SUMMARY
Background
Intravenous (IV) formulations of proton pump inhibitors are effective for patients in whom oral therapy is not appropriate.
Aim
To compare IV esomeprazole and IV lansoprazole for the control of intragastric pH.
Methods
In this open-label crossover study, healthy, Helicobacter pylori-negative adults were randomized to one of two treatment sequences, each consisting of two 5-day dosing periods of IV esomeprazole 40 mg or IV lansoprazole 30 mg. Twenty-four-hour intragastric pH monitoring was conducted on days 1 and 5 of each dosing period.
Results
On days 1 and 5, intragastric pH was >4.0 significantly longer with esomeprazole than lansoprazole (least-squares means: day 1, 40.0% vs. 33.6%; day 5, 61.9% vs. 45.4%; both P < 0.0001). During the first 4 h of pH monitoring, intragastric pH was >4.0 significantly longer on days 1 and 5 with esomeprazole than lansoprazole (P < 0.0001). Kaplan-Meier estimates of median hours to stable pH >4.0 were 4.92 for esomeprazole and 5.75 for lansoprazole (P = 0.0014 for test on Gehan scores).
Conclusion
In healthy adults, IV esomeprazole 40 mg controlled intragastric acidity faster and more effectively than IV lansoprazole 30 mg.
INTRODUCTION
Gastric acid suppression with proton pump inhibitors (PPIs) is an effective therapy for the treatment of GERD.1 Intravenous (IV) formulations of the PPIs pantoprazole, lansoprazole and esomeprazole are currently approved by the US Food and Drug Administration (FDA) for short-term treatment (≤7 days) of erosive oesophagitis in patients unable to take oral PPI formulations. Although not approved by the FDA for use in the critical care setting, IV PPIs are commonly used as a prophylactic against stress-induced ulcers or gastrointestinal bleeding for patients undergoing surgery.2 Currently, the only PPI approved for use in the critical care setting is immediate-release oral omeprazole. The feasibility of using IV PPIs in critical care settings is uncertain because the mechanism of action is thought to require postprandial acid stimulation.3, 4
Because IV PPI formulations are typically used short term, clinical efficacy of erosive oesophagitis healing and GERD symptom control cannot be directly compared between different PPIs; however, a positive relationship has been demonstrated between intragastric and intra-oesophageal acid control and the clinical outcomes of healing of erosive oesophagitis and GERD symptom control.5, 6 In the absence of clinical evaluations, pharmacodynamic effects of PPIs can be compared by measuring the time that intragastric pH is >4.0 during 24 h. Once-daily dosing of IV esomeprazole has been shown to provide a faster intragastric acid control on day 1 and greater intragastric acid control on days 1 and 5 of dosing compared with IV pantoprazole.7 Superior intragastric acid control of oral esomeprazole over oral lansoprazole has been previously reported.8–11 This study is the first to compare the pharmacodynamic effects of IV formulations of esomeprazole and lansoprazole.
The purpose of this study was to compare the efficacy of intragastric acid control of once-daily IV dosing of esomeprazole 40 mg with lansoprazole 30 mg in healthy adults.
METHODS
Study design
The protocol for this randomized, open-label, two-treatment crossover study (D9612L00080; ClinicalTrials.gov Identifier NCT00230516) was approved by the Institutional Review Boards associated with each of the two US study centers [National Institutes of Health-Funded Clinical Research Center at the University of California, Los Angeles (JRP) and the Oklahoma Foundation for Digestive Research (PBM)]. Each subject provided written informed consent before any study-specific procedure was conducted. Study procedures were performed in accordance with the ethical principles of the Declaration of Helsinki and consistent with Good Clinical Practice.
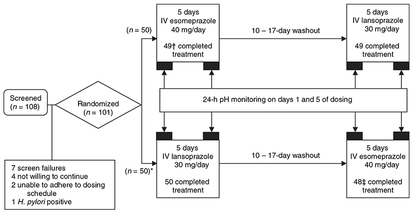
At screening, demographic information and medical history were recorded, a physical examination and electrocardiogram were performed, blood and urine samples were collected for routine laboratory analysis, and Helicobacter pylori infection was tested by serology. Within 21 days of screening, subjects were randomized in a 1:1 ratio to one of two treatment sequences, each consisting of two 5-day treatment periods of IV esomeprazole 40 mg or IV lansoprazole 30 mg separated by a 10- to 17-day washout period (Figure 1). Study medication was administered intravenously over 30 min, 4 h before breakfast on dosing days 1 and 5 and 30 min before breakfast on dosing days 2–4. Subjects remained at the study site for 24-h pH monitoring only on days 1 and 5. Subjects returned to the study site on days 2, 3 and 4 for IV administration of study medication.
