Abstract
Background & Aims:
To safely manage the hypersecretory state in patients with Zollinger–Ellison syndrome, both upper endoscopy and gastric analysis are required to titrate optimal medical therapy. Conventional gastric analysis requires more than 1 hour to perform and results in significant patient dissatisfaction. In this study, we have validated endoscopic gastric analysis as a novel technique that can effectively replace conventional gastric analysis.
Methods:
In a prospective, crossover study, 12 patients with Zollinger–Ellison syndrome underwent gastric analysis, first by the conventional method and then by an endoscopic technique performed on the same day. Acid concentration was determined by titration, volume output was recorded, and acid output was calculated using standard methodologies and formulas. Agreement was assessed following the Bland–Altman method. To assess repeatability, both techniques were repeated on the same day in a subset of patients.
Results:
Excellent agreement was reported between acid output (95% limits of agreement, −1.27 to 1.61 mEq/h) and acid concentration (95% limits of agreement, −0.01 to 0.01 mEq/mL), although poor agreement was observed between volume output measured. Endoscopic gastric analysis showed greater reproducibility regarding acid and volume output measured.
Conclusions:
We introduce a new, rapid, reproducible, and accurate endoscopic technique to measure acid output in patients with Zollinger–Ellison syndrome who require both annual endoscopy and gastric analysis. The data presented here suggest that endoscopic gastric analysis would be equally effective in determining acid output in other hypersecretory states. Additional analysis of cost effectiveness is needed to evaluate its use as a screening tool in select populations.
Historically, the popularity of gastric secretory studies as a test of choice for hyperchlorhydria has fluctuated since its development in 1871 when Wilhelm Otto Leube first recognized the diagnostic potential of gastric chemical analysis.1 Recently, interest in gastric acid analysis has again surfaced because of its critical role in differentiating appropriate from inappropriate hypergastrinemia with the increasing use of potent acid inhibitory agents such as proton pump inhibitors (PPIs).2,3 Although an increased serum gastrin level alone has little significance, simultaneous measurement of serum gastrin with gastric acid output together can distinguish appropriate from inappropriate fasting hypergastrinemia.4
Gastric analysis traditionally has been considered the gold standard in the diagnosis of Zollinger–Ellison syndrome (ZES), a less common cause of inappropriate fasting hypergastrinemia.5,6 To both diagnose and manage the high acid output (AO) state seen in ZES, patients often initially require gastric analysis at 3-month intervals to assess medical therapeutic success. In these patients in particular, it is absolutely critical to develop both a highly accurate and easily used AO assay to follow up disease progression clinically.
Despite its clinical use, the gastric acid secretory study has intermittently fallen out of favor with clinicians as a result of difficulty with the examination.7 Typically, conventional testing with a nasogastric tube is burdensome and complex, demanding a reliable collection of samples in specific timed intervals. It requires longer than an hour to collect gastric acid and involves significant patient discomfort, making gastric secretory studies extremely difficult to repeat longitudinally in a large series of patients.8 In many cases the adequacy of the collection is overly dependent on the correct placement of the tube in the fundic area of the stomach. There is also an issue with accessibility because the examination, as a result of these difficulties, is generally not available to the majority of practitioners. Common complications resulting from nasogastric tube insertion include pain, sinusitis, epistaxis and other mucosal erosions, induced gag reflex and vomiting and less commonly pleural effusion, esophageal perforation, and a rare but lethal nasogastric tube syndrome characterized by acute upper airway obstruction resulting from bilateral vocal cord paralysis.9,10
Given the difficulties, limitations, and complications of conventional gastric analysis, we sought to validate a new, rapid, simple endoscopic technique (endoscopic gastric analysis [EGA]) whereby a single 15-minute sample of gastric juice can be collected under direct endoscopic visualization as part of a routine upper endoscopy and used to calculate acid secretion in 12 patients with ZES, who required repeated-interval assessment of acid output. In this study, we compared results including volume output (VO), acid concentration (AC), and AO obtained by conventional gastric analysis (GA) with those obtained by EGA. In addition, in an effort to evaluate reproducibility, 5 patients underwent repeat examinations with both procedures completed on the same day.
