Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is known to regulate gastric acid secretion and intestinal motility. In the present study, the pattern of distribution of PACAP and PACAP type 1 receptor (PAC1) immunoreactivities were examined in the rat stomach and distal colon using a specific polyclonal antibody raised against rat/human PAC1. Western blot of the membrane preparations of NIH/3T3 cells transfected with the human PAC1 obtained by using rabbit polyclonal anti-PAC1 antibody showed a protein band with a molecular mass of ~ 50 kDa. NIH/3T3 cells transfected with the human PAC1 and incubated with the anti-PAC1 antibody displayed surface cell-type immunoreactivity, which was internalized following ligand exposure. In gastric or colonic longitudinal muscle/myenteric plexus (LMMP) whole mount preparations as well as cryostat sections, PACAP immunoreactivity was observed in cell bodies within the myenteric ganglia and nerve fibers in the muscle layers and mucosa. PAC1 immunoreactivity was confined mainly on the surface of the nerve cells. PACAP and PAC1 immunoreactivities showed a similar pattern of distribution in gastric and colonic tissues. Adjacent sections or LMMP whole mount preparations labeled with protein gene product 9.5 (PGP 9.5) revealed the neuronal identity of myenteric cells bearing PAC1. The neuronal localization of PACAP and PAC1 receptors supports their role in the neural regulation of gastric acid secretion and gastrointestinal motor function.
Keywords: Pituitary adenylate cyclase activating polypeptide, Pituitary adenylate cyclase activating polypeptide type 1 receptor, Enteric nervous system, Protein gene product 9.5, Immunohistochemistry, Rat
1. Introduction
It is well established that numerous peptides present in the enteric nervous system (ENS) of the gastrointestinal (GI) tract are involved in the regulation of digestive functions [1]. Among these functions, gastric acid secretion has been described to be dependent upon a paracrine-related release of mediators and/or neural-derived mediators in both animals and humans [2]. For example, the pituitary adenylate cyclase activating polypeptide (PACAP) present in the ENS [3–5] is known to stimulate gastric acid secretion via the release of histamine from enterochromaffin-like (ECL) cells [6–9]. Conversely, PACAP inhibits gastric acid secretion in rats via a local release of secretin, somatostatin and prostaglandin E2 [10]. Likewise, the calcitonin gene-related peptide, a neuropeptide that coexists with PACAP in the sensory nervous system in rats [11], inhibits gastric acid secretion via the release of somatostatin [12].
PACAP is a member of the secretin/glucagon/vasoactive intestinal polypeptide (VIP) family of peptides and occurs in two biological forms, PACAP-27 and PACAP-38 [13,14], which are widely distributed in the central and peripheral nervous systems as well as non-neural tissues [15]. Recent studies indicate that the stimulatory effect of PACAP on gastric acid secretion involves the PACAP type 1 receptor (PAC1) expressed on the surface of ECL cells [8]. The PAC1 implicated in the regulation of gastric acid secretion by the ligands PACAP-27 or PACAP-38 has been cloned and classified as a member of the class II receptor super-family [16]. The receptor displays a high affinity for PACAP-27 and PACAP-38 but a very low affinity for the other family peptides such as VIP and secretin [16].
Besides its action on gastric acid secretion, PACAP is known to play a role in the regulation of GI motor function [17]. In guinea pig large intestine, PACAP immunoreactive (IR) cell bodies, mostly of the Dogiel type-I morphology, are present in the myenteric ganglia [4]. In the human colon, PACAP inhibits phasic contractions and causes a concentration-dependent relaxation [18]. In rats, both PACAP and VIP are reported to be the main determinants of the descending relaxation phase of the peristaltic reflex in the colon [19].
Since its recent discovery, several studies have demonstrated the peripheral and/or central effect of PACAP on gastric acid secretion and GI motor function [6,20–25]. However, only a few studies have addressed the distribution of PACAP and PAC1 in the GI tract [7–11,17–19]. In order to gain more insights into the regulatory role of PACAP on the GI functions, we have investigated by confocal microscopy analysis the distribution of PACAP and PAC1 in the rat stomach and distal colon. A special emphasis was made on the presence of PACAP and its receptor in the ganglionic myenteric neurons due to their well-established role in the GI motor activity [18–24]. A preliminary report of this study, in abstract form, has been previously published [26].
2. Materials and methods
2.1. Generation of polyclonal anti-PAC1 antibodies
Polyclonal antibodies raised against PAC1 were developed in rabbits immunized with the amino acid sequence (YQLRMSSLPADNLAT) corresponding to the C-terminus region of the receptor that is conserved between the rat and human PAC1 [27]. Once produced, the antisera were affinity-purified using a Protein G-Sepharose column and screened by Western blot and immunohistochemistry. All procedures were performed in collaboration with the CURE Antibody Core at the University of California, Los Angeles.
2.2. Characterization of the specificity of anti-PAC1 antibodies
NIH/3T3 cells stably transfected with the human PAC1 receptor were used to characterize the specificity of the polyclonal antibodies directed against PAC1 (#95129). The pCDL-SRα/Neo containing the human PAC1 insert was linearized at its Aat II restriction site within the ampicillin-resistant gene as described previously [28]. It was then stably transfected into NIH/3T3 fibroblasts using electroporation (BTX model T820, San Diego, CA) at 475 V/ms for four pulses on 2 × 107 cells in 0.25 ml containing 20 μg vector, Wt cDNA and 500 μg/ml salmon sperm. Geneticin® (250 mg/ml, Gibco, Gaithersburg, MD) was used to select the clones. Ten clones were subjected to radioligand binding assays to examine the binding affinity. The clones exhibiting the highest efficacy for adenylyl cyclase and radioligand binding inhibition were selected for further studies. The clone of transfected cells used for screening the specificity of the PAC1 antibody (#95129) expressed approximately 500,000 receptors per cell as described previously [27,28]. To characterize the antibody, the stably transfected cells were washed twice with phosphate-buffered saline (PBS, pH 7.4), pelleted by low speed centrifugation and lysed with Laemmli buffer. Membrane lysates (approximately 20 μg per lane) were separated electrophoretically using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gel. Following separation, the gels were blotted onto nitrocellulose membranes blocked with 4% bovine serum albumin in TBS with 0.05% Tween-20 (TTBS) for 1 h at room temperature. Subsequently, the blots were washed twice with TTBS and the nitrocellulose membranes were incubated overnight at room temperature with the anti-PAC1 antiserum (1:1000). The blots were then washed with TTBS and incubated for 1 h at room temperature with a goat anti-rabbit antibody conjugated to horseradish peroxidase (1:5000). Detection of the bands was performed using an Amersham ECL detection kit (Arlington Heights, IL). NIH/3T3 cells, which had not been transfected with human PAC1, were used as a control.
