrogens on cell proliferation, the expression of Ki67, a marker of proliferation, was quantified using immunohistochemistry in mammary samples from females at PND24, puberty, and adulthood (Figure 3A). At PND24, Ki67 expression was significantly lower in females exposed to 2 μg BPS/kg/day compared to controls (Figure 3B). Females developmentally exposed to 200 μg BPS/kg/day also exhibited lower Ki67 expression, although this difference was not statistically significant (p = 0.068, Bonferroni posthoc). In contrast, developmental exposure to EE2 had no significant effect on proliferation rates at PND24 (Figure 3B).
Figure 3: Proliferation is altered by developmental BPS exposure in an age-specific manner.
A) Representative images demonstrating changes in the expression of Ki67, a marker of proliferation, during development. All images are from control females. Arrowheads indicate positive cells. Scale bar = 20 μm. B) Quantification of proliferation at PND24. C) Quantification of proliferation during puberty. D) Quantification of proliferation in adulthood. Ki67 in control animals was low (typically in the range of 1–2%). Inset shows higher levels of Ki67 expression in a gland from an adult female exposed to 1 μg EE2/kg/day. E) Examples of intraductal hyperplasias from H&E stained images. Scale bar = 20 μm. F) Examples of ducts with epithelial cells located within the lumen. Scale bar = 20 μm. In all panels, * p < 0.05, Bonferroni posthoc after significant ANOVA. Sample sizes (depending on age): control = 15–17; 2 μg BPS/kg/day = 9–14; 200 μg BPS/kg/day = 8–12; 0.01 μg EE2/kg/day = 8–10; 1 μg EE2/kg/day = 8–9.
Mammary glands from females collected at puberty were similarly evaluated. BPS-treatment induced almost 3-times more cell proliferation compared to controls, although these differences were not statistically significant (Figure 3C). Similarly, developmental exposure to 1 μg EE2/kg/day also increased rates of cell proliferation in pubertal glands (Figure 3C), but this increase was also not statistically significant.
Finally, Ki67 expression was evaluated in mammary glands at 9 weeks of age. Proliferation rates were low in controls (approximately 2.3%), and exposure to 2 μg BPS/kg/day but not 200 μg BPS/kg/day significantly increased proliferation in the mammary epithelium (Figure 3D). Both doses of EE2 increased proliferation, although these effects were not statistically significant (Figure 3D).
3.5. Both BPS and EE2 exposures increase the incidence of intraductal hyperplasias in adult mammary glands
To determine if the increases in Ki67 expression observed in adult mammary glands were consistent with changes in the histomorphology of mammary tissue, all ducts within a single longitudinal section of each mammary gland were examined. Intraductal hyperplasias, defined using the Annapolis criteria as an increase in cell number without cytologic atypia [57], were observed in just one control animal, but in significantly more mammary glands from females exposed to both doses of BPS and both doses of EE2 (Table 2, Figure 3E). Importantly, ducts with epithelial cells within the lumen were also observed in BPS- and EE2-treated females (Figure 3F). Although these observations may be an artifact of the histological methods used, they are consistent with hyperplastic lesions.
Table 2.
Intraductal hyperplasias are observed in BPS- and EE2-treated females in adulthood
| Treatment group | Number of females evaluated | Incidence of intraductal hyperplasia (%) | p-value, Chi Square compared to controls |
|---|---|---|---|
| Controls | 23 | 1/23 (4%) | |
| 2 μg BPS/kg/day | 16 | 8/16 (50%) | p<0.01 |
| 200 μg BPS/kg/day | 13 | 8/13 (62%) | p<0.01 |
| 0.01 μg EE2/kg/day | 16 | 9/16 (56%) | p<0.01 |
| 1 μg EE2/kg/day | 11 | 7/11 (64%) | p<0.01 |
3.6. Developmental stage specific effects of BPS and EE2 on ERα and PR Expression
The expression of two hormone receptors, ERα and PR, was assessed in all treatment groups at PND24, puberty, and in adulthood (Figure 4A). At PND24, neither BPS nor EE2 significantly altered expression of ERα (Figure 4B). BPS also did not alter expression of PR, although developmental exposure to EE2 increased its expression, with significant effects observed at 0.01 μg EE2/kg/day compared to the control group (p < 0.05, Bonferroni posthoc, Figure 4C).
