Abstract
Background
Patients with early-stage pancreatic neuroendocrine tumors (PNETs) may develop metastatic recurrences despite undergoing potentially curative pancreas resections. We sought to identify factors predictive of metastatic recurrences and develop a prognostication strategy to predict recurrence-free survival (RFS) in resected PNETs.
Methods
Patients with localized PNETs undergoing surgical resection between 1989 and 2015 were identified. Univariate and multivariate analysis were used to identify potential predictors of post-resection metastasis. A score-based prognostication system was devised using the identified factors. The bootstrap model validation methodology was utilized to estimate the external validity of the proposed prognostication strategy.
Results
Of the 140 patients with completely resected early-stage PNETs, overall 5- and 10-year RFS were 84.6% and 67.1%, respectively. The median follow-up was 56 months. Multivariate analysis identified tumor size > 5 cm, Ki-67 index 8–20%, lymph node involvement, and high histologic grade (G3, or Ki-67 > 20%) as independent predictors of post-resection metastatic recurrence. A scoring system based on these factors stratified patients into three prognostic categories with distinct 5-year RFS: 96.9%, 54.8%, and 33.3% (P <0.0001). The bootstrap model validation methodology projected our proposed prognostication strategy to retain a high predictive accuracy even when applied in an external dataset (validated c-index of0.81).
Conclusions
The combination of tumor size, LN status, grade, and Ki-67 was identified as the most highly predictive indicators of metastatic recurrences in resected PNETs. The proposed prognostication strategy may help stratify patients for adjuvant therapies, enhanced surveillance protocols and future clinical trials.
Keywords: Pancreas, Neuroendocrine tumors, Neoplasm recurrence, Surgical oncology
Condensed Abstract
A score-based prognostication strategy identifies patients most at risk for metastatic recurrence after surgical resection of pancreatic neuroendocrine tumors. The proposed prognostication strategy may help stratify patients for adjuvant therapies and enhanced surveillance protocols and future clinical trials.
Introduction
The incidence of pancreatic neuroendocrine tumors (PNETs) has risen significantly in recent years. Improvement in diagnostic capabilities and increased awareness of this relatively rare disease likely explains the 2–3-fold increase in its incidence over the past decade.1–3 While most PNETs have relatively indolent biology with most tumors amenable to potentially curative surgical resection, a small fraction exhibits highly aggressive biological behaviors.4 Clinically, this can manifest as a future metastatic recurrence despite prior surgery with curative intent. In practice, the identification of those patients who have the greatest likelihood for the development of recurrence is of paramount importance, because interventions such as adjuvant chemotherapy, somatostatin analogues, or peptide receptor radiotherapy (PRRT) would be considered for those patients.
The American Joint Committee on Cancer (AJCC) and the European Neuroendocrine Tumor Society (ENETS) have proposed staging classification systems specific for PNETs.5,6 Although their prognostic performances for overall survival have been well recognized, their abilities to predict for the development of post-resection distant relapses remain suboptimal.7 Prior studies have reported on a number of potential clinical and pathologic factors with prognostic implications in PNETs, including patient age, tumor size, lymph node status, tumor grade, and Ki-67 proliferation index.2,8–13 However, a prognostication strategy that predicts for the risk of metastatic recurrence following PNET resection remains ill-defined, especially for intermediate (G2)-grade tumors that are known to have high prognostic uncertainty.
Adjuvant therapy in patients with resected PNETs is not recommended in the current clinical practice given the lack of data to support its use. However, its role and efficacy in the subgroup of patients at high risk for metastatic recurrences would be considered if there was a validated prognostication system. A simplified, clinically useful prognostication strategy that identifies this high-risk subgroup may allow for an improved decision-making with respect to the delivery of adjuvant therapy as well as postoperative surveillance strategies.
Accordingly, our aim was to identify clinicopathologic factors that are prognostic for metastatic recurrences in resected PNETs. Using these prognostic factors, we further sought to devise a clinically useful prognostication strategy to identify the subgroup of postoperative patients who are at an increased risk for future metastatic recurrence.
