Abstract
Mortality due to opioid use has grown to the point where, for the first time in history, opioid-related deaths exceed those caused by car accidents in many states in the United States. Changes in the prescribing of opioids for pain and the illicit use of fentanyl (and derivatives) have contributed to the current epidemic. Less known is the impact of opioids on hippocampal neurogenesis, the functional manipulation of which may improve the deleterious effects of opioid use. We provide new insights into how the dysregulation of neurogenesis by opioids can modify learning and affect, mood and emotions, processes that have been well accepted to motivate addictive behaviours.
The endogenous opioid system consists of approximately 30 different opioid peptides, including β-endorphins, Met5enkephalin and Leu5-enkephalin, orphanin FQ (also known as nociceptin) and dynorphins. These opioid peptides bind to their cognate G protein-coupled receptors, namely, the μ-opioid peptide receptor (MOP; also known as MOR), δ-opioid peptide receptor (DOP; also known as DOR), κ-opioid peptide receptor (KOP; also known as KOR) and nociceptin opioid peptide receptor (NOP; also known as OPRL1). The endogenous opioids and their receptors are expressed by various cell types and widely distributed throughout the body, including the central and peripheral nervous systems, immune cells, the adrenal medulla and the gonads, with the potential to modulate many different physiological and psychological processes (FIG. 1). From the distribution of the opioid peptides throughout various brain regions, it is clear that the endogenous opioid system has functions beyond the modulation of pain perception. For example, in addition to their actions at the spinal cord and periaqueductal grey matter in modulating pain transmission, enkephalins are located within the amygdala, where they regulate emotional responses. Their presence in the autonomic nuclei of the hypothalamus is important for the regulation of cardiovascular and/or respiratory function, and they exert broad effects on anterior (and posterior) pituitary hormone secretion, including stimulating the release of prolactin, growth hormone and adrenocorticotropic hormone and inhibiting the release of luteinizing hormone, oxytocin and arginine vasopressin.
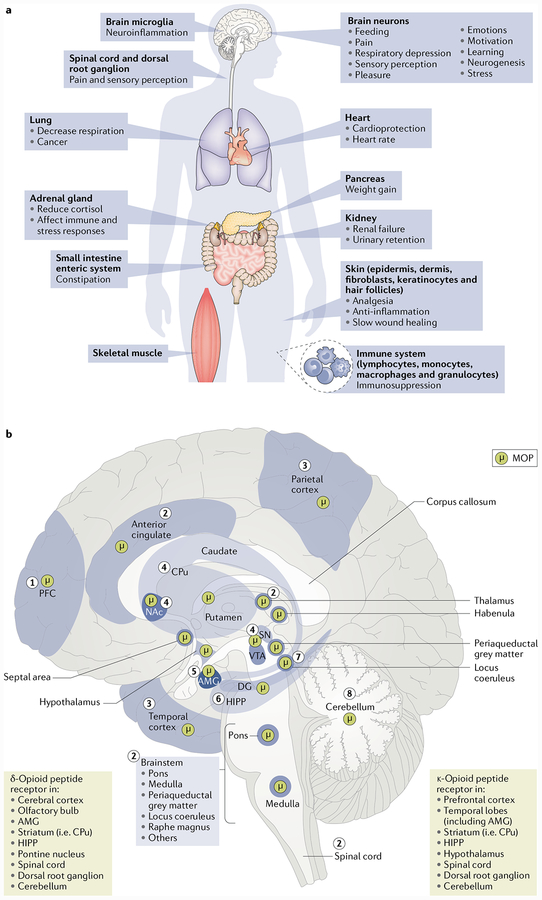
Fig. 1 |. Opioid actions throughout the body.
a | The human body naturally synthetizes opioids, which are used as neurotransmitters to regulate many vital functions. Endogenous and exogenous opioids act through receptors found peripherally on nerve terminals innervating the adrenal glands, pancreas and gastrointestinal tract and on immune, epidermal and dermal cells140,141, thus modulating steroid production, body weight via insulin secretion, opioid-induced constipation, inflammation and wound healing140,142–145. b | Opioids also activate centrally located receptors, such as the μ-opioid peptide receptor (MOP), that play a variety of roles in brain function. Control of food and drug intake is regulated by MOP in the prefrontal cortex (PFC) (1); analgesia, slow breathing and relaxation can be induced by the activation of MOP in the anterior cingulate, thalamus, brainstem nuclei, spinal cord and dorsal root ganglia (2); sensory perception can be influenced by MOP in parietal and temporal cortices (3); motivation, desire and associative learning involve stimulation of MOP in the nucleus accumbens (NAc), caudate-putamen (CPu) nuclei, ventral tegmental area (VTA) and substantia nigra (SN), key structures of the reward system (4); MOP is expressed in the amygdala (AMG), which is required for emotional conditioned learning and responses (5); MOP activation in the hippocampus (HIPP) can alter learningand neurogenesis (6); and MOP is present in both the locus coeruleus, a structure important in stress and drug withdrawal (7), and the cerebellum (8)2,6,61,141,146,147. In general, the expression of opioid peptides in projection neurons, immune cells, or epidermal and dermal cells overlaps with the location of opioid receptor expression, reflecting the autocrine, paracrine and endocrine mechanism of action of endogenous opioids61,140. DG, dentate gyrus.
The hippocampus is a key brain structure crucial in learning and memory, especially in contextual memory. The opioid peptides and their receptors are expressed in all hippocampal regions1–3, including the dentate gyrus (DG). The action of exogenous opioid drugs in the hippocampus is likely involved in the effects of opioids on learning and memory. Indeed, opioid drugs can impair anterograde and retrograde recall in patients with pain4. The other effect of opioids that is clearly linked to learning and memory is the development of addiction. Addiction and relapse have been interpreted as resulting from an abnormally robust memory formed during the opioid-taking experience5. Because the hippocampus is also included in the reward circuitry (Fig. 2), the opioid-dependent mnesic effects are also associated with the extensively studied positive (that is, pleasure) and negative (that is, stress, depression and anxiety related to withdrawal) affect and emotion, as well as the incentive salience characterizing the consumption of opiates6. The role of opioids in regulating both memory and affect, as well as emotion, is important in view of the current epidemiological context of opioid drug addiction, which is at an all-time high in North America, where opioid overdose is one of the leading causes of death. Indeed, the concept that both affective dysregulation and learning are the driving forces behind addictive behaviours, where the disruption of consolidating the contextual memory associated with drug experiences and rewards may have therapeutic potential to prevent relapse6–8, has gained attention in the past decade5.
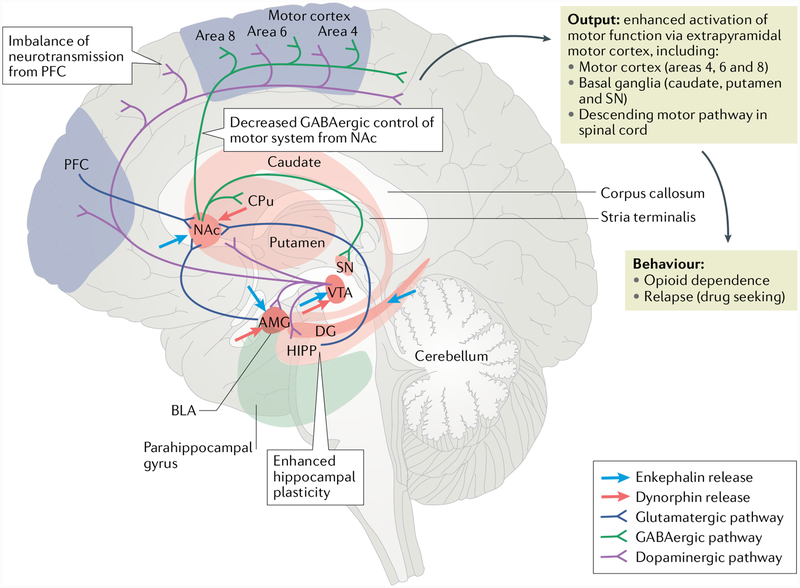
Fig. 2 |. Neural pathways involved in opioid-mediated mnesic changes.
Aberrant neuroadaptations in three associative memory systems have been described to contribute to drug addiction6. These systems include conditioned-incentive learning supported by the nucleus accumbens (NAc) and amygdala (AMG); habit learning depending on the caudateputamen (CPu); and declarative and contextual memory encoded by the hippocampus (HIPP)6. The NAc, AMG and CPu are also key components of the reward-salience-emotion circuit. The centrally localized NAc processes context and cue information from the HIPP — probably conveyed by glutamatergic projections — and is associated with the reinforcing effects of opioids, reflected by increased dopaminergic transmission from the ventral tegmental area (VTA). Opioids facilitate dopamine release in the NAc both directly and indirectly by inhibiting GABAergic control over the dopaminergic neurons in the VTA148. The opioid system influences the memory systems via enkephalinergic projections into the HIPP, NAc, AMG and VTA and via dynorphin projections into the NAc and/or AMG2,6,146. Additionally, the NAc integrates glutamatergic inputs from the basolateral amygdala (BLA), which encodes the environmental affective value during a drug experience. After long-term opioid exposure, the summed information resulting from the convergence of all these afferences131 exits the NAc and is converted into the behaviour of opioid dependence or relapse to opioid seeking via enhanced extrapyramidal motor system activity133,148. Decreased GABAergic control of the motor system from the NAcand imbalance in neurotransmission in and from the prefrontal cortex (PFC) are hypothesized to contribute to the loss of inhibitory control and poor decision-making, leading to drug-induced reinstatement6,148. Opiate-withdrawal-related memory, reflected by enhanced hippocampal plasticity during opiate withdrawal, can also facilitate relapse5. DG, dentate gyrus; SN, substantia nigra. Adapted from REF149, CC-BY-2.0 (https://creativecommons.org/licenses/by/2.0/uk/).