2.3. Immunofluorescent detection of PAC1 expression on NIH/3T3 cells
NIH/3T3 cells transfected or not with human PAC1 were grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% calf serum containing the selection antibiotic, G418 (Gibco). Cells were plated overnight at a low density on poly-lysine-treated cover slips. They were then washed with PBS, fixed with 4% paraformaldehyde and permeabilized for 15 min at room temperature with PBS containing 0.01% Triton X-100 (PBS-T) in the presence of 10 mg/ml goat immunoglobulin G (IgG) to block nonspecific binding and subsequently incubated overnight at 4 °C with the polyclonal anti-PAC1 antibody (1:1000). Following incubation for 2 h at 4 °C with Alexa 488 conjugated to goat anti-rabbit IgG (Fab2) (Molecular Probes, Eugene, OR) at the dilution of 1:5000, the cells were washed in PBS, mounted in Vectashield medium (Vector Laboratories, Burlingame, CA) and sealed with cover slips for fluorescence microscopy. Mounted cells were examined using a Zeiss LSM510 laser scanning microscope (Carl Zeiss, Thornwood, NY), and the images were captured using the computerized system coupled to the laser scanning microscope.
To determine whether the antibody directed against PAC1 cross-reacts with other receptors of the VPAC-family, NIH/3T3 cells transfected with human VPAC1 were incubated with the rabbit polyclonal anti-PAC1 antibody and processed as above.
To examine the specificity of the antibody raised against PAC1, the internalization of the ligand–receptor complex was studied using NIH/3T3 cells transfected with or without human PAC1. Cells were plated on glass cover slips and incubated for 1 h at 4 °C with 0.1 μmol/l of PACAP 27 (Bachem, Torrance, CA). They were then warmed to 37 °C for 30 min, fixed, permeabilized with PBS-T and stained with the polyclonal antibody raised against PAC1, followed by goat anti-rabbit IgG conjugated to Alexa 488. The internalization of PAC1 was visualized using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss). Untransfected NIH/3T3 cells were used as a negative control and processed as above.
2.4. Animals
Male Sprague–Dawley rats (Harlan, San Diego, CA) weighing 250–300 g were housed under controlled environmental conditions (22 ± 1 °C; 12:12 light–dark cycle and regular rat chow and water ad libitum) for at least 5 days before the experiment. All studies were carried out in fed animals between 9:00 AM and 12:00 P.M. under Protocol #9710–037 approved by the Animal Research Committee of the Veterans Affairs Greater Los Angeles Healthcare System.
2.5. Tissue preparation
Rats (n = 16) were sacrificed by decapitation and the tissues were collected for frozen sections or longitudinal muscle/myenteric plexus (LMMP) whole mount preparations as described previously [29,30]. The stomach was opened along the lesser curvature and the distal colon along the mesenteric border into the ice-cold saline. For frozen sections, the tissues were fixed overnight in Zamboni’s fixative (pH 7.4) at 4 °C, cryoprotected in PBS containing 20% sucrose and embedded in the optimum cutting temperature (OCT) medium (Sakura Finetek, Torrance, CA). Segments of gastric corpus and distal colon were cryostat-sectioned at 12 Am, separately taw-mounted on microscope slides, dried at room temperature and stored at 4 °C for immunohistochemistry.
For the LMMP whole mount preparations, gastric and colonic tissues were pinned flat in a Sylgard-coated Petri dish (Sylgard 184, Dow Corning, Midland, MI) and fixed overnight in Zamboni’s fixative (pH 7.4) at 4 °C. LMMP whole mount preparations of approximately 0.25 cm2 were each dissected from the gastric corpus and distal colon as reported previously [29,30]. Gastric and colonic LMMP whole mount preparations were collected separately in PBS (pH 7.4) supplemented with 0.1% sodium azide as preservative and stored at 4 °C for immunohistochemistry.
2.6. PACAP, PAC1 and protein gene product 9.5 (PGP 9.5) immunolabeling
Adjacent cryostat sections from the gastric corpus or distal colon were incubated for 30 min at room temperature with 3% normal goat serum in PBS containing 0.3% Triton X-100 (PBS-T, pH 7.4) (Sigma, St. Louis, MO). Sections were then incubated separately for 48 h at 4 °C with rabbit polyclonal antibodies directed against: (i) PACAP-38 (1:1000; Peninsula Laboratories, San Carlos, CA), (ii) PAC1 (1:1000; CURE: Digestive Diseases Research Center, Antibody Core Facility, Los Angeles, CA) or (iii) protein gene product 9.5 (1:1000; PGP 9.5, Ultraclone, Wellow Isle of Wight, UK). As controls, pre-absorbed primary antibodies (5 μg antigen per 1 μg of antibody) were used with exception for PGP 9.5. After the first incubation, the sections were washed (3 × 10 min) with PBS-T and incubated for 1 h at room temperature with tetramethylrhodamine isothiocyanate (TRITC) or fluorescein isothiocyanate (FITC) conjugated to goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA) diluted 1:100 and 1:50, respectively. After further PBS washing (3 × 10 min), the sections were mounted in bicarbonate-buffered glycerol (pH 8.6) and examined using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss).
Gastric and colonic LMMP whole mount preparations were rinsed in PBS (pH 7.4) and processed as above for PACAP, PAC1 and PGP 9.5 immunolabeling. PGP 9.5 is a marker for nerve cell bodies and axons in the central and peripheral nervous system [31]. The antibody raised against PGP 9.5 was successfully used to identify myenteric nerve cell bodies in the rat stomach and colon [30,32].
2.7. Image analysis and microscopy
Immunofluorescence labeling of PACAP, PAC1 or PGP 9.5 in NIH/3T3 cells, gastric or colonic tissues was viewed using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss). Confocal microscope images showing PACAP, PAC1 or PGP 9.5 immunoreactivity were captured via the computerized image analysis system coupled to the laser scanning microscope.