Methods
Patient Selection and Data Acquisition
We reviewed records of patients undergoing surgical resection for PNETs between 1989 and 2015 with the approval from the UCLA School of Medicine Institutional Review Board. Clinical and pathologic data obtained included age, gender, tumor location, type of surgery, tumor pathologic features, tumor recurrences, and overall survival. Tumor pathologic characteristics were obtained from pathology reports. Grade was determined according to the World Health Organization (WHO) 2010 Classification System. Ki-67 proliferation index was determined from pathology reports. For cases prior to 2010 in which Ki-67 proliferation index was not routinely examined, archived tissues were reassessed for Ki-67 proliferation index. Patients were excluded from analysis if they had positive margins, metastatic disease at the time of initial surgery, underwent enucleation, or had missing information on key tumor pathologic features. Patients were followed in the postoperative period at 3–6-month interval initially and then 6–12-month interval afterwards. Radiographic imaging was obtained for the surveillance of recurrence at the time of follow-up. All post-resection metastatic recurrence was defined as the evidence of metastatic disease noted on radiographic evaluation.
Identification of Prognostic Factors Predicting Recurrence-Free Survival
Follow-up time was defined as the time to the last known date the patient was alive (for overall survival)/disease free (for recurrence free survival) or the date of death/metastatic recurrence. Overall survival (OS) and recurrence-free survival (RFS) were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate Cox regression analysis was performed to evaluate individual factors for their associations with metastatic recurrence. For continuous variables, including the Ki-67 cutoff, the optimal cutoff point was determined by fitting the dichotomized variable to a Cox proportional hazard model, and then determining the point with the most significant split in RFS as defined by the log-rank test.14 Multivariate backward stepwise Cox regression analysis was used to identify independent prognostic factors for RFS in post-resection PNETs. Akaike information criterion (AIC) values were used for variable selection. Harrell’s concordance index (c-index) was employed for quantification of prognostic factors’ predictive accuracies.15 The c-index measures the accuracy of predicted outcomes compared to actual outcomes. For any random pairs of patients selected from a sample, the c-index is the probability that a patient who has recurrence first also had a shorter predicted RFS (higher predicted risk) as determined by the prognostic factor. An index of 0.5 indicates that a predictive factor has no predictive power over chance alone, whereas a c-index of 1 suggests correct outcome prediction in all cases.
Development of a Prognostic Score System
Prognostic factors identified from above were used to devise a prognostic scoring system. Point value was assigned to each factor based on the regression coefficients (B coefficients) of the Cox regression analysis. The resulting scores were then used to categorize patients into three distinct prognostic groups based on 5-year RFS. Harrell’s c-index was used to determine the predictive accuracy of this prognostication strategy.
Validation of the Prognostic Factors and the Score System
Performance evaluation of a prediction model on the same original dataset used to develop the model itself (“apparent performance”) results in optimism bias from overfitting. This leads to a significant overestimation of the predictive accuracy of the model when it is applied to a new set of external validation samples. To correct this bias and to estimate the true predictive accuracy of the prognostic factors/score system when they are applied to an external dataset, we utilized the bootstrap methodology with 1000 resamples for validation. Bootstrapping methodology replicates the process of sampling from a population, by drawing samples with replacement from the original dataset. Each “bootstrap” sample is then used as a “training” set to develop its own prediction model, allowing for prognostic factor selection and model fitting at every resample. The original dataset is then used as a “testing” set to evaluate the performance of the model generated from the “training” (bootstrap sample) set. The difference in prediction performance between the “training” set and the “testing” set is defined as the “optimism” error. This process is repeated 1000 times and the optimism errors are averaged. The true predictive accuracy of a model is then defined as apparent performance – optimism error = true performance. Bootstrapping methodology has been described to provide a nearly unbiased estimate of predictive model performance in an external dataset and is often employed to validate a prediction model when a validation cohort is not available.15
Statistical Analysis
Continuous variables were examined using Student t test. Categorical variables were evaluated with the chi-squire test. P values <0.05 were defined as statistically significant. All statistical analyses were performed using the R program (version 2.12.0: www.r-project.org).
Results
Patient Demographics and Tumor Characteristics
A total of 171 patients underwent pancreatic resections for PNETs between 1989 and 2015. Of these patients, 31 were excluded from the current study for having metastatic disease at the time of initial surgery, positive margins in resected surgical specimens, and/or underwent an enucleation procedure. The final study group consisted of 140 patients who underwent pancreatic resections for localized PNETs with negative margins. Table 1 summarizes the patient demographics and tumor characteristics. The median age was 58 (interquartile range (IQR), 46–68). The mean tumor size was 3.2 cm (0.4–12.5 cm), and tumors were more commonly located in body/tail compared to the head of pancreas (57.1% vs. 42.9%). The majority of tumors were nonfunctional (85.7%). Applying the WHO grade classification system, 50.7% were low grade (G1), 45.0% were intermediate grade (G2), and 4.3% were high grade (G3). All G3 tumors had Ki67 index >20%. Lymph node (LN) involvement was found in 31 patients (22.1%).