The presence of opioid sites of action in the hippo-campus also explains the opioid modulation of neurogenesis, which is a phenomenon that occurs in two neurogenic brain regions, one of them being the DG9–16. Adult neurogenesis refers to the generation of new neurons in adult brain17 (FIC. 3a), and it has been widely recognized to influence cognitive processes18 such as affect, mood, emotion19–21, learning and memory22,23. Activity of the new neurons selectively integrated into existing circuitry can be influenced by animal experiences24 and may modify the hippocampal synaptic plasticity involved in learning and memory (that is, long-term potentiation (LTP))25. Thus, neurogenesis presents itself as a common mechanism through which opioids can alter affect, mood, emotion, learning and memory. Targeting neurogenesis in adults will provide new perspectives for the development of antiaddiction therapies. The other non-nociceptive opioid effects, such as respiratory depression, have not been correlated to learning and memory, affect and emotion, or neurogenesis to our knowledge. This Review highlights how opioid drugs can modulate adult neurogenesis in order to change learning and affect (that is, anxiety and depression). The term ‘opioids’ is often used instead of ‘opiates’ because of the difficulty in distinguishing between the direct effect of opiates on biological processes and the opiate-dysregulated endogenous opioid system, which in turn can regulate the same processes.
Opioid effects on neurogenesis
Many reports describe the opioid-receptor-mediated regulation of neurogenesis17 in both the embryonic and adult brain, along with in vitro studies using neural stem and progenitor cells (NSPCs) extracted from the brains of mice and rats. The results suggest the universal modulation by opioids of both embryonic and adult neurogenesis in a variety of brain sites. The difference between exogenous and endogenous opioids is noteworthy, because the modulatory effects of endogenous opioids are considered physiological, while the addictive effects of exogenous opioids, which are the primary focus of this Review, represent the pathological status.
Neural progenitor proliferation.
Although the majority of NSPCs in the CNS undergo differentiation and finally lose their capacity to divide, actively dividing cells in both embryonic and adult brains have nonetheless been observed. As an indispensable process in the maintenance of the stem pool and the generation of functional differentiated cells17,26,27, the proliferation of NSPCs is a crucial step in the regulation of neurogenesis (FIG. 3a). Thus far, the regulation of NSPC proliferation is con-sistently observed in response to many factors, including MOP-targeting opioid drugs17.
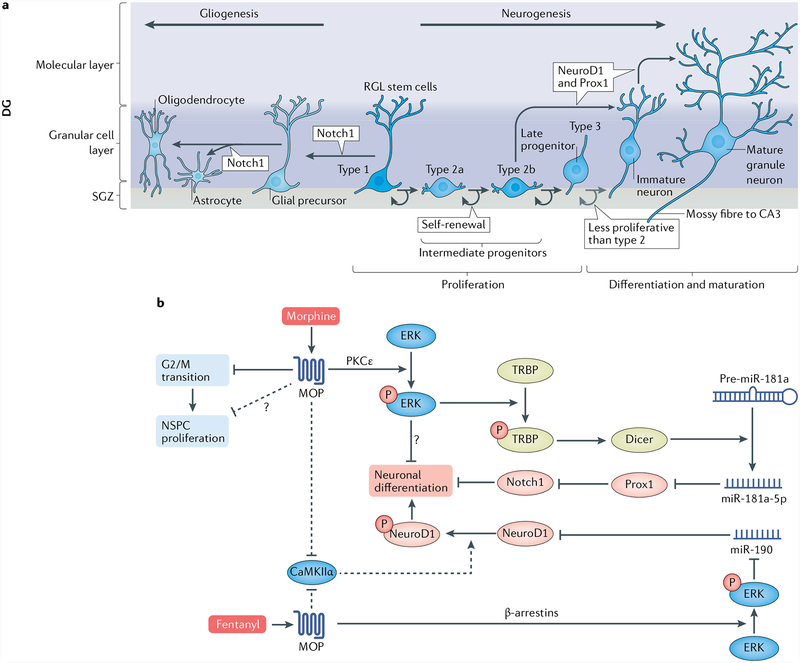
Fig. 3 |. Neurogenesis and biased MOP agonism in NSPC proliferation and differentiation.
a | The subgranuίar zone (SGZ) of the dentate gyrus (DG) provides a unique microenvironment where adult neural stem and progenitor cells (NSPCs) undergo several consecutive developmental stages characterized by different cell types17. NSPCs are derived from embryonic stem cells that survived in the adult SGZ. NSPCs self-renew and include type 1 radiaί-gίia-ίike (RGL) stem cells, which give rise to intermediate progenitor cells type 2a and 2b. These type 2 cells can generate type 3 progenitors (neuroblasts), which are capable of differentiating into immature neurons. During maturation, immature neurons become granule neurons and are functionally integrated into the DG pre-existing circuit. Type 2b and type 3 cells, immature and mature neurons, express the transcription factors NeuroD1 and Prox1, which are specific to granule cell development32. Notch 1 promotes astroglial but not neuronal differentiation17. b | μ-Opioid peptide receptor (MOP) agonists (such as morphine) inhibit NSPC proliferation mainly by slowing the cell cycle G2/M phase transition (prolonged S phase) in mouse embryonic NSPCs28, though details regarding the mechanism remain elusive. Other pathways leading to stagnating proliferation (indicated by the dashed arrow and question mark) might exist. Morphine induces extraceίίuίar-signaί-reguίated kinase (ERK) phosphorylation in a PKCε-dependent manner, and the activated ERK in turn phosphorylates TRBP, which is a stabilizer of Dicer, the essential enzyme for microRNA maturation43. The mature miR-181a-5p then inhibits the transcription factor Prox1, an inhibitor of another transcription factor, Notch1. Thus, increased Notch1 expression mediates the inhibition of neuronal differentiation15. The inhibition of neurogenic differentiation 1 (NeuroD1) phosphorylation is another pathway by which morphine affects the transition of NSPCs into immature neurons in adult mice16. It is inferred that although both morphine and fentanyl affect NeuroD1 phosphorylation by inhibiting CaMKIIα, a crucial enzyme that catalyses NeuroD1 phosphorylation, fentanyl was able to activate NeuroD1 via the β-arrestin-ERK-miR-190 pathway, thus reflecting the biased antagonism of MOP agonists44. Involvement of this pathway has been demonstrated only in cultured primary neurons74.
The antiproliferative effects of opioids on embryonic NSPC proliferation were shown in the dorsal telencephalon of the E15.5 embryonic mouse. Acute in utero morphine treatment resulted in a prolonged C2/M phase in both radial glial and basal progenitor cells, suggesting a role of morphine in inhibiting cell cycle progression and embryonic neurogenesis in the developing cortex28. More recently, it was found that acute morphine exposure inhibited the proliferation of NSPCs separated from cortices of E14 mice, which was reversed by the addition of naloxone, a non-selective antagonist of opioid receptors29. Similar results were discovered in NSPCs isolated from embryonic rat telencephalon, as chronic morphine treatment inhibited the proliferation rate of NSPCs in a concentration-dependent manner, and opioid receptor involvement was confirmed by a naloxone blockade.
The modulating effect of the opioid system on adult NSPC proliferation has been widely shown. This effect was first discovered by the labelling of 5-bromo-2’-deoxyuridine (BrdU)-positive cells in the DG of adult rats, which indicated an antiproliferative effect of both morphine and heroin9. Similarly, the cell cycle modulation of NSPCs in the adult mouse subgranular zone (SGZ) by morphine was further supported by using two endogenous cell cycle markers, proliferating cell nuclear antigen (PCNA) and phosphorylated histone H3 (pHisH3), along with BrdU labelling10. The impaired NSPC proliferation in adult animals was shown to be reversible, and drug withdrawal resulted in a rebound increase in proliferating cells, which was rescued to the normal level after 2 weeks of withdrawal11.
There are two noteworthy aspects of adult NSPC proliferation inhibition by morphine. First, the administration paradigm determines the effect of modulating NSPC proliferation. For example, pellet implantation, which produces a high and stable morphine concentration in the blood, resulted in a significant decrease in NSPC proliferation, whereas intermittent, single daily morphine injections had no significant effect13. Second, morphine modifies adult NSPC proliferation in a stagespecific manner14. For example, chronic morphine inhibited the proliferation of type 2b and type 3 NSPCs rather than cells of other stages30. The results above sup-port the antiproliferative effect of MOP-targeting opioid receptor agonists in both embryonic and adult NSPCs. In human fetal brain-derived NSPCs, a robust expression of KOP was observed and, in contrast to morphine, κ-agonists such as U50,488 and dynorphin 1–17 stimulated the proliferation and migration of these cells31. The effect of endogenous dynorphin remains unclear. The data point to differential effects of targeting different opioid receptor types.
NSPC differentiation and maturation.