Materials and Methods
The study was conducted from 2001 to 2004 as a prospective, nonrandomized, single-center, cross-over study in 12 patients with active ZES. Patients met the criteria for the diagnosis of ZES based on an increased serum gastrin level (>200 pg/mL), gastric acid hypersecretion (>15 mEq/h), and/or a positive secretin test or biopsy examination–proven gastrinoma. The study was conducted in patients with active ZES because these individuals frequently require serial interval assessment of AO and therefore would benefit from an accurate, less-invasive procedure. All patients gave their informed consent to participate in the study. This study was approved by the Office for Protection of Research Subjects at the David Geffen School of Medicine at UCLA.
After an overnight fast of at least 12 hours, each of the 12 patients first underwent a standard GA using a nasogastric tube. The same group of patients subsequently underwent an EGA completed that same day. Both GA and EGA were completed by the same physician, a highly experienced endoscopist based at a major academic institution. Patients were allowed to continue taking antisecretory medications as prescribed except on the morning of the procedure. Six patients predetermined to undergo repeat analysis at a 6-month interval were not allowed to alter medication dosages throughout the study; ultimately, however, 1 patient had worsening symptoms requiring a medication change and was excluded from repeat analysis. All patients were encouraged not to swallow any saliva throughout both GAs.
Conventional GA was performed with the patient in the left lateral decubitus position using a standard 16F nasogastric tube with an antireflux filter (Anderson Products, Haw River, NC). The nasogastric tube was passed into the stomach, and the tip then was positioned into the most dependent portion of the stomach. Placement of the nasogastric tube was verified by injecting air through the tube and auscultating for bubbling sounds and by water recovery of more than 90% of injected 100 mL sterile water. Gastric fluid was aspirated continuously for 60 minutes, and the total VO collected was divided into 4 sequential 15-minute collections and measured in milliliters. The acid concentration of each 15-minute sample was determined using a Radiometer Copenhagen Titrator (Copenhagen, Denmark), with titration of the gastric juice to a pH of 7.0 using 0.1 N NaOH titrant. The 15-minute collection of VO was recorded and multiplied by the AC of that collection to calculate a 15-minute AO. The hourly AO was derived from adding the four 15-minute AO values.
After a brief resting period lasting 1 hour, all 12 patients then underwent EGA with a Pentax endoscope (model 2700 or 2900; Pentax, Lincoln Park, NJ). Endoscopy was performed without the use of anticholinergic agents, and sedation was achieved with meperidine and/or midazolam given preoperatively and/or perioperatively. The mean meperidine dose given was 95.6 mg (range, 0–175 mg) and the mean midazolam dose given was 4.1 mg (range, 0–8 mg). One patient, allergic to meperidine, was given fentanyl at a dose of 300 mcg. The endoscope was passed into the stomach and all gastric contents were aspirated and discarded. A single 15-minute collection of gastric acid then was aspirated and collected under direct visualization into a Lukens trap (Sherwood Medical, St Louis, MO). Gastric fluid aspiration was performed as part of a routine endoscopic examination of the stomach and duodenal bulb and care was taken to avoid overdistention of the stomach. The AC was determined by titration in the earlier-mentioned fashion. The VO was recorded and used to calculate a 15-minute AO that then was multiplied by 4 to calculate the hourly AO.
Six patients underwent repeat examination at least once including both conventional GA and EGA at a mean follow-up period of 6.4 months after the initial examination to assess repeatability in techniques. Two patients had repeat EGA and GA performed on 2 and 3 separate occasions, respectively (accounting for the 21 total data points in this study); repeatability in these patients was assessed by analyzing data from the 2 dates closest in time. Both repeat EGA and conventional GA procedures were completed in the earlier-described manner and under the same conditions to assess for reproducibility regarding VO, AC, and AO.
Results of VO, AC, and AO by EGA and GA were recorded. Agreement was assessed by Bland–Altman methodology. The level of agreement was evaluated by calculating the bias (mean difference and SD of the differences) and the 95% confidence interval for the bias to determine the 95% limits of agreement (mean difference ± 2 SD), representing the limits within which 95% of the differences (EGA vs GA) in the sample will be found. To determine with 95% certainty the range of values that contain the mean difference in the whole population (from which the sample was obtained), 95% confidence intervals (mean difference ± 2 standard error of the mean) for the lower and upper limits of agreement were calculated. Least-squares regression analysis of the differences between EGA vs GA on the average of EGA and GA was completed to evaluate the relationship between the mean difference and the magnitude of the measurement itself. Repeatability for both procedures was assessed by evaluating agreement between the first and repeat test in a similar manner with calculation of repeatability coefficients (calculated as twice the SD of the paired differences).