Figure 1.
Study design and subject disposition. *One subject was assigned to receive intravenous (IV) lansoprazole 30 mg followed by IV esomeprazole 40 mg, but received IV lansoprazole 30 mg in both treatment periods. The subject was discontinued after two doses of IV lansoprazole 30 mg were administered in the second treatment period. † One subject was discontinued from the intention-to-treat population due to study drug non-compliance, insufficient washout and lack of evaluable pH data. ‡ Two subjects were discontinued from the intention-to-treat population due to study drug non-compliance, insufficient washout and lack of evaluable pH data.
Subjects
Healthy adults aged 18–70 years, inclusive, who were negative for infection with H. pylori and weighed within 40% of their ideal body weight were included in this study. Non-pregnant, non-lactating women of childbearing potential were eligible, if they were using a medically acceptable form of birth control, as determined by the investigator, throughout the study and had a negative serum pregnancy test result on the first day of study medication dosing. Subjects who had a history of a significant disease (e.g. renal, hepatic and cardiovascular disease) that might affect the pharmacokinetics of PPIs were excluded. Subjects were not permitted any prescription strength H2-receptor antagonists, prokinetic drugs, PPIs or drugs that could alter the pharmacokinetics of PPIs within 2 weeks before the first dose of study drug. Subjects who required concomitant medication that is dependent on the presence of gastric acid for optimal absorption (e.g. ketoconazole, iron salts, digoxin and ampicillin esters) were excluded. Subjects could not have current or past (within 6 months of screening) endoscopic evidence of oesophageal pathology or a history of gastric or oesophageal surgery.
Intragastric pH assessments
Intragastric pH monitoring was conducted on days 1 and 5 of each dosing period (Figure 1). A dual-channel ComforTEC Plus disposable pH probe attached to a GERDcheck data logger (Sandhill Scientific, Inc., Highlands Ranch, CO, USA) was used for pH recording. The calibrated pH microelectrode was positioned in the stomach 10 cm below the lower oesophageal sphincter (LES) using the LES locator and/or by formal manometric localization. pH recording began immediately before study drug administration, 1–2 h after placement of the pH probe. Intragastric pH was recorded every 5 s for 24 h. Subjects were permitted as much as 16 fluid ounces (473 mL) of water during the first 4 h of the fasting period after dosing. Subjects were prohibited from eating, drinking or taking antacids after midnight. Standardized meals that were not high in fat or calories were provided on each day of pH recording. The first meal (lunch) was provided approximately 4 h after dosing was completed. Subjects also were provided dinner and an evening snack. Meals were administered in an identical fashion during both treatment sequences. Subjects were instructed to maintain a consistent amount of nicotine consumption (if appropriate), not to lie down until 10 pm, and not to start any new physical training activities during pH monitoring. At the end of the pH monitoring period on the mornings of days 2 and 6, the pH probe was removed.
The primary outcome measure was the percentage of time with pH >4.0 on day 5 (steady state). Secondary outcome measures included the percentage of time with pH >2.5, 4.0 and 6.0 on day 1 and the percentage of time with pH >2.5 and 6.0 on day 5. Post hoc analyses evaluated the onset of intragastric acid control and control of intragastric acidity during the first 4 h of pH monitoring while subjects were in a fasting state.
Safety assessments
Adverse events (AEs) were recorded spontaneously by the subject in response to an open question or revealed by observation from the first administration of study drug until the follow-up telephone contact 10–14 days after the last study visit. Serious AEs were collected from the time the subject signed the informed consent agreement until 14 days after the last dose of study drug.