Differentiation is the process that occurs when proliferating NSPCs give rise to offspring with different lineages and phenotypes. Regarding neurogenesis, neuronal differentiation of NSPCs and the following maturation of neuroblasts are crucial steps in determining the rate of newborn neuron generation32. The initiation of differentiation and line-age determination occurs at a stage as early as that of type 2 cells that feature limited self-renewal and transient amplification33, which is therefore a crucial stage in the study of neurogenesis modulated by opioids.
It was reported that enkephalin and U69,593, agonists of MOP and KOP, respectively, promoted the differentiation of embryonic stem cells (ESCs) to neuronal-oriented precursors via the activation of extracellular-signal-regulated kinase (ERK)34. However, both opioid agonists inhibited NSPC-derived neurogenesis and astrogenesis via their corresponding receptors by means of p38-mediated and ERK-mediated pathways, respectively35. This report was supported by a recent in vitro study, as neuronal differentiation of primary embryonic NSPCs was inhibited by morphine sulfate at both early and late stages of cellular differentiation, implicating the role of opioids in fetal brain development36. Findings from studies using neural ESCs support the role of the third type of opioid receptor, DOP, in embryonic neurogenesis and neuroprotection, as its selective agonist SNC80 could promote neural differentiation through the activation of Trk-dependent tyrosine kinase, along with the association of PI3K, PKC, CaMKII and MEK37. Moreover, buprenorphine, a partial agonist of MOP and antagonist of DOP and KOP, was found to decrease the pro-liferation and differentiation of cultured rat embryonic NSPCs by inhibiting brain-derived neurotrophic factor (BDNF) expression38. The effects of opioids on NSPC differentiation may rely on the administration paradigm, the targeted opioid receptors and the origins of embryonic NSPCs.
The role of opioids in the differentiation of adult NSPCs and the maturation of new neurons and glia has been clearly demonstrated by recent studies. The phenotypes of DG granule cells were shown to be substantially altered by repeated morphine administration, and a marked rebound was detected after 1 week of withdrawal, suggesting that morphine inhibits the neuronal differentiation of DG granule cells11. Moreover, it was found that chronic morphine administration pre-vented neuronal maturation by increasing the percent-age of type 2b NSPCs while decreasing that of type 3 cells31. By contrast, in vitro experiments showed that MOP and DOP antagonists were capable of promoting neuronal differentiation while inhibiting glial differentiation, supporting the involvement of opioid agonists in adult NSPC differentiation39. In the sub ventricular zone-olfactory bulb (SVZ-OB) system, however, it was found that naloxone inhibited neuronal differentiation induced by paced mating in female rats, indicating a role of endogenous opioids in facilitating, rather than inhibiting, the maturation of adult-born neurons40. In a recent study in mice, KOP agonists were found to promote oligodendrocyte differentiation and myelination, with therapeutic implications for multiple sclerosis41. Thus, it is clear that the endogenous opioid system, which modulates neural growth and development, is mimicked by exogenous opioids that disrupt neurogenesis by inhibiting the maturation of neurons42.
Our recent research further elucidated the effects of opioids on the differentiation and maturation of adult NSPCs. Although morphine and fentanyl are both agonists of MOP, only morphine can modulate NSPC differentiation by inducing astrocyte-preferential differentiation. This ability of morphine to control mechanisms of cell fate determination is attributed to its regulation of the miR-181a-Prox1-Notch1 pathway, which is a result of different signalling mechanisms of the two agonists leading to MAPK activation15 (FIG. 3b). Furthermore, the effect of morphine is due to its ability in activating the PKCε-TRBP-Dicer pathway, which modulates the processing of microRNA maturation43. Fentanyl, however, activates ERK via a β-arrestin-dependent pathway, thus resulting in decreased expression of miR-190, which targets and inhibits neurogenic differentiation 1 (NeuroD1), a transcriptional factor implicated in neuronal differentiation and maturation44 (FIG. 3b). NeuroD1 also participates in opioid mediated neurogenesis, as its overexpression rescues the morphine-induced loss of late-stage progenitors and immature neurons16. These studies reveal the complexity of mechanisms that control NSPC differentiation and exemplify the mechanism of biased agonism in the regulation of adult neurogenesis by opioids.
The findings discussed above indicate several critical signalling cascades that are responsible for the alterations of neurogenesis induced by opioids. These cascades include MAPKs and other signalling cascades of transcriptional factors, such as the Pax6-Ngn2-Tbr2-NeuroD1-Tbr1 and Prox1-Notch1 signalling pathways17. More recently, it was discovered that the regulator of G protein signalling 4 (RGS4) could regulate STAT5B-directed responses, which in turn modulate opioid-induced neurite outgrowth and neuronal differentiation45. These signalling cascades, along with biased agonism (the ligand-dependent functional selectivity of a receptor for certain pathways), represent common mechanisms underlying opioid effects on neurogenesis.
Adult neurogenesis in humans and rodents.
The role of adult neurogenesis disruption by long-term opioids has mainly been studied using addiction-related behaviours such as reward, opioid-associated contextual memory and mood changes46·47. Manipulations that increase adult hippocampal neurogenesis, either by environ-mental enrichment, chronic antidepressant treatment or exercise, are correlated with reduced drug taking and relapse. Conversely, manipulations that decrease neurogenesis, such as stress and schizophrenia, are associated with increased drug taking and relapse47. Most of these behavioural data have been shown in rodents. However, the importance could be disputed because of the current controversy regarding whether the adult human brain, like the rodent brain, continues to generate new neurons. Indeed, a study published in March 2018 by Sorrells et al.48 concluded that neurogenesis in the hippocampus decreases throughout childhood and stops in the adult (after adolescence). Research on neurogenesis-regulated processes with animals would be useless for medical advances if the adult human brain could not make new neurons. However, a more recent report from April 2018 by Boldrini et al.49 showed the contrary, namely, that neurogenesis is preserved in 79-year-old healthy adult humans. None of the patients presented psychiatric or chronic illness or drug and/or alcohol history, which, like their lifestyle, can influence the birth of new cells. The known history of the analysed post-mortem brains in the more recent study and the more accurate methodology tend to add more validity to the persistence of neurogenesis throughout human ageing. Moreover, over the past two decades, birth dating studies in different brain areas using BrdU50 or 14C (REF.51) have supported the existence of adult human neurogenesis52. Despite the controversy, there is still the possibility that activities such as exercise and/or the administration of neurogenic molecules could reactivate an endogenous program of neurogenesis and generate new neurons in the human brain, as it has been demonstrated in rodents53,54 and monkeys55. Therefore, strategies that target neurogenesis to rescue opioid-dependent learning and memory impairments should continue to be explored.
Opioid system, learning and memory
The strong association of environmental, and possibly interoceptive, cues with various hedonic aspects of drug experience is considered maladaptive. Indeed, this pathological opioid-associated memory undoubtedly renders abstinent patients more vulnerable to relapse when exposed to opioid-associated cues and/or context.
Opioid-associated memory.
The reinforcing effects of opioids and the underlying motivational circuit charac-terize opioid-associated learning. The opioid hedonic effect increases the motivation for further consumption by reinforcing the memories of the association between the opioid’s feeling of relief (negative reinforcement) and/or pleasure (positive reinforcement), the drug taking and the exteroceptive and interoceptive cues56. These opioid-associated memories are also reinforced by a negative affect following dissipation of the opioid hedonic effect and precipitation of physical and emotional withdrawal, which motivate the individual to carry out compulsive drug seeking6. The rewarding and/or reinforcing effects of opioids influence memory processes, from encoding and storage to retrieval (Supplementary Box 1), with the opioid reward circuitry sharing the same major neural substrates as the memory system6 (FIG. 2).
Opioid-associated learning necessitates primarily MOP activation, as demonstrated by the abolishment of morphine57·58 and heroin59 conditioned place preference (CPP) in MOP−/− mice and by the reduction of morphine self-administration in these mutant mice60 (BOX 1; Supplementary Table 1). DOP mediates drug-context learning, as indicated by impaired morphine CPP in DOP−/− animals61. However, DOP is involved in the contextual encoding but not the motivational aspect of a drug-associated experience because non-spatial cues predicting drug reward (for example, sound) restored morphine CPP in DOP−/− mice62. KOP activation does not appear to be directly involved in opioid-associated memory, as suggested by unchanged morphine CPP in KOP−/− mice63 and dynorphin knockout mice64. Regarding endogenous opioids, it remains unclear how they control maladaptive opioid-associative learning and memory, although dysregulation of a β-endorphin basal inhibitory tone may be involved in contextual-associated learning, as suggested by increased morphine CPP in β-endorphin−/− mice61,65,66 (BOX 2).
Box 1 |. Opioid-associated and non-opioid-associated learning and memory tasks.
Opioid-associated learning
Associative learning in opioid and drug abuse in animals is measured by conducting either classical (Pavlovian) or operant (instrumental) conditioning with opioids as reinforcers132.
Classical conditioning.
The conditioned place preference assesses drug (including opioid) contextual-associated memoryand is usually utilized to examine drug reward. The animal learns to associate an environment or context (conditioned stimulus) with opioid taking (unconditioned stimulus). Through this association, if the drug is hedonic, the animal is expected to spend more time in the drug-paired environment to seek the drug (conditioned response)133. If withdrawal is associated with the context, such as in the conditioned place aversion task, the subject will avoid spending time in that context134.
