SUMMARY
A long-term cure is now possible in more than 30% of selected patients with Zollinger-Ellison syndrome who undergo gastrinoma resection. The need, however, for continued gastric acid antisecretory therapy in these patients remains controversial. The current study was designed to determine whether post-operative antisecretory therapy is needed in patients who have undergone successful gastrinoma resection and, if so, to attempt to define criteria with which to identify patients who require therapy. Twenty-eight consecutive patients who had previously undergone curative gastrinoma resection were prospectively studied. When antisecretory therapy was discontinued, 43% (12/28) of these patients developed gastro-oesophageal reflux, diarrhoea, acid-peptic symptoms or endoscopic evidence of acid-peptic disease within 2 weeks and were deemed to have failed a trial of antisecretory drug withdrawal. The remaining 57% (16/28) of patients who successfully discontinued antisecretory therapy were followed for a mean time of 31 months after withdrawal of therapy. Analysis of acid output studies pre-operatively, as well as at the time of drug withdrawal, demonstrated that patients who were unable to discontinue antisecretory therapy exhibited higher pre-operative maximal acid output values and higher basal acid output values at the time of attempted drug withdrawal than patients who were able to discontinue therapy. Despite these findings, there was significant overlap in acid output values between groups so that it was not possible to define specific acid output criteria for successful drug withdrawal. Pre-operative clinical characteristics, such as the presence or absence of gastro-esophageal reflux or acid-peptic disease, or post-operative laboratory values, such as the fasting serum gastrin level, did not correlate with the ability to discontinue antisecretory therapy. We conclude that following successful curative gastrinoma resection, 40 % of patients still require antisecretory therapy and that both symptom evaluation as well as upper endoscopy should be used to guide attempted drug withdrawal. Although patients who are not able to discontinue therapy have significantly higher acid output measurements than those who are able to discontinue therapy, neither acid output criteria nor any other laboratory or clinical characteristics are able to predict the need for continued antisecretory therapy in these patients.
INTRODUCTION
Recent studies demonstrate that long-term cure is possible in more than 30% of selected patients with Zollinger-Ellison syndrome who undergo gastrinoma resection.1–3 However, the long-term management of gastric acid secretion in these patients remains controversial,1, 3–9 Some recommend stopping all anti-secretory therapy in all patients immediately following successful surgery.4 Others maintain that a proportion of patients who have undergone successful curative resection continue to need antisecretory medication.5–8 Some authorities9 suggest that all patients undergoing attempts at curative gastrinoma resection should also undergo a parietal cell vagotomy at the same time to reduce the need for post-operative antisecretory therapy especially in those who are not cured but possibly also in those who are cured.
The primary reason for the lack of consensus in how to manage these patients is that the need for gastric acid antisecretory therapy, postoperatively, in patients who have undergone curative gastrinoma resection has not been systematically studied previously. A recent study5 analysed the gastric secretory function of Zollinger-Ellison syndrome patients following curative gastrinoma resection and showed that both the short-term secretory effects as well as the long-term trophic effects of hyper-gastrinaemia are rapidly reversed within 3–6 months of curative gastrinoma resection. The mean basal acid output (BAO) decreased by 75% and then remained unchanged for the next 4 years of f0llow-up.5 Despite this reduction, two-thirds of cured patients remained low-level hypersecretors of gastric acid (i.e. BAO values of greater than 10 mm0l/h).5 In this study,5 a majority of the patients were maintained on low-dose gastric acid antisecretory agents post-resection : thus it remains unclear whether all gastric acid antisecretory therapy could still have been withdrawn from these patients. The answer to this problem has important clinical implications for the management of these patients because it affects the frequency of follow-up visits, the choice of what procedures to perform at follow-up visits, the assessment of when to evaluate patients for possible gastrinoma recurrence and the need for the continued expense of long-term maintenance antisecretory therapy.
In order to address these concerns, the present study was designed to determine whether a need for postoperative gastric antisecretory therapy exists in patients who have undergone curative gastrinoma resection, and if so, to attempt to define criteria by which to identify those patients who will require ongoing antisecretory therapy. An additional aim was to determine whether there were any criteria which could be used to predict a need for post-operative antisecretory therapy thereby enabling a pre-operative decision to be made as to whether to perform a parietal cell vagotomy, as has been suggested by some9 but not other1,3–7 investigators.
MATERIALS
Patients
All patients with Zollinger-Ellison syndrome, who had undergone gastrinoma resection at the National Institutes of Health, were included in the present study provided that they were assessed as being disease-free post-operatively. The study group, all of whom had positive histological evidence of gastrinoma at the time of surgery, was composed entirely of patients with the sporadic form of Zollinger-Ellison syndrome and there were no patients with Zollinger-Ellison syndrome associated with multiple endocrine neoplasia type-I (MEN-I). The study group forms part of the larger cohort of patients with Zollinger-Ellison syndrome undergoing ongoing active study as approved by the clinical research committee of the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health.
Diagnosis of Zollinger-Ellison syndrome
The pre-operative diagnostic criteria for Zollinger-Ellison syndrome were as described previously1,7,10,11 and included an elevated fasting serum gastrin (greater than 100pg/ml) in the presence of a BAO of greater than 15 mmol/h (greater than 5 mmol/h if the patient had undergone previous gastric acid reducing surgery), positive provocative testing (an increase in serum gastrin of greater than 200 pg/ml following secretin stimulation or greater than 395 pg/ml calcium infusion), a positive histological diagnosis of gastrinoma, or a combination of these criteria.