Statistical analysis
The per protocol (PP) population included all subjects who received at least one dose of study drug, had evaluable pH data on day 5 of both treatment periods, and had no major protocol violations or deviations that might affect gastric pH. The safety analyses included all subjects who received at least one dose of study medication. pH data were considered to be evaluable, if subjects had ≥20 h of valid pH data within the reference range of 0–9, did not have one continuous hour or more of pH data outside the reference range, and had data judged to be evaluable by the central reader. The pH tracings and other relevant data were generated and reviewed by the central reader in a blinded manner.
The primary and secondary efficacy outcomes were analysed by comparing the percentage of time that the intragastric pH was higher than a threshold between treatment groups on days 1 and 5. The percentage of time with pH higher than a threshold was defined as 100 times the proportion of the records with pH higher than the threshold divided by the total number of records (up to 24 h). Least-squares mean (LSM) percentages and 95% confidence intervals (CIs) of time during the 24-h monitoring period that pH was above 2.5, 4.0 and 6.0 on days 1 and 5 of each dosing period were calculated for each treatment and analysed using a mixed model with fixed effects for treatment, sequence and period. Safety data were summarized descriptively.
A post hoc analysis was conducted such that the mixed model used in the primary analysis was refit using only the first 4 h of data on days 1 and 5. An additional post hoc analysis was performed to determine when pH was maintained at >4.0 for 1 consecutive hour (stable pH >4.0) for each patient. As many as 5 min of missing data was allowed in accumulation of the 60 min of consecutive readings of pH >4.0. The time to stable pH >4.0 was transformed using Gehan scores12 to account for right censoring in the data. The transformed data were fit to a mixed model with fixed effects for treatment, period and sequence. Treatment effects were tested at the a-level of 0.05. Kaplan-Meier estimates of the median time to stable pH >4.0 were calculated on the untransformed data. A sample size of 78 subjects was estimated to be required to provide 95% power to detect a difference of 7.5 percentage points between treatment groups in the number of hours out of 24 with pH >4.0.
RESULTS
A total of 101 subjects were enrolled, and 97 completed the study (Figure 1). The PP population consisted of 96 subjects who had evaluable data for both day 5 dosing periods. Subjects were predominantly men (64.6%) and white (93.8%), with a mean age of 29 years (Table 1).
Table 1.
Patient demographics and baseline characteristics (per protocol population)
| Characteristic | No. of patients (total 96) |
|---|---|
| Men, n (%) | 62 (64.6) |
| Women, n (%) | 34 (35.4) |
| Mean (s.d.) age, years | 28.8 (11.1) |
| Range | 18–65 |
| White/black/other, n (%) | 90 (93.8)/2 (2.1)/4 (4.2) |
| Mean (s.d.) body mass index (kg/m2) | 25.5 (4.9) |
| Range | 16.3–42.0 |
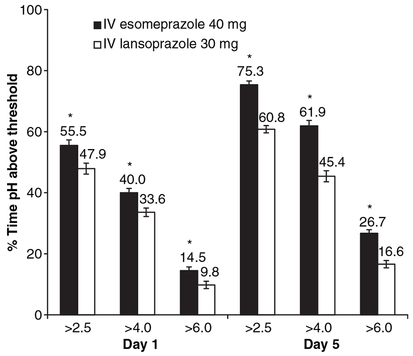
The mean time with evaluable pH data on day 5 was 23.9 h for IV esomeprazole 40 mg and 24 h for IV lansoprazole 30 mg. The analysis of the primary outcome showed that the LSM (±S.E.M.) percentage of time with pH >4.0 was significantly (P ≤ 0.0001) greater with IV esomeprazole than with IV lansoprazole on dosing day 5 (Figure 2). Analysis of the secondary outcomes showed that the LSM (±S.E.M.) percentages of time with pH >2.5, 4.0 and 6.0 on day 1 and >2.5 and 6.0 on day 5 were significantly longer with IV esomeprazole than with IV lansoprazole (all P ≤ 0.0001; Figure 2).
Figure 2.
Least-squares mean (±S.E.M.) percentages of the 24-h monitoring period on days 1 and 5 with pH >2.5, 4.0 and 6.0 (n = 96). *P ≤ 0.0001. IV, intravenous.