Figure 4. Expression of ERα and PR in the mammary gland changes during development.
A) Representative images illustrating ERα and PR expression in the mammary gland at PND24, at puberty, and in adulthood. All images are from control females. Arrowheads indicate positive cells. Scale bar = 20 μm. B) Quantification of ERα at PND24, puberty, and in adulthood. C) Quantification of PR at PND24, puberty, and in adulthood. In all panels, * p < 0.05, Bonferroni posthoc after significant ANOVA. Sample sizes (depending on age): control = 15–17; 2 μg BPS/kg/day = 9–14; 200 μg BPS/kg/day = 8–12; 0.01 μg EE2/kg/day = 8–10; 1 μg EE2/kg/day = 8–9.
At puberty, expression patterns of both ERα and PR were different from what was observed in the pre-pubertal period. ERα-expression was increased in all four treatment groups compared to controls, although statistical significance was only observed in females exposed to 1 μg EE2/kg/day (p < 0.05, Bonferroni posthoc, Figure 4B). Compared to controls, developmental exposure to either dose of BPS significantly increased the expression of PR (Figure 4C). In contrast, EE2 had no significant effect on the percentage of epithelial cells expressing PR at this age (Figure 4C).
Finally, the percentage of epithelial cells expressing ERα and PR was evaluated in adults. BPS exposure induced decreases in expression of ERα (p = 0.08, 2 μg BPS/kg/day versus controls, Bonferroni posthoc; p < 0.05, 200 μg BPS/kg/day versus controls, Bonferroni posthoc; see Figure 4B). In contrast, EE2 treatment increased the expression of ERα (p < 0.05, 1 μg EE2/kg/day versus controls, Bonferroni posthoc; Figure 4B). PR expression was not affected by developmental BPS exposure, and although more epithelial cells in EE2-treated females expressed PR compared to controls, these differences were not statistically significant (Figure 4C).
4. Discussion
This study examined the effects of developmental exposure to low doses of BPS and EE2 on the mammary gland of female CD-1 mice at three stages of development: pre-puberty, puberty, and early adulthood. There are several broad conclusions that can be drawn from this study. First, the developing mammary gland is sensitive to low doses of several xenoestrogens, with effects that manifest in later life after exposures have ceased. Second, the effects of these two xenoestrogens, evaluated side-by-side with the same route of exposure and evaluative tools, are distinct at every period of development we examined. Finally, this study reveals that BPS induces long-lasting effects on the mammary gland that may increase the animal’s risk for mammary cancer. Additional studies are needed to evaluate the long-term consequences of BPS exposure, including its ability to induce or promote carcinogenesis.
Here, we found that developmental BPS exposure, but not EE2 exposure, alters the morphology of the mammary gland prior to puberty. BPS-exposed females had larger TEBs and more area of the gland comprised of TEBs (Figure 1) even though these same females also had fewer epithelial cells expressing Ki67, a proliferation marker (Figure 3). Although these results might appear to be contradictory, they suggest that BPS may advance some aspects of gland development while delaying others. Similar effects have been described for BPA-treated animals, where developmental exposures increased the number and size of TEBs while decreasing the length of ducts at puberty [58], and embryonic BPA was shown to both advance development of the fat pad while decreasing lumen formation in the epithelium [59]. At PND24, developmental EE2 treatment increased the expression of PR without altering the percentage of epithelial cells expressing ERα. In the mammary gland, PR expression is mediated by ERα [60, 61], suggesting that EE2 may alter development of the mammary gland via activation of ER-dependent pathways prior to PND24. Increased expression of PR may alter the mammary gland’s sensitivity to progesterone or induce an increase in future branching points, an endpoint that is progesterone-sensitive [62–64]. In fact, the more dense mammary glands observed in EE2-treated females in adulthood are consistent with such an increase in branching points. Prior studies have shown that developmental exposures to BPA increase the expression of PR in the mouse mammary gland in adulthood and also sensitize the gland to progesterone [65]; these results may be consistent with the effects of EE2 observed here.