Table 1.
Patient demographics and tumor characteristic
| Characteristics | |
|---|---|
| Age (years), median (IQR) | 58 (46–68) |
| Gender (%) | |
| Male | 67 (47.5%) |
| Female | 73 (51.8%) |
| Tumor size (cm); mean (SD), range | 3.2 (2.6), 0.4–12.5 |
| Tumor location | |
| Head | 60 (42.9%) |
| Body/Tail | 80 (57.1%) |
| Tumor type (%) | |
| Functional | 20 (14.3%) |
| Insulinoma | 16 (11.4%) |
| VIPoma | 2 (1.4%) |
| Glucagonoma | 1 (0.7%) |
| Gastrinoma | 1 (0.7%) |
| Non-functional | 120 (85.7%) |
| Operation type (%) | |
| Pancreaticoduodenectomy | 60 (42.9%) |
| Distal pancreatectomy | 80 (57.1%) |
| WHO Grade (%) | |
| 1 | 71 (50.7%) |
| 2 | 63 (45.0%) |
| 3 | 6 (4.3%) |
| AJCC T stage (%) | |
| 1 | 67 (47.9%) |
| 2 | 49 (35.0%) |
| 3 | 24 (17.1%) |
| 4 | 0 (0%) |
| Lymph node status (%) | |
| Positive | 31 (22.1%) |
| Negative | 109 (77.9%) |
| Lymphovascular invasion | |
| Present | 42 (30.0%) |
| Absent | 98 (70.0%) |
| Perineural invasion | |
| Present | 24 (17.1%) |
| Absent | 116 (82.9%) |
| MEN 1 status | |
| Positive | 6 (4.3%) |
| Negative | 134 (97.1%) |
AJCC, American Joint Committee on Cancer; WHO, World Health Organization; MEN 1, multiple endocrine neoplasia type 1; SD, standard deviation; IQR, interquartile range
Follow-up and Outcomes
The median follow-up of survivors was 56 months from the time of surgical resection. Overall 5-year and 10-year survival rates were 90.6% and 83.5%, respectively. Metastatic recurrences were found in 23 (16.4%) patients. All cases with metastatic recurrences occurred within the liver, with additional peritoneal carcinomatosis and bone metastasis in 2 patients. The 5-year and 10-year RFS were 84.6% and 67.1%, respectively. Median time to metastatic recurrence was 3.84 years (IQR, 1.89–6.67).
Prognostic Factors for PNET Post-Resection Metastatic Recurrences
On univariate analysis (Table 2), factors predictive of post-resection metastatic recurrences included the following: tumor size > 5 cm (hazard ratio (HR), 4.85; 95% confidence interval (CI), 2.05–11.5), T-stage 3 (HR, 16.5; 3.66–74.7), high grade (G3) (HR, 16.0; 5.66–44.9), Ki-67>8% (HR, 9.6; 4.05–22.8), positive LN (HR, 4.93; 2.19–11.3), presence of LVI (HR, 3.88; 1.90–10.7), and PNI (HR, 2.65; 1.08–6.52). Patient age and tumor functionality, although previously found to be relevant prognostic factors for overall survival, were not significant predictors for RFS.
Table 2.