Results
Twelve patients with active ZES who met entrance criteria were evaluated by conventional GA followed by EGA. The mean age of the study group was 55.3 ± 10.2 years, ranging from 39 to 69 years of age. Of the 12 enrolled patients, 7 were men (mean age, 52.4 ± 9.2 y; range, 39–63 y) and 5 were women (mean age, 59.4 ± 11.1 y; range, 43–69 y). Despite surgical intervention, all enrolled patients had active disease that was well controlled on PPI therapy. The demographics for this cohort of patients were typical of that for patients with a diagnosis of ZES.11
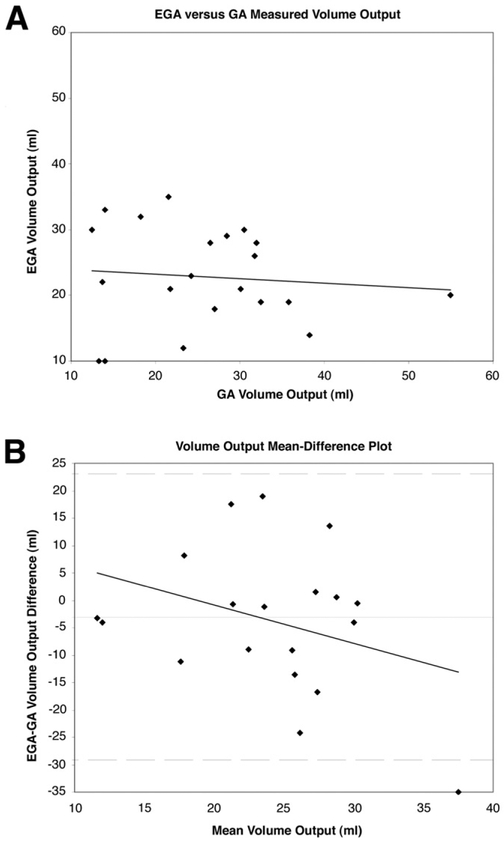
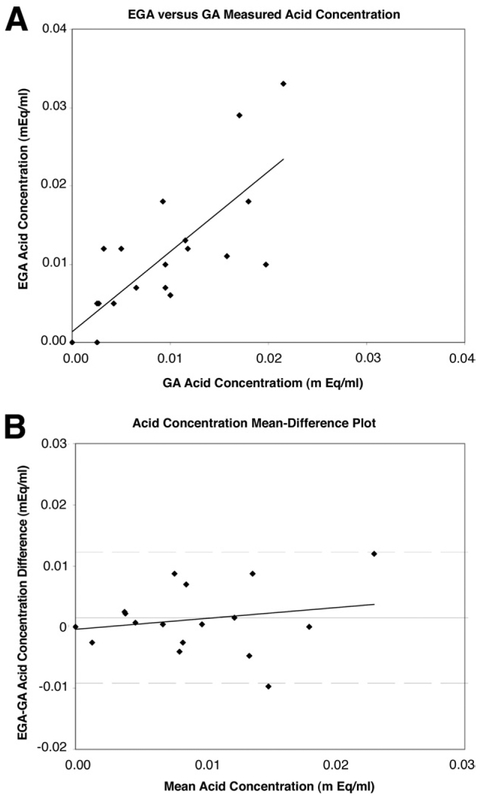
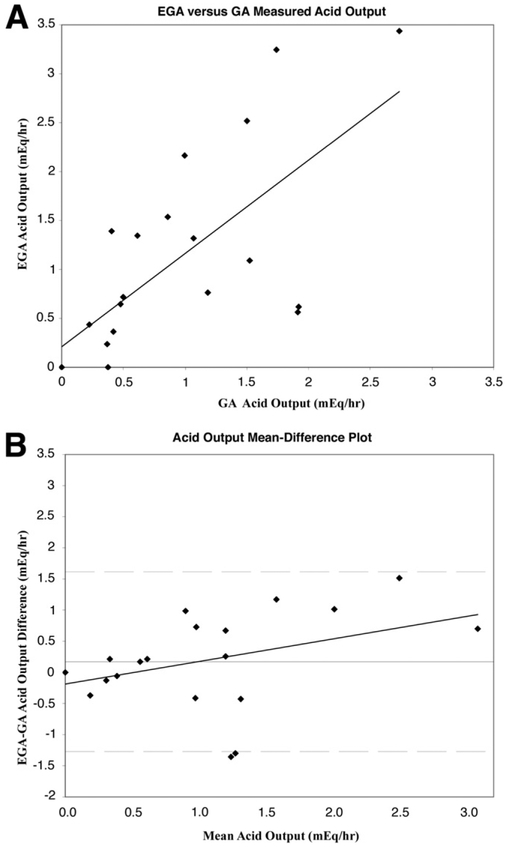
Agreement between EGA and GA was assessed using the method of Bland and Altman.12-14 These results are shown graphically in Figures 1, 2, and 3, which illustrate a comparison of VO, AC, and AO, respectively. In Figures 1A, 2A, and 3A, EGA values are plotted against GA values with a least-squares or regression line shown. In Figures 1B, 2B, and 3B, a mean-difference plot is shown with the mean plotted along with the 95% limits of agreement and a least-squares line. Excellent agreement is reported between AO (limits of agreement −1.27 to 1.61 mEq/h) and AC (limits of agreement, −.01 to .01 mEq/mL), although this is limited by the variable repeatability of both EGA and GA (Table 1), whereas poor agreement is seen for VO recorded (limits of agreement, −29.20 to 23.07 mL). In Table 1, the mean of EGA and GA differences (column 1) and averages (column 2) are shown for all 21 data points included in the study. The mean differences expressed as a percentage of the corresponding mean averages for VO, AC, and AO were 12.7%, 19.3%, and 17.3%, respectively.
Figure 1.
(A) Plot of EGA-measured volume output (VO) against GA-measured VO with least-squares regression analysis. (B) Bland–Altman plot assessing agreement between EGA and GA VO with least-squares regression analysis. The mean difference (horizontal solid line) is plotted along with the 95% limits of agreement (dashed lines).
Figure 2.
(A) Plot of EGA-measured AC against GA-measured AC with least-squares regression analysis. (B) Bland–Altman plot assessing agreement between EGA and GA AC with least-squares regression analysis. The mean difference (horizontal solid line) is plotted along with the 95% limits of agreement (dashed lines).