3. Results
3.1. Western blot analysis of PAC1 expression from NIH/3T3 cells
Western blot analysis of the membrane preparations showed a protein band of ~ 50 kDa in the lane loaded with extract of NIH/3T3 cells transfected with the human PAC1 (Fig. 1). No immunoreactivity was detected in the lane loaded with membrane preparations of untransfected NIH/3T3 cells (Fig. 1), as well as the ones incubated with the anti-PAC1 antibody pre-absorbed with the specific antigen (data not shown). The Western blot analysis performed on immunoprecipitates from untransfected and PAC1-transfected NIH/3T3 cells confirmed the presence of the band of ~ 50 kDa mentioned above (data not shown).
Fig. 1.
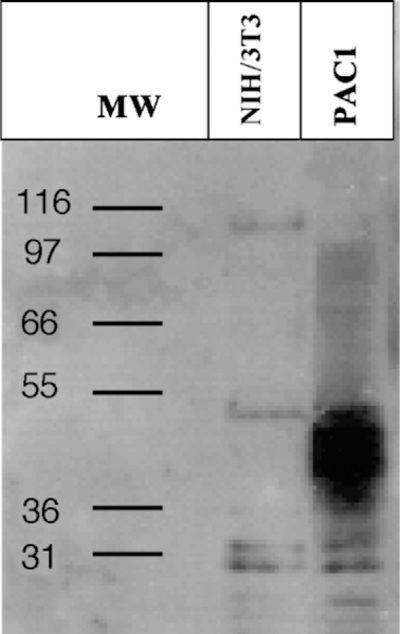
Western blot using a rabbit polyclonal antibody directed against PAC1 (1:1000). A protein band of ~ 50 kDa corresponding to the molecular mass of PAC1 is visible in the lane loaded with the extract of NIH/3T3 cells transfected with the human PAC1. No signal is detected in the lane loaded with extract of untransfected NIH/3T3 cells.
3.2. Immunocytochemical labeling of NIH/3T3 cells
NIH/3T3 cells transfected with the human VPAC1 or PAC1 were labeled with the anti-PAC1 polyclonal antibody. PAC1 immunoreactivity was absent in NIH/3T3 cells transfected with the human VPAC1 (Fig. 2A), demonstrating that the antibody does not cross-react with other receptors of the VPAC-family. In contrast, PAC1 positive staining was detected on the surface of NIH/3T3 cells transfected with the human PAC1 (Fig. 2B). Following PACAP-27 exposure at 37 °C, the internalization of PAC1 immunoreactivity was seen in NIH/3T3 cells transfected with human PAC1. This process was rapid and showed the translocation of PAC1 immunoreactivity from the cell surface to the perinuclear area (Fig. 3A and B). No PAC1 immunoreactivity was seen in untransfected NIH/3T3 cells incubated with the PAC1 antibody (data not shown).
Fig. 2.
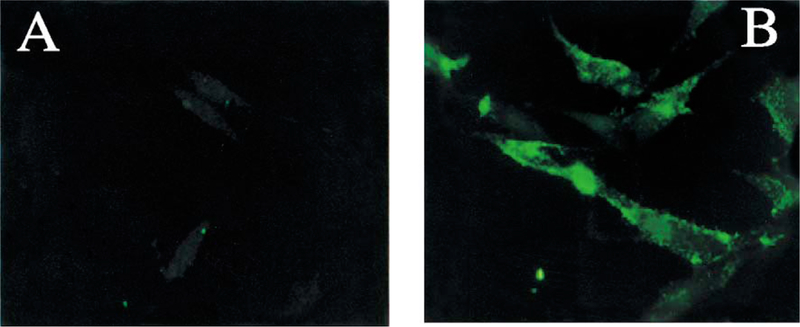
NIH/3T3 cells transfected with the human VPAC1 (A) or PAC1 (B) and stained with a rabbit polyclonal PAC1 antibody for demonstration of the lack of cross-reactivity with VPAC1 and specificity for PAC1. (A) PAC1 immunoreactivity is absent in NIH/3T3 cells transfected with the human VPAC1. (B) A surface cell-type PAC1 immunoreactivity is clearly visible in NIH/3T3 cells transfected with the human PAC1. Magnification: × 100.
Fig. 3.
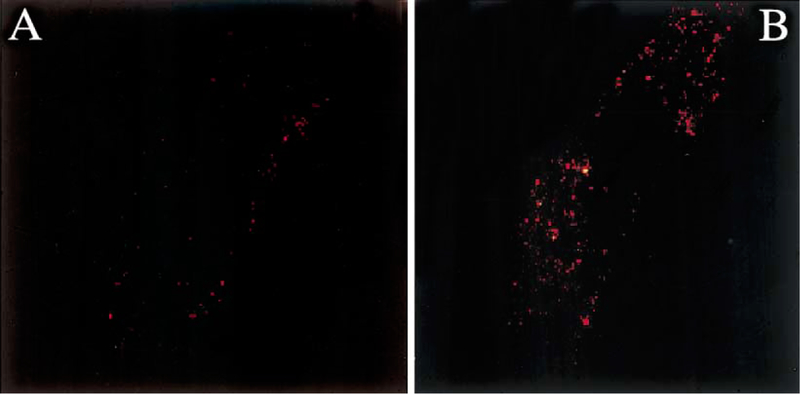
(A) Confocal microscope image of an NIH/3T3 cell transfected with the human PAC1 showing surface cell-type PAC1 immunoreactivity after being exposed to a rabbit polyclonal PAC1 antibody. (B) Following exposure to PACAP-27 at 37 °C, the internalization of the PAC1 immunoreactivity seen on the membrane of NIH/3T3 cells transfected with the human PAC1 (A) is visible 10 min later. Magnification: × 100.
