Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1) is a heptahelical, G protein-coupled receptor that has been shown to be expressed by non-squamous lung cancer and breast cancer cell lines, and to be coupled to the growth of these tumors. We have previously shown that PACAP and its receptor, PAC1, are expressed in rat colonic tissue. In this study, we used polyclonal antibodies directed against the COOH terminal of PAC1, as well as fluorescently labeled PACAP, Fluor-PACAP, to demonstrate the expression of PAC1 on HCT8 human colonic tumor cells, using FACS analysis and confocal laser scanning microscopy. Similarly, anti-PACAP polyclonal antibodies were used to confirm the expression of PACAP hormone by this cell line. We then investigated the signal transduction properties of PAC1 in these tumor cells. PACAP-38 elevated intracellular cAMP levels in a dose-dependent manner, with a half-maximal (EC50) stimulation of approximately 3 nM. In addition, PACAP-38 stimulation caused an increase in cytosolic Ca2+ concentration [Ca2+]i, which was partially inhibited by the PACAP antagonist, PACAP-(6–38). Finally, we studied the potential role of PACAP upon the growth of these tumor cells. We found that PACAP-38, but not VIP, increased the number of viable HCT8 cells, as measured by MTT activity. We also demonstrated that HCT8 cells expressed the Fas receptor (Fas-R/CD95), which was subsequently down-regulated upon activation with PACAP-38, further suggesting a possible role for PACAP in the growth and survival of these tumor cells. These data indicate that HCT8 human colon tumor cells express PAC1 and produce PACAP hormone. Furthermore, PAC1 activation is coupled to adenylate cyclase, increase cytosolic [Ca2 +]i, and cellular proliferation. Therefore, PACAP is capable of increasing the number of viable cells and regulating Fas-R expression in a human colonic cancer cell line, suggesting that PACAP might play a role in the regulation of colon cancer growth and modulation of T lymphocyte anti-tumoral response via the Fas-R/Fas-L apoptotic pathway.
Keywords: Pituitary adenylate cyclase-activating polypeptide; Pituitary adenylate cyclase-activating polypeptide type 1 receptor, HCT8 cells; Colon cancer growth
1. Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a member of the secretin/glucagon/vasoactive intestinal peptide (VIP) superfamily that includes secretin, glucagon, calcitonin, parathyroid hormone, and glucagonlike peptide [1]. PACAP was first isolated from ovine hypothalamic extracts in 1989 via its ability to stimulate cAMP production in the anterior pituitary of rats [1]. Two biologically active forms of the peptide, PACAP-27 and PACAP-38 [1,2], are products of post-translational processing of a PACAP precursor molecule. Both forms include a 27-amino-acid polypeptide sequence, having 70% homology with VIP. However, PACAP-38 contains an additional 11-amino-acid extension at the C-terminal [1–3]. Receptors for VIP and PACAP have been recently cloned and a pharmacological classification has been established, based on the relative affinities of PACAP and VIP [4]. PAC1 receptor has a high affinity for only PACAP-27 and PACAP-38 and a 1000-fold less affinity for the peptides VIP and helodermin.
Early radioligand studies have suggested the existence of distinct high-affinity receptors for PACAP, VIP, and helodermin on specific tumor cell lines, such as the pancreatic acinar cancer cell line AR42J, human neuroblastoma NB-OK1 cell line, an astrocytoma cell line, and other tumor cell lines [5,6]. PACAP and VIP receptors have been detected in several cancer cell lines, including those of the breast, gastric, pancreatic, and lung, as well as melanoma and neuroblastoma [7–10]. In early studies, VIP and PACAP have been shown to have regulatory roles in cellular proliferation and cellular linked growth factor pathways [10–12]. In lung and breast cancer cell lines, PACAP stimulates the induction of the immediate-early genes and increases proliferation indices [8,10,12]. In native cell systems, PACAP has been shown to stimulate proliferation [13], differentiation [14,15], and cell survival [16–18] in the nervous system and in the gastric mucosa [19–21].
In a recent study, PACAP-immunoreactive nerve fibers and expression of PAC1 in the mucosa have been identified in the normal rat stomach and colon, suggesting a potential role for PACAP in these tissues [22]. Furthermore, expression of Fas-R, a cell surface “death receptor” that mediates apoptosis upon activation by its ligand (Fas-L), has been identified in normal colon mucosa, as well as in various adenomas and colon carcinomas [23]. The down-regulation of Fas-R expression or signaling in colon cancer cells have been implicated as a cause of tumor immune escape [24–26]. In various cells of T lineage, Fas-R [22,26,27] has been shown to play a significant role in the maintenance of immunological homeostasis and peripheral tolerance by deletion of activated T lymphocytes [28–31]. Therefore, we investigated Fas-R expression on HCT8 human colonic tumor cell line to elucidate a potential role for PACAP in regulating expression of the Fas-R, as well as in the growth of tumor cells. To date, in human colonic tumors, no studies have been conducted to specifically identify PACAP or PAC1 expression, nor PACAP’s effect on Fas-R expression has been investigated.