Figure 3.
(A) Plot of EGA-measured AO against GA-measured AO with least-squares regression analysis. (B) Bland–Altman plot assessing agreement between EGA and GA AC with least-squares regression analysis. The mean difference (horizontal solid line) is plotted along with the 95% limits of agreement (dashed lines).
Table 1.
Comparison of Mean Differences and Repeatability Coefficients Between Endoscopic (EGA) and Gastric Analysis (GA)
| Mean of EGA-GA differences | Mean of EGA and GA averages | EGA repeatability coefficient | GA repeatability coefficient | |
|---|---|---|---|---|
| AO, mEq/h | 0.17 ± 0.73 | 0.98 ± 0.82 | 1.63 | 2.22 |
| VO, mL | −3.07 ± 13.34 | 24.12 ± 6.33 | 16.76 | 35.01 |
| AC, mEq/mL | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.02 | 0.01 |
NOTE. Data are presented as means ± SD.
To analyze the relationship between EGA and GA further, we investigated each specific 15-minute interval collection of VO, AC, and AO measured during GA and compared these with the 15 minute results by EGA (all measurement expressed in hourly values). Greatest agreement in AO measured was seen over the third interval (minutes, 30–45) of GA, whereas the poorest agreement was seen over the second interval (minutes, 15–30). Overall, poor agreement is reported between the VO recorded by EGA and by GA. The best of these values was seen again over the third interval (minutes, 30–45), whereas the worst agreement was seen over the first interval (minutes, 0–15). Finally, excellent agreement in AC was seen over all 4 time intervals.
To study the repeatability of the 2 methods, 6 patients underwent repeat analysis with both GA and EGA at least once at a mean follow-up period of 6.4 months. One patient was excluded because of repeat EGA and GA values significantly different from initial values as a result of worsening symptoms requiring a medication dosage change. For those patients who underwent repeat analysis more than once, results from the 2 dates closest in time were used for comparison. Repeatability was determined by Bland–Altman methodology whereby the repeatability coefficient was calculated as twice the SD of the paired differences. In a limited assessment, EGA showed greater repeatability regarding AO and VO (Table 1).