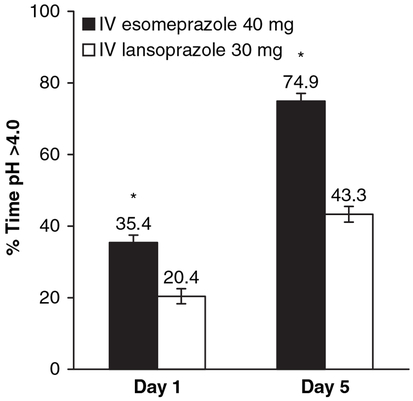
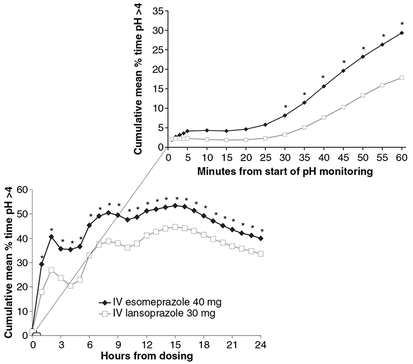
In the post hoc analyses, the LSM (±S.E.M.) percentage of time with pH >4.0 during the first 4 h of pH monitoring was significantly longer on days 1 and 5 with IV esomeprazole than IV lansoprazole (P < 0.0001; Figure 3). Significant differences in cumulative time with pH >4.0 occurred between IV esomeprazole and IV lansoprazole beginning 30 min after the start of pH monitoring (Figure 4). The time to achieve a stable pH was significantly faster for esomeprazole than lansoprazole (P =0.0014; from mixed model on Gehan scores). The variables ‘period’ and ‘treatment sequence’ were not significant in the mixed model for time to achieve stable pH. The Kaplan-Meier estimates of the median time to achieve stable pH >4 were 4.9 h for IV esomeprazole 40 mg and 5.8 h for IV lansoprazole 30 mg.
Figure 3.
Least-squares mean (±S.E.M.) percentages of the first 4 h of monitoring on days 1 and 5 with pH >4.0 (n = 96). *P < 0.0001. IV, intravenous.
Figure 4.
Cumulative mean percentage of time with pH >4.0 on day 1; inset shows cumulative mean percentage of time with pH >4.0 by minute for the first hour after start of pH monitoring (n = 96). *P < 0.0065. IV, intravenous.
A similar proportion of subjects reported AEs during treatment with IV esomeprazole 40 mg and IV lansoprazole 30 mg (24.5% and 22.0% respectively). The most common AEs during esomeprazole and lansoprazole dosing were injection site reactions (11% and 8% respectively) and upper respiratory tract infections (1% and 3% respectively). Two AEs were considered treatment related: (i) moderate headache reported by a subject while receiving IV esomeprazole 40 mg that lasted for 6 days; and (ii) mild chest discomfort reported by a subject while receiving IV lansoprazole 30 mg that continued for 17 days. No deaths, serious AEs or discontinuations from the study occurred due to an AE.
DISCUSSION
In healthy adults, IV esomeprazole 40 mg controlled intragastric acidity more effectively than IV lansoprazole 30 mg, as demonstrated by the larger percentage of time that intragastric pH was greater than 2.5, 4.0 and 6.0 on dosing days 1 and 5. In addition, IV esomeprazole 40 mg controlled intragastric acidity faster on day 1 than IV lansoprazole 30 mg, showing significantly greater acid suppression within 30 min of dosing. Both agents were well tolerated and had similar safety profiles.