Operant (instrumental) conditioning.
Self-administration is the traditional model of drug abuse used to investigate the associative habit learning of drug taking. Self-administration is based on the reinforcing property of a drug to strengthen the learning of a stimulus (pressing a lever) and response (drug taking) habit that will gradually become a maladaptive, drug-directed form of habitual behaviour135.
Non-opioid-associated (or any addictive drug) learning
Non-opioid-associated (or any addictive drug) learning refers to learning that is motivated by reinforcers other than opioids (or any other addictive drugs). For example, the measurement of spatial learning with the Barnes maze, radial arm maze, T-maze or Y-maze involves a positive reward, such as food, as an incentive to learn the area layout136. The Morris water maze also assesses spatial learning and relies on the water semi-aversive property to motivate learning the location of an escaping platform thanks to distant spatial cues136. Passive and active avoidance tests and fear conditioning are fear-motivated associative avoidance tasks during which subjects learn to avoid an environment by associating a cue or the environment itself with an aversive stimulus (for example, foot shock) previously delivered136. The novel object recognition task involves exploiting rodents’ innate preference for novelty to evaluate recognition memory136.
Box 2 |. Transient dysregulation of the endogenous opioid system homeostasis.
Long-term opiate consumption and cessation cause transient disruption of the endogenous opioid system homeostasis. Therefore, the organism initiates allostatic changes in order to re-establish the neuronal systems7,137. Indeed, continuous circulation of exogenous opioids during chronic use overstimulates the endogenous opioid system. Consequently, inhibitory mechanisms take place to physiologically and psychologically oppose the maladaptive hedonic opioid effects and maintain homeostasis7,137. upon opiate removal, the compensatory actions against the endogenous opioid system effects become particularly important and unopposed and may interfere with the endogenous opioid system inhibitory control of, for example, aversive learning or stress, which can render abstinent patients with opiate dependence more susceptible to relapse.
For instance, following chronic morphine treatment in rodents, preproenkephalin transcription is decreased in structures responsible for reward and habit learning, such as the caudate-putamen (CPu) and nucleus accumbens (NAc), and increased in the hippocampus and cortex, both of which are involved in declarative learning and memory61. Endogenous opioids attenuate maturing neuron survival12. Hippocampal preproenkephalin upregulation may interfere with neurogenesis and thus cue-drug association learning. Under morphine withdrawal, preproenkephalin is downregulated in the CPu, NAc and pons, which are components of the basal ganglia, which controls voluntary motor movements and procedural learning61. By contrast, the dysphoric prodynorphin is upregulated following withdrawal from chronic intermittent injections in the CPu and rostral NAc61. Both enkephalin and β-endorphin inhibit the hypothalamic-pituitary-adrenal (HPA) axis response to stress88,138. Enkephalin may also mediate a basal hedonic tone and basal-ganglia-mediated motor control65. Therefore, both the preproenkephalin downregulation and prodynorphin upregulation during opioid abstinence and withdrawal could lead to a decrease in enkephalin’s inhibitory control over the HPA axis and increased anxiety, respectively88. These phenomena may contribute to stress-related impulsive behaviour, which in turn may impair the executive function, working memory and fluid intelligence observed in early abstinence in patients with opioid dependence79.
During chronic morphine exposure, prepro enkephalin upregulation in the hippocampus67 or other endogenous opioids may interfere with hippocampal neurogenesis functioning, which potentially under-lies opioid-associated memory16,46 (FIG. 4). Endogenous opioids decrease maturing neuron survival, as observed in MOP−/− mice12. Opiates such as morphine reduce the proliferation of progenitors and stimulate gliogenesis at the expense of neuronal differentiation15. To draw the mechanisms by which the modulation of neurogenesis could underlie the development of strong but maladaptive opioid-associated memories, we must consider neurogenesis modulation in correlation with every step of an opioid-associated memory task and during withdrawal. Indeed, neurogenesis modulation affects drug-associated learning processes in a time-dependent manner53,68. In one of the rare correlative studies, morphine was shown to decrease NeuroD1 activity, possibly via inhibition of CaMKIIα, the day of the CPP test (day 15 — one day after the conditioning session)69. During that day, the mice did not receive a morphine injection. Thus, it is unclear whether the NeuroD1 decrease in activity is related to morphine context acquisition during training or to the beginning of abstinence or extinction (that is, the new association ‘context-no morphine’). Furthermore, an absence of reducing effect of fentanyl on NeuroD1 activity69 reflects a biased agonism that adds more complexity to the role of neurogenesis in opioid-associated learning and/or memory.
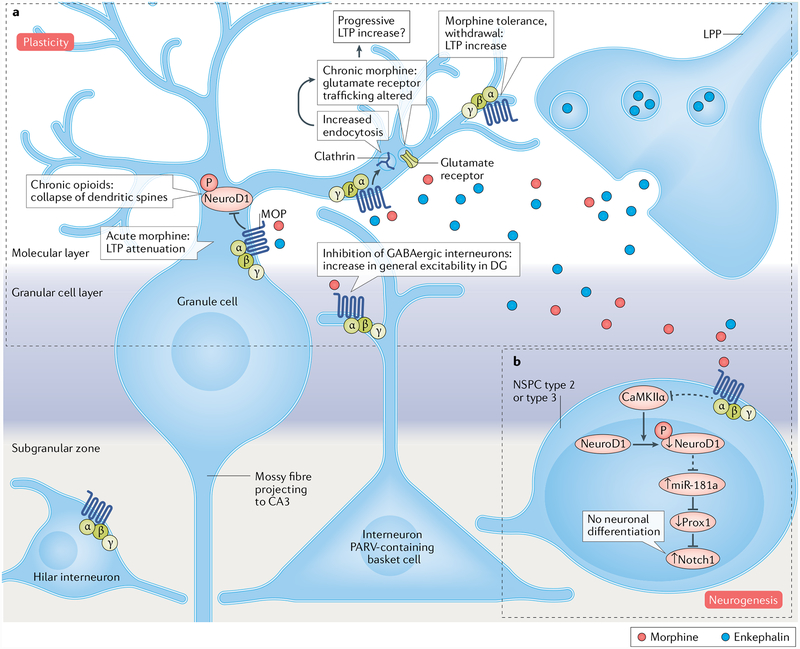
Fig. 4 |. Opioids alter normal learning by potentially regulating neurogenesis and plasticity.
a | In the dentate gyrus(DG) of the hippocampus, opioid receptors are located in the molecular layer, the granule cell layer3,150 and the hilus150 (not included in part a). The μ-opioid peptide receptor (MOP) is predominantly in distinct subpopulations of GABAergic interneurons known to inhibit granule cells: for example, the MOP is common in parvaίbumin (PARV)-containing basket cell terminals that form inhibitory-type synapses with granule cell somata and dendrites, occurs occasionally in interneurons that innervate other interneurons (not represented) and appears in a modest number of hilar interneurons150. MOP immunoreactivity is also seen in some dendrites of granule cells150. Enkephalins are released by the lateral perforant path (LPP) projecting into the molecular layer150. Acute morphine infusion induces long-term potentiation (LTP) attenuation in LPP-granuίe cell synapses75. Prolonged morphine exposure and the endogenous opioid tone decrease dendritic spine density possibly via reduced activity of neurogenic differentiation 1 (NeuroD1)70,71,74,151, a neurogenic transcription factor expressed in cells such as the immature postmitotic granule neurons. Chronic morphine treatment (48 hours) also stimulates clathrin-mediated endocytosis, which alters glutamate receptor trafficking73,78, thus leading to glutamatergic neurotransmission and LTP changes. After chronic administration (72 hours), tolerance to the effects of morphine enhances LTP in the same way as for opioid antagonists75. Opioid-agonist-mediated or opioid-antagonist-mediated LTP modulations may cause learning impairment or improvement, respectively. b | In the sub granular zone, opioids are assumed to directly act on neural stem and progenitor cells (NSPCs) with MOP on the cell surface39,47. Morphine disrupts neurogenesis by attenuating the activity of NeuroD1, which is also expressed in late-stage dividing type 2b and type 3 NSPCs. Reduced NeuroD1 activity is correlated with up regulation of the microRNA miR-181a (dashed line, potential pathway), which silences Prox1 expression. The decrease in Prox1, being an inhibitor of Notch1, results in an increase in the levels of Notch1, whose role is to block neuronal differentiation15, thus affecting normal learning.
Chronic morphine decreases dendritic spine stability in rodent primary hippocampal neuronal cultures (a downstream event modulated by NeuroD1)70–74 and also modulates hippocampal-dependent LTP71. LTP, a substrate of learning and memory, is influenced by neurogenesis5,25. There is confusion in interpreting the role of LTP in opioid-associated memory because there are few studies that correlate the time course of the LTP modulation by the opioids with each process of associative learning75,76. However, one of the few reports demonstrated that acute morphine infusion (1 hour) in lateral perforant path (LPP)-granule cell synapses of the DG attenuated LTP induction, while chronic morphine infusion for 72 hours augmented LTP75. Similarly, hippocampal slices from morphine-dependent rats maintained in artificial cerebrospinal fluid (ACSF) with morphine (to prevent spontaneous withdrawal) did not express CA1 LTP. By contrast, hip-pocampal slices from morphine-dependent rats maintained in ACSF with naloxone (precipitated withdrawal) or ACSF alone (spontaneous withdrawal) displayed LTP76. Therefore, the morphine-mediated enhanced LTP may not be involved in so-called opioid-cue association learning but may be the result of the dissipation of the morphine’s effects during withdrawal. This hypothesis of increased LTP reflecting morphine tolerance is supported by the fact that, unlike acute morphine, endogenous opioid blockade by naloxone, CTAP (MOP-targeting) or nor-BNI (KOP-targeting) increases mossy fibre tetanus-induced LTP in wild-type rodents77. Moreover, morphine decreases glutamatergic transmission in primary hippocampal neurons78.