Generally, all procedures completed were well tolerated. Minor complaints of discomfort and headache were noted during GA, and 1 patient developed an esophageal submucosal hematoma secondary to trauma on insertion and placement of a nasogastric tube (Figure 4). The hematoma subsequently was visualized on endoscopic examination completed later that day. All patients tolerated EGA well and without any complications.
Figure 4.
Endoscopic images of a (A) submucosal hematoma secondary to trauma on insertion/placement of a (B) nasogastric tube that healed spontaneously after 2 weeks. (A) Submucosal hematoma confirmed during subsequent EGA. (B) Healed submucosal hematoma after 2 weeks.
Discussion
Previously, multiple attempts made to enhance gastric secretory studies, including efforts to abbreviate the conventional 1-hour study down to a 20-minute15 or 10-minute secretory test, have lead to increased errors in VO estimation as measurements were extrapolated to hourly values.16 Others have attempted to measure AO indirectly to decrease the length and invasiveness of the conventional examination.17,18 Endoscopic approaches to measure basal acid output directly in protocols similar to the one presented here also have been attempted. Previously, one study attempted to compare an endoscopic protocol that took 2 hours to complete with conventional GA completed within a 1-month period.19 More recently, a second group devised a protocol to compare AO measured endoscopically and conventionally with tetragastrin stimulation in 15 patients.20 In the past, other studies have relied on correlation coefficients and regression analysis methods now considered inappropriate in the assessment of agreement.12-14 Consequently, we developed a new, rapid, simple endoscopic procedure to directly measure AO and compared this with conventional testing using a Bland–Altman approach to validate EGA in patients with ZES who require frequent gastric analysis to monitor disease progression.
On enrollment, all 12 patients had active disease despite surgical intervention that was well managed (pre-enrollment mean AO, 1.09 ± .59 mEq/h) on high-dose PPI therapy. EGA and GA were performed in these patients as part of a scheduled routine examination to monitor disease progression. To assess agreement, we predetermined how far apart measurements between EGA and GA could be to conclude good agreement between the 2 techniques. In our experience, it has not been uncommon to see GA AO values fluctuate by at least a few mEq/h from one measurement to another even when performed on the same patient, under the same conditions, over a short interval period. In addition, small differences within a few milliequivalents per hour alone would never alter the medical management of these patients; that is, for instance, a patient with an AO value of even 10 mEq/h would not be treated differently if this value instead ranged from 7.5 to 12.5 mEq/h. Therefore, we set our upper limit of differences between EGA and GA AO to be ±2.5 mEq/h, arguing that differences that are less than this arbitrary number would be both clinically insignificant and difficult to differentiate from the natural variation that occurs with repeat examination.
In this study of 12 patients comprising 21 data points, we found that the limits of agreement between EGA and GA AO ranged from −1.27 to 1.61 mEq/h with a standard error of the limit of 0.27 mEq/h (Figure 3B). Based on calculated confidence intervals, on the most optimistic interpretation, the differences between EGA and GA ranged from −0.69 to 1.03 mEq/h, whereas on the worst interpretation, these differences ranged from −1.84 to 2.18 mEq/h, an interval nonetheless within our predetermined limits. Furthermore, as seen in Figure 3B, in patients with highly suppressed AO values (mean AO, <1 mEq/h), there is an even narrower range of differences between EGA and GA, suggesting even greater agreement. In these 12 patients with active ZES and highly suppressed AO, greater agreement at low AO measurements becomes even more important. Regression analysis of the mean difference (EGA-GA) on the average of EGA and GA AO (Figure 3B) reveals that the former increases as the latter or magnitude increases, suggesting, in this study, that EGA may be less accurate in patients with increased AO. However, given limited measurements of increased AO in this select population of patients with well-controlled ZES via either EGA or GA, additional studies are required to evaluate EGA efficacy accurately in patients with increased AO.
By a similar analysis, there is excellent agreement between EGA and GA regarding acid concentration (Figure 2B) and poor agreement regarding VO (Figure 1B). Regression analysis in the former suggests minimal effect of magnitude on the mean difference, whereas in the latter, GA measurements of volume increase compared with EGA measurements of volume at greater magnitudes. High correlation between EGA and GA measurements of AC and AO are reported in Figures 2A and 3A, whereas poor correlation is seen in Figure 1A.