Definition of cure
Following gastrinoma resection all patients were assessed both biochemically and by imaging studies in order to ascertain whether they were apparently cured of Zollinger-Ellison syndrome. As described previously,2, 5, 6, 12 patients were placed in the cured category only if their fasting serum gastrin concentration, in the presence of known gastric acid secretion, was less than 100 pg/ml, if they had a negative secretin provocative test (an increase in serum gastrin concentration of less than 200 pg/ml following intravenous injection of 2 U/kg of Kabi secretin), and if all imaging studies were negative for recurrent tumour (i.e. they were assessed as having no evidence of Zollinger-Ellison syndrome).
Investigations
The BAO and maximal acid output (MAO) were determined as described previously.13 Briefly, a nasogastric tube was positioned in the stomach and the gastric contents were continuously aspirated. The BAO was measured after discontinuing all oral antisecretory medication (greater than 30 h for histamine H2-receptor antagonists and greater than 7 days for omeprazole) by collecting four consecutive 15-min samples after first discarding the overnight residual secretions. Each sample was titrated to pH 7.0 with 0.01 N NaOH and the BAO was determined by adding the number of millimoles of acid in each of the four samples obtained in this manner. MA0 was measured by collecting four 15-min samples following subcutaneous administration of pentagastrin (6 μg/kg). Normal values for acid output measurements were as described previously.5, 14, 15 The upper limits of normal for BAO are 10.5 mmol/h for men without previous gastric acid reducing surgery,15 5.6 mmol/h for women without previous gastric acid reducing surgery15 and 5 mmol/h for all patients with previous gastric acid reducing surgery.5, 14 The upper limits of normal for MA0 are 48 mmol/h for men and 30 mmol/h for wornen15 and the upper limit of normal for the BAO/MAO ratio is 0.47.5,14
Upper gastrointestinal endoscopy was performed in the left lateral decubitus position using a Fujinon video-endoscopy system (Fujinon Inc., Wayne, NJ, USA) following sedation with meperidine (Demerol, Winthrop Pharmaceuticals, New York, NY, USA) and midazolam (Versed, Roche Laboratories, Nutley, NJ, USA). The upper gastrointestinal mucosa was carefully examined and biopsies were taken if any abnormalities were seen.
Abdominal imaging studies were performed both pre and postoperatively in all patients, as described previously, these studies included selective arteriography,16 CT scan,17 ultrasonography18 and magnetic resonance imaging.19
Fasting serum gastrin values were determined by Bioscience Laboratories (New York, NY, USA) and all samples were diluted into the normal range for accurate determination of higher values as described previously.5
Study design
There were 44 patients who were eligible for the study by virtue of being disease-free post-operatively. Sixteen of these patients were excluded from the protocol because they had relapsed at or prior to the withdrawal of antisecretory medication (n = 14) or because they had been lost to follow-up (n = 2). The remaining 28 patients were a11 included in the present protocol.
Gastric acid antisecretory therapy had been stopped in eight of the 28 patients after the initial post-operative evaluation because their BAO values were < 10 mmol/h as described previously.5 In the remaining 20 patients, all antisecretory therapy was withdrawn at least 2 weeks prior to a scheduled National Institutes of Health admission. Patients were instructed to be especially aware of the development of recurrent symptoms of gastric acid hypersecretion. They were advised to contact us immediately if peptic symptoms or diarrhoea occurred so that we could determine whether they should restart their maintenance antisecretory medication or whether they should continue taking no medication up until admission. On the morning following admission all patients were carefully interviewed for symptoms of gastric acid hypersecretion and then underwent acid output measurements followed immediately by upper gastrointestinal endoscopy. Successful antisecretory drug withdrawal was defined as an absence of symptoms and an upper gastrointestinal endoscopy without evidence of active peptic ulceration or severe mucosal disease, as defined by the presence of at least multiple mucosal erosions that had not been present previously in the presence of gastric acid antisecretory therapy. Patients who developed persistent symptoms (e.g. heart-burn, abdominal pain or diarrhoea) at any time after antisecretory therapy withdrawal, which responded to re-institution of antisecretory therapy, were deemed to have failed the protocol. Acid output measurements were not included in the determination of whether antisecretory therapy withdrawal was successful or not. During the course of their admission, each patient was evaluated with serial fasting serum gastrin determinations, a secretin stimulation test and abdominal imaging studies to determine whether they remained disease-free. Patients who no longer fulfilled the criteria for being cured were removed from the protocol. All patients who successfully discontinued gastric acid antisecretory therapy were followed as out-patients. If persistent symptoms, which resolved with re-institution of therapy, developed at any time in these patients, they were deemed to have failed antisecretory therapy withdrawal.
Statistics
All values are expressed as mean ± 1 S.E.M. The statistical methods employed include the paired t-test for comparisons within a group such that patients acted as their own controls and the Mann-Whitney U- or Fisher’s exact tests for comparisons between two different groups of patients. P-values of < 0.05 were considered statistically significant.
RESULTS
Characteristics of the 28 patients (16 males and 12 females), none of whom had MEN-1, are shown in Table 1. Analysis of pre-operative fasting serum gastrin levels, acid output measurements and antisecretory drug requirements (Table 1) reveals that the patients were representative of the general population with the sporadic form of Zollinger-Ellison syndrome in terms of the severity of their gastric acid hypersecretion, as described in a number of previous studies.1, 6, 7, 14, 9,20–28 Thus the results obtained can be extrapolated to the sporadic form of Zollinger-Ellison syndrome in general. Approximately three-quarters of all patients with Zollinger-Ellison syndrome have the sporadic form of the disease.1
Table 1.