Non-opioid-associated memory.
In humans, opioid drug administration affects daily task performances requiring forms of memory that use reinforcers (that is, motivators) different from opioids4,79,80 (Box 1; Supplementary Box 1 and Supplementary Table 2). In rodents, acute and chronic morphine and/or heroin impairs hippocampus-dependent spatial acquisition81,82 and retention and retrieval when injected after acquisition83. The same retention deficits were observed after β-endorphin, endomorphin 1 and endomorphin 2 administration84,85. By contrast, the opioid antagonist, either naloxone or naltrexone, facilitates spatial acquisition86 and retention87. These results suggest that there is an endogenous opioid basal tone that inhibits learning, similar to that observed in β-endorphin−/− mice tested for morphine-associated memory65. The naltrexone-mediated or naloxone-mediated improved learning might result from enhanced stress generated from the endogenous basal tone removal88. The role of the endogenous opioid tone inhibition in enhancing spatial learning is questioned by the behavioural discrepancies between the genetic backgrounds and mutations of knockout animals. Studies using MOP−/− mice with a 100% C57BL/6 background and MOP exon 1 excised exhibited intact acquisition and first retrieval86,89–91. By contrast, MOP−/− mice with a hybrid genetic background (that is, 50% 129SVJ and 50% C57BL/6 or 129/Ola-C57BL/6) or MOP exons 2and 3 deleted displayed impaired spatial learning57,92,93. These discrepancies between the different genetic backgrounds need to be clarified.
The aforementioned opioid-induced mnesic deficits in tasks using reinforcers other than opioids may involve neuronal plasticity and neurogenesis modulation. Less well described is the opioid-antagonist-mediated learning facilitation in 100% C57BL/6 mice, which could be of interest for the development of promnesic treatments. One of the few proposed mechanisms suggests that chronic naltrexone increases hippo-campal glutamatergic neurotransmission to facilitate learning77,86. However, studies using MOP−/− mice with a 129SVJ background showed, by contrast, impaired learning associated with attenuated LTP in the CA3 (REF92) and the DG94. The lower level of neurogenesis in the 129SVJ strain of mice compared with the 100% C57BL/6 mice should be taken into account when considering the behavioural and electrophysiological differences95. Again, the contradicting results linked to the differences between genetic backgrounds must be resolved. The other hypothesis relies on the fact that naltrexone increases the number of new neurons39,96. The inhibition of the MOP endogenous tone may stimulate neurogenesis to positively influence long-term memory or even prophylactically delay or improve neuronal dysfunction in neurodegenerative disorders. No studies have assessed the effects of an opioid antagonist on neurogenesis in relation to both memory and neurodegeneration (for example, such as observed in dementia), but it is noteworthy that depot naltrexone treatment of opioid use disorder is having unanticipated success in reducing opioid craving97.
Treatment of maladaptive memory.
Current treatments such as methadone replacement therapy or naltrexone maintenance treatment do not address the issue of addiction in opioid addicts, who must follow these therapies throughout their lives to maintain permanent abstinence98. One of the strategies adopted by researchers to prevent relapse in patients recovering from opioid dependence is to pharmacologically enhance cue-exposure therapy (CET). During CET, subjects are exposed to repeated extinction trials that result in cues and context previously associated with drug taking becoming associated with the lack of drug98–100. Drug candidates for the development of anti-relapse treatments combined with CET act through direct modulation of the neurotransmission systems. A partial coagonist of the glutamatergic NMDA receptor, D-cycloserine, and an uncompetitive NMDA antagonist, memantine, are among these candidates and have the advantage of having been clinically tested101,102. D-Cycloserine facilitates the extinction of morphine-withdrawal-associated place aversion in morphine-dependent rats102 and has already achieved positive results in CET for people with fear and anxiety disorders102. Memantine was clinically effective in reducing physical withdrawal signs in patients with heroin dependence101 and attenuates the maintenance of morphine self-administration in mice103.
Another approach focuses on the phenomenon of reconsolidation occurring in parallel to learning the new ‘cue-no drug’ association during extinction and CET100,104. Reconsolidation is the process in which the formerly acquired cue-drug memory becomes labile when reactivated or retrieved during extinction and subsequently restabilized into long-term storage through new protein synthesis after being subjected to reminders100,104. Treatments that interfere with reconsolidation would lessen the cue-drug association, its drug-seeking triggering effect and the probability of relapse. A few attempts to disrupt reconsolidation in opioid addiction were made by inhibiting ERK, protein synthesis with rapamycin, muscarinic acetylcholine receptors with scopolamine, NMDA receptors with ketamine and β-adrenergic receptors with propranolol99,105. All abolished the previously learned morphine CPP when administered after re-exposure to a drug-paired environment. Other potential new treatments that interrupt reconsolidation, such as blocking PKA activity with the inhibitor Rp-cAMPs, were effective in attenuating cue-induced reinstatement in cocaine self-administration99 but need to be tested in opioid addiction.
An emerging anti-relapse strategy corresponds to the time-dependent manipulation of adult hippocampal neurogenesis via a pharmacological agent. Adult hippocampal neurogenesis has a time-dependent differential effect on drug-associated memory68. Our recent data published in 2017 regarding a novel synthetic compound, KHS101, which is known to specifically accelerate neuronal differentiation54, showed that KHS101 injected before morphine CPP training prolonged the CPP extinction whereas when administered after morphine-induced CPP training, KHS101 shortened extinction53. The increase in neurogenesis that occurs after learning a cue-drug association or that occurs during a period of abstinence weakens the retention of the cue-drug association and the propensity of relapse. Molecules such as KHS101 or surgical interventions that stimulate neurogenesis such as NSPC transplantation106 may have therapeutic potential in drug or opioid addiction. These strategies based on modulating neurogenesis have therapeutic potential, not only because they can weaken drug-associated memory and behaviour but also because of the involvement of neurogenesis in regulating emotional disorders such as depression and anxiety.
Anxiety and depression
Opioids are superior analgesics for treating moderate-to-severe pain because of their ability to diminish not only the sensory experience (pain intensity) but also the emotional, affective dimension (that is, how much it bothers the individual) pain. Thus, opioid analgesics have the ability to allow one to dissociate from their pain such that they no longer care about it, in addition to decreasing pain intensity. This ability of opioids to modulate affect transcends to mood disorders, and MOP agonists, at least acutely, have been recognized for decades to have antidepressant effects107. MOP agonists, with the exception of tianeptine, are not generally prescribed for depression108. Buprenorphine (a partial MOP agonist with KOP antagonist proper-ties) is effective in alleviating symptoms of treatmentresistant depression109, and the KOP antagonism may contribute to its success in treating the negative affect experienced during addiction cycles. Chronic pain is often comorbid with mood disorders, which can range from 30–100% depending on the painaetiology. This co-occurrence of psychopathology in patients with chronic pain negatively influences their pain intensity, pain-related disability and the effectiveness of treatment (BOX 3). Additionally, long-term opioid use can precipitate depressive episodes110. We recently proposed that learning and memory processes are engaged in creating the association between opioid use and negative reinforcement (relief of negative affect, including during withdrawal) and that this memory contributes to drug relapse following prolonged periods of abstinence, because the learned association between opioid use and negative reinforcement may be generalizable to different stressors7. We surmise that in patients with chronic pain, the salient value associated with initial opioid use is pain relief, but it transitions to the alleviation of stress or depressed mood causative, or not, to the occurrence of pain. We proposed that subsequent stressors will trigger memories of the learned associations between opioid use and drug relief of dysphoric states, which could trigger drug relapse7. Many elegant studies have demonstrated that adult hippocampal neurogenesis contributes to the effectiveness of antidepressants18,111, and it is not unreasonable that the antidepressant actions of opioids and the learned association between opioid use and negative reinforcement may engage adult neurogenesis. Although most studies suggest that MOP agonists negatively affect or disrupt hippocampal neurogenesis9–11,15–17,28,29,31,35,36,43,46,112, the opposite may occur during cycles of withdrawal or via KOP, as KOP antagonists have antidepressant actions113. Furthermore, KOP is expressed on neural stem cells and NSPCs in the hippocampus, suggesting that KOP may modulate neurogenesis114.
Box 3 |. Risks associated with opioid misuse.