At puberty, we were surprised to find that there were no significant effects of either BPS or EE2 on any of the morphological endpoints we evaluated (Figure 1). Proliferation rates were also not affected by developmental exposures to either BPS or EE2. Instead, only subtle effects were observed on the percentage of cells expressing ERα (significantly increased only in females exposed to 1 μg EE2/kg/day) and expression of PR (significantly increased by both doses of BPS). These results deviate from previous studies of BPA, where developmental exposures produced visible morphological effects at puberty [58, 66] by altering the response of the mammary gland to both estrogen and progesterone [65, 67]. However, the effects we observed in adulthood suggest similarities in long-term responses of the mammary gland to developmental BPA, BPS and EE2 exposures. All three compounds increased the overall density of the adult mammary gland, with increases in ductal side-branching (producing more terminal ends) and advanced differentiation of the gland (producing more alveolar buds).
TEBs are a characteristic of the pubertal mammary gland, yet we also observed these structures in adult mammary glands in females exposed to xenoestrogens during development (Figure 2). Although prior studies have reported that developmental xenoestrogen exposures can shift the timing of puberty, including the appearance of TEBs [68–70], to our knowledge, the presence of TEBs in adult mouse mammary glands have not been reported previously. The retention of TEBs is consistent with the appearance of intraductal hyperplasias in BPS-treated females, as well as the increased proliferation rates observed in females exposed to 2 μg BPS/kg/day.
Why might the effects of two xenoestrogens on the mammary gland be different? EE2 is widely considered a prototypical estrogen, although it has features that distinguish it from the endogenous estrogen 17β-estradiol; for example, EE2 preferentially binds ERα over ERβ and never achieves maximal agonist activity via ERβ [71]. BPS, in contrast, preferentially binds to ERβ [72] and may also alter progesterone signaling [73]. Because ERβ does not appear to be expressed in the mouse mammary gland until later adulthood ([74]; and Vandenberg, unpublished observation), it is surprising that BPS induces more severe effects than EE2. EE2 and BPS are also known to act via non-genomic signaling pathways including binding to the membrane ER [75, 76]. Thus, the characterization of these compounds as ‘xenoestrogens’ is quite limiting and it should perhaps not be unexpected that EE2 and BPS can induce different effects on estrogen-sensitive organs like the mammary gland. Another possibility requires consideration of the biochemical pathway by which estrogens act. Estrogens circulate in the blood, cross the cell membrane, and bind to their receptors. Once bound, receptors can activate other transcription factors (e.g., AP1 and SP1) or translocate into the nucleus where they bind to estrogen response elements (EREs), recruit co-activators and co-repressors, promoting (or repressing) gene expression [77]. Thus, it is plausible that BPS and EE2 promote different dimerization patterns; for example, one might promote ERα homodimers, whereas another might promote ERα/ERβ heterodimers. It is also plausible that the different ligands promote the binding of receptors to different EREs [78] or that the binding of different ligands promotes the recruitment of different coregulators, which influences the expression of downstream genes [79, 80]. More work is needed to understand the molecular basis for the divergent effects of BPS, BPA and EE2 exposures.
We chose to study BPS because it is a common replacement chemical for BPA, found in many consumer products, with what appears to be widespread human exposures [35, 36]. Although BPA is well-studied, and the effects of developmental BPA exposure on the rodent mammary gland have been well elucidated [19, 20], BPS and other common replacements remain poorly evaluated [30]. Recent work demonstrated that mice exposed to BPS during pregnancy and lactation have modest disruptions to maternal behavior and altered expression of ERα in specific regions of the maternal brain [41]. The lactating mammary gland was disrupted by BPS exposures during this time period, with effects consistent with BPS-induced early involution [42]. The exposed offspring were also affected by developmental BPS exposures, with abnormal maternal behaviors including infanticide [41], abnormal social behaviors [43], and decreased body weight [42, 43].