3.3. Distribution of PACAP and PAC1 immunoreactivity in the gastric corpus
PACAP immunoreactive (IR) nerve fibers were detected within the mucosa of the gastric corpus (Fig. 4C), muscle layer and myenteric plexus (Fig. 4B and D). Gastric corpus LMMP whole mount preparations, which display the integrity of myenteric ganglia, showed ganglionic nerve cell bodies positive for PACAP (Fig. 4B). Similar features were seen in the myenteric plexus in frozen cross-sections of the gastric corpus (Fig. 4D). PACAP-IR nerve fibers were also seen in the muscle layer above the myenteric plexus (Fig. 4D). In the gastric corpus LMMP whole mount preparations, PAC1 and PACAP immunoreactivity showed a similar pattern of distribution in the myenteric ganglionic nerve cell bodies (Fig. 5C and D). Adjacent gastric corpus LMMP whole mount preparations labeled with the polyclonal PGP 9.5 antibody showed the presence of nerve cell bodies (Fig. 5A) similar in size and shape to ganglionic myenteric cells expressing PAC1 and PACAP (Fig. 5C and D). The similarity in the distribution of PGP 9.5, PAC1 and PACAP immunoreactivity in the myenteric ganglia (Fig. 5) suggests that PAC1 and PACAP were present in the myenteric neuronal component of the gastric corpus. A detailed analysis of the gastric corpus LMMP whole mount preparations labeled with the anti-PAC1 antibody showed wide-spread PAC1 staining on the surface of myenteric ganglionic cells resembling nerve cell bodies (Fig. 6B and C, arrow). In addition, PAC1 immunoreactivity was present into the cytoplasm of these ganglionic nerve cell bodies (Fig. 6B and C). The presence of PAC1 immunoreactivity into the gastric corpus myenteric ganglia (Fig. 6B and C) was also seen in cryostat sections showing PAC1-positive ganglia as bulbous structures lying along the longitudinal muscle (Fig. 6D, arrow).
Fig. 4.
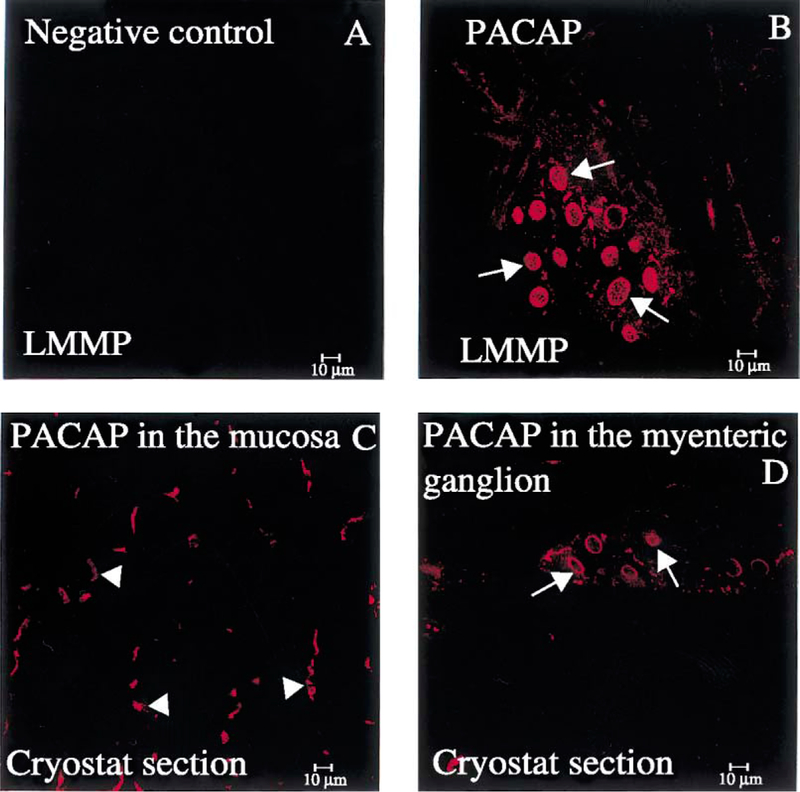
(A) The immunolabeling of gastric corpus LMMP whole mount preparations with a pre-adsorbed rabbit polyclonal PACAP antiserum does not display any immunoreactivity. (B) When incubated with rabbit polyclonal anti-PACAP antibody, myenteric ganglionic nerve cell bodies positive to PACAP are visible (arrows). (C) Cryostat sections of the gastric corpus labeled with the above antiserum show nerve fibers-IR to PACAP in the mucosa. (D) PACAP-positive ganglionic nerve cell bodies seen in (B) are also detected in the cryostat sections (arrows), while PACAP-positive nerve fibers are barely visible in the circular muscle above the myenteric plexus. Scale bar: 10 μm.
Fig. 5.
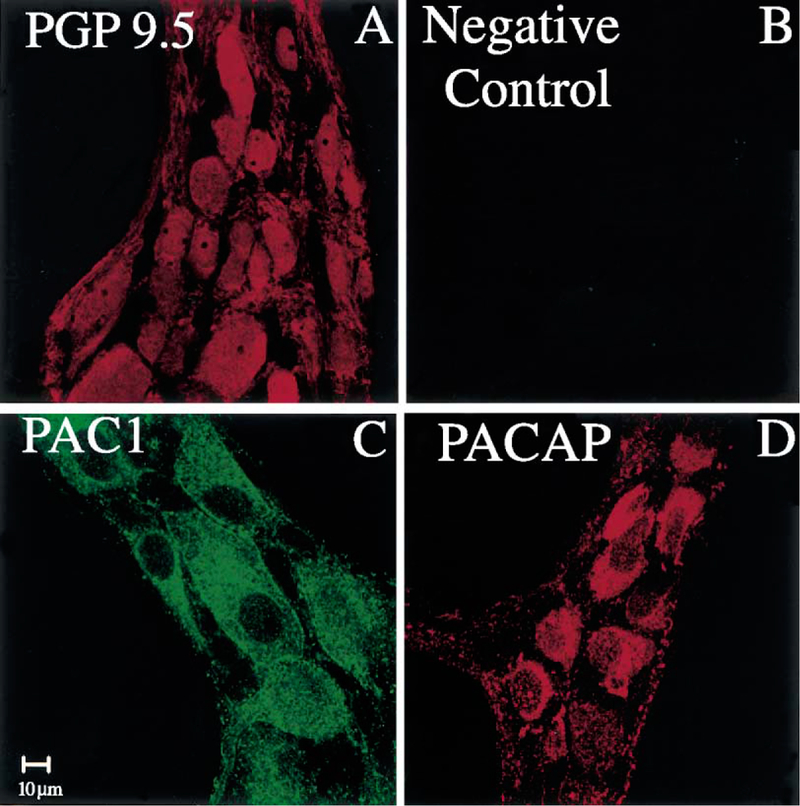
(A) A gastric corpus LMMP whole mount preparation stained with a rabbit polyclonal PGP 9.5 antibody shows myenteric ganglionic nerve cell bodies-IR to PGP 9.5. (B) No immunoreactivity is seen in the adjacent piece of the gastric corpus LMMP whole mount preparation incubated with the antibody diluent in place of the primary antibody (anti-PGP 9.5). (C) In the presence of rabbit polyclonal anti-PAC1 antibody, the myenteric ganglionic nerve cell bodies seen in (A) display PAC1 immunoreactivity. (D) The myenteric ganglionic nerve cell bodies seen in (A) are also displaying PACAP immunoreactivity in the presence of a rabbit polyclonal PACAP antibody. Scale bar: 10 μm.