Risks associated with opioid misuse
High catastrophizing scores in chronic pain are associated with craving
Distress intolerance (the perceived or actual inability to manage negative emotional and somatic states)
Previous history of illicit drug use
Anxiety, depression or anger
Aberrant drug-related behavior
Sleep disturbance/low average number of hours of sleep
Sex (males)
Factors that decrease opioid misuse
For every 1-hour increase in the average number of hours of nightly sleep, the risk of misuse decreased by 20%139
Patients with chronic pain with notable psychopathology are more likely to report the following:
Heightened pain intensity
Increased pain-related disability
More negative mood
Reduced effectiveness of opioid analgesics compared with the effectiveness in patients with pain and no psychopathology
The link between neuropsychiatric disorders and adult DG neurogenesis has been widely recognized115. Stress and anxiety phenotypes are associated with a transient reduction in neurogenesis116. Humans with major depressive disorder have smaller hippocampi and fewer mature granule cells in the DG, which may be a result of reduced neurogenesis117. Supporting this association is the observation that the ablation of neurogenesis gives rise to anxiety and depression-like behaviour in rodents118. Moreover, recent studies revealed the requirement of new DG neurons for antidepressant efficacy119,120. More direct evidence supporting the association between adult neurogenesis and mood disorders was obtained by manipulating new DG neurons in the absence of substantial stress, showing an anti-anxiety effect after the stimulation of mature DG neurons121.
The KOP exemplifies one of the most extensively studied systems involved in both mood disorders and adult neurogenesis107. KOP agonists and antagonists elicit depressive and antidepressive-like effects, respectively122,123. Various mechanisms and circuits involving monoamine transmitters have been pro-posed to account for these phenotypes, one of which is the involvement of KOP in adult neurogenesis. Buprenorphine, an antagonist of KOP, is a negative modulator of NSPC proliferation112. The antidepressant effects of KOP antagonist nor-BNI have been proposed to be due partly to an increase in BDNF expression in the hippocampus124, which is well known to be a mechanism associated with the antidepressant effects of selective serotonin reuptake inhibitors (SSRIs). The DOP system also regulates affective-like behaviour, where constitutive knockout ofthe DOP leads to an anxiogenic phenotype107. Like KOP antagonists, DOP agonists produce antidepressant effects in rodent models, and this phenotype has been associated with increases in BDNF expression125.
The role of the endogenous opioid system, especially the human MOP system, in depressive disorder has been underlined by a growing number of studies in recent years. For example, patients with depression showed MOP deactivation in the amygdala during social rejection, whereas healthy controls showed MOP activation in multiple brain regions126. This observation suggests the involvement of altered endogenous opioid activity in depression, which may help us yield novel therapeutics by using MOP agonists with a low risk of abuse127. Thus, we hypothesize the association between the endogenous opioid system, adult neurogenesis and mood and consider opioid-regulated neurogenesis and gliogenesis as novel targets for the treatment of psychiatric disorders.
Conclusion and perspectives
Knowledge of the impact of long-term opioid use on multiple biologic functions gives insight into how complex and challenging the treatment of the adverse effects of opioids can be. The most effective current medical intervention for individuals with opioid addiction is to substitute short-acting, euphoric opioids (for example, heroin) with long half-life and partial agonists such as buprenorphine or methadone to keep patients in a state where they are prevented from experiencing the reward that occurs following the administration of short-acting opioids, as well as alleviating withdrawal128. However, opioid maintenance therapy is defined as a harm reduction strategy aimed at relieving the cravings and symptoms of withdrawal rather than the prevention of opioid use128. The problem of relapse remains. Long-term exogenous opioid use alters the communication between the endogenous opioid system and the circuits involved in learning, memory and affect and the process of neurogenesis. The fact that addiction involves multiple brain functions and their underlying circuits is important when considering therapy. The treatment of addiction cannot be undertaken by only one modality.
A novel strategy that targets simultaneously opioidassociated maladaptive learning and memory, mood alteration and opioid reward via a common mechanism such as neurogenesis is worth studying for its antiaddiction therapeutic potential. Newborn healthy cells have the advantage of being able to rebuild a healthy environment, which may help to rebalance brain chemistry in patients with addiction106,129 and lead to complete healing. Moreover, therapies based on promoting in situ neuronal generation may offer more healing potential than strategies based on modulators of neurotransmitters such as memantine and D-cycloserine. NSPC transplantation106 (that is, neural stem cell therapy) into specific brain areas involved in addictive behaviours could hypothetically have the same benefits as neurogenesis-based approaches. Indeed, in contrast to ESCs, the risk of tumour formation with NSPCs is reduced because NSPC differentiation is restricted to the neuronal and glial lineages130. The only limitation of both the neurogenesis-based strategy and NSPC transplantation is the possibility of propagation of the altered brain chemistry due to chronic opioid use from host to new cells or grafts such as in the case of Parkinson disease treatment130. Overall, cell replacement therapy has the potential to be the future of addiction therapy practice. Additionally, a holistic approach integrating personalized counselling, nutritional support, lifestyle modifications and/or mindfulness-based interventions131 is required to avoid psychological and environmental causes and risk factors for relapse.
Supplementary Material
Acknowledgements
Funding from the National Institutes of Health DA031442 (P-Y.L. and C.K.), the National Natural Science Foundation of China 81701313 (C.X.) and the Shirley and Stefan Hatos Neuroscience Research Foundation DA005010 (C.J.E. and C.M.C.) is gratefully acknowledged.
Glossary
- Opioid
A broad term used to designate all substances, natural (for example, morphine) and synthetic (for example, fentanyl), that bind to opioid receptors in the nervous system
- Hippocampus
A major anatomical structure located in the medial temporal lobe of the mammalian brain that processes a unidirectional flow of information via a trisynaptic loop
- Learning
The process by which we integrate sensory information from our interaction with our environment for behavioural adaptation
- Memory
The record left by a learning process
- Affect
A broad range of feelings that people can experience, embodying both emotions and moods
- Emotion
An intense feeling that is short term and is typically directed at a source, often with facial expressions and body language
- Opiates
The natural alkaloid compounds found in the opium poppy plant Papaver somniferum
- Neurogenic brain regions
in the adult mammal, these include the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zone-oflactory bulb system
- Mood
A less specific and less intense state of mind than emotion that is less likely to be provoked by a particular event but lasts longer
- G2/M phase
A period of protein synthesis and rapid cell growth (C2) transitioning into division (M)
- Cell cycle
A series of consecutive phases —Cap 1 (C1) phase, DNA synthesis (S) phase, gap 2 (C2) phase (growth) and mitosis or meiosis (M) phase — that lead to the duplication and division of genetic information into two daughter cells
- 5-bromo-2’-deoxyuridine
(BrdU). A synthetic analogue of thymidine and marker of proliferating cells
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neuroscience thanks Z. Georgoussi and the other anonymous reviewers for their contribution to the peer review of this work.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41583-018-0092-2.
References
- 1.Arvidsson U et al. Distribution and targeting of amu-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci 15, 3328–3341 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansour A, Khachaturian H, Lewis ME, Akil H & Watson SJ Anatomy of CNS opioid receptors. Trends Neurosci 11, 308–314 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Stengaard-Pedersen K Comparative mapping of opioid receptors and enkephalin immunoreactive nerve terminals in the rat hippocampus. A radiohistochemical and immunocytochemical study. Histochemistry 79, 311–333 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Kamboj SK, Tookman A, Jones L & Curran HV The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain 117, 388–395 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Kutlu MG & Gould TJ Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn. Mem 23,515–533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent review examines the relationshipbetween the effects of various drugs, includingopiates, on hippocampus-dependent learning and memory and on drug addiction.
- 6.Koob GF & Volkow ND Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This key review describes the brain neurocircuitry that is engaged at each stage of the addiction cycle, how it changes with increasing involvement with drugs of abuse and how it produces the pathological state of addiction.
- 7.Evans CJ & Cahill CM Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res 5, 1748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent review presents arguments in supportof an addiction disease model whereby a learned association of drug relief from an aversive mental state, either pre-existing (such as depression or anxiety) or created by withdrawal, drives addictive-like behaviours in susceptible individuals.
- 8.Lee JL, Di Ciano P, Thomas KL & Everitt BJ Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47, 795–801 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Eisch AJ, Barrot M, Schad CA, Self DW & Nestler EJ Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl Acad. Sci. USA 97,7579–7584 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report showing that opiatesregulate neurogenesis in the adult rat hippocampus and suggesting that the decrease in neurogenesis may be one mechanism by which opiates influence hippocampal functions.
- 10.Mandyam CD, Norris RD & Eisch AJ Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J. Neurosci. Res 76, 783–794 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Kahn L, Alonso G, Normand E & Manzoni OJ Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur. J. Neurosci 21, 493–500 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Harburg GC et al. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience 144, 77–87 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer SJ et al. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administration paradigm. Neuroscience 151, 1217–1224 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Arguello AA et al. Effect of chronic morphine on the dentate gyrus neurogenic microenvironment. Neuroscience 159, 1003–1010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C, Zhang Y, Zheng H, Loh HH & Law PY Morphine modulates mouse hippocampal progenitor cell lineages by upregulating miR-181a level. Stem Cells 32, 2961–2972 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Xu C, Zheng H, Loh HH & Law PY Morphine modulates adult neurogenesis and contextual memory by impeding the maturation of neural progenitors. PLOS ONE 11, e0153628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Loh HH & Law PY Effects of addictive drugs on adult neural stem/progenitor cells. Cell. Mol. Life Sci 73, 327–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent review synthesizes knowledge on the different stages and cell types in adultneurogenesis and discusses the effects of various addictive drugs on progenitor cells, as well as the current understanding of the major signalling pathways underlying such effects.