In general it appears that EGA overestimates GA AO and AC by 0.17 ± 0.73 mEq/h and 0.0016 ± 0.0054 mEq/mL, respectively, and underestimates VO by 3.07 ± 13.34 mL (Table 1). The mean differences expressed as a percentage of the corresponding mean averages for volume, AC, and AO were all very similar. To evaluate these mean biases further, we analyzed each 15-minute VO, AC, and AO measurement by GA and compared these with EGA values. Not surprisingly, the worst agreement between VO aspirated by GA and EGA occurred over the first 15-minute interval (mean, −13.07 ± 23.81 mL), during which copious volume given to ease nasogastric tube insertion may have falsely increased residual gastric volume before GA. Conversely, conventional GA that relies on blind insertion and suctioning of gastric contents also can be decreased falsely by incomplete aspiration of diffuse gastric pooling or by high pyloric loss. In contrast, direct visualization and capture allows EGA to provide a highly accurate and reproducible recording of gastric VO. AC had excellent agreement over all 4 collection intervals, with the best agreement seen over the first interval. AO, consequently, had greatest agreement over the third and fourth interval, during which VO and AC had the greatest agreement overall.
The amount of agreement between 2 methods is limited by the reproducibility of each method of measurement.12-14 To assess reproducibility more accurately, 5 patients underwent repeat analysis at a mean of 6.4 months (1 patient who required a medication dosage increase was excluded). Overall, EGA showed greater repeatability regarding AO and VO measured compared with GA (Table 1). The calculated 95% repeatability coefficients for EGA (± 1.63 mEq/h) and GA (±2.22 mEq/h) AO are similar to the 95% limits of agreement of EGA-GA plotted in Figure 3B (−1.27 to 1.61 mEq/h), suggesting that any differences observed between EGA and GA may be explained by lack of repeatability in either EGA or GA alone. If the limits of agreement were considerably wider than the calculated repeatability coefficients, then poor repeatability alone would not be the sole explanation of a decreased agreement.
Limitations of using EGA include the sedation required for endoscopic analysis and the effects of sedation on inhibiting gastric acid secretion. Sedation was achieved in the study population with intravenous meperidine, midazolam, or a combination given preoperatively and/or perioperatively. In rats, anesthetic agents, including chloralose, pentobarbitone, and urethane, have mixed effects on gastric acid secretion,21,22 whereas a combination of fluanisone, fentanyl, and midazolam inhibited acid secretion by reducing enterochromaffin-like cell histamine release.23 In human beings, both benzodiazepines24 and meperidine25 have been found to inhibit acid secretion, although this inhibition does not appear to occur immediately on drug administration. Meeroff et al25 reported data on AO inhibition by meperidine beginning at 1 hour when given intramuscularly. In our procedure, time from the onset of sedative delivery, which also was given perioperatively, to the end of examination generally was rapid, occurring within minutes, likely explaining why AO values did not appear to be affected in the endoscopic procedure. Despite the potential effects of sedation on gastric acid secretion, the results presented here show that the gastric output as determined by EGA did not appear to be inhibited significantly compared with the GA group.
A second limitation to the study lies with the nonrandom order of testing of GA and EGA in this study. Patients who underwent GA first potentially could have had measured AO values that were decreased by a residual amount of antisecretory medication that may not have affected EGA values measured 1 hour later. In addition, GA values were measured in the morning after a 12-hour fast and before breakfast when AO levels are known to be at their lowest state.26 Despite this, however, analysis of the interval collection data show that AC and AO levels did not increase (as would have been expected) but actually decreased over the 1-hour collection. Most likely, the nonrandom order of testing did not affect measured AO levels because all 12 patients were in a steady-state equilibrium as a result of the long duration of PPI effect.