Fig. 6.
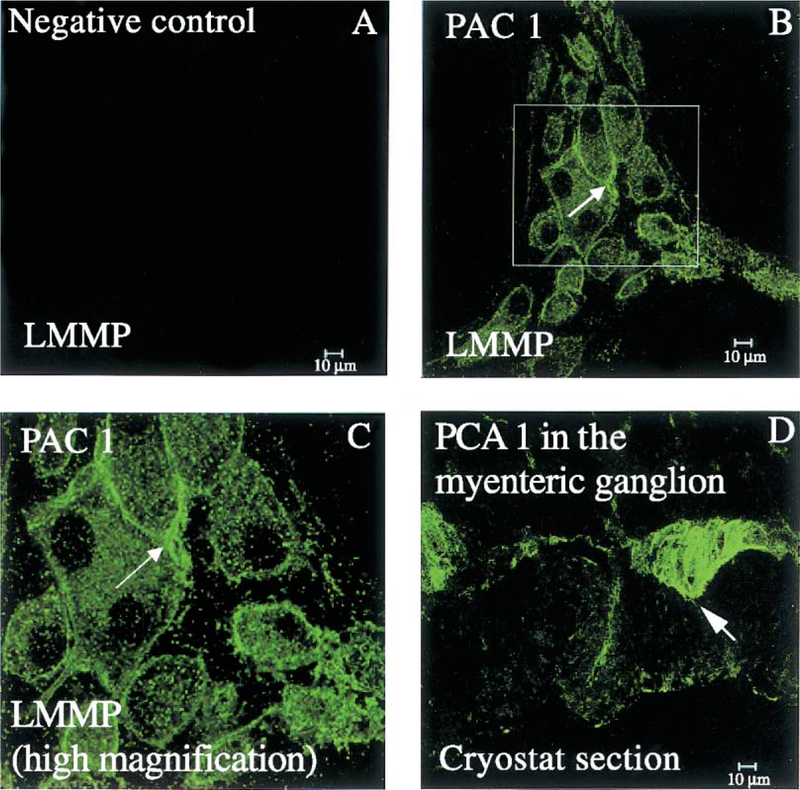
(A) Absence of any immunoreactivity in the gastric corpus LMMP whole mount preparation labeled with pre-adsorbed rabbit polyclonal anti-PAC1 antibody. (B) Following incubation with a rabbit polyclonal PAC1 antibody, PAC1 immunostaining is seen on the surface (arrow) as well as into the cytoplasm of myenteric ganglionic nerve cell bodies. (C) A higher magnification of the framed region shown in (B). (D) PAC1 immunostaining in the myenteric ganglia (arrow) as well as nerve fibers in the circular and longitudinal muscle from a frozen gastric corpus cross-section incubated with a rabbit polyclonal PAC1 antibody. Scale bar: 10 μm.
3.4. Distribution of PACAP and PAC1 immunoreactivity in the distal colon
LMMP whole mount preparations from the distal colon labeled with the polyclonal PACAP antibody showed a pattern of distribution of PACAP immunoreactivity similar to that seen in the gastric corpus (Fig. 7). There was no PACAP immunoreactivity in the colonic preparations incubated with the pre-absorbed antibody (Fig. 7A). Adjacent colonic LMMP whole mount preparations labeled with the polyclonal anti-PGP 9.5 antibody showed positive nerve cell bodies resembling PACAP-IR cells (Fig. 7B and D). In distal colon LMMP whole mount preparations, positive PACAP staining was seen in the ganglionic myenteric cells (Fig. 7D). This pattern of distribution of PGP 9.5 was similar to that observed for PAC1 (Fig. 7C). A detailed analysis of PAC1 immunoreactivity in LMMP whole mount preparations of the distal colon showed PAC1 immunoreactivity on the surface of ganglionic myenteric neurons (Fig. 8B and C, arrows). A similar staining was observed in the myenteric plexus and muscle layer in cross-sections of the distal colon (Fig. 8D, arrows). In the colonic LMMP whole mount preparations, the distribution of PAC1 as well as PGP 9.5 immunoreactivity was similar to that seen in the gastric corpus LMMP whole mount preparations.
Fig. 7.
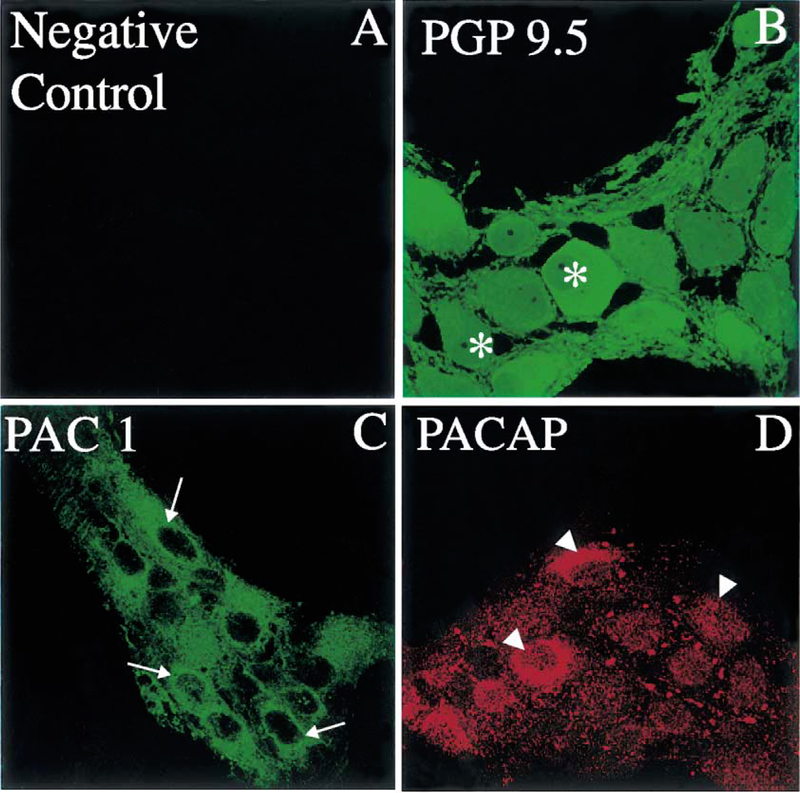
(A) Absence of immunoreactivity in the distal colon LMMP whole mount preparation incubated with an antibody diluent in place of the primary antibody (rabbit anti-PGP 9.5). (B) A distal colon LMMP whole mount preparation incubated with a rabbit polyclonal PGP 9.5 antibody showing ganglionic nerve cell bodies-IR to PGP 9.5 (asterisks). (C) The PGP 9.5-positive nerve cell bodies seen in (B) display PAC1 immunoreactivity (arrows) when the colonic tissue is incubated with a rabbit PAC1 antibody. (D) The ganglionic nerve cell bodies seen in (B) are also PACAP-positive (arrowheads) in the presence of a rabbit PACAP antibody. Scale bar: 10 μm.