In the current study, we demonstrate that the HCT8 cell line expresses PACAP and PAC1, and that the activation of PAC1 is coupled to both an increase of intracellular cAMP and Ca2 + levels. Moreover, PACAP’s addition to the culture appears to stimulate the proliferation of HCT8 cells. Furthermore, we show that HCT8 cells, under baseline conditions, express surface Fas-R, which is down-regulated upon stimulation with PACAP. Our results suggest that PACAP might play a role in the regulation of colon cancer growth and in the modulation of T lymphocyte anti-tumoral responses via the Fas-R/Fas-L apoptotic pathway.
2. Materials and methods
2.1. Cell culture
HCT8 were cultured in RPMI (Hyclone, Logan, UT) added with 10% fetal bovine serum (FBS), kanamycin (Gibco, Carlsbad, CA) and gentamicin (Sigma, St. Louis, MO) antibiotics. Adherent confluent cells were washed with PBS and treated with trypsin/EDTA, then the cells were pelleted, resuspended in fresh medium, and incubated at 37 °C in 5% CO2. The cells, studied during their exponential growth phase, were mycoplasma-free, as determined by immunocytofluorescence staining with DAPI.
2.2. FACS analysis
The expression of PAC1 and PACAP was characterized in HCT8 cells using FACS analysis. Approximately 1 million cells per sample were grown in RPMI containing 10% FBS, then trypsinized, and fixed with 4% formaldehyde and 0.1% Triton-X for 15 min at 4 °C. The fixed cells were washed twice with PBS and incubated with rabbit polyclonal anti-PAC1 (1:1000) or anti-PACAP (1:1000) (Peninsula Laboratories, San Carlos, CA) antibodies in PBS/1% BSA/goat serum.
Cells were also incubated with rabbit IgG (10 mg/ml) as a negative control. The following day, the samples were washed twice with PBS and then incubated with goat anti-rabbit FITC-conjugated antibodies (Molecular Probes, Eugene OR) for 1 h at 4 °C in PBS/1% BSA. The samples were analyzed with a fluorescence-activated cell sorter (BD Bioscience, Franklin Lakes, NJ) and data collected.
2.2.1. Confocal microscopy
To further confirm the expression of PAC1 and PACAP by HCT8 cells, confocal microscopy was used to characterize the receptor and hormone expression in the cells, using rabbit polyclonal anti-PAC1 antibodies or anti-PACAP anti-bodies, respectively. HCT8 cells were plated overnight at a low density on polylysine-coated coverslips. The following day, the cells were fixed for 30 min with 4% paraformaldehyde and 0.1% Triton-X. Then, the cells were incubated at 4 °C with rabbit polyclonal anti-PAC1 (1:1000) or anti-PACAP antibodies (1:1000) overnight. Cells were also incubated with rabbit IgG (10 Ag/ml) as a negative control. The next day, the cells were washed twice with PBS and then incubated with goat anti-rabbit Alexa-488 antibodies (Molecular Probes) in PBS/1% BSA/goat serum. Samples were observed using a Zeiss LSM 510 Laser Scanning Microscope (Carl Zeiss, Thornwood, NY).
2.2.2. Fluorescent PACAP (Fluor-PACAP)
Alexa-488-conjugated PACAP-27 (Fluor-PACAP) was utilized for imaging studies as described previously [31]. Fluor-PACAP was shown to have a pharmacological and biological activity identical to the native peptide [33]. HCT8 cells were cultured overnight as described above, then washed twice in PBS and placed in a phenol red-free culture medium. At 4 °C, 10 nM of Fluor-PACAP was added. The cells were then warmed to 37 °C and studied by confocal microscopy as described above.
2.3. Adenylyl cyclase stimulation
To demonstrate that PAC1 receptors expressed on HCT8 are functionally coupled to intracellular mediators, such as adenylyl cyclase, HCT8 cells were stimulated with PACAP-38 in a dose-dependent manner and the cAMP levels were measured, as previously described [32]. Briefly, HCT8 cells were seeded on 24-well culture dishes overnight (~ 2.0 × 105 cells/well) with RPMI containing 10% FBS, kanamycin, and gentamicin, in presence of [3H]-adenine (Amersham, Arlington Heights, IL) at a concentration of 2 mCi/ml. The cells were then washed with RPMI/1% BSA, incubated with or without the indicated concentration of PACAP-38 in RPMI/ 1% BSA and 2.5 mM 3-isobutyl,1–1-methyl-xanthine (IBMX) (Sigma) for 30 min and then aspirated. The cells were lysed by a 100-μl 2% SDS, 1 mM cAMP solution. Total [3H]-cAMP was assayed by consecutive Dowex AG-50W-X4 resin (Bio-Rad, Richmond, CA) and aluminum oxide (Sigma) column chromatography, according to a modification of the procedure described by Salomon et al. [34]. Eluates were collected in vials and counted using a Beckman Liquid Scintillation Counter (Beckman, Fullerton, CA).