Patient and tumor factors associated with metastatic recurrences in localized PNETs following potentially curative surgical resection
| Patient and tumor variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate analysis | ||
| Age | 0.50 (0.21–1.21) | 0.125 |
| Tumor type | ||
| Functional | 1.0 (reference) | |
| Nonfunctional | 0.66 (0.20–2.26) | 0.51 |
| Tumor sizea | 1.26 (1.13–1.41) | < 0.001 |
| Tumor sizeb | ||
| < 5 cm | 1.0 (reference) | |
| > 5 cm | 4.85 (2.05–11.5) | < 0.001 |
| Tumor location | ||
| Distal/body | 1.0 (reference) | |
| Head | 1.93 (0.83–4.46) | 0.125 |
| T-stage | ||
| 1 | 1.0 (reference) | |
| 2 | 4.06 (0.88–18.9) | 0.073 |
| 3 | 16.5 (3.66–74.7) | < 0.001 |
| WHO grade | ||
| G1/2 | 1.0 (reference) | |
| G3 | 6.0 (5.66–44.93) | < 0.001 |
| Ki-67a | 1.06 (1.04–1.08) | < 0.001 |
| Ki-67b | ||
| ≤ 8% | 1.0 (reference) | |
| >8% | 9.6 (4.05–22.8) | < 0.001 |
| Lymph node status | ||
| Negative | 1.0 (reference) | |
| Positive | 4.93 (2.19–11.3) | < 0.001 |
| Lymphovascular invasion | ||
| Absent | 1.0 (reference) | |
| Present | 3.83 (1.90–10.7) | 0.002 |
| Perineural invasion | ||
| Absent | 1.0 (reference) | |
| Present | 2.65 (1.08–6.52) | 0.034 |
| MENI | ||
| Absent | 1.0 (reference) | |
| Present | 0.79 (0.11–5.89) | 0.813 |
| Multivariate analysis | ||
| Tumor size | ||
| ≤ 5 cm | 1 (reference) | |
| > 5 cm | 2.64 (1.02–6.86) | 0.046 |
| Ki-67 | ||
| <8% | 1 (reference) | |
| 8–20% | 3.46 (1.27–9.40) | 0.015 |
| >20% (WHO grade G3) | 18.54 (5.08–67.61) | < 0.0001 |
| Lymph node | ||
| Negative | 1 (reference) | |
| Positive | 4.28 (1.52–12.10) | 0.0059 |
Analyzed as a continuous variable
The optimal cutoff point was determined by fitting the dichotomized variable to a Cox proportional hazard model, and then determining the point with the most significant split in RFS as defined by the log-rank test
AJCC, American Joint Committee on Cancer; WHO, World Health Organization; LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion; MEN 1, multiple endocrine neoplasia type 1
On multivariate analysis (Table 2), tumor size > 5 cm (HR, 2.64; 1.02–6.86), positive LN (HR, 4.28; 1.52–12.10), Ki-67 index 8–20% (HR, 3.46; 1.27–9.40), and WHO grade 3 (Ki-67 > 20%) (HR, 18.54; 5.08–67.61) were independent predictors of post-resection metastatic recurrence.
Accuracy of Recurrence-Free Survival Stratification by the Identified Prognostic Factors
The predictive accuracy of the identified prognostic factors for RFS was assessed using Harrell’s c-index. Individually, tumor size (c-index, 0.67; 95% CI, 0.57–0.77), LN positivity (c-index, 0.68; 0.59–0.77), and Ki-67 (c-index, 0.70; 0.62–0.78) were each associated with a moderate predictive accuracy. However, when these prognostic factors were combined into a single Cox regression model, the c-index improved significantly to 0.86 (CI, 0.77–0.95). To adjust for the optimism bias and to estimate the predictive accuracy of the combined prognostic factors in an external dataset, bootstrap methodology with 1000 resamples was utilized for validation. Corrected c-index of the combined prognostic factors adjusting for the optimism error was 0.83.