Fig. 8.
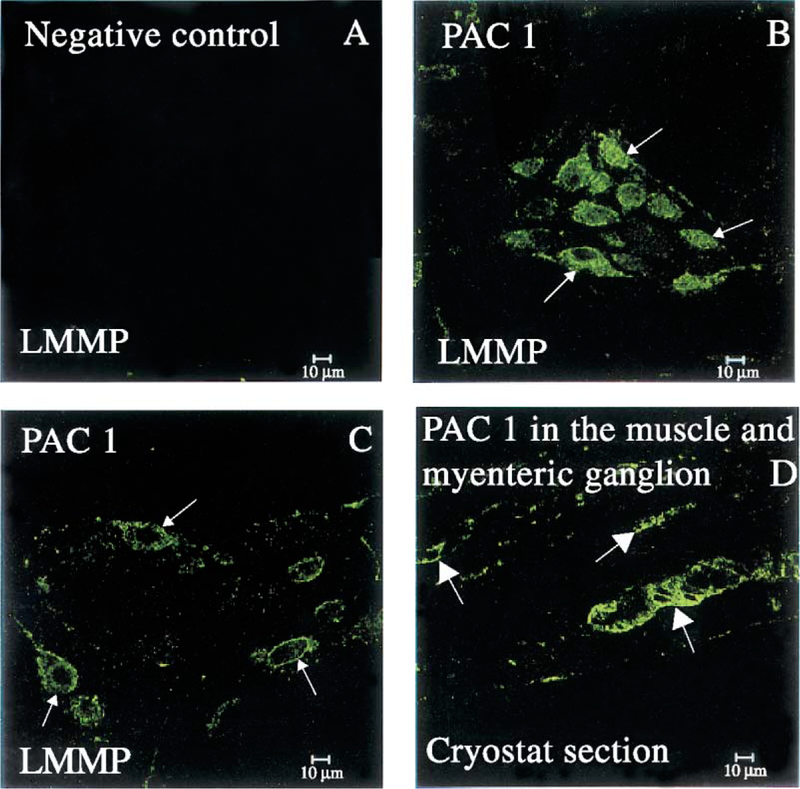
(A) The distal colon LMMP whole mount preparation incubated with a pre-absorbed rabbit polyclonal PAC1 antibody does not show positive staining. (B and C) Colonic tissues incubated with a rabbit PAC1 antibody showing PAC1 immunostaining on the membrane and cytoplasm of ganglionic nerve cell bodies (arrows). (D) The PAC1 immunoreactivity detected in the colon distal LMMP whole mount preparation (B and C) is also detectable in the cryostat section clearly showing PAC1-positive myenteric ganglia as well as nerve fibers in the circular muscle (arrows). Scale bar: 10 μm.
4. Discussion
In the present study, we examined the distribution of PACAP and PAC1 immunoreactivity in the rat gastric corpus and distal colon using frozen cross-sections and LMMP whole mount preparations. The LMMP whole mount preparations preserve the integrity of ganglionic myenteric cells and allow a clear identification of the cell surface staining. For the detection of PAC1 immunoreactivity in rat stomach and distal colon, the PAC1 antibody used showed a positive reaction in NIH/3T3 cells transfected with human PAC1 but not VPAC1. NIH/3T3 cells transfected with human PAC1 express the PAC1 protein [27,28] and are a suitable tool to test the specificity of an antibody directed against PAC1. Western blot analysis of the membrane preparations incubated with the polyclonal anti-PAC1 antibody showed a broad protein band of approximately 50 kDa in the lane loaded with the extract of NIH/3T3 cells transfected with the human PAC1. This is consistent with the deduced size of the PAC1 protein fromNIH/3T3 cells stably transfected with PAC1 [16,27]. Immunoprecipitation of NIH/3T3 cells transfected with the human PAC1 using the polyclonal anti-PAC1 antibody PAC1 confirmed the above data, whereas no immunoreactivity was detected with the untransfected NIH/3T3 cells.
Using immunohistochemical techniques, NIH/3T3 cells transfected with human PAC1 and incubated with anti-PAC1 antibody displayed immunoreactivity on the cell surface, a typical localization for G protein-coupled membrane receptors [28,33]. Following ligand exposure at 37 °C, the detected immunoreactivity appeared to have translocated towards the perinuclear area. This suggests that intra-cytoplasmic processes could be important for receptor signaling and supports the role of ligand-induced receptor internalization. Taken together, the obtained results clearly demonstrated the specificity of the antibody raised against rat/human PAC1.