2.4. Ca2+ imaging
To further investigate whether PAC1, expressed by HCT8 cells, could mediate an increase in cytosolic [Ca2+]i, calcium imaging was performed as previously described [35]. HCT8 cells were plated overnight at a density of 50–100 thousand cells/ml on glass coverslips. The cells were then washed with a physiological salt solution (PSS: 127 mM NaCl, 1.8 mM CaCl2, 5 mM KCl, 2 mM MgCl2, 0.5 mM NaH2PO4, 5 mM NaHCO3, 10 mM glucose, and 10 mM HEPES, pH 7.4) added with 0.1% bovine serum albumin (BSA). Cells were incubated with PSS containing 2 μM fura-2 acetoxymethy ester (fura-2/ AM) for 30 min at 37 °C and the excess of dye was washed with PSS thereafter. Cells were then imaged in a chamber thermostatically controlled at 37 °C within an hour from the loading. Addition of hormone or antagonist was accomplished by pipetting 4 ml of PSS solution containing either PACAP-38 (10 nM) or PACAP-(6–38) (1 μM), into the incubation chamber in which the fluid volume was maintained at ~ 500 μl by means of an aspirator. Ionomycin (10 μM) was used as a positive control and administered in the same manner. Dynamic video imaging was performed using the MagiCal hardware and Tardis software provided by Applied Imaging (Newcastle, UK) and averaging 16 frames with an analogue hardware averager. In all experiments, background subtraction was employed before ratioing using a frame of 256 × 256 pixels. The ratio of light emitted at 510 nm, from cells excited alternately at 340 and 380 nm, was calculated from averaged video frames on a pixel-by-pixel basis and was proportional to the cytosolic Ca2 + concentration. Calibration was performed using a dissociation constant of 225 nM for fura-2 and Ca2 + at 37 °C [36]. Th minimal and maximal fluorescence at 340 and 380 nm were determined in cells loaded with dye, and made permeable to extracellular Ca2+ with 10 μM ionomycin in a solution containing either 10 mM Ca2+ or 10 mM EGTA. To reduce the variance between data sets, the average basal concentration of Ca2 + was subtracted from all data points for each individual experiment. This ensures that any effect caused by addition of stimulus would not be lost to the variance between each individual trial, as a result of differences in initial Ca2+ concentrations in the cells.
2.5. Fas-R (CD95) expression
To confirm Fas-R expression in HCT8 cells, confocal microscopy was used to characterize receptor expression on the cell surface using anti-CD95-FITC antibodies (Dako, Carpinteria, CA). HCT8 cells were plated overnight at a low density on polylysine-coated cover slips. The following day, the cells were incubated with anti-CD95-FITC antibodies (1:1000) for 4 h on ice. After washing with PBS, the cells were analyzed using a Zeiss LSM 510 Laser Scanning Microscope. To determine the effect of PACAP on Fas-R expression, HCT8 cells were incubated with or without 1 μM PACAP on coverslips for 24 h. Subsequently, the cells were incubated with anti-CD95-FITC antibody for 4 h on ice, washed with PBS, and analyzed by confocal microscopy.
2.6. MTT proliferation assay
To investigate the effect of PACAP on HCT8 growth, cells ( ~ 20,000 cells/well) were plated in 96-well plates overnight at 37 °C. The next day, the cells were activated with specific concentrations of PACAP-38, or VIP, in absence of FBS, but in the presence of 1 × insulin-trans-ferrin-selenium A (Gibco) for 24 h. Ten microliters of 12 mM MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide) (Molecular Probes) was added to each well and incubated for 4 h at 37 °C. Following this incubation, 100 μl of 0.01 M HCl/10% SDS solution was added to each well and the cells incubated overnight at 37 °C. The reaction was read the day after at 570 nm in an MRX Revelation ELISA Reader (Dynex Technologies, Vista, CA). MTT is proportional to the number of viable cells in each well. Hence, the proliferation of HCT8 cells, induced by PACAP stimulation, was compared to that of unstimulated cells.
3. Results
3.1. FACS analysis
To confirm the presence of PAC1 and PACAP in HCT8, FACS analysis was performed using rabbit polyclonal anti-PAC1 and anti-PACAP antibodies, respectively (Fig. 1A and B). Incubation with rabbit IgG was also used as a negative control. FACS analysis showed that approximately 60% of the gated HCT8 cells express PAC1 receptor (panel A) and 89% express PACAP (panel B). As a positive control, rabbit polyclonal anti-PAC1-incubated NIH-3T3 cells, stably transfected with PAC1 (panel C), showed a positive shift of fluorescence intensity, whereas untransfected NIH-3T3 cells (panel D), which were used as a PAC1 negative control, failed to shift. These results indicate the specific expression of PAC1 and PACAP by HCT8 cells as assessed by FACS analysis.
Fig. 1.