Prognostication Strategy for Predicting Metastatic Recurrence in PNETs Following a Potentially Curative Pancreas Resection
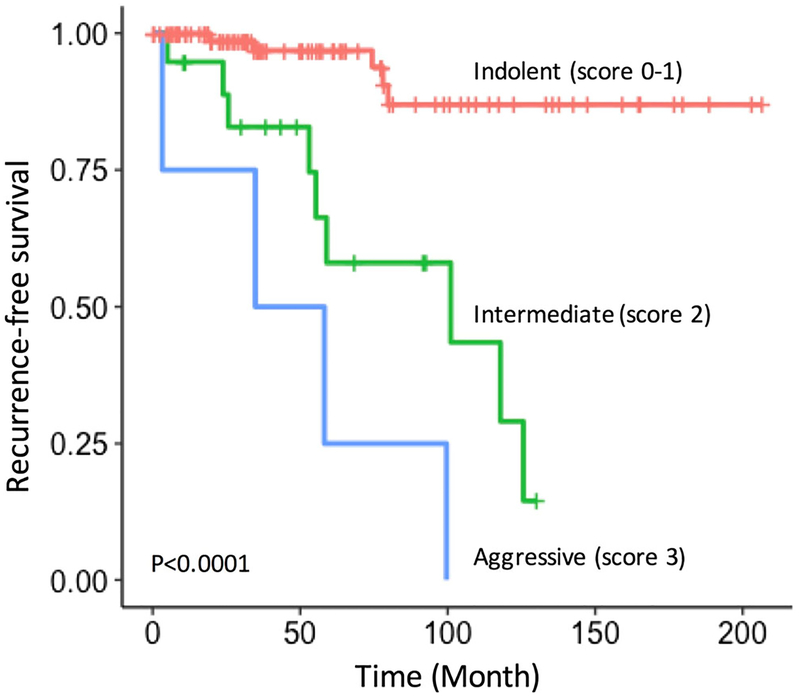
In order to devise a clinically applicable prognostication strategy, we developed a score system for post-resection metastatic recurrence risk stratification. This system assigns 1 point each for tumor size > 5 cm, Ki-67 index 8–20%, and positive LN and 2 point for Ki-67 index > 20% (equivalent to high (G3) grade). Point values were assigned based on the regression coefficients. As determined by the summation of points, patients were categorized into three prognostic groups: indolent (score, 0–1; 5-year RFS, 96.9%), intermediate (score, 2; 5-year RFS, 54.8%), or aggressive (score, 3–4; 5-year RFS, 33.3%). This prognostication strategy effectively stratified patients according to their risks of post-resection metastatic recurrence (Fig. 1). Of note, applying this prognostication strategy to non-functional tumor-only group (i.e., excluding all functional tumors) yielded similar prognostication capabilities: indolent (5-year RFS, 96.3%), intermediate (52.8%), and aggressive (33.3%).
Fig. 1.
Kaplan-Meier estimates of recurrence free survival (RFS) by the score-based prognostic categories
On Cox regression analysis, patients in the intermediate (score 2) and aggressive (scores 3–4) groups had a significantly elevated risk of metastatic recurrence (intermediate: HR, 9.36, 3.19–27.4; aggressive: HR, 26.1, 8.23–82.6; P <0.0001 for all) compared to patients in the indolent group (score, 0–1) (Table 3). In a multivariate Cox regression model including the score-based prognostic grouping and clinicopathological factors not used for scoring, the score-based prognostic group was the only variable that remained as an independent predictor of RFS (Table 4).
Table 3.
5-year disease-free survival associated with score-based prognostic groups
| Prognostic factors | Points | Score | Prognostic group |
5-year RFS |
|---|---|---|---|---|
| Tumor size | ||||
| < 5 cm | 0 | 0–1 | Indolent | 96.9% |
| ≥ 5 cm | 1 | |||
| LN involvement | ||||
| Negative | 0 | 2 | Intermediate | 54.8% |
| Positive | 1 | |||
| Ki-67 index/grade | ||||
| <8% | 0 | 3–4 | Aggressive | 33.3% |
| 8–20% | 1 | |||
| > 20% (or WHO grade G3)a | 2 |
WHO 2010 grade classification defines G3 (high grade) as Ki-67 index >20%
RFS, recurrence-free survival; LN, lymph node
Table 4.
Multivariate Cox regression analysis evaluating the score-based prognostic groups and clinicopathological factor
| Prognostic factors | Hazard ratio (95% CI) | P value |
|---|---|---|
| Prognostic groups by score | ||
| Indolent (score: 0–1) | 1 (reference) | |
| Intermediate (score: 2) | 7.23 (1.75–29.79) | 0.00611 |
| Aggressive (score: 3–4) | 28.49 (5.77–140.61) | < 0.0001 |
| Age | 1.00 (0.96–1.05) | 0.972 |
| Tumor type | ||
| Functional | 1 (reference) | |
| Nonfunctional | 0.762 (0.16–3.71) | 0.735 |
| Tumor location | ||
| Distal/body | 1 (reference) | |
| Head | 0.60 (0.19–1.94) | 0.394 |
| T-stage | ||
| T1 | 1 (reference) | |
| T2 | 1.55 (0.23–10.35) | 0.65 |
| T3 | 2.17 (0.24–20.04) | 0.49 |
| Lymphovascular invasion | ||
| Absent | 1 (reference) | |
| Present | 2.35 (0.88–6.27) | 0.0879 |
| Perineural Invasion | ||
| Absent | 1 (reference) | |
| Present | 0.87 (0.24–3.10) | 0.826 |
| MEN1 | ||
| Absent | 1 (reference) | |
| Present | 4.46 (0.33–60.13) | 0.260 |
Italic – p-value < 0.05
The predictive accuracy of the score-based prognostication strategy, as measured by the c-index, was 0.82 (CI, 0.72–0.92). Using this integrated strategy for prognosis prediction resulted in a superior predictive accuracy compared to using individual prognostic factors alone (Table 5).