EE2 is a pharmaceutical estrogen that is widely used in oral contraceptives. The US Centers for Disease and Control and Prevention (CDC) reported in 2012 that approximately 10.2 million women in the US were using the pill as a form of contraceptive [81]. The CDC also notes that approximately 9% of women will conceive within the first year of using oral contraceptives, mostly due to missed doses. A recent study capitalizing on the availability of extensive birth registries in Denmark questioned whether oral contraceptive use before or during pregnancy would lead to an increased risk for birth defects [82]. Charlton and colleagues found that the use of oral contraceptive during or before pregnancy had no association with the frequency of major birth defects. Unfortunately, to our knowledge, no study has evaluated whether inadvertent exposure of embryos and fetuses to EE2 alters disease risk later in life. The tragic history of the pharmaceutical estrogen diethylstilbestrol demonstrated that individuals exposed to synthetic estrogens during gestation can appear ‘normal’ at birth, but develop diseases at or after puberty [83, 84]. In a large multi-generational study conducted by the National Toxicology Program (NTP), rats were orally exposed to a range of doses of EE2 over four generations [85]. Adverse effects induced by EE2 included decreased body weights, altered estrous cycles in the females, male mammary gland hyperplasia and some mineralization of renal tubules. Our study examined doses similar and lower to those evaluated in the NTP study (i.e., doses in the NTP study ranged from 0.1 – 6 μg EE2/kg/day).
Many studies of endocrine disrupting chemicals historically use EE2 as a positive control. Although these studies have revealed many effects of EE2, they have also shown that the effects of other xenoestrogens do not always replicate the effects from EE2 exposure. For example, one study observed how a mixture of xenoestrogens collected from the Douro River were distinct from the effects of 100 ng/L EE2 on gametogenesis in fish [86]. Fish exposed to the mixture developed higher levels of ovarian follicle atresia compared to the EE2 exposed group. In our own studies, we have observed striking differences between the effects of BPS and EE2 on a range of maternal behavior and brain endpoints [41, 42, 53, 54]. For example, females exposed to BPS during early development spent significantly less time on the nest compared to untreated controls but this was not observed in EE2 treated females; in contrast, EE2 treated females displayed decreases in the number of dopaminergic neurons in the ventral tegmental area of the midbrain, but this was not observed in BPS treated females. Here, the striking increase in retained TEBs in the adult mammary glands of BPS-exposed females may be a unique feature of this xenoestrogen, whereas the presence of intraductal hyperplasias is shared by females exposed to either BPS or EE2 and has also been observed after developmental BPA exposures [56]. Addressing the mechanisms by which different estrogenic compounds induce divergent effects on hormone sensitive endpoints, including the mammary gland, is an important area for future work.
5. Conclusions
Here, we exposed CD-1 mice to BPS or EE2 during pregnancy and lactation, and examined exposed offspring prior to puberty (PND24), in puberty (PND32–25), and in adulthood (9 weeks of age). Both xenoestrogens altered mammary gland morphology, cell proliferation, and the expression of hormone receptors. Our results suggest that, like BPA, BPS alters the morphology of the mammary gland, with the most overt effects manifesting in adulthood. BPS and EE2 induce different effects, providing further evidence that compounds can have a similar mode of action (e.g., ER agonists) and yet contribute differently to disease risk.
Highlights:
Perinatal BPS exposures alter mammary gland morphology at pre-puberty and adulthood
Adult mice exposed to BPS during development have retained TEBs
Developmental BPS or EE2 exposures induce intraductal hyperplasias in adulthood
The effects of BPS are distinct from EE2, a common estrogenic positive control
Acknowledgements
The authors thank other members of the Vandenberg lab for their assistance and feedback on this work including Mary Catanese, Charlotte LaPlante, Archana Gopal, Anupama Singh, and Michael Lemieux. The authors acknowledge support from the University of Massachusetts Commonwealth Honors College (Honors Grant to SK) and Award Number K22ES025811 from the National Institute of Environmental Health Sciences of the National Institutes of Health (to LNV). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Massachusetts.
Disclosures
LNV has received travel reimbursement from Universities, Governments, NGOs and Industry, to speak about endocrine-disrupting chemicals. SK, MM and BM have nothing to disclose.