Pre-operative patient characteristics
| Patients | 28 |
| Gender | |
| Male/female | 16/12 |
| Previous partial gastrectomy | 3 |
| Age at operation (years) | |
| Mean ± S.E.M. | 51 ± 2 |
| Range | 32–69 |
| Disease duration at operation (years) | |
| Mean ± S.E.M. | 8.4 ± 1.3 |
| Range | 0.8–26.5 |
| Pasting serum gastrin (pg/ml) | |
| Mean ± S.E.M. | 1153 ± 260 |
| Range | 107–5800 |
| Basal acid output (mmol/h) | |
| Mean ± S.E.M. | 35 ± 4 |
| Range | 2–95 |
| Maximal acid output (mmol/h) | |
| Mean ± S.E.M. | 51 ± 5 |
| Range | 4–127 |
| Antisecretory drug requirements | |
| Histamine H2-receptor antagonist | 19 |
| Ranitidine equivalent dose* (g/24 h) | |
| Mean ± S.E.M. | 1.74 ± 0.2 |
| Range | 0.3 −3.6 |
| Omeprazole | 9 |
| Dose (mg/24 h) | |
| Mean ± S.E.M. | 71 ± 12 |
| Range | 20–120 |
The ranitidine equivalent dose is the dose of cimetidine or famotidine expressed in the equivalent ranitidine dose as outlined in Ref. 21.
Fifty-seven per cent (16/28) of the patients were able to discontinue taking all antisecretory medications as part of the present protocol. Table 2 lists the gender, pre- and post-operative acid output measurements and durations of disease, cure and drug withdrawal for the 16 patients who were able to discontinue all antisecretory therapy successfully. As expected, there was a significant decrease in all acid output parameters in the 16 cured patients who were subsequently able to discontinue all anti-secretory medication. When assessed 42 ± 7 months (mean 5 S.E.M.) after curative surgery, BAO values were in the normal range in 63% (10/16) of these patients (Table 2). MAO values were also in the normal range in 63% (10/16) of patients and the BAO/MAO ratios were in the normal range in 94% (15/16) of patients (Table 2). The mean BAO, MA0 and BAO/MAO ratio decreased from 30 ± 4 mmol/h, 45 ± 5 mmol/h and 0.66 ± 0.06 to 5 ± 1 mmol/h (P = 0.0001), 30 ± 4 mmol/h (P = 0.0005) and 0.2 ± 0.04 (P = 0.0001), respectively (Table 2). These 16 patients were followed for a further 31 ± 9 months (means ± S.E.M. : range 2–109 months) and during this period, three have restarted antisecretory medication. Two patients (1 and 16, Table 2) developed recurrent Zollinger-Ellison syndrome and one patient (8, Table 2) developed a high lesser curve gastric ulcer in the presence of normal acid output measurements. The remaining 13 patients continue to not require any antisecretory therapy.
Table 2.
Gender, pre- and post-operative acid output measurements and durations of disease, cure and drug withdrawal in 16 patients who were able to discontinue all antisecretory therapy successfully
| Pre-operative | At withdrawal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Disease duration (years) |
BAO (mmol/h) |
MAO (mmol/h) |
BAO/MAO ratio | Time after surgery (months) |
BAO (mmol/h) |
MAO (mmol/h) |
BAO/MAO ratio | Time off all medicine (months) |
| 1 | F | 0.8 | 30 | 75 | 0.4 | 24 | 1 | 34 | <0.1 | 29† |
| 2 | F | 4.5 | 30 | 33 | 0.9 | 104 | 1 | 27 | <0.1 | 105 |
| 3 | M | 10 | 24 | 58 | 0.4 | 17 | 13 | 48 | 0.2 | 11 |
| 4* | M | 15.5 | 8 | 11 | 0.7 | 69 | 1 | 5 | 0.2 | 75 |
| 5* | M | 17.5 | 10 | 19 | 0.5 | 80 | 1 | 4 | 0.3 | 109 |
| 6 | M | 14.9 | 2 | 4 | 0.5 | 46 | 0 | 2 | 0 | 70 |
| 7 | F | 5.8 | 44 | 44 | 1.0 | 21 | 3 | 30 | 0.1 | 11 |
| 8 | F | 2.6 | 25 | 31 | 0.8 | 55 | 6 | 16 | 0.4 | 12‡ |
| 9 | F | 6.3 | 15 | 50 | 0.3 | 16 | 8 | 50 | 0.2 | 10 |
| 10 | M | 9.9 | 25 | 41 | 0.6 | 41 | 5 | 24 | 0.2 | 10 |
| 11 | F | 5.6 | 44 | 54 | 0.8 | 4 | 3 | 51 | <0.1 | 10 |
| 12 | F | 3.1 | 50 | 50 | 1.0 | 6 | 6 | 47 | 0.1 | 11 |
| 13 | M | 14.9 | 60 | 65 | 0.9 | 59 | 4 | 41 | 0.1 | 6 |
| 14 | M | 3.0 | 43 | 71 | 0.6 | 5 | 6 | 36 | 0.2 | 7 |
| 15 | F | 3.3 | 19 | 58 | 0.3 | 58 | 9 | 35 | 0.3 | 2 |
| 16 | M | 8.5 | 50 | 58 | 0.9 | 61 | 17 | 23 | 0.7 | 12† |
| Mean ± S.E.M. | 7.9 ± 1.3 | 30 ± 4 | 45 ± 5 | 0.66 ± 0.06 | 42 ± 7 | 5 ± 1 | 30 ± 4 | 0.2 ± 0.04 | 31 ± 9 | |
| Range | 0.8 ± 17.5 | 2–60 | 4–75 | 0.3–1.0 | 4–104 | 0–17 | 2–58 | 0–0.7 | 2–109 | |
Previous partial gastrectomy.
Developed evidence of gastrinoma recurrence during follow-up
Restarted medication after the diagnosis of a gastric ulcer.