Table 5.
Predictive performance of the score-based risk stratification compared to its component prognostic factors
| Prognostic factor | c-index | P valuea |
|---|---|---|
| Score-based prognostic groups | 0.82 (95% CI 0.82–0.92) | - |
| Size | 0.67 (95% CI 0.57–0.77) | 0.0431 |
| Lymph node involvement | 0.68 (95% CI 0.59–0.77) | 0.0408 |
| Ki67 | 0.70 (95% CI 0.62–0.78) | 0.056 |
P value in comparison against the score-based prognostic groups
Lastly, bootstrapping with 1000 resamples was utilized for validation. The corrected c-index after adjusting for the optimism error remained at 0.81. This was a significant improvement compared to the AJCC (c-index, 0.70), ENETS (0.69), and WHO (0.72) classifications.
Predictive Performance of the Score-Based Prognostication Strategy for WHO Low- (G1) and Intermediate-Grade (G2) Tumors
In our current series, we found metastatic recurrences in 5 of 6 (83.3%) patients with G3 tumors, compared to 4 of 72 (5.56%) patients with G1 tumors and 14 of 62 (22.6%) patients with G2 tumors. The current WHO classification effectively identifies the small number of patients with G3 tumors that have an extremely high likelihood of developing metastatic recurrence. However, identifying patients at risk for recurrence within the G1/G2 histologic groups remains a significant challenge and leads to patient/physician anxiety. Notably, despite the fact that only 13.4% (18 of 134) of G1/G2 tumors developed recurrence while 83.3% (5 of 6) of G3 tumors developed recurrence, G1/G2 tumors still comprised approximately 80% (18 of 23) of all metastatic recurrence observed in our current cohort. This highlights the importance of further risk stratification within the G1/G2 groups.
Given the high prognostic uncertainty associated with the low-/intermediate (G1/G2)-grade tumors, we evaluated the performance of our prognostication strategy applied specifically to this subgroup. By categorizing patients into the indolent vs. intermediate vs. aggressive groups using the score-based strategy as described above, patients with G1/G2 tumors were effectively stratified according to their risks for post-resection metastatic recurrences (5-year RFS, indolent: 96.9% vs. intermediate: 58% vs. aggressive: 25.0%; P < 0.0001; Fig. 2). Notably, the score-based prognostic groups applied to G1/G2-only cohort achieved a similar 5-year RFS split as when it was applied to all tumor grades. C-index of the score-based prognostication strategy applied specifically to patients with G1/G2 tumors was 0.82 (CI, 0.68–0.96). This was a significant improvement compared to using the low- (G1) vs. intermediate (G2)-grade categories for risk stratification (c-index, 0.65).
Fig. 2.
Kaplan-Meier estimates of recurrence free survival (RFS) by the score-based prognostic categories applied to patients with G1/G2 tumors
Discussion
As the incidence of PNET rises each year, there is a strong demand to better understand the natural history to guide optimal management strategies. Prior studies have largely focused on overall survival and included patients with highly varied stages of the disease. In a disease with a broad spectrum of indolent to aggressive biology, as well as a long overall survival, a meaningful and measureable endpoint becomes disease recurrence after a surgery that is meant to be curative. In the current study, we analyzed patients with early stage PNETs who underwent presumed curative formal pancreatic resections. We identified and validated four prognostic factors for post-resection metastatic recurrence: tumor size > 5 cm, positive LN, Ki-67 8–20%, and high grade (G3, or Ki-67 > 20%). We further devised and validated an integrated prognostic scoring system, which effectively stratified patients according to their risks of metastatic relapses following potentially curative pancreas resections (c-index, 0.82).