Abbreviations:
- BPA
bisphenol A
- BPS
bisphenol S
- EE2
ethinyl estradiol
- ER
estrogen receptor
- H&E
hematoxylin and eosin
- PND
postnatal day
- PR
progesterone receptor
- TEB
terminal end bud
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hennighausen L, Robinson GW, Think globally, act locally: the making of a mouse mammary gland, Genes Dev 12 (1998) 449–55. [DOI] [PubMed] [Google Scholar]
- [2].Hennighausen L, Robinson GW, Signaling pathways in mammary gland development, Developmental Cell 1 (2001) 467–75. [DOI] [PubMed] [Google Scholar]
- [3].Nandi S, Endocrine control of mammary gland development and function in the C3H/ He Crgl mouse, J Nat Cancer Inst 21 (1958) 1039–63. [PubMed] [Google Scholar]
- [4].Richert MM, Schwertfeger KL, Ryder JW, Anderson SM, An atlas of mouse mammary gland development, Journal of Mammary Gland Biology and Neoplasia 5 (2000) 227–41. [DOI] [PubMed] [Google Scholar]
- [5].Brisken C, Ataca D, Endocrine hormones and local signals during the development of the mouse mammary gland, Wiley interdisciplinary reviews. Developmental biology 4(3) (2015) 181–95. [DOI] [PubMed] [Google Scholar]
- [6].Brisken C, O’Malley B, Hormone action in the mammary gland, Cold Spring Harb Perspect Biol 2(12) (2010) a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wadia PR, Cabaton NJ, Borrero MD, Rubin BS, Sonnenschein C, Shioda T, Soto AM, Low-Dose BPA Exposure Alters the Mesenchymal and Epithelial Transcriptomes of the Mouse Fetal Mammary Gland, PLoS ONE 8(5) (2013) e63902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clarke R , Introduction and overview: sex steroids in the mammary gland, Journal of Mammary Gland Biology and Neoplasia 5 (2000) 245–50. [DOI] [PubMed] [Google Scholar]
- [9].Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O, Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene, Proc Natl Acad Sci U S A 90 (1993) 11162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA, Estrogen receptors a and b in the rodent mammary gland, Proc Natl Acad Sci U S A 97 (2000) 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fenton SE, The mammary gland: a tissue sensitive to environmental exposures, Rev Environ Health 24(4) (2009) 319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Macon MB, Fenton SE, Endocrine disruptors and the breast: early life effects and later life disease, J Mammary Gland Biol Neoplasia 18(1) (2013) 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bern HA, Edery M, Mills KT, Kohrman AF, Mori T, Larson L, Long-term alterations in histology and steroid receptor levels of the genital tract and mammary gland following neonatal exposure of female BALB/cCrgl mice to various doses of diethylstilbestrol, Cancer Res 47(15) (1987) 4165–72. [PubMed] [Google Scholar]
- [14].Bern HA, Mills KT, Jones LA, Critical period of neonatal estrogen exposure in occurence of mammary gland abnormalities in adult mice, Proc Soc Exp Biol Med 172 (1983) 239–42. [DOI] [PubMed] [Google Scholar]
- [15].Hoshino K, Connolly MT, Development and growth of mammary glands of mice prenatally exposed to estradiol benzoate, The Anatomical record 157 (1967) 262. [Google Scholar]
- [16].Padilla-Banks E, Jefferson WN, Newbold RR, Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels, Endocrinology 147(10) (2006) 4871–82. [DOI] [PubMed] [Google Scholar]
- [17].Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A, Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring, Reprod Toxicol 18(6) (2004) 803–11. [DOI] [PubMed] [Google Scholar]
- [18].Heindel JJ, Vandenberg LN, Developmental origins of health and disease: a paradigm for understanding disease etiology and prevention, Curr Opin Pediatr 27(2) (2015) 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soto AM, Brisken C, Schaeberle C, Sonnenschein C, Does cancer start in the womb? Altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors, J Mammary Gland Biol Neoplasia 18(2) (2013) 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang HS, Vom Saal FS, Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies, Endocrine Disruptors 1(1) (2013) e25078. [Google Scholar]
- [21].Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA, A review of the carcinogenic potential of bisphenol A, Reprod Toxicol 59 (2016) 167–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, Human exposure to bisphenol A (BPA), Reprod Toxicol 24(2) (2007) 139–77. [DOI] [PubMed] [Google Scholar]