Table 3 lists the gender, pre- and post-operative acid output measurements and durations of disease, cure and drug withdrawal for the 12 patients who were unable to discontinue all antisecretory therapy successfully. When assessed 44 ± 8 months (mean ± S.E.M.) after curative surgery, BAO values were in the normal range in 33 % (4/12) of patients, MAO values were in the normal range in 36% (4/11) of patients and BAO/MAO ratios were in the normal range in all 11 patients (Table 3). The mean BAO, MAO and BAO/MAO ratio decreased from 41 ± 7 mmol/h, 70 ± 8 mmol/h and 0.58 ± 0.06 to 11 ± 1 mmol/h (P = 0.0014). 46 ± 6 mmol/h (P = 0.0077) and 0.3 ± 0.03 (P = 0.0007), respectively (Table 3). Two of these 12 patients (23 and 26, Table 3) were unable to discontinue all antisecretory medications for the required 2-week period before evaluation at the National Institutes of Health because of the development of severe symptoms of gastric acid hypersecretion which resolved with the re-introduction of antisecretory therapy. The remaining 10 patients were all evaluated after 2 full weeks of antisecretory drug withdrawal.
Table 3.
Gender, pre- and post-operative acid output measurements and durations of disease, cure and drug withdrawal in 12 patients who were unable to discontinue all antisecretory therapy
| Pre-operative | At withdrawal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Disease duration (years) |
BAO (mmol/h) |
MAO (mmol/h) |
BAO/MAO ratio | Time after surgery (months |
BAO (mmol/h) |
MAO (mmol/h) |
BAO/MAO ratio | Time off all medicine (months) |
| 17 | M | 9.5 | 66 | 97 | 0.7 | 77 | 6 | ND† | — | 0.5 |
| 18 | F | 17.8 | 28 | 60 | 0.5 | 17 | 6 | 35 | 0.2 | 0.5 |
| 19 | M | 5.9 | 41 | 65 | 0.6 | 16 | 16 | 62 | 0.3 | 0.5 |
| 20 | M | 1.2 | 63 | 105 | 0.6 | 5 | 19 | 74 | 0.3 | 0.5 |
| 21* | F | 1.5 | 15 | 42 | 0.4 | 19 | 6 | 16 | 0.4 | 0.5 |
| 22 | M | 10 | 95 | 127 | 0.7 | 40 | 7 | 53 | 0.1 | 0.5 |
| 23 | M | 22.3 | 24 | 80 | 0.3 | 14 | 17 | 80 | 0.2 | 0.25‡ |
| 24 | M | 2.3 | 16 | 56 | 0.3 | 70 | 13 | 42 | 0.3 | 0.5 |
| 25 | F | 3.7 | 22 | 40 | 0.6 | 62 | 10 | 40 | 0.3 | 0.5 |
| 26 | F | 1.3 | 38 | 38 | 1.0 | 83 | 11 | 29 | 0.4 | 0.1‡ |
| 27 | M | 26.5 | 52 | 81 | 0.6 | 53 | 10 | 51 | 0.4 | 1 |
| 28 | M | 7.5 | 31 | 46 | 0.7 | 68 | 6 | 26 | 0.2 | 0.5 |
| Mean ± S.E.M. | 9.1 ± 2.5 | 41 ± 7 | 70 ± 8 | 0.58 ± 0.06 | 44 ± 8 | 11 ± 1 | 46 ± 6 | 0.3 ± 0.03 | 0.48 ± 0.02 | |
| Range | 1.2–26.5 | 15–95 | 23–127 | 0.3–1.0 | 5–83 | 6–19 | 16–80 | 0.1–0.4 | 0.1–1 | |
Previous partial gastrectorny.
Not done—MEN-2 with medullary thyroid carcinoma, pentagastrin injection deemed to be unsafe.
Unable to tolerate antisecretory drug withdrawal for 2 full weeks due to the development of severe symptoms of gastric acid hypersecretion.
Persistent symptoms of gastric acid hypersecretion developed in 50% of the patients (6/12) who failed antisecretory drug withdrawal. In contrast, upper gastrointestinal endoscopy was abnormal in 83% of the patients (10/12) who failed antisecretory drug withdrawal (including six patients who were symptom-free at the time of evaluation). Only 33 % of the patients (4/12) who failed antisecretory drug withdrawal exhibited both symptoms as well as endoscopic changes of gastric acid hypersecretion at the time of evaluation. Heartburn was the most common symptom occurring in 42 %, abdominal pain developed in 33 % and diarrhoea developed in 25% of the 12 patients who failed antisecretory therapy withdrawal. Endoscopic findings included severe peptic oesophagitis in 42%, multiple duodenal erosions in 33% and frank duodenal ulcers in 17% of the 12 patients who failed antisecretory therapy withdrawal. Of the six patients who failed antisecretory drug withdrawal on the basis of endoscopic findings alone, two had severe oesophagitis (patients 17 and 28; Table 3), three had multiple duodenal erosions (patients 18, 24 and 25; Table 3) and one had both oesophagitis and duodenitis (patient 19; Table 3). BAO values were elevated in four of these patients (patients 18, 19, 24 and 25; Table 3). The remaining two patients (patients 17 and 28; Table 3) had BAO values in the normal range. Both the symptoms as well as the endoscopic abnormalities of gastric acid hypersecretion resolved in all 12 patients following re-introduction of antisecretory therapy. None of the 16 patients in whom antisecretory therapy was successfully withdrawn manifested abnormalities in either symptoms of gastric acid hypersecretion or endoscopic findings.
Tables 4 to 6 present analyses of the change in acid output criteria following surgery comparing the group that was able to discontinue antisecretory medication with that which was not. There was no significant difference in the percentage of patients whose BAO (Table 4), MAO (Table 5) or BAO/MAO ratio (Table 6) had returned to the normal range between the group of patients that was and the group of patients that was not able to discontinue all antisecretory therapy.