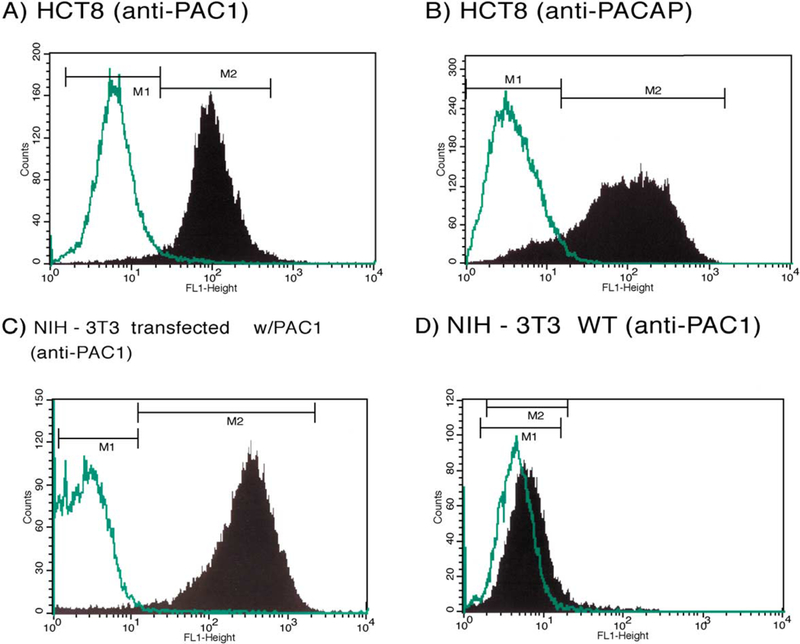
FACS analysis. Detection of PAC1 and PACAP using rabbit polyclonal anti-PACl and anti-PACAP antibodies. Panels A and B show 60% and 89% positivity in gated HCT8 cells for the detection of PAC1 receptor and PACAP hormone, respectively (M2). PACl-transfected NIH-3T3 cells and untransfected NIH-3T3 WT cell lines were used as positive and negative controls for PAC1, respectively (C, D). Rabbit IgG were used as a negative control for unspecific binding (M1).
3.1.1. Confocal laser scanning microscopy
To further confirm that the HCT8 cell line expresses PAC1 and PACAP, confocal laser scanning microscopy was used to image cells that were incubated with rabbit polyclonal anti-PAC1 or rabbit anti-PACAP antibodies, or rabbit IgG, and then goat anti-rabbit FITC-conjugated antibodies (Fig. 2). Panels A and C show that HCT8 were positive for PAC1 and PACAP, respectively, which confirmed cell surface expression of the receptor as well the presence of PACAP hormone in their cytoplasms. Panels B and D show rabbit IgG negative controls. In addition, NIH-3T3 cells transfected with the PAC1 receptor were used as a positive control for PAC1 (panel E). Untransfected NIH-3T3 cells were used as a negative control (panel F), which stained negative under similar conditions. Fluorescent PACAP ligand was also used to detect membrane expression of PAC1 on HCT8 and revealed a positive staining (data not shown).
Fig. 2.
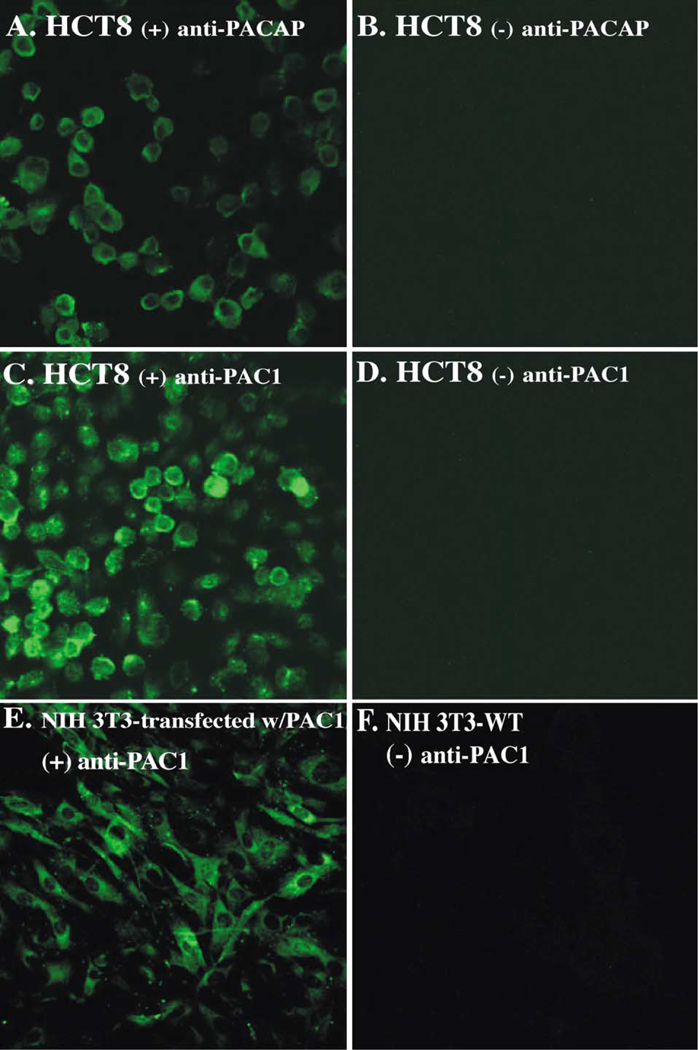
Confocal analysis. Confocal images show the expression of PACAP (panel A) and PAC1 (panel C) in HCT8 cells. Anti-PACAP or anti-PAC1 rabbit polyclonal Abs, followed by Alexa-488-conjugated goat anti-rabbit IgG, were used. In panels B and D, cells were incubated with rabbit IgG, followed by Alexa-488-conjugated goat anti-rabbit IgG. PAC1-transfected NIH-3T3 cells (panel E) and untransfected NIH-3T3 (panel F) were used as PAC1 positive and negative controls, respectively.