An endoscopic approach to measuring gastric acid output offers multiple advantages over the traditional examination including improved cost effectiveness by combining 2 examinations into 1 procedure and by decreasing the time required to complete gastric acid analysis from the normal 1-hour period to a collection time of only 15 minutes. Accuracy also may be improved by an endoscopic technique that allows direct visualization of volume for aspiration, thereby decreasing loss of secretions through the pylorus. Previous studies using phenol red as a marker report a nearly 10% loss of gastric contents through the pylorus in the conventional examination.27 Finally, and perhaps most importantly, the ease of endoscopic examination will facilitate future serial and longitudinal study on the effects of PPIs and Helicobacter pylori eradication on gastric acid secretion in a growing number of patients.8
Endoscopically obtained gastric acid analysis is tremendously more patient-friendly and less discomforting given the sedation used. Although we did not use patient satisfaction surveys to gauge the response to EGA vs GA, it can be inferred anecdotally from patients participating in this study that when given the option of a 1-hour unsedated GA to a sedated EGA, that all prefer the latter. Nasogastric tube insertion is associated with both minor complications (trauma, gagging, vomiting, nose bleed, and headache) and severe complications (pleural effusion, esophageal perforation, laryngeal injury, and nasogastric tube syndrome).9,10 In our study of 12 patients undergoing a total of 21 conventional gastric analyses, we report a patient developing an esophageal submucosal hematoma as a result of trauma from nasogastric tube insertion identified hours later on endoscopy as part of the EGA (Figure 4). Although the literature reports a few cases of esophageal perforation,28-31 no instances of submucosal hematoma were found. In this instance, the hematoma was identified only because of the subsequent endoscopy as part of the study protocol within 2 hours after conventional GA. In contrast, all endoscopic gastric analyses were well tolerated without complications.
Disadvantages of EGA are multiple and include indirect and direct expenses associated with the examination. In those patients, including patients with ZES, who require both interval esophagogastroduodenoscopy and gastric analysis, EGA is clearly more cost effective. In other patients who may not require upper endoscopy, additional cost analysis is needed to determine in which population EGA can succeed as a cost-effective screening replacement for conventional GA. At our institution, the high cost of conventional gastric analysis, including costs for physician and nursing time (> 1 h), analysis of multiple specimens, cost of complications, and cost of postprocedure monitoring make EGA appear even more appealing, especially given that patients with ZES require annual endoscopic surveillance of peptic disease by upper endoscopy.
In the present study, we introduce a new, rapid, simple endoscopic technique to measure gastric acid secretion in 12 patients with ZES and report excellent agreement, although limited by repeatability, between EGA and GA regarding AO and AC measured. Although this study was completed only in ZES patients with highly suppressed AO who required frequent interval assessment, the results presented here suggest that EGA deserves additional analysis in other hypersecretory conditions including patients with reflux disease on and off medical treatment to define its precise role and accuracy further. Since its discovery, conventional gastric acid analysis has been accepted anecdotally as the gold standard for measuring AO despite multiple flaws that likely limit its repeatability. In contrast, endoscopy allows for direct visualization of aspiration and should be considered as accurate if not more accurate than blind conventional gastric analysis. Additional study is required to evaluate both conventional GA and EGA in hypersecretory and nonhypersecretory conditions to determine which test is more accurate, more repeatable, and more likely to be the gold standard.
Abbreviations used in this paper
- AC
acid concentration
- AO
acid output
- EGA
endoscopic gastric analysis
- GA
gastric analysis
- PPI
proton pump inhibitor
- VO
volume output
- ZES
Zollinger–Ellison Syndrome
References
- 1.Ingelfinger FJ. Gastric analysis: ancient but going strong. N Engl J Med 1973;288:964–965. [DOI] [PubMed] [Google Scholar]
- 2.Spindel E, Harty RF, Leibach JR, et al. Decision analysis in evaluation of hypergastrinemia. Am J Med 1986;80:11–17. [DOI] [PubMed] [Google Scholar]
- 3.Waldum HL, Fossmark R, Bakke I, et al. Hypergastrinemia in animals and man: causes and consequences. Scand J Gastroenterol 2004;39:505–509. [DOI] [PubMed] [Google Scholar]