Table 4.
Correlation between the basal acid output (BAO) and the ability to successfully withdraw antisecretory therapy after curative gastrinoma resection*
| Number of patients | |||
|---|---|---|---|
| BAO (mmol/h) |
Withdrawn | Not withdrawn | Total |
| Normal† | 10 | 4 | 14 |
| Elevated | 6 | 8 | 14 |
| Total | 16 | 12 | 28 |
Values indicate the number of patients in whom antisecretory medication was or was not successfully withdrawn in relation to the BAO at the time of attempted drug withdrawal.
Normal BAO is < 10.5 mmol/h for men without previous gastric resection, < 5.6 mmol/h for women without previous gastric resection and < 5 mmol/h in patients with previous gastric resections (see references 5, 19 and 28).
There is no significant difference between the proportion of patients with normal or elevated BAO who were successfully withdrawn from antisecretory therapy (P = 0.10, Fisher’s exact test).
Table 6.
Correlation between the BAO/MAO ratio and the ability to successfully withdraw antisecretory therapy after curative gastrinoma resection*
| Number of patients | |||
|---|---|---|---|
| BAO/MAO (mmol/h) |
Withdrawn | Not withdrawn | Total |
| Normal† | 15 | 11 | 26 |
| Elevated | 1 | 0 | 1 |
| Total | 16 | 11 | 27‡ |
Values indicate the number of patients in whom antisecretory medication was or was not successfully withdrawn in relation to the BAO/MAO ratio at the time of attempted drug withdrawal.
One patient excluded (see Table 3).
There is no significant difference between the proportion of patients with normal or elevated BAO/MAO ratio who were successfully withdrawn from antisecretory therapy (P = 0.60, Fisher’s exact test).
Table 5.
Correlation between the maximal acid output (MAO) and the ability to withdraw antisecretory therapy successfully after curative gastrinoma resection*
| Number of patients | |||
|---|---|---|---|
| MAO (mmol/h) |
Withdrawn | Not withdrawn | Total |
| Normal† | 11 | 4 | 15 |
| Elevated | 5 | 7 | 12 |
| Total | 16 | 11 | 27‡ |
Values indicate the number of patients in whom antisecretory medication was or was not successfully withdrawn in relation to the maximal acid output at the time of attempted drug withdrawal.
Normal MA0 is < 48 mmol/h for men and < 30 mmol/h for women (see reference 28).
One patient excluded (see Table 3).
There is no significant difference between the proportion of patients with normal or elevated MAO who were successfully withdrawn from antisecretory therapy (P = 0.08, Fisher’s exact test).
One aim of the present study was to attempt to identify acid output criteria that would enable a distinction to be made, either pre- or post-operatively, between surgically cured patients with Zollinger-Ellison syndrome who do and who do not require ongoing maintenance anti-secretory therapy. To this end, we compared the measurements of BAO, MA0 and the BAO/MAO ratio, both pre- and post-operatively, between the group of 16 patients who were and the 12 patients who were not able to discontinue all antisecretory medications in the present study. In order for these comparisons to have validity, it was important to first confirm that the two patient groups were similar in terms of gender distribution, age at operation, duration of disease prior to curative surgery and duration of cure prior to attempted drug withdrawal. There were no significant differences noted in any of these possibly confounding variables between the two groups (Tables 2 and 3; specific analyses not shown). In addition, the above comparisons were repeated after having excluded the three patients with previous partial gastrectomies from the analysis and no differences from the original observations were noted.
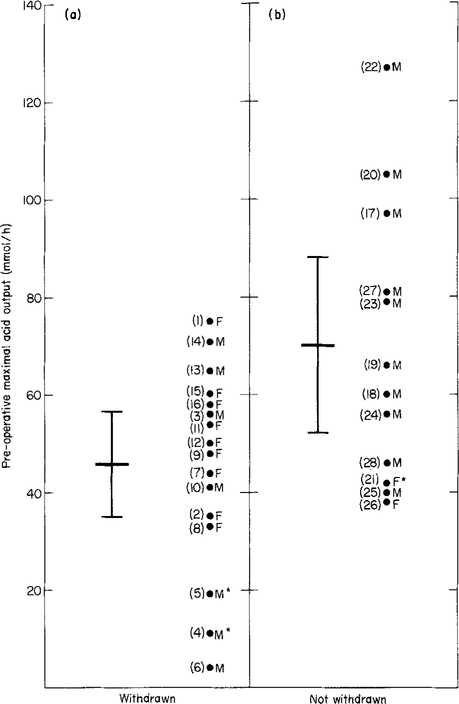
With regard to pre-operative criteria, neither the BAO values nor the BAO/MAO ratios differed significantly between the two groups (Tables 2 and 3; specific analyses not shown) but MAO values (Tables 2 and 3 and Figure 1) were significantly higher in the group that could not be withdrawn from medication. As illustrated in Figure 1, despite there being a significant difference in the mean pre-operative MAO value between patients who were and were not able to discontinue all antisecretory medication (P = 0.045), there also was a significant overlap between the two groups thereby preventing us from identifying a useful cut-off value with which to predict the need for long-term antisecretory therapy following curative resection.
Figure 1.
Preoperative MA0 values in 28 patients with Zollinger-Ellison syndrome who underwent curative gastrinoma resection. (a) Data for the 16 patients who subsequently were able to discontinue all antisecretory medication and (b) data for the 12 patients who subsequently were unable to discontinue all antisecretory medication. The difference in means (each represented by the longer horizontal bars) between the two groups was significant using the Mann Whitney U-test (P = 0.045). The shorter horizontal bars represent the 95% confidence limits for each respective mean value. The numbers in parentheses refer to each individual patient’s number (see Tables 2 and 3). F = Female, M = Male, *Previous partial gastrectomy.