In the rat gastric corpus, PACAP and PAC1 immunoreactivities were found in nerve fibers within the mucosa and muscle layers and myenteric ganglionic nerve cell bodies as confirmed by PGP 9.5, a widely used marker for neuronal cell bodies and axons in the central and peripheral nervous system [30,31]. Although the PACAP and PAC1 immunoreactivity was mainly confined on the cell surface, positive vesicle-like structures were also detected into the cytoplasm of neurons in the examined gastric corpus and colonic LMMP whole mount preparations. Interestingly, we have previously observed PAC1 immunoreactivity in the cytoplasm of NIH/3T3 cells transfected with human PAC1 and incubated with an anti-PAC1 antibody [33]. However, the presence of PAC1 on or into the myenteric ganglionic neurons supports the role of PACAP in the neuronal regulation of gastric motility. Indeed, it has been shown that intracerebroventricular or intracisternal injection of PACAP in rats increases gastric motility through the vagal pathway. Similarly, microinjection of PACAP in the dorsal vagal complex and nucleus obscurus significantly increases the intragastric pressure, which is abolished by bilateral vagotomy [23]. Conversely, the intravenous administration of PACAP decreases gastric motility independently of vagal or adrenergic mechanisms [21]. Peripheral PACAP inhibits gastric motility through an unknown pathway [21]. The observation that PAC1 is expressed in the gastric ganglionic myenteric neurons could contribute towards the understanding of mechanisms underlying the effect of peripheral PACAP on the gastric motility. Although the present study, as designed, cannot give functional insight into the possible regulation of gastric motor activity by peripheral PACAP, the implication of PAC1 present on the enteric neurons cannot be excluded as a possibility. Besides the gastric motor function, PACAP plays an important role in the neurohumoral regulation of gastric acid secretion [6–10,34,35]. The role of the detected ganglionic myenteric PAC1 on gastric acid secretion remains unknown. However, the presence of PACAP immunoreactivity in nerve fibers within the gastric acid-secreting mucosa and myenteric plexus would suggest a role in the regulation of gastric acid secretion. As demonstrated for PACAP (present study), several peptide-containing nerve fibers and nerve cell bodies have been reported in the gastric wall [36], and the electron microscopic evidence of synaptic junctions on parietal cells indicates that hydrochloric acid secretion is influenced directly by the nervous system [37]. Indeed, the nerves terminating at the parietal cells originate exclusively from the myenteric plexus, where the vagus fibers already terminate without entering the mucosa [37]. Due to the complexity and diversity of the biochemical coding in the enteric nervous system, the functional implications of enteric PACAP and PAC1 in the rat stomach deserve extensive investigation.
In the rat distal colon, the pattern of distribution of PACAP and PAC1 was similar to that found in the gastric corpus. PACAP- and PAC1-positive labeling was present in nerve fibers in the mucosa, muscle and myenteric plexus, where intense staining was detected in the ganglionic nerve cell bodies. The wide distribution of PAC1 immunoreactivity in the examined enteric neuronal elements implies that by binding to its specific receptor, PACAP could be involved in a wide range of physiological events in the GI tract including motor activity. This corroborates the previous findings indicating multiple origins and functions for PACAP in the rat gastrointestinal tract [3]. In the gastrointestinal tract, PACAP is co-localized with several regulatory peptides such as CGRP, VIP, gastrin-releasing peptide or the neurotransmitter nitric oxide synthase [3]. The presence of PACAP and PAC1 in the enteric neurons and the reported co-localization of PACAP with other regulatory neuropeptides [3,38] strengthen the diversity of PACAP physiological properties in the GI tract [6,7,9,10,17,39–41]. It remains unclear whether PACAP and PAC1 immunoreactivity detected in the ganglionic myenteric neurons is present on the sensory, motor or interneurons. Further investigations are warranted to classify the ganglionic neuron(s) that contain(s) PACAP and express PAC1 in the gastric corpus and distal colon.
In conclusion, the present study demonstrates for the first time the presence of both PACAP and PAC1 in the ganglionic myenteric neurons of the rat gastric corpus or distal colon using specific polyclonal antibodies directed against PACAP or PAC1. In the gastric corpus and distal colon, PACAP and PAC1 immunoreactivities were found on the ganglionic myenteric cell surface as well as in nerve fibers within the mucosa and muscle layers. The pattern of distribution of PACAP and PAC1 in the examined ENS strengthens the hypothesis that PACAP could bind to the neuronal elements expressing PAC1 to regulate the gastric and colonic motor function. In the future, a classification of enteric neurons bearing PAC1 based on their biochemical coding and/or electrophysiological behavior will bring new insight into the mechanisms leading to the regulation of gastrointestinal secretory and motor functions by PACAP.
Acknowledgements
This study was supported by the VA Career Development Award, VA Merit Review Award (J. Pisegna) and DK-41301 (Animal and Imaging Cores, J. Pisegna, Y. Taché and G. Sachs).
References
- [1].Taylor GS, Bywater RA. Novel autonomic neurotransmitters and intestinal function. Pharmacol Ther 1989;40:401–38. [DOI] [PubMed] [Google Scholar]
- [2].Walsh JH. Gastrointestinal hormones In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1994. p. 1–129. [Google Scholar]
- [3].Hannibal J, Ekblad E, Mulder H, Sundler F, Fahrenkrug J. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract of the rat: distribution and effects of capsaicin or denervation. Cell Tissue Res 1998;291:65–79. [DOI] [PubMed] [Google Scholar]
- [4].Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase-activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res 1995;279:385–92. [DOI] [PubMed] [Google Scholar]
- [5].Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase-activating polypeptide innervation of the rat female reproductive tract and the associated paracervical ganglia: effect of capsaicin. Neuroscience 1996;73: 1049–60. [DOI] [PubMed] [Google Scholar]
- [6].Lindstrom E, Bjorkqvist M, Boketoft A, Chen D, Zhao CM, Kimura K, et al. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept 1997;71:73–86. [DOI] [PubMed] [Google Scholar]