The relative efficacy of esomeprazole and lansoprazole seen in this study is in agreement with previous comparisons of oral esomeprazole 40 mg and oral lansoprazole 30 mg in healthy subjects and in patients with symptoms of GERD. LSM percentages of time with pH >4.0 on day 5 were 58–65% for esomeprazole vs. 48–53% for lansoprazole and were similar to those reported in this study with IV administration (62% for esomeprazole vs. 45% for lansoprazole).8–11, 13 In addition, the steady-state LSM percentage of time with pH >4.0 for IV esomeprazole in this study (62%) was similar to results from previous studies in healthy adults of the same dosage of IV esomeprazole (58–66%).7, 13
One of the key findings in this study was that esomeprazole and lansoprazole provided significant acid suppression during the first 4 h after dosing, when subjects were still fasting. Because it is believed that stimulation of acid secretion (i.e. activation of proton pumps), such as that which occurs after a meal, is required for antisecretory activity of PPIs,3, 4 this result was unexpected. Although a basal level of gastric acid secretion via pathways involving vagal stimulation, acetylcholine and histamine-2 is always present,4 it was not thought that sufficient acid stimulation existed to observe an antisecretory effect of PPIs. The results of this study demonstrate that PPIs do have pharmacodynamic activity without meal stimulation of gastric acid secretion. In addition, the study protocol was not designed to evaluate the rapidity of acid suppression following IV administration of PPIs; however, the 1- to 2-h delay between insertion of the pH probe and administration of study medication provided sufficient time for subjects to acclimate to the recording conditions and to alleviate stress-induced gastric acid secretion that may result from insertion of the pH microelectrode. The results therefore demonstrated a rapid onset of action with increased intragastric pH within 30 min after dosing, as shown by the increase in the cumulative mean percentage of time with pH >4.0 and substantial acid control within 1 h (Figure 4). These findings suggest that PPIs may be useful in situations that require rapid acid control, such as emergency care situations. Future studies designed to evaluate the onset of effect of PPIs are necessary to determine the timing of intragastric acid suppression following IV PPI treatment.
The present results are consistent with those from a study of similar design that compared IV esomeprazole 40 mg with IV pantoprazole 40 mg in healthy subjects.7 In that study, the time that intragastric pH was >4.0 during 24 h on days 1 and 5 was significantly (P < 0.001) greater with esomeprazole (8.3 and 13.9 h respectively) than pantoprazole (5.3 and 9.0 h respectively). The onset of effect was not as fast as observed in the current study; however, importantly, during the first 4 h after dosing on day 1, when subjects were still fasting, time that intragastric pH was >4.0 was 1.7 and 0.6 h for esomeprazole and pantoprazole respectively (P < 0.0001).
Although the mechanism for fasting preprandial PPI activation in these two studies is not clear, as mentioned previously, several known pathways exist for acid stimulation other than food stimulation.4 In a study by Zeng et al.14 in rats, pituitary adenylate cyclase activating polypeptide (PACAP) was shown to be a potential mediator of neural regulation of gastric acid secretion. In addition, it has been suggested that a certain number of proton pumps are always active and continuously secrete low levels of acid.4, 15 An additional increase in cumulative acid suppression was observed with esomeprazole and lansoprazole after the meal stimulus at approximately 5 h, suggesting that the additional acid stimulation increased the antisecretory effect of the PPIs. However, when interpreting the graph of the cumulative mean percentage of time pH >4.0 (Figure 4), each successive data point represents the cumulative effect of acid suppression from the beginning of pH monitoring up to that point and not only the instantaneous effect at that time.
In clinical practice, patients likely to receive IV PPI treatment are those in a critical care setting. This study was conducted with healthy volunteers who do not accurately reflect the demographic characteristics and comorbidities present in critically ill patients in intensive care units.
In conclusion, the results of this study suggest that IV esomeprazole provides faster time to stable pH above 4.0 and faster onset of and greater acid suppression than IV lansoprazole. Moreover, the efficacy of PPIs under fasting conditions questions and provides further insight into our understanding of gastrin-stimulated acid secretion as a prerequisite for effective PPI pharmacology and supports their use in clinical settings with patients who are unable or not permitted to eat and require immediate onset of acid suppression.