3.2. cAMP response to PACAP-38
To investigate the coupling of PAC1 to adenylate cyclase, intracellular cAMP levels were measured. PACAP-38 elevated cAMP levels in a dose-dependent manner with detectable stimulation observed at basal peptide concentrations of 0.01 nM and maximum stimulation at 1 μM of peptide. A 12fold increase in cAMP levels was measured for maximal PACAP-38 stimulation with the half-maximal effective dose (EC50) at 3 nM (Fig. 3). These results were consistent with the previously described pharmacology of the PAC1 receptors and indicate that the receptors exhibit signal transduction coupled to adenylate cyclase. The data shown represented the means of three experiments performed in triplicate.
Fig. 3.
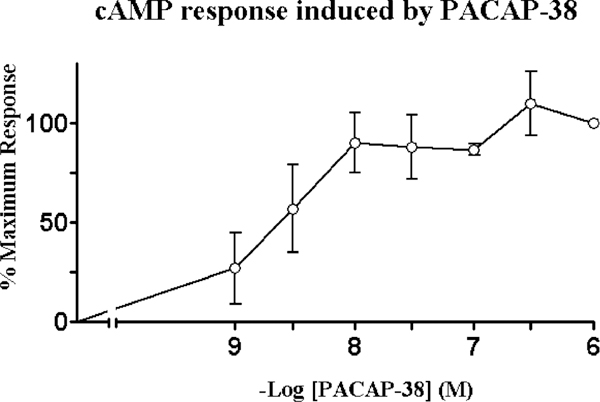
Stimulation of adenylyl cyclase. cAMP response was determined in HCT8 cell line as a function of PACAP-38 concentration. The percent maximal responses above basal is shown. A 12-fold increase in cAMP levels were observed for maximal 1 aM PACAP-38 stimulation with a half-maximal effective dose (EC50) of 3.0 nM. These data represent the means of three experiments performed in triplicate.
3.2.1. Ca2+ response to PACAP-38
To investigate the hypothesized coupling between PAC1 and [Ca2+]i, fura-2-loaded HCT8 cells were stimulated with 200 nM PACAP-38. In an entire field of cells imaged ( ~ 60 cells), only 5% of HCT8 cells had a robust increase in intracellular [Ca2 +]i, whereas the majority of the cells were nonresponsive. Fig. 4A represents the average [Ca2 +]i response of responsive cells, where intracellular [Ca2 +]i was increase to a maximal level of 96 ± 49 nM (n = 22) over basal. Fig. 4B and C shows the [Ca2 +]i responsive profile of two different HCT8 cells imaged, indicating that of the responsive cells imaged, each had varying magnitude of responses. Fig. 4D shows a [Ca2+]i profile of a non-responsive cell, which made up 95% of the entire field of cells imaged.
Fig. 4.
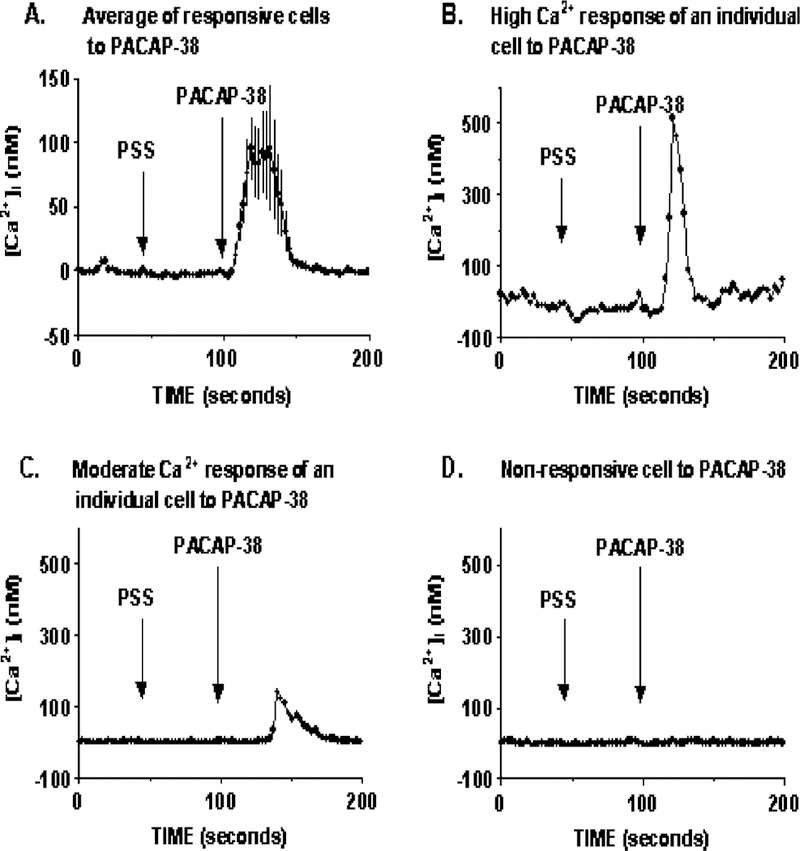
Ca2 + response to PACAP stimulation. In an average field of 60 HCT8 cells imaged, 200 nM PACAP-38 caused a robust increase in intracellular [Ca2 +]i of 96 ± 49 (n = 22) in 5% of the imaged cells (A). Panels B and C represent Ca2+ response profiles of two different HCT8 cells responsive to PACAP stimulation, demonstrating that not all responsive cells increase [Ca2 +]i to the same degree. Panel D is the [Ca2 +]i response profile of one nonresponsive cell. Ninety-five percent of the cells imaged were nonresponsive to PACAP-38 stimulation. PSS addition was used as a control for any response occurring as a result in change of medium.