- 18.Anacker C & Hen R Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci 18, 335–346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anacker C et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol. Psychiatry 79, 840–849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucassen PJ, Stumpel MW, Wang Q & Aronica E Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58, 940–949 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Revest JM et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 14, 959–967 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y & Gould E Neurogenesis may relate to some but notall types of hippocampal-dependent learning. Hippocampus 12, 578–584 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myhrer T Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res 41,268–287 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Kee N, Teixeira CM, Wang AH & Frankland PW Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci 10, 355–362 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Snyder JS, Kee N & Wojtowicz JM Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol 85, 2423–2431 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Lopez S, Lerner RG & Petritsch C Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell. Mol. Life Sci 71, 575–597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernal A & Arranz L Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell. Mol. Life Sci 75, 2177–2195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sargeant TJ, Day DJ, Miller JH & Steel RW Acute in utero morphine exposure slows G2/M phase transition in radial glial and basal progenitor cells in the dorsal telencephalon of the E15.5 embryonic mouse. Eur. J. Neurosci 28, 1060–1067 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Willner D et al. Short term morphine exposurein vitro alters proliferation and differentiation of neural progenitor cells and promotes apoptosis via mu receptors. PLOS ONE 9, e103043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arguello AA et al. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience 157, 70–79 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng WS et al. Human neural precursor cells express functional kappa-opioid receptors. J. Pharmacol. Exp. Ther 322, 957–963 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Kempermann G, Song H & Gage FH Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol 7, a018812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review describes the complex multistep process of adult neurogenesis and the restriction points at which regulation occurs.
- 33.Kempermann G, Jessberger S, Steiner B & Kronenberg G Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27, 447–452 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kim E et al. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J. Biol. Chem 281, 33749201333760 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn JW et al. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J. Neurochem 112, 1431–1441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dholakiya SL, Aliberti A & Barile FA Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol. Lett 247,45–55 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Narita M et al. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem 97, 1494–1505 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Wu CC et al. Prenatal buprenorphine exposure decreases neurogenesis in rats. Toxicol. Lett 225, 92–101 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Persson AI et al. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur. J. Neurosci 17, 1159–1172 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Santoyo-Zedillo M, Portillo W & Paredes RG Neurogenesis in the olfactory bulb induced by paced mating in the female rat is opioid dependent. PLOS ONE 12, e0186335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du C et al. Kappa opioid receptor activation alleviates experimental autoimmune encephalomyelitis and promotes oligodendrocyte-mediated remyelination. Nat. Commun 7, 11120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser KF & Knapp PE Opiate drugs with abuse liability hijack the endogenous opioid system to disrupt neuronal and glial maturation in the central nervous system. Front. Pediatr 5, 294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C, Zheng H, Loh HH & Law PY Morphine promotes astrocyte-preferential differentiation of mouse hippocampal progenitor cells via PKCε-dependent ERK activation and TRBP phosphorylation. Stem Cells 33, 27625–2772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H et al. μ-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol. Pharmacol 77, 102–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pallaki P et al. A novel regulatory role of RGS4 in STAT5B activation, neurite outgrowth and neuronal differentiation. Neuropharmacology 117, 408–421 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Bortolotto V & Grilli M Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front. Pharmacol 8, 254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Loh HH & Law PY Effect of opioid on adult hippocampal neurogenesis. ScientificWorldJournal 2016, 2601264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorrells SF et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boldrini M et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22,589–599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Investigators of this new study analyse whole post-mortem hippocampi from brains of healthy human individuals ranging from 14 to 79 years of age and provide morphological evidence of the persistence of neurogenesis throughout human ageing.
- 50.Eriksson PS et al. Neurogenesis in the adult human hippocampus. Nat. Med 4, 1313–1317 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Ernst A et al. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Kempermann G et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23, 25–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Kibaly C, Xu C, Loh HH & Law PY Temporal effect of manipulating NeuroD1 expression with the synthetic small molecule KHS101 on morphine contextual memory. Neuropharmacology 126, 58–69 (2017). [DOI] [PubMed] [Google Scholar]; This recent study shows that an increase in adult hippocampal neurogenesis has a time-dependent differential effect on morphine-associated memory.
- 54.Wurdak H et al. A small molecule accelerates neuronal differentiation in the adult rat. Proc. Natl Acad. Sci. USA 107, 16542–16547 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perera TD et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLOS ONE 6, e17600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise RA & Koob GF The development and maintenance of drug addiction. Neuropsychopharmacology 39, 254–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthes HW et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Sora I et al. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology 25, 41–54 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Contarino A et al. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur. J. Pharmacol 446, 103–109 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Becker A et al. Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn Schmiedebergs Arch. Pharmacol 361, 584–589 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Le Merrer J, Becker JA, Befort K & Kieffer BL Reward processing by the opioid system in the brain. Physiol. Rev 89, 1379–1412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Merrer J, Faget L, Matifas A & Kieffer BL Cues predicting drug or food reward restore morphineinduced place conditioning in mice lacking delta opioid receptors. Psychopharmacology 223, 99–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simonin F et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488 H and attenuates morphine withdrawal. EMBO J 17, 886–897 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmer A et al. Absence of Δ−9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J. Neurosci 21, 9499–9505 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skoubis PD, Lam HA, Shoblock J, Narayanan S 83. & Maidment, N. T. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur. J. Neurosci 21, 1379–1384 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Charbogne P, Kieffer BL & Befort K 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76(Suppl. B), 204–217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basheer R & Tempel A Morphine-induced reciprocal alterations in Gαs and opioid peptide mRNA levels in discrete brain regions. J. Neurosci. Res 36, 551–557 (1993). [DOI] [PubMed] [Google Scholar]
- 68.Noonan MA, Bulin SE, Fuller DC & Eisch AJ Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci 30, 304–315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that the suppression of adult hippocampal neurogenesis via cranial irradiation after drug taking enhances resistance to the extinction of drug-seeking behaviour, suggesting that pro-neurogenic treatments during abstinence may prevent relapse.
- 69.Zheng H, Zhang Y, Li W, Loh HH & Law PY NeuroD modulates opioid agonist-selective regulation of 89. adult neurogenesis and contextual memory extinction. Neuropsychopharmacology 38, 770–777 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows that, via differential control of miR-190 levels, morphine and fentanyl exhibit differential regulation of NeuroD activity, thereby resulting in differential modulation of adult neurogenesis and the extinction and reinstatement of the CPP response.
- 70.Liao D, Lin H, Law PY & Loh HH Muopioid receptors modulate the stability of dendritic spines. Proc. Natl Acad. Sci. USA 102, 1725–1730 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao D, Grigoriants OO, Loh HH & Law PY Agonist-dependent postsynaptic effects of opioids on miniature excitatory postsynaptic currents in cultured hippocampal neurons. J. Neurophysiol 97,1485–21494 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao D et al. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol. Cell. Neurosci 35, 456–469 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moron JA et al. Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins: a proteomics study focusing on endocytic proteins. Mol. Cell. Proteomics 6, 29–42 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Zheng H et al. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J. Neurosci 30, 8102–8110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito Y, Tabata K, Makimura M & Fukuda H Acute and chronic intracerebroventricular morphine infusions affect long-term potentiation differently in the lateral perforant path. Pharmacol. Biochem. Behav 70, 353–358 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Salmanzadeh F, Fathollahi Y, Semnanian S & Shafizadeh, M. Dependence on morphine impairs the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Brain Res 965, 108–113 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Harrison JM, Allen RG, Pellegrino MJ, Williams JT & Manzoni OJ Chronic morphine treatment alters endogenous opioid control of hippocampal mossy fiber synaptic transmission. J. Neurophysiol 87, 2464–2470 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Kam AY, Liao D, Loh HH & Law PY Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J. Neurosci 30, 15304–15316 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rapeli P et al. Cognitive function during early abstinence from opioid dependence: a comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry 6, 9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McLellan J, Marshman LAG & Hennessy M Anterograde amnesia and disorientation are associated with in-patients without traumatic brain injury taking opioids. Retrograde amnesia (RA) is absent. RA assessment should be integral to post-traumatic amnesia testing. J. Clin. Neurosci 44, 184–187 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Spain JW & Newsom GC Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology 105, 101–106 (1991). [DOI] [PubMed] [Google Scholar]
- 82.Tramullas M, Martinez-Cue C & Hurle MA Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology 54, 640–652 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Ma MX, Chen YM, He J, Zeng T & Wang JH Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 147, 1059–1065 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Introini-Collison IB, Ford L & McGaugh JL Memory impairment induced by intraamygdala betaendorphin is mediated by noradrenergic influences. Neurobiol. Learn. Mem 63, 200–205 (1995). [DOI] [PubMed] [Google Scholar]
- 85.Ukai M, Watanabe Y & Kameyama T Endomorphins 1 and 2, endogenous mu-opioid receptor agonists, impair passive avoidance learning in mice. Eur. J. Pharmacol 421, 115–119 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Kibaly C, Kam AY, Loh HH & Law PY Naltrexone facilitates learning and delays extinction by increasing AMPA receptor phosphorylation and membrane insertion. Biol. Psychiatry 79, 906–916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallagher M Naloxone enhancement of memory processes: effects of other opiate antagonists. Behav. Neural. Biol 35, 375–382 (1982). [DOI] [PubMed] [Google Scholar]
- 88.Bali A, Randhawa PK & Jaggi AS Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci. Biobehav. Rev 51,138–150 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Lubbers ME, van den Bos R & Spruijt BM Mu opioid receptor knockout mice in the Morris Water Maze: a learning or motivation deficit? Behav. Brain Res 180, 107–111 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Olmstead MC, Ouagazzal AM & Kieffer BL Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLOS ONE 4, e4410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cominski TP, Ansonoff MA, Turchin CE & Pintar JE Loss of the mu opioid receptor induces strain-specific alterations in hippocampal neurogenesis and spatial learning. Neuroscience 278, 11–19 (2014). [DOI] [PubMed] [Google Scholar]; This study illustrates the importance of genetic backgrounds in the differences in spatial learning performances and hippocampal cell survival between two strains of MOP-knockout mice (C57BL/6J and 129S6).