Recent studies have evaluated a number of potential prognostic factors for PNET.1,8,10,13 Consistent with our finding, tumor size, LN status, Ki-67 proliferative index, and tumor grade have all been individually implicated in predicting PNET prognosis.9,10,13 However, their collective role in prognostication remained undefined except within a few prior reports. For instance, Ballian et al. have proposed a prognostication system for disease recurrence based on size, grade, node, and margin (SGNM).16 Although this study differed from ours in evaluating for all recurrences (both local and metastatic) and included patients with R1 resections, we shared similar findings with regard to size, grade, and nodal status being important predictors of cancer recurrence. One important difference is that our current analysis included the evaluation of Ki-67 index in all tumors tested. Addition of Ki-67 may offer an additional prognostic power, as it has been shown to correlate with malignant potentials independent of histologic grade.17–19 Furthermore, using Ki-67 > 8% as a score criterion allows for patient stratification within the prognostically uncertain WHO intermediate-grade (G2) group.
Our proposed prognostication system differs from the currently available PNET staging classification systems in two important ways. First, unlike the currently available AJCC, ENETS, and WHO classifications that consider TNM and histological grade information separately for prognostication, we combined the components of TNM staging (tumor size and lymph node status) with Ki-67 (determinant of tumor grade) to form a single integrated classification system. Secondly, our proposed prognostication system was developed using post-resection metastasis as the primary outcome, as opposed to patient mortality. This may have helped achieve its high predictive accuracy for recurrence-free survival with a c-index of 0.82. In a report by Liu et al., the AJCC, WHO 2010, and ENETS classification systems were evaluated for their roles in predicting recurrence-free survival.7 The reported c-indices of these systems for the prediction of disease recurrence were 0.65 (AJCC), 0.67 (WHO 2010), and 0.66 (ENETS). Similarly, in a study by Strosberg et al., the AJCC and ENETS TNM classifications were shown to have some prognostic potential for predicting recurrence-free survival, yet they could not achieve statistical significance as independent predictors of disease recurrence on multivariate analysis.20 These findings suggest a need for an improved prediction of disease recurrence using our proposed system compared to the currently available staging systems.
Currently, no recommendation for adjuvant therapies in resected early-stage PNET exists. Our current study, as well as prior studies, shows that a subset of patients indeed suffers from metastatic recurrences despite R0 resections.9,10,21 Available studies show overall 5-year recurrence-free survival of approximately 60–70%, with an estimated 5-year overall survival of 60%.2,20 These findings suggest that a significant subset of patients remain at risk for having a more aggressive form of pNETs that manifest as distant recurrence with worse overall prognosis. Adjuvant therapy to prevent the development of metastatic disease may be indicated specifically in these high-risk patients. Given the overall indolent biology associated with most localized PNETs, identifying these high-risk patients likely to benefit from adjuvant therapies as well as intensified follow-up strategies is highly challenging. Therefore, effective uses of adjuvant therapies must rely on a well-defined prognostication system to identify high-risk patients. We show that our proposed prognostication strategy has the potential to serve this role, as it enables stratification of patients into three very distinct groups: indolent (score, 0–1; 5-year RFS, 96.9%), intermediate (score, 2; 5-year RFS, 54.8%), or aggressive (score, 3–4; 5-year RFS, 33.3%). This score system may be utilized in identifying appropriate patients for future adjuvant therapies or enrollment in enhanced surveillance protocols, especially in patients with low-/intermediate-grade (G1/G2) tumors that are known to have high prognostic uncertainly.
There is currently no data on the effectiveness of adjuvant therapy in patients with resected pNETs. However, potential role of adjuvant therapy has been suggested in poorly differentiated neuroendocrine tumors with high risk for relapse. Recent North American NeuroEndocrine Tumor Society guideline recommends adjuvant therapy with 4–6 cycles of cisplatin or carboplatin plus etoposide in poorly differentiated NETs.22 Our proposed score system identifies a subset of well-differentiated tumors with similar prognosis to G3 (high-grade, poorly differentiated) tumors. This subset of tumors may potentially benefit from adjuvant therapies and at minimum prompt an adjuvant therapy discussion and potentially be offered a more vigilant follow-up plan over the ensuing 5 years. Further clinical trials targeting specifically the high-risk PNETs patients are needed.
Our current study is limited by its retrospective nature and the use of a single institution experience. Given that routine Ki-67 analysis in PNETs has only recently become widespread in clinical laboratories, we currently will need to wait for few years to ensure adequate follow-up prior to an external validation of our score-based prognostication strategy. In lieu of a mature external data set for validation, the bootstrapping methodology has been described to provide a nearly unbiased estimate of predictive model performance in an external dataset.15 This computational strategy is often employed to validate a prediction model when a validation cohort is not available. Even after adjusting for the optimism error through bootstrapping with 1000 resamples, we found both the PNET prognostic factors as well as the score-based prognostication strategy to retain their predictive accuracies for post-resection metastatic recurrences.