3.2.2. PACAP-(6–38) inhibits PACAP-38-induced Ca2+ response
To demonstrate that the increase in [Ca2+]i was PAC1-specific, the PACAP-(6–38) antagonist was administered ~ 100 s before PACAP-38 stimulation. Fig. 5A shows the averaged of five independent [Ca2 +]i imaging experiments, using 10 nM PACAP-38, which resulted in an increase in the [Ca2+]i to 45 ± 14 nM (n = 5) over basal for the entire field of cells imaged ( ~ 60 cells). This effect of increase in [Ca2+]i caused by PACAP-38 stimulation was partially blocked by 1 μM of PACAP-(6–38) antagonist, resulting in a reduction of the [Ca2 +]i response to 16 ± 14 nM (n = 5) over basal level (Fig. 5B). The mean response of the entire field of cells imaged is presented here, because lack of a response to PACAP treatment in individual cells cannot be identified as a result of the nonresponsive nature of the cell or the effect of the antagonist. Hence, the average response of all the cells imaged will allow for an indication of the antagonist effect.
Fig. 5.
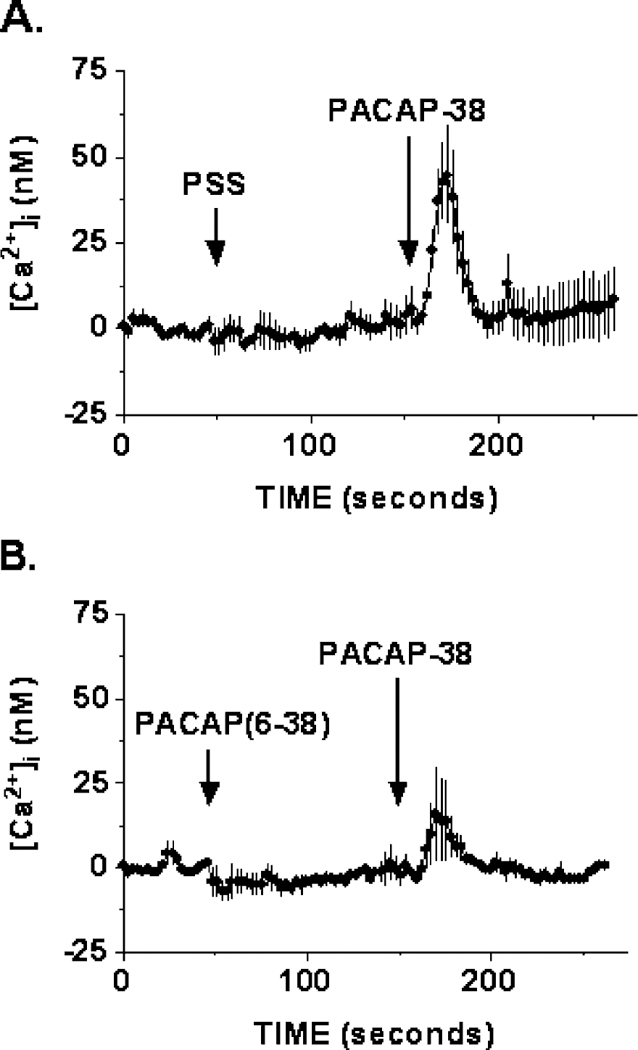
Inhibition of Ca2 + response. Data represent mean Ca2 + responses of the entire field of HCT8 cells imaged. (A) A robust increase in intracellular [Ca2 +]i of 45 ± 14–10 nM (n = 5) PACAP-38 stimulation was observed. (B) PACAP-(6–38) antagonist (1 μM) reduced the effect of PACAP-induced Ca2 + response to 16 ± 14 nM (n = 5).
3.3. Down-regulation of Fas-R expression by PACAP
The expression of Fas-R on the surface of HCT8 tumor cells was determined by laser scanning confocal microscopy (Fig. 6). Panel A shows confocal image of Fas-R expression detected on HCT8 cell line using monoclonal mouse anti-CD95 FITC-conjugated antibodies. Following incubation with PACAP-38 for 24 h, a decrease in fluorescence was observed, indicating the down-regulation of Fas receptor expression (panel B). In cells not exposed to PACAP, Fas-R expression was visualized, as shown in the panel A. These data suggest that PACAP may play a role in modulating lymphocyte immune responses against colon cancer cells by down-regulating Fas-R expression in colonic tumors.