- 4.Roy PK, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis—a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine 2001;80:189–222. [DOI] [PubMed] [Google Scholar]
- 5.Metz DC, Starr JA. A retrospective study of the usefulness of acid secretory testing. Aliment Pharmacol Ther 2000;14:103–111. [DOI] [PubMed] [Google Scholar]
- 6.Ishimori A, Miura Y, Sakurada H, et al. Significance of gastric analysis with particular reference to the diagnosis of Zollinger-Ellison syndrome. Tohoku J Exp Med 1975;117:15–21. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld L Gastric tubes, meals, acid, and analysis: rise and decline. Clin Chem 1997;43:837–842. [PubMed] [Google Scholar]
- 8.Iijima K, Ohara S, Sekine H, et al. Changes in gastric acid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut 2000;46:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanaka M, Kishida S, Yoritaka A, et al. Acute upper airway obstruction induced by an indwelling long intestinal tube: attention to the nasogastric tube syndrome. J Clin Gastroenterol 2004; 38:913. [DOI] [PubMed] [Google Scholar]
- 10.O’Neil R, Krishnananthan R. Intrapleural nasogastric tube insertion. Australas Radiol 2004;48:139–141. [DOI] [PubMed] [Google Scholar]
- 11.Metz DC, Soffer E, Forsmark CE, et al. Maintenance oral pantoprazole therapy is effective for patients with Zollinger-Ellison syndrome and idiopathic hypersecretion. Am J Gastroenterol 2003;98:301–307. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician 1983;32:307–317. [Google Scholar]
- 14.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160. [DOI] [PubMed] [Google Scholar]
- 15.Johnston D, Jepson K. Use of pentagastrin in a test of gastric acid secretion. Lancet 1967;2:585–588. [DOI] [PubMed] [Google Scholar]
- 16.Baron JH. Timing of peak acid output after pentagastrin. Gastroenterology 1969;56:641–643. [PubMed] [Google Scholar]
- 17.Eisenband RM, Wenger J. Endoscopic estimate of gastric acid secretion. J Clin Gastroenterol 1981;3:287–290. [DOI] [PubMed] [Google Scholar]
- 18.Segawa K, Nakazawa S, Tsukamoto Y, et al. Estimate of gastric acid output by evaluation of fasting gastric juice collected endoscopically. Hepatogastroenterology 1991;38:79–82. [PubMed] [Google Scholar]
- 19.Yamaguchi H, Nakazawa S, Segawa K, et al. Precise gastric secretory studies using a new fiber-gastroscope. Hepatogastroenterology 1987;34:215–218. [PubMed] [Google Scholar]
- 20.Iijima K, Ohara S, Sekine H, et al. A new endoscopic method of gastric acid secretory testing. Am J Gastroenterol 1998;93: 2113–2118. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe K, Yano S, Lin WC. Stimulatory effect of pentobarbital and some anesthetics on gastric secretion in the continuously perfused stomach in rats under urethane anesthesia. Jpn J Pharmacol 1987;44:63–69. [DOI] [PubMed] [Google Scholar]
- 22.Bastaki SM, Waton G, Garner A. Effects of anaesthetic agents on basal and histamine-stimulated acid secretion in the fistula rat. Eur J Gastroenterol Hepatol 1995;7:1199–1202. [DOI] [PubMed] [Google Scholar]
- 23.Cui GL, Sandvik AK, Munkvold B, et al. Effects of anaesthetic agents on gastrin-stimulated and histamine-stimulated gastric acid secretion in the totally isolated vascularly perfused rat stomach. Scand J Gastroenterol 2002;37:750–753. [DOI] [PubMed] [Google Scholar]
- 24.Schurizek BA. The effects of general anaesthesia on antroduodenal motility, gastric pH and gastric emptying in man. Dan Med Bull 1991;38:347–365. [PubMed] [Google Scholar]
- 25.Meeroff JC, Fernandez GG, Gaon D. Potent reduction of basal acid output produced by meperidine. Am J Dig Dis 1978;23:696–698. [DOI] [PubMed] [Google Scholar]
- 26.Kwiecien S, Konturek SJ. Gastric analysis with fractional test meals (ethanol, caffeine, and peptone meal), augmented histamine or pentagastrin tests, and gastric Ph recording. J Physiol Pharmacol 2003;54:69–82. [PubMed] [Google Scholar]
- 27.Baron JH. The clinical use of gastric function tests. Scand J Gastroenterol 1970;6:9–46. [PubMed] [Google Scholar]
- 28.Jackson RH, Payne DK, Bacon BR. Esophageal perforation due to nasogastric intubation. Am J Gastroenterol 1990;85:439–442. [PubMed] [Google Scholar]
- 29.Tiller HJ, Rhea WG Jr. Iatrogenic perforation of the esophagus by a nasogastric tube. Am J Surg 1984;147:423–425. [DOI] [PubMed] [Google Scholar]
- 30.Norman EA, Sosis M. Iatrogenic oesophageal perforation due to tracheal or nasogastric intubation. Can Anaesth Soc J 1986;33: 222–226. [DOI] [PubMed] [Google Scholar]
- 31.Seefelder C, Elango S, Rosbe KW, et al. Oesophageal perforation presenting as oesophageal atresia in a premature neonate following difficult intubation. Paediatr Anaesth 2001;11:112–118. [DOI] [PubMed] [Google Scholar]