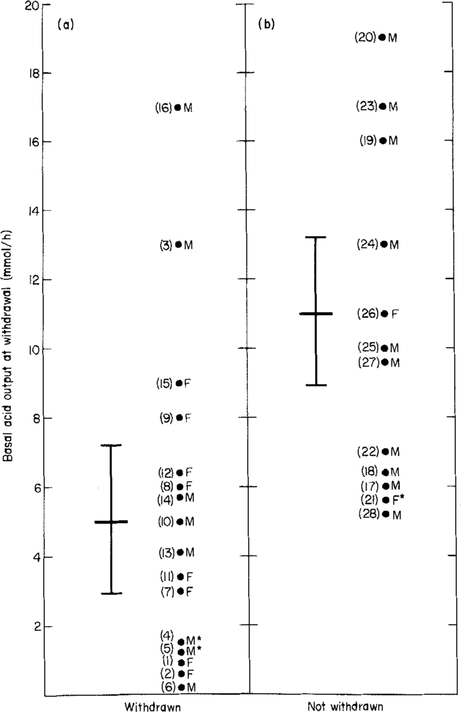
With regard to criteria at the time of drug withdrawal, BAO values (Tables 2 and 3 and Figure 2) were significantly higher in the group that could not be withdrawn from medication while the MAO values and BAO/MAO ratios were not significantly different (Tables 2 and 3; specific analyses not shown). As illustrated in Figure 2, despite there being a significant difference in the mean BAO value between patients who were and who were not able to discontinue all antisecretory medication (P = 0.005), there also was a significant overlap between the two groups thereby preventing us from identifying a useful cut-off value with which to predict the need for long-term antisecretory therapy following curative re-section. For example, had we chosen a BAO value of > 10 mmol/h to predict the need for antisecretory therapy (the currently accepted criterion in patients who have not been cured surgically1, 7, 13, 21–28), less than half of the patients who actually needed therapy would have received it and 12% of those who did not require therapy would have been treated unnecessarily (Figure 2).
Figure 2.
Basal Acid Output (BAO) values at the time of antisecretory therapy withdrawal in 28 patients with Zollinger-Ellison syndrome who had previously undergone curative gastrinoma resection. (a) Data for the 16 patients who were able to discontinue all antisecretory medication and (b) data for the 12 patients who were unable to discontinue all antisecretory medication. The difference in means (each represented by the longer horizontal bars) between the two groups was significant using the Mann Whitney U-test (P = 0.005). The shorter horizontal bars represent the 95% confidence limits for each respective mean value. The numbers in parentheses refer to each individual patient’s number (see Tables 2 and 3). F = Female, M = Male, *Previous partial gastrectomy.
The presence or absence of specific pre-operative clinical characteristics, such as symptoms of oesophageal reflux, abdominal pain or diarrhoea or previous endoscopic evidence of erosive oesophagitis or active duodenal ulcers, did not correlate with the likelihood of successful drug withdrawal after curative gastrinoma resection (data not shown). Furthermore, post-operative fasting serum gastrin levels at the time of attempted anti-secretory drug withdrawal did not correlate with the likelihood of successful drug withdrawal (data not shown).
DISCUSSION
The primary aim of this study was to determine whether a need for post-operative gastric antisecretory therapy exists in patients who have undergone successful curative gastrinoma resection. The present study demonstrates that long-term antisecretory therapy is necessary in about 40% of such patients because, within 2 weeks of antisecretory therapy withdrawal, they develop symptoms of gastric acid hypersecretion and/or endoscopic evidence of acid-peptic disease. A number of factors can be considered responsible for the failure to withdraw gastric acid antisecretory therapy successfully in this sub-group of patients. It is possible that these patients were in fact not cured of Zollinger-Ellison syndrome and thus the failure to withdraw antisecretory therapy is due to persistent or recurrent disease. However, no evidence could be found to support this assertion because, at the time of evaluation, all patients who failed the present study protocol had normal fasting serum gastrin levels, negative secretin provocative tests and negative abdominal imaging studies, Furthermore, these patients were evaluated at a mean time of 44 months after surgery, with seven of the 12 patients having had greater than 2 years of follow-up so that there was sufficient follow-up in these patients to establish long-term disease-free status. It could also be argued that a greater percentage of patients could have discontinued antisecretory therapy successfully had these studies been performed at a later time post-operatively. This is also unlikely because the effects of curative resection on BAO, MA0 and BAO/MAO ratio are complete within 3–6 months and that during further follow-up of up to 4 years, no further reduction in these parameters occurs.5 Because all the patients in the present study were evaluated at least 3–6 months post-operatively, it is unlikely that any further changes in acid secretory drive would occur in these patients. Lastly, it could be argued that the present study used inadequate criteria for the definition of what constituted failure of antisecretory therapy withdrawal. Half of the patients who failed antisecretory therapy did so on the basis of persistent symptoms of gastric acid hypersecretion and in all cases, when gastric acid antisecretory therapy was restarted, the symptoms of gastric acid hypersecretion resolved. Therefore, these six patients clearly required antisecretory therapy for control of symptoms. The remaining six patients who failed antisecretory therapy did so based on endoscopic findings alone. It can be argued that these patients may have been able to continue to not take any antisecretory medication because, despite endoscopic abnormalities, they remained free of symptoms. However, the present study was designed in such a way as to ensure that patients who failed antisecretory drug withdrawal, based purely on endoscopic findings, had to have definitive evidence of moderate to severe mucosal disease that had not been present prior to attempted drug withdrawal and that resolved completely after re-institution of therapy. With this study design, it was clear that the endoscopic changes were a direct result of the antisecretory therapy withdrawal because of the time-course of their development, We felt that it was unsafe to expose patients with known endoscopic changes to the potential risks of continued therapy withdrawal while waiting for them to develop symptoms in association with their endoscopic findings.