ACKNOWLEDGEMENTS
Declaration of personal interests: (i) J. R. Pisegna is an advisory board member for Alza, Johnson & Johnson and Wyeth, is a member of the speakers bureau for Novartis and Wyeth, and receives grant support from AstraZeneca LP, Novartis, TAP and Janssen. (ii) P. B. Miner is a consultant to Abbott, ARYx, AstraZeneca, ISIS, Janssen, Novartis and Solvay; is a member of the speakers bureau for AstraZeneca, Axcan, Centocor, ISIS, Janssen, Novartis and Solvay; is a patent holder with ISIS; is a member of scientific advisory boards with Alza, ARYx, AstraZeneca, Forest, ISIS, Janssen, Novartis, Romark, Solvay and UCB; and receives grant/research support from Abbott, ARYx, AstraZeneca, Axcan, Berlex, Centocor, Elan, GlaxoSmithKline, ISIS, Janssen, Novartis, Otsuka, Procter & Gamble, Salix, Solvay, Takeda, TAP and Wyeth/Ayerst. (iii) M. B. Sostek and J. T. Monyak are employees of and own stock in AstraZeneca. (i) All authors provided substantial contributions to the concept and design of the study and interpretation of the data. Each author critically reviewed, revised and approved the submitted version of the manuscript. (ii) J. R. Pisegna and P. B. Miner recruited patients. (iii) J. T. Monyak provided the statistical analysis plan and analysed the data. Declaration of funding interests: (i) This study was supported by AstraZeneca LP. The Clinical Research Center at the David Geffen School of Medicine at UCLA was also supported in part by General Clinical Research Centers Program, grant no. M01-RR00865. (ii) Writing support was provided by Lisa M. Klumpp and Judy E. Fallon of Scientific Connexions and funded by AstraZeneca LP, with editorial assistance from John Tumas. (iii) Radu Tutuian of the University Hospital of Zurich served as the central reader of the pH studies. (iv) Assunta Cuccia of AstraZeneca LP was in charge of clinical study management.
REFERENCES
- 1.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100: 190–200. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong D Intravenous proton pump inhibitor therapy: a rationale for use. Rev Gastroenterol Disord 2005; 5(Suppl. 2): S18–30. [PubMed] [Google Scholar]
- 3.Hatlebakk JG, Katz PO, Camacho-Lobato L, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment Pharmacol Ther 2000; 14: 1267–72. [DOI] [PubMed] [Google Scholar]
- 4.Pisegna JR. Pharmacology of acid suppression in the hospital setting: focus on proton pump inhibition. Crit Care Med 2002; 30(Suppl.): S356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz PO, Ginsberg GG, Hoyle PE, Sostek MB, Monyak JT, Silberg DG. Relationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2007; 25: 617–28. [DOI] [PubMed] [Google Scholar]
- 6.Bell NJV, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 1992; 51(Suppl. 1): 59–67. [DOI] [PubMed] [Google Scholar]
- 7.Wilder-Smith CH, Röhss K, Bondarov P, Hallerbäck B, Svedberg L-E, Ahlbom H. Esomeprazole 40 mg i.v. provides faster and more effective intragastric acid control than pantoprazole 40 mg i.v.: results of a randomized study. Aliment Pharmacol Ther 2004; 20: 1099–104. [DOI] [PubMed] [Google Scholar]
- 8.Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol 2003; 98: 2616–20. [DOI] [PubMed] [Google Scholar]
- 9.Miner P Jr, Katz PO, Chen Y, Sostek M. Reanalysis of intragastric pH results based on updated correction factors for Slimline® and Zinetics™ 24 single-use pH catheters [letter to the editor]. Am J Gastroenterol 2006; 101: 404–5. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DA, Stacy T, Ryan M, et al. A comparison of esomeprazole and lansoprazole for control of intragastric pH in patients with symptoms of gastrooesophageal reflux disease. Aliment Pharmacol Ther 2005; 22: 129–34. [DOI] [PubMed] [Google Scholar]
- 11.Wilder-Smith CH, Röhss K, Nilsson-Pieschl C, Junghard O, Nyman L.Esomeprazole 40 mg provides improved intragastric acid control as compared with lansoprazole 30 mg and rabeprazole 20 mg in healthy volunteers. Digestion 2003; 68: 184–8. [DOI] [PubMed] [Google Scholar]
- 12.Feingold M, Gillespie BW. Cross-over trials with censored data. Stat Med 1996; 15: 953–67. [DOI] [PubMed] [Google Scholar]
- 13.Wilder-Smith CH, Bondarov P, Lundgren M, et al. Intravenous esomeprazole (40 mg and 20 mg) inhibits gastric acid secretion as effectively as oral esomeprazole: results of two randomized clinical studies. Eur J Gastroenterol Hepatol 2005; 17: 191–7. [DOI] [PubMed] [Google Scholar]
- 14.Zeng N, Athmann C, Kang T, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 1999; 104: 1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois A Control of gastric acid secretion In: Brandt LJ, ed. Clinical Practice of Gastroenterology, Vol. 1, Chapter 20 Philadelphia, PA: Churchill Livingstone, 1999: 180–8. [Google Scholar]