- [23].Onundi Y, Drake BA, Malecky RT, DeNardo MA, Mills MR, Kundu S, Ryabov AD, Beach ES, Horwitz CP, Simonich MT, Truong L, Tanguay RL, Wright LJ, Singhal N, Collins TJ, A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water, Green Chemistry (2017). [Google Scholar]
- [24].Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM, Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption, Endocrine Reviews 30(1) (2009) 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Watson CS, Jeng YJ, Guptarak J, Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways, J Steroid Biochem Mol Biol 127(1–2) (2011) 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses, Endocr Rev 33(3) (2012) 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vandenberg LN, Prins GS, Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA), Andrology 4(4) (2016) 561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM, Prins GS, An evaluation of evidence for the carcinogenic activity of bisphenol A, Reprod Toxicol 24(2) (2007) 240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vandenberg LN, Luthi D, Quinerly D, Plastic bodies in a plastic world: multi-disciplinary approaches to study endocrine disrupting chemicals, J Cleaner Production 140(1) (2017) 373–85. [Google Scholar]
- [30].Rochester JR, Bolden AL, Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes, Environ Health Perspect 123(7) (2015) 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liao C, Liu F, Kannan K, Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues, Environ Sci Technol 46(12) (2012) 6515–22. [DOI] [PubMed] [Google Scholar]
- [32].Liao C, Kannan K, Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure, J Agric Food Chem 61(19) (2013) 4655–62. [DOI] [PubMed] [Google Scholar]
- [33].Liao C, Kannan K, A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States, Arch Environ Contam Toxicol 67(1) (2014) 50–9. [DOI] [PubMed] [Google Scholar]
- [34].Simoneau C, Valzacchi S, Morkunas V, Van den Eede L, Comparison of migration from polyethersulphone and polycarbonate baby bottles, Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28(12) (2011) 1763–8. [DOI] [PubMed] [Google Scholar]
- [35].Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K, Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures, Environ Sci Technol 46(12) (2012) 6860–6. [DOI] [PubMed] [Google Scholar]
- [36].Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM, Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014, Environ Sci Technol 49(19) (2015) 11834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boucher JG, Gagne R, Rowan-Carroll A, Boudreau A, Yauk CL, Atlas E, Bisphenol A and Bisphenol S Induce Distinct Transcriptional Profiles in Differentiating Human Primary Preadipocytes, PloS one 11(9) (2016) e0163318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM, Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish, Proc Natl Acad Sci U S A 112(5) (2015) 1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gao X, Ma J, Chen Y, Wang HS, Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: evidence of female-specific proarrhythmic effects, Environmental health perspectives 123(6) (2015) 571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Catanese MC, Suvorov A, Vandenberg LN, Beyond a means of exposure: a new view of the mother in toxicology research, Toxicol Res 4 (2015) 592–612. [Google Scholar]
- [41].Catanese MC, Vandenberg LN, Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters, Endocrinology 158(3) (2017)516–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].LaPlante CD, Catanese MC, Bansal R, Vandenberg LN, Bisphenol S alters the lactating mammary gland and nursing behaviors is mice exposed during pregnancy and lactation, Endocrinology 10.1210/en.2017-00437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim B, Colon E, Chawla S, Vandenberg LN, Suvorov A, Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight, Environ Health 14 (2015) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hill CE, Sapouckey SA, Suvorov A, Vandenberg LN, Schumacher U, Developmental exposures to bisphenol S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: a pilot study, Cogent Medicine (2017) 1317690. [PMC free article] [PubMed] [Google Scholar]