Having determined that a need for antisecretory therapy exists in certain patients following curative gastrinoma resection, a second aim of the present study was to establish whether there were any clinical or acid output criteria (obtained either pre- or post-operatively) that might distinguish between patients who were likely to need or not need ongoing antisecretory therapy. Two potentially useful gastric acid output criteria, the pre-operative MAO and the BAO at the time of drug withdrawal, were found to correlate significantly with the likelihood of successful antisecretory drug withdrawal. It is unclear why there should be a difference in the pre-operative MAO values between the patients who are and who are not able to discontinue antisecretory therapy following curative gastrinoma resection. This is especially so because this difference cannot be explained in terms of a longer duration of hypergastrinaemia-induced trophic stimulation of the parietal cell mass.5, 14, 20, 29–31 Moreover, the difference in MAO had disappeared by the time therapy was discontinued many months after curative surgery. It is far easier to understand how the second distinguishing criterion, the BAO at the time of withdrawal, may relate to the inability to withdraw antisecretory therapy in some patients after curative gastrinoma resection. Many studies have shown that if basal acid secretion is adequately reduced, no symptoms or mucosal disease occur.1, 13, 25, 26 The data from the present study suggests that if all patients with post-operative BAO values of > 10 mmol/h receive anti-secretory medication, only 12 % will receive therapy unnecessarily, which suggests that this criterion may be of some clinical benefit in predicting who needs therapy. However, the present study also suggests that this cannot be the sole criterion on which to base the need for therapy because BAO values of < 10 mmol/h were found in over 50% of those patients who required therapy. Thus, although both these criteria were shown to be of statistical significance, because of the considerable overlap between groups, we were unable to identify specific values for either criterion that could provide adequate sensitivity and specificity for their use in the determination of which patients would and would not require long-term therapy. Therefore, we conclude that acid output values alone cannot be used to determine the need for antisecretory therapy following curative gastrinoma resection, and that evaluation for symptoms of gastric acid hypersecretion and especially performance of upper gastrointestinal endoscopy are also required.
We were also unable to identify any pre-operative clinical criteria such as the presence or absence of oesophageal reflux symptoms, diarrhoea or abdominal pain, or previous endoscopic findings that correlated with the ability to withdraw antisecretory therapy. The failure to find a correlation with the presence or absence of oesophageal disease is particularly unexpected because recent studies of gastro-oesophageal reflux disease, in both Zollinger-Ellison syndrome patients3’ as well as in idiopathic acid-peptic disease,33 demonstrate that a greater degree and duration of acid inhibition is required to control symptoms and heal oesophagitis than in those with non-oesophageal acid-peptic disease. In addition, the lack of correlation between the post-operative fasting serum gastrin level and the likelihood of successful antisecretory therapy withdrawal suggests that correlation between acid output values and the failure of antisecretory therapy withdrawal is not due to a persistent gastrin drive in those patients who failed anti-secretory therapy withdrawal.
Controversy exists regarding the potential value of performing a parietal cell vagotomy at the time of curative gastrinoma resection.1, 7, 9, 23 Proponents of the procedure point out that it reduces the post-operative antisecretory drug requirements in all patients,9 especially those who are not rendered disease-free. Opponents of routine parietal cell vagotomy1, 6, 7 cite, as reasons for not performing the procedure, the additional anaesthesia time required to perform the procedure, its low likelihood of long-term success, its potential side-effects, and the efficacy and safety of available medical therapy for gastric acid hypersecretion. Most importantly, as was shown in the present study, more than half the patients who are cured by gastrinoma resection do not require any antisecretory therapy at all. Thus, it could be argued, that the performance of a parietal cell vagotomy in these patients would constitute an entirely unnecessary procedure. In the present study, we found that 40% of cured patients continue to require long-term antisecretory therapy. Had we been able to demonstrate specific pre-operative acid output criteria, which could have allowed us to predict accurately which cured patients would require long-term antisecretory therapy after successful curative surgery, then the present study would have provided criteria that could have been used at exploratory laparotomy to determine the need for the addition of a parietal cell vagotomy. However, the present study was unable to identify any such criteria. These findings support the previous recommendation that a parietal cell vagotomy should be considered in all patients at the time of initial surgery.9 If it can be shown that the addition of a parietal cell vagotomy at the time of curative surgery results in all patients being able to discontinue all gastric antisecretory therapy then the procedure should be routinely performed.