Fig. 6.
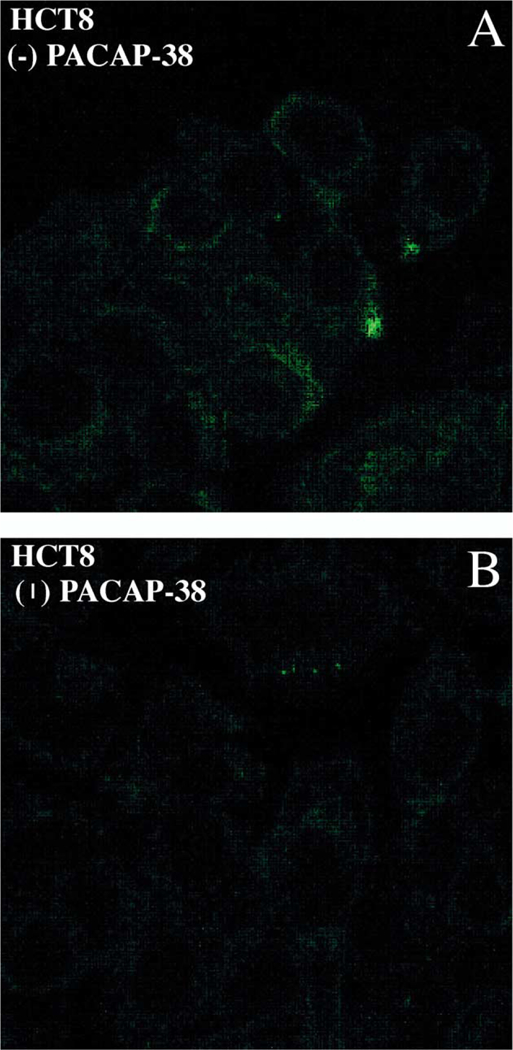
Fas expression and regulation. (A) Confocal microscope images show expression of Fas-R detected with anti-Fas-R-FITC mouse anti-bodies in HCT8 cells. (B) Down-regulation of Fas-R is seen upon 24 h, 1 AM PACAP-38 activation.
3.4. PACAP stimulation increases the proliferation ofHCT8 cells
The effect of PACAP on the growth of HCT8 cells was investigated using the MTT assay. Following a 24-h incubation of HCT8 cells with PACAP-38 or VIP, cellular proliferation was assessed by MTT assay, which provides an indication of the number of viable cells (Fig. 7). In serum-free media, containing only insulin-transferrin-selenium A, an increase in the number of viable cells was detected with 10−9 M PACAP-38 stimulation; a maximal MTT activity was observed at 10−7 M (EC50= 10 nM). A 25% increase in the number of viable cells after stimulation compared to unstimulated cells was observed in PACAP-38-treated HCT8 cells, whereas no increase, and in some cases, a decreased number, was detected with VIP-treated cells, suggesting that the effects were specifically mediated by PAC1.
Fig. 7.
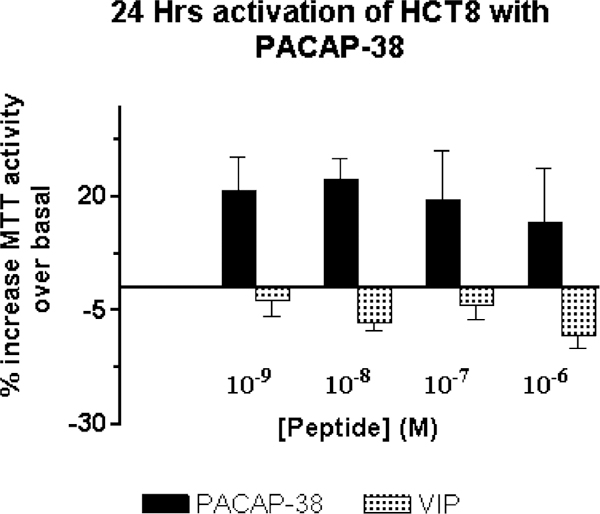
MTT proliferation assay. A ~ 25% average increase in MTT activity, over basal, was observed in HCT8 cells activated with varying concentration of PACAP for 24 h. The increase in MTT activity correlates with the increase in the number of viable cells. VIP-treated cells did not show an increase in MTT activity, and hence in the cell number. Cells were stimulated in media containing no fetal bovine serum, but added with insulin-transferrin-selenium A.