In conclusion, we show that size > 5 cm, positive LN, Ki-67 8–20%, and high grade tumors (G3, or Ki-67 > 20%) are independent predictors of metastatic recurrence following a potentially curative pancreas resection. Our proposed score-based prognostication strategy provides an enhanced risk stratification for cancer recurrence and has the potential to guide adjuvant therapy decisions as well as tailor surveillance protocols.
Synopsis.
We describe factors predictive of metastatic recurrences in resected pancreas neuroendocrine tumors (PNETs) and develop a prognostication strategy to predict recurrence-free in resected PNETs
Acknowledgements
We thank Daniela Markovic from UCLA Department of Biomathmatics for her consultation on statistical methodology.
Funding Information This work was funded by the UCLA Jonsson Comprehensive Cancer Center Impact Grant.
Footnotes
Disclosure The authors declare that they have no conflict of interest.
References
- 1.Bilimoria KY, Tomlinson JS, Merkow RP, Stewart AK, Ko CY, Talamonti MS et al. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg 2007;11(11):1460–7; discussion 7–9. doi: 10.1007/s11605-007-0263-3. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008;247(3):490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 3.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional Neuroendocrine Carcinoma of the Pancreas: Incidence, Tumor Biology, and Outcomes in 2,158 Patients. J Gastrointest Surg 2010;14(3):541–8. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15(2): 409–27. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The Pathologic Classification of Neuroendocrine Tumors: A Review of Nomenclature, Grading, and Staging Systems. Pancreas 2010;39 (6):707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 6.Kloppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Archiv. 2010;456(6):595–7. doi: 10.1007/s00428-010-0924-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2013;37(6):853–9. doi: 10.1097/PAS.0b013e31827fcc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toste PA, Kadera BE, Tatishchev SF, Dawson DW, Clerkin BM, Muthusamy R et al. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg 2013;17(12):2105–13. doi: 10.1007/s11605-013-2360-9. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton NA, Liu TC, Cavatiao A, Mawad K, Chen L, Strasberg SS et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery 2012;152(1):107–13. doi: 10.1016/j.surg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boninsegna L, Panzuto F, Partelli S, Capelli P, Delle Fave G, Bettini R et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer 2012;48(11):1608–15. doi: 10.1016/j.ejca.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF et al. Pancreatic neuroendocrine tumors. Cancer 2009;115(4):741–51. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Tang LH, Klimstra DS. Effect of Tumor Heterogeneity on the Assessment of Ki67 Labeling Index in Well-differentiated Neuroendocrine Tumors Metastatic to the Liver: Implications for Prognostic Stratification. Am J Surg Pathol 2011;35(6):853–60. 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 13.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF et al. Determining Prognosis in Patients With Pancreatic Endocrine Neoplasms: Can the WHO Classification System Be Simplified? J Clin Oncol 2007;25(35):5609–15. doi: 10.1200/JC0.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 14.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S et al. C utoff Finder: A Com prehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLOS ONE 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4):361–87. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Ballian N, Loeffler AG, Rajamanickam V, Norstedt PA, Weber SM, Cho CS. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford). 2009;11(5):422–8. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamali M, Chetty R. Predicting Prognosis in Gastroentero-Pancreatic Neuroendocrine Tumors: An Overview and the Value of Ki-67 Immunostaining. Endocr Pathol 2008;19(4):282. doi: 10.1007/s12022-008-9044-0. [DOI] [PubMed] [Google Scholar]
- 18.Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P et al. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: A comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol. 1996;27(11):1124–34. doi: 10.1016/S0046-8177(96)90303-2. [DOI] [PubMed] [Google Scholar]
- 19.Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer 2013;108(9):1838–45. doi: 10.1038/bjc.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strosberg JR, Cheema A, Weber JM, Ghayouri M, Han G, Hodul PJ et al. Relapse-Free Survival in Patients With Nonmetastatic, Surgically Resected Pancreatic Neuroendocrine Tumors: An Analysis of the AJCC and ENETS Staging Classifications. Ann Surg 2012;256(2):321–5. doi: 10.1097/SLA.0b013e31824e6108. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134(6):1057–63. doi: 10.1016/j.surg.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas 2013;42(4):557–77. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]