- [45].vom Saal FS, Akingbemi BT, Belcher SM, Crain DA, Crews D, Guidice LC, Hunt PA, Leranth C, Myers JP, Nadal A, Olea N, Padmanabhan V, Rosenfeld CS, Schneyer A, Schoenfelder G, Sonnenschein C, Soto AM, Stahlhut RW, Swan SH, Vandenberg LN, Wang HS, Watson CS, Welshons WV, Zoeller RT, Flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research, Toxicol Sci 115(2) (2010) 612–3. [DOI] [PubMed] [Google Scholar]
- [46].Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, Vanlandingham M, Churchwell MI, Twaddle NC, McLellen M, Chidambaram M, Bryant M, Woodling K, Gamboa da Costa G, Ferguson SA, Flaws J, Howard PC, Walker NJ, Zoeller RT, Fostel J, Favaro C, Schug TT, NIEHS/FDA CLARITY-BPA research program update, Reprod Toxicol 58 (2015) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petitti DB, Clinical practice. Combination estrogen-progestin oral contraceptives, N Engl J Med 349(15) (2003) 1443–50. [DOI] [PubMed] [Google Scholar]
- [48].Pletzer BA, Kerschbaum HH, 50 years of hormonal contraception-time to find out, what it does to our brain, Front Neurosci 8 (2014) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Della Seta D, Minder I, Dessi-Fulgheri F, Farabollini F, Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats, Brain Res Bull 65(3) (2005) 255–60. [DOI] [PubMed] [Google Scholar]
- [50].Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA, National S Birth Defects Prevention, Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk, Pharmacoepidemiol Drug Saf 22(9) (2013) 1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].vom Saal FS, Richter CA, Ruhlen RR, Nagel SC, Timms BG, Welshons WV, The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A, Birth Defects Res (Part A) 73 (2005) 140–5. [DOI] [PubMed] [Google Scholar]
- [52].Ohta R, Takagi A, Ohmukai H, Marumo H, Ono A, Matsushima Y, Inoue T, Ono H, Kanno J, Ovariectomized mouse uterotrophic assay of 36 chemicals, J Toxicol Sci 37(5) (2012) 879–89. [DOI] [PubMed] [Google Scholar]
- [53].Catanese MC, Vandenberg LN, Low doses of 17a-ethinyl estradiol alter the maternal brain and induce stereotypies in CD-1 mice exposed during pregnancy and lactation, Reprod Toxicol doi: 10.1016/j.reprotox.2017.07.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Catanese MC, Vandenberg LN, Developmental estrogen exposures and disruptions to maternal behavior and brain: effects of ethinyl estradiol, a common positive control, Horm Behav doi: 10.1016/j.yhbeh.2017.10.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM, The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization?, Journal of Steroid Biochemistry and Molecular Biology 101(4–5) (2006) 263–74. [DOI] [PubMed] [Google Scholar]
- [56].Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM, Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice, Reprod Toxicol 26 (2008) 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE, The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting, Oncogene 19 (2000) 968–88. [DOI] [PubMed] [Google Scholar]
- [58].Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM, In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland, Biol Reprod 65(4) (2001) 1215–23. [DOI] [PubMed] [Google Scholar]
- [59].Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM, Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland, Endocrinology 148(1) (2007) 116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Haslam SZ, The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones, Endocrinology 125 (1989) 2766–72. [DOI] [PubMed] [Google Scholar]
- [61].Kariagina A, Aupperlee MD, Haslam SZ, Progesterone receptor isoform functions in normal breast development and breast cancer, Crit Rev Eukaryot Gene Expr 18(1) (2008) 11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang S, Counterman LJ, Haslam SZ, Progesterone action in normal mouse mammary gland, Endocrinology 127 (1990) 2183–9. [DOI] [PubMed] [Google Scholar]
- [63].Haslam SZ, Shyamala G, Progesterone receptors in normal mammary glands of mice: characterization and relationship to development, Endocrinology 105(3) (1979) 786–95. [DOI] [PubMed] [Google Scholar]