- 92.Jamot L, Matthes HW, Simonin F, Kieffer BL & Roder JC Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav 2, 80–92 (2003). [DOI] [PubMed] [Google Scholar]
- 93.Jang CG et al. Impaired water maze learning performance in mu-opioid receptor knockout mice. Brain Res. Mol. Brain Res 117, 68–72 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Matthies H et al. Lack of expression of long-term potentiation in the dentate gyrus but not in the CA1 region of the hippocampus of mu-opioid receptor-deficient mice. Neuropharmacology 39, 952–960 (2000). [DOI] [PubMed] [Google Scholar]
- 95.Cominski TP, Turchin CE, Hsu MS, Ansonoff MA & Pintar JE Loss of the mu opioid receptor on different genetic backgrounds leads to increased bromodeoxyuridine labeling in the dentate gyrus only after repeated injection. Neuroscience 206, 49–59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holmes MM & Galea LA Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav. Neurosci 116,160–168 (2002). [PubMed] [Google Scholar]
- 97.Tanum L et al. Effectiveness of injectable extended release naltrexone versus daily buprenorphinenaloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry 74, 1197–1205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bailey CP & Husbands SM Novel approaches forthe treatment of psychostimulant and opioid abuse — focus on opioid receptor-based therapies. Expert Opin. DrugDiscov 9, 1333–1344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torregrossa MM, Corlett PR & Taylor JR Aberrant learning and memory in addiction. Neurobiol. Learn. Mem 96, 609–623 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xue YX et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336, 241–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bisaga A et al. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology 157, 1–10 (2001). [DOI] [PubMed] [Google Scholar]
- 102.Myers KM & Carlezon WA Jr. D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol. Psychiatry 71, 947–955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Semenova S, Danysz W & Bespalov A Low-affinity NMDA receptor channel blockers inhibit acquisition of intravenous morphine self-administration in naive mice. Eur. J. Pharmacol 378, 1–8 (1999). [DOI] [PubMed] [Google Scholar]
- 104.Sorg BA Reconsolidation of drug memories. Neurosci. Biobehav. Rev 36, 1400–1417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin J et al. Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int. J. Neuropsychopharmacol 17, 127–136 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Kazma M, Izrael M, Revel M, Chebath J & Yanai J Survival, differentiation, and reversal of heroin neurobehavioral teratogenicity in mice by transplanted neural stem cells derived from embryonic stem cells. J. Neurosci. Res 88, 315–323 (2010). [DOI] [PubMed] [Google Scholar]
- 107.Lutz PE & Kieffer BL Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36,195–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review highlights genetic and pharmacological approaches that reveal the distinct roles of MOP,DOP and KOP in mood control and theantidepressant potential of DOP agonists and KOP antagonists.
- 108.Samuels BA et al. The behavioral effects of the antidepressant tianeptine require the mu-opioid receptor. Neuropsychopharmacology 42,2052–2063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This investigation demonstrates that the acute and chronic antidepressant-like behavioural effects of tianeptine, used mainly in the treatment of major depressive disorder, require MOP.
- 109.Stanciu CN, Glass OM & Penders TM Use of buprenorphine in treatment of refractory depression —a review of current literature. Asian J. Psychiatr 26,94–98 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Salas J et al. New-onset depression following stable, slow, and rapid rate of prescription opioid dose escalation. Pain 158, 306–312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song NN et al. Divergent roles of central serotonin in adult hippocampal neurogenesis. Front. Cell. Neurosci 11, 185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pettit AS, Desroches R & Bennett SA The opiate analgesic buprenorphine decreases proliferation of adult hippocampal neuroblasts and increases survival of their progeny. Neuroscience 200, 211–222 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Carroll FI & Carlezon WA Jr Development of kappa opioid receptor antagonists. J. Med. Chem 56, 2178–2195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tan KZ, Cunningham AM, Joshi A, Oei JL & Ward MC Expression of kappa opioid receptors in developing rat brain — implications for perinatal buprenorphine exposure. Reprod. Toxicol 78, 81–89 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Yun S, Reynolds RP, Masiulis I & Eisch AJ Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med 22, 1239–1247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yun S et al. Stress-induced anxietyand depressive-like phenotype associated with transient reduction in neurogenesis in adult nestin-CreERT2/diphtheria toxin fragment A transgenic mice. PLOS ONE 11, e0147256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boldrini M et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 38, 1068–1077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller BR & Hen R The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol 30, 51–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Surget A et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depressionand of antidepressant reversal. Biol. Psychiatry 64,293–301 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Surget A et al. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry 16, 1177–1188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kheirbek MA et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mague SD et al. Antidepressant-like effects of kappaopioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther 305, 323–330 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Shirayama Y et al. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem 90, 1258–1268 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Zhang H, Shi YG, Woods JH, Watson SJ & Ko MC Central kappa-opioid receptor-mediated antidepressant-like effects of nor-binaltorphimine: behavioral and BDNF mRNA expression studies. Eur. J. Pharmacol 570, 89–96 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Torregrossa MM et al. The delta-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology 29, 649–659 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Hsu DT et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry 20, 193–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pecina M et al. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol. Psychiatry 10.1038/s41380-018-0117-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crnic KB & Todorovic MM Recidivism with opiate addicted patients on buprenorphine substitution treatment: case report. Hospital Pharmacol 4,533–541 (2017). [Google Scholar]
- 129.Israel Y et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol 52, 1–4 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Fu MH et al. Stem cell transplantation therapy in Parkinson’s disease. SpringerPlus 4, 597 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garland EL & Howard MO Mindfulness-based treatment of addiction: current state of the field and envisioning the next wave of research. Addict. Sci. Clin. Pract 13, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodriguiz RM & Wetsel WC in Animal Models of Cognitive Impairment Ch. 12 (eds Levin ED & Buccafusco JJ) (CRC Press/Taylor & Francis, Boca Raton, 2006). [PubMed] [Google Scholar]
- 133.Peters J & De Vries TJ Glutamate mechanisms underlying opiate memories. Cold Spring Harb. Perspect. Med 2, a012088 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lynch WJ, Nicholson KL, Dance ME, Morgan RW & Foley PL Animal models of substance abuse and addiction: implications forscience, animal welfare, and society. Comp. Med 60, 177–188 (2010). [PMC free article] [PubMed] [Google Scholar]
- 135.Ostlund SB & Balleine BW On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov. Today Dis. Models 5, 235–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Savage S & Ma D III. Animal behaviour testing: memory. Br. J. Anaesth 113, 6–9 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Cahill CM, Walwyn W, Taylor AMW, Pradhan AAA & Evans CJ Allostatic mechanisms of opioid tolerance beyond desensitization and downregulation. Trends Pharmacol. Sci 37, 963–976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Drolet G et al. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. Biol. Psychiatry 25,729–741 (2001). [DOI] [PubMed] [Google Scholar]
- 139.Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y & Mackey SC Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J. Pain Res 10, 979–987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stein C & Kuchler S Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci 34, 303–312 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Peng J, Sarkar S & Chang SL Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend 124, 223–228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kapas S, Purbrick A & Hinson JP Action of opioid peptides on the rat adrenal cortex:stimulation of steroid secretion through a specificmu opioid receptor. J. Endocrinol 144, 503–510 (1995). [DOI] [PubMed] [Google Scholar]
- 143.Wen T, Peng B & Pintar JE The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol. Endocrinol 23, 671–678 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Galligan JJ & Akbarali HI Molecular physiology of enteric opioid receptors. Am. J. Gastroenterol Suppl.2, 17–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bodnar RJ Endogenous opiates and behavior: 2015. Peptides 88, 126–188 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Nestler EJ Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci 25, 210–218 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Zubieta JK, Dannals RF & Frost JJ Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am. J. Psychiatry 156, 842–848 (1999). [DOI] [PubMed] [Google Scholar]
- 148.Volkow ND, Wang GJ, Fowler JS, Tomasi D & Telang F Addiction: beyond dopamine reward circuitry. Proc. Natl Acad. Sci. USA 108, 15037–15042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arias-Carrion O et al. Dopaminergic reward system: a short integrative review. Int. Arch. Med 3, 24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drake CT, Chavkin C & Milner TA Opioid systems in the dentate gyrus. Prog. Brain Res 163, 245–263 (2007). [DOI] [PubMed] [Google Scholar]
- 151.Robinson TE, Gorny G, Savage VR & Kolb B Widespread but regionally specific effects of experimenterversus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 46, 271–279 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.