REFERENCES
- 1.Jensen RT, Gardner JD. Zollinger-Ellison syndrome : clinical presentation, pathology, diagnosis and treatment In: Zakim D, Dannenberg A, eds. Peptic ulcer and other acid-related diseases. New York: Spectrum, 1991; 117–212. [Google Scholar]
- 2.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome : Results of a 10-year prospective study. Ann Surg 1992; 215: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with Zollinger-Ellison syndrome. World J Surg 1991; 15: 151–9. [DOI] [PubMed] [Google Scholar]
- 4.Thompson NW Discussion of paper by Fraker D, Norton JA, Saeed Z, Maton PN, Gardner JD, Jensen RT. Surgery 1988; 104: 1063. [PubMed] [Google Scholar]
- 5.Pisegna JR, Norton JA, Slimak GG, et al. Effects of curative gastrinoma resection on gastric secretory function and antisecretory drug requirements in the Zollinger-Ellison syndrome. Gastroenterology 1992; 102: 767–78. [DOI] [PubMed] [Google Scholar]
- 6.Fraker D, Norton JA, Saeed Z, Maton PN, Gardner JD, Jensen RT. A prospective study of pre- and postoperative control of acid hypersecretion in patients with Zollinger-Ellison syndrome. Surgery 1988: 104: 1054–63. [PubMed] [Google Scholar]
- 7.Jensen RT, Gardner JD. Gastrinoma In: Go VLW, Gardner JD, Lebenthal E, DiMagno EP, Reber H, Scheele GA, eds. The exocrine pancreas : biology, pathobiology and diseases. 2nd edn. New York: Raven Press; (in press). [Google Scholar]
- 8.Mignon M, Ruszniewski P, Haffar S, Rigaud D, Rene E, Bonfils S. Current approach to the management of the tumoral process in patients with gastrinoma. World J Surg 1986; 10: 703–10. [DOI] [PubMed] [Google Scholar]
- 9.Richardson CT, Peters MN, Feldman M, et al. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists : a prospective study. Gastroenterology 1985; 89: 357–67. [DOI] [PubMed] [Google Scholar]
- 10.Frucht H, Howard JM, Slaff JI, et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome. Ann Intern Med 1989; 111: 713–22. [DOI] [PubMed] [Google Scholar]
- 11.Stage JG, Stadil R The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol 1979; 14 (Suppl. 53): 79–91. [PubMed] [Google Scholar]
- 12.Norton JA, Doppman JL, Collen MJ, et al. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg 1986; 203 : 468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raufman J-P, Collins SM, Pandol SJ, et al. Reliability of symptoms in assessing control of gastric acid secretin in patients with Zollinger-Ellison syndrome. Gastroenterology 1983; 84: 108–13. [PubMed] [Google Scholar]
- 14.Aoyagi T, Summerskill WHJ. Gastric secretion with ulcerogenic islet cell tumor. Arch Intern Med 1966; 117: 667–72. [PubMed] [Google Scholar]
- 15.Feldman M Gastric secretion In: Sleisenger MH, Fordtran JS, eds. Gastrointestinal disease, 3rd edn. Philadelphia: W B Saunders, 1983, 541–58. [Google Scholar]
- 16.Maton PN, Miller DL, Doppman JL, et al. Role of selective angiography in the management of patients with Zollinger-Ellison syndrome. Gastroenterology 1987; 92 : 905–12. [DOI] [PubMed] [Google Scholar]
- 17.Wank SA, Doppman JL, Miller DL, et al. Prospective study of the ability of computed axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology 1987; 92: 905–12. [DOI] [PubMed] [Google Scholar]
- 18.London JF, Shawker TH, Doppman JL, et al. Prospective assessment of abdominal ultrasound in patients with Zollinger-Ellison syndrome. Radiology 1991; 178 : 763–7. [DOI] [PubMed] [Google Scholar]
- 19.Frucht H, Doppman JL, Norton JA, et al. Gastrinomas: comparison of MR imaging with CT, angiography, and US. Radiology 1989; 171: 713–17. [DOI] [PubMed] [Google Scholar]
- 20.Polacek MA, Ellison EH. Parietal cell mass and gastric acid secretion in the Zollinger-Ellison syndrome. Surgery 1966; 60: 606–14. [PubMed] [Google Scholar]
- 21.Howard JM, Chremos AN, Collen MJ, et al. Famotidine, a new potent, long-acting histamine H,-receptor antagonist : comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology 1985; 88 : 1026–33. [DOI] [PubMed] [Google Scholar]
- 22.Jensen RT, Collen MJ, McArthur KE, et al. Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric hypersecretory states. Am J Med 1984; 77 (Suppl. B): 90–105. [PubMed] [Google Scholar]
- 23.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RT. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. In: Norton JA ed. Progress symposium on the treatment of islet cell tumors. World J. Surg (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mctavish D, Buckley MM-T, Heel RC. Omeprazole: An updated review of its pharmacology and therapeutic use in acid-related disorders. Drugs 1991: 42: 138–70. [DOI] [PubMed] [Google Scholar]
- 25.Maton PN, Vinayek R, Frucht H, et al. Long term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology 1989; 97: 827–36. [DOI] [PubMed] [Google Scholar]
- 26.Frucht H, Maton PN, Jensen RT. Use of omeprazole in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1991; 36: 394–404. [DOI] [PubMed] [Google Scholar]
- 27.Lehy T, Mignon M, Cadiot G, et al. Gastric endocrine cell behavior in Zollinger-Ellison patients upon long-term anti-secretory treatment. Gastroenterology 1989. : 96: 1029–40. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Davies KA, Rutgersson SL. Omeprazole in the treatment of Zollinger-Ellison syndrome: a 4 year international study. Aliment Pharmacol Ther 1988; 2 : 13–37. [DOI] [PubMed] [Google Scholar]
- 29.Sum P, Perey BJ. Parietal cell mass (PCM) in a man with Zollinger-Ellison syndrome. Can J Surg 1969; 12: 285–88. [PubMed] [Google Scholar]
- 30.Card WI, Marks IN. The relationship between the acid output of the stomach following “maximal” histamine stimulation and the parietal cell mass. Clin Sci 1960; 19: 147–63. [PubMed] [Google Scholar]
- 31.Neuburger P, Lewin M, Bonfils S. Parietal and chief cell population in four cases of the Zollinger-Ellison syndrome. Gastroenterology 1972; 63: 937–42. [PubMed] [Google Scholar]
- 32.Miller LS, Vinayek R, Frucht H, Gardner JD, Jensen RT, Maton PN. Reflex oesophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology 1990; 98 : 341–6. [DOI] [PubMed] [Google Scholar]
- 33.Klinkenberg-Knol EC The role of omeprazole in healing and prevention of reflux disease. Hepatogastroenterology 1992; 37 (SUPPI. 1): 27–30. [PubMed] [Google Scholar]