- [7].Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 1999;104:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeng N, Kang T, Lyu RM, Wong H, Wen Y, Walsh JH, et al. The pituitary adenylate cyclase-activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann N Y Acad Sci 1998;865:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zeng N, Athmann C, Kang T, Walsh JH, Sachs G. Role of neuropeptide-sensitive L-type Ca(2+) channels in histamine release in gastric enterochromaffin-like cells. Am J Physiol 1999;277:G1268–80. [DOI] [PubMed] [Google Scholar]
- [10].Li P, Chang TM, Coy D, Chey WY. Inhibition of gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin, and PGE(2). Am J Physiol: Gastrointest Liver Physiol 2000;278:G121–7. [DOI] [PubMed] [Google Scholar]
- [11].Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, et al. Pituitary adenylate cyclase-activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience 1993;57:725–32. [DOI] [PubMed] [Google Scholar]
- [12].Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology 1995;109:701–6. [DOI] [PubMed] [Google Scholar]
- [13].Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase-activating polypeptide with 38 residues (PACAP-38). Biochem Biophys Res Commun 1990;170:643–8. [DOI] [PubMed] [Google Scholar]
- [14].Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue—hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989;164:567–74. [DOI] [PubMed] [Google Scholar]
- [15].Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 2000;21:619–70. [DOI] [PubMed] [Google Scholar]
- [16].Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A 1993;90:6345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lauff JM, Modlin IM, Tang LH. Biological relevance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract. Regul Pept 1999;84:1–12. [DOI] [PubMed] [Google Scholar]
- [18].Schworer H, Clemens A, Katsoulis S, Kohler H, Creutzfeldt W, Schmidt WE. Pituitary adenylate cyclase-activating peptide is a potent modulator of human colonic motility. Scand J Gastroenterol 1993;28:625–32. [DOI] [PubMed] [Google Scholar]
- [19].Grider JR, Katsoulis S, Schmidt WE, Jin JG. Regulation of the descending relaxation phase of intestinal peristalsis by PACAP. J Auton Nerv Syst 1994;50:151–9. [DOI] [PubMed] [Google Scholar]
- [20].Yamamoto H, Kuwahara A, Fujimura M, Maeda T, Fujimiya M. Motor activity of vascularly perfused rat duodenum: 2. Effects of VIP, PACAP-27 and PACAP-38. Neurogastroenterol Motil 1999;11:235–41. [DOI] [PubMed] [Google Scholar]
- [21].Ozawa M, Aono M, Moriga M. Central effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gastric motility and emptying in rats. Dig Dis Sci 1999;44:735–43. [DOI] [PubMed] [Google Scholar]
- [22].Onaga T, Harada Y, Okamoto K. Pituitary adenylate cyclase-activating polypeptide (PACAP) induces duodenal phasic contractions via the vagal cholinergic nerves in sheep. Regul Pept 1998;77:69–76. [DOI] [PubMed] [Google Scholar]
- [23].Krowicki ZK, Arimura A, Nathan NA, Hornby PJ. Hindbrain effects of PACAP on gastric motor function in the rat. Am J Physiol 1997;272:G1221–9. [DOI] [PubMed] [Google Scholar]
- [24].Katsoulis S, Schmidt WE. Role of PACAP in the regulation of gastro-intestinal motility. Ann N Y Acad Sci 1996;805:364–78. [DOI] [PubMed] [Google Scholar]
- [25].Mungan Z, Hammer RA, Akarca US, Komaki G, Ertan A, Arimura A. Effect of PACAP on gastric acid secretion in rats. Peptides 1995;16:1051–6. [DOI] [PubMed] [Google Scholar]
- [26].Miampamba M, Germano PM, Taché Y, Fahrenkrug JA, Wong H, Walsh JH, et al. Immunohistochemical assessment of PACAP hormone and its receptor PAC1 in the gastric and colonic enteric nervous system. Gastroenterology 2000;118:A307. [Google Scholar]
- [27].Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase-activating polypeptide receptor: evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem 1996;271:17267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lyu RM, Germano PM, Choi JK, Le SV, Pisegna JR. Identification of an essential amino acid motif within the C terminus of the pituitary adenylate cyclase-activating polypeptide type I receptor that is critical for signal transduction but not for receptor internalization. J Biol Chem 2000;275:36134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miampamba M, Sharkey KA. c-Fos expression in the myenteric plexus, spinal cord and brainstem following injection of formalin in the rat colonic wall. J Auton Nerv Syst 1999;77:140–51. [PubMed] [Google Scholar]
- [30].Miampamba M, Yang H, Sharkey KA, Taché Y. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am J Physiol 2001;280:G979–91. [DOI] [PubMed] [Google Scholar]
- [31].Krammer HJ, Karahan ST, Rumpel E, Klinger M, Kuhnel W. Immunohistochemical visualization of the enteric nervous system using antibodies against protein gene product (PGP) 9.5. Anat Anz 1993;175:321–5. [DOI] [PubMed] [Google Scholar]
- [32].Miampamba M, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor (CRF) increases Fos expression in the colonic myenteric plexus and fecal pellet output in rats. Neurogastroenterol Motil 1999;11:A-276. [Google Scholar]
- [33].Germano PM, Stalter J, Le SV, Wu M, Yamaguchi DJ, Scott D, et al. Characterization of the pharmacology, signal transduction and internalization of the fluorescent PACAP ligand, fluor-PACAP, on NIH/3T3 cells expressing PAC1. Peptides 2001;22:861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sandvik AK, Cui G, Bakke I, Munkvold B, Waldum HL. PACAP stimulates gastric acid secretion in the rat by inducing histamine release. Am J Physiol 2001;281:G997–G1003. [DOI] [PubMed] [Google Scholar]
- [35].Ozawa M, Aono M, Mizuta K, Moriga M, Okuma M. Central administration of PACAP stimulates gastric secretion mediated through the vagal pathway in anesthetized rats. Dig Dis Sci 1997;42:2552–9. [DOI] [PubMed] [Google Scholar]
- [36].Ekblad E, Ekelund M, Graffner H, Hakanson R, Sundler F. Peptide-containing nerve fibers in the stomach wall of rat and mouse. Gastro-enterology 1985;89:73–85. [DOI] [PubMed] [Google Scholar]
- [37].Radke R, Stach W, Weiss R. Innervation of the gastric wall related to acid secretion: a light and electron microscopy study on rats, rabbits and guinea pigs. Acta Biol Med Ger 1980;39:687–96. [PubMed] [Google Scholar]
- [38].Sundler F, Ekblad E, Absood A, Hakanson R, Koves K, Arimura A. Pituitary adenylate cyclase-activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience 1992;46:439–54. [DOI] [PubMed] [Google Scholar]
- [39].Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides 2000;21:1565–82. [DOI] [PubMed] [Google Scholar]
- [40].Katsoulis S, Schmidt WE. Role of PACAP in the regulation of gastrointestinal motility. Ann N Y Acad Sci 1996;805:364–78. [DOI] [PubMed] [Google Scholar]
- [41].Shen Z, Larsson LT, Malmfors G, Absood A, Hakanson R, Sundler F. A novel neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), in human intestine: evidence for reduced content in Hirschsprung’s disease. Cell Tissue Res 1992;269:369–74. [DOI] [PubMed] [Google Scholar]