- [64].Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ, Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development, Endocrinology 150 (2009) 1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, Tanos T, Lefebvre G, Rougemont J, Yalcin-Ozuysal O, Brisken C, Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number, Mol Endocrinol 25(11) (2011) 1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM, Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice, Endocrinology 146(9) (2005) 4138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM, Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains, Environ Health Perspect 115(4) (2007) 592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fenton SE, Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences, Endocrinology 147(6 Suppl) (2006) S18–24. [DOI] [PubMed] [Google Scholar]
- [69].Rayner JL, Wood C, Fenton SE, Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine, Toxicol Appl Pharmacol 195 (2004) 23–34. [DOI] [PubMed] [Google Scholar]
- [70].Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE, The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure, Reprod Toxicol 54 (2015) 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S, Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists, Mol Pharmacol 54(1) (1998) 105–12. [DOI] [PubMed] [Google Scholar]
- [72].Molina-Molina JM, Amaya E, Grimaldi M, Saenz JM, Real M, Fernandez MF, Balaguer P, Olea N, In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors, Toxicol Appl Pharmacol (2013). [DOI] [PubMed] [Google Scholar]
- [73].Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM, Are structural analogues to bisphenol a safe alternatives?, Toxicol Sci 139(1) (2014) 35–47. [DOI] [PubMed] [Google Scholar]
- [74].Couse JF, Korach KS, Estrogen receptor null mice: what have we learned and where will they lead us?, Endocrine Reviews 20 (1999) 358–417. [DOI] [PubMed] [Google Scholar]
- [75].Alonso-Magdalena P, Ropero AB, Soriano S, Garcia-Arevalo M, Ripoll C, Fuentes E, Quesada I, Nadal A, Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways, Mol Cell Endocrinol 355(2) (2012) 201–7. [DOI] [PubMed] [Google Scholar]
- [76].Vinas P, Watson CS, Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions, Environ Health Perspect 121(3) (2013) 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nadal A, Fuentes E, Ripoll C, Villar-Pazos S, Castellano-Munoz M, Soriano S, Martinez-Pinna J, Quesada I, Alonso-Magdalena P, Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus?, J Steroid Biochem Mol Biol 176 (2018) 16–22. [DOI] [PubMed] [Google Scholar]
- [78].Henley DV, Korach KS, Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function, Endocrinology 147(6 Suppl) (2006) S25–32. [DOI] [PubMed] [Google Scholar]
- [79].Li Y, Burns KA, Arao Y, Luh CJ, Korach KS, Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro, Environ Health Perspect 120(7) (2012) 1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hall JM, McDonnell DP, Korach KS, Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements, Mol Endocrinol 16(3) (2002) 469–86. [DOI] [PubMed] [Google Scholar]
- [81].Daniels K, Daugherty J, Jones J, Mosher W, Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15–44: United States, 2011–2013, Natl Health Stat Report (86) (2015) 1–14. [PubMed] [Google Scholar]
- [82].Charlton BM, Molgaard-Nielsen D, Svanstrom H, Wohlfahrt J, Pasternak B, Melbye M, Maternal use of oral contraceptives and risk of birth defects in Denmark: prospective, nationwide cohort study, Bmj 352 (2016) h6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].McLachlan JA, Commentary: prenatal exposure to diethylstilbestrol (DES): a continuing story, Int J Epidemiol 35(4) (2006) 868–70. [DOI] [PubMed] [Google Scholar]
- [84].Newbold RR, Lessons learned from perinatal exposure to diethylstilbestrol, Toxicol Appl Pharmacol 199(2) (2004) 142–50. [DOI] [PubMed] [Google Scholar]
- [85].National Toxicology Program, Multigenerational reproductive toxicology study of ethinyl estradiol (CAS No. 57-63-6) in Sprague-Dawley rats, Natl Toxicol Program Tech Rep Ser (547) (2010) 1–312. [PubMed] [Google Scholar]
- [86].Silva P, Rocha MJ, Cruzeiro C, Malhao F, Reis B, Urbatzka R, Monteiro RA, Rocha E, Testing the effects of ethinylestradiol and of an environmentally relevant mixture of xenoestrogens as found in the Douro River (Portugal) on the maturation of fish gonads--a stereological study using the zebrafish (Danio rerio) as model, Aquatic toxicology 124–125 (2012) 1–10. [DOI] [PubMed] [Google Scholar]