4. Discussion
PACAP is one of the most recently discovered peptides in the secretin/glucagon/vasoactive intestinal peptide (VIP) family of peptide hormones [2]. Since its discovery, many studies have identified specific PACAP binding receptors in normal [22] and tumoral tissues [8,37]. The recent cloning of the rat and human PACAP receptor, PAC1 [38–40], has lead to the development of molecular and antibody probes to specifically localize PAC1. PACAP receptors have been detected on several cancer cell lines, derived from breast, gastric, pancreatic, and lung tumors, in which they appear to regulate cellular proliferation [10–12]. In a previous study, we have showed that PACAP and PAC1 are expressed in normal colonic mucosa [22], leading us to postulate that PACAP may play an important role in the regulation of colonic tumor growth. In this study, we report the presence of PAC1 and PACAP in HCT8 human colonic tumor cell line, and show PACAP’s effects on signal transduction and proliferation of these cells. Furthermore, we demonstrate that Fas-R is expressed by HCT8 tumor cells, and is down-regulated following stimulation with PACAP. Since Fas-R has been reported to be present in normal colonic epithelium and to play a role in anti-tumoral immune responses [23], we hypothesize that PACAP-38, by down-regulating surface Fas-R expression, promotes tumor immune escape, blocking Fas-R-mediated apoptosis in colonic transformed cells. This idea is supported by similar observations described in pertinent hepatocellular carcinoma studies, which demonstrated that down-regulation of Fas-R on these tumor cells constituted a mechanism of immune evasion, leading to their survival [24]. In addition, PACAP, found to be present in the cytoplasm of HCT8 tumor cells, may stimulate their growth through autocrine pathways.
Immunocytochemical analysis, performed in HCT8 cells, using specific rabbit polyclonal anti-PACl and anti-PACAP antibodies, provided evidence of both PAC1 and PACAP expression by confocal laser scanning microscopy. Similarly, using a fluorescent PACAP ligand, Fluor-PACAP, PACAP receptors localization was also confirmed by con-focal microscopy. The expression of PAC1 and PACAP in HCT8 tumor cells was further demonstrated by flow cytometry; the results of FACS analysis showed that 60% of the HCT8 gated were positive for PAC1 and 89% for PACAP. This is consistent with the results obtained by confocal microscopy studies. Although internalization experiments were not performed in the current investigation, we have previously demonstrated that PAC1 undergoes rapid internalization in transfected NIH-3T3 cells [33,41]. Future studies aimed at characterizing the kinetics of PAC1 internalization in HCT8 cells using Fluor-PACAP will elucidate the potential differences in internalization and/or desensitization of native PAC1 expressed on tumor cells compared to transfected receptors.
To investigate the signal transduction pathways of PACAP receptors in HCT8 cells, we evaluated the ability of PACAP to activate adenylyl cyclase by measuring cAMP levels. PACAP-38 increased cAMP levels in a dose-dependent manner with a half-maximal stimulation at 3 nM, which is consistent with the pharmacology of cloned PAC1 [32]. In addition, PACAP-38 causes a robust increase of intracellular [Ca2 +]i of 96 ± 49 nM above basal in 5% of the cells imaged. The low number of cells that were Ca2 +-responsive upon PACAP treatment may be attributed to the fact that in a given population analyzed by FACS, only 60% of the HCT8 cells stained positive for PAC1 receptor. Moreover, expression of PAC1 receptors does not confer functionality of the receptor in all of the HCT8 cells. Other factors such as number of receptor expressed by a single cell, Ca2 + mediators and receptors, plus extracellular factors, may need to be in the right distribution for a Ca2 + response to occur. Hence, the cells that responded may have all these factors balanced. Further studies are definitely needed to better understand why certain cells do not elicit a Ca2 + response to PACAP stimulation.
Previous studies have shown that differential expression of the four PACAP receptor splice variants (SV) by various cell types results in differential coupling to adenylyl cyclase and/or phospholipase C [32,38]. SV-2 has been shown to be coupled to both effectors, whereas SV-1 is predominantly coupled to adenylyl cyclase [38]. Our failure to measure an IP response thus far (unpublished observation) indicates that the [Ca2 +]i response seen in HCT8 cells may not be phospholipase C-mediated, which would support the hypothesis that SV-1 PAC1 receptor are mainly expressed in these cells. Although our study was not aimed specifically at identifying which splice variants were expressed in HCT8 human colonic tumors, to have identified the signaling profile in HCT8 would be significant in providing insights into what role PACAP plays as a cancer growth modulator.
It would be of particular interest to investigate whether PACAP stimulation in HCT8 cells can induce phosphorylation of mitogen activated protein kinase (MAPK) through a cAMP-mediated process. It fact, it has been shown in several cell lines, including cerebellar granular and lung cancer cells, that PACAP has the ability to induce phosphorylation of MAPK through a cAMP-dependent pathway [17,42]. Therefore, investigating the ability of PACAP to activate MAPK in colon cancer cells will clarify the mechanism utilized by PACAP to promote the growth of colonic tumors. Thus, further characterization of the signal transduction of the PACAP receptor in these tumors is required.
We also explored PACAP-induced effects on the growth of HCT8 colonic tumor cells. Our proliferation data suggest that PACAP-38 was capable of inducing a 25% increase in the number of viable cells, compared to unstimulated cells, after 24 h of culture in media containing no serum. On the other hand, VIP hormone failed show a positive effect on HCT8 cells proliferation, and, instead, caused a slight decrease in the cell number. These findings, in addition to the localization of endogenous PACAP in this cell line, suggest that PACAP may promote the growth and survival of human colonic tumors in vivo, possibly acting through an autocrine mechanism. Future studies using PACAP-(6–38) partial antagonist in biological assays would confirm the role of the PACAP hormone in colonic tumors.
Lastly, Fas-R and its ligand, Fas-L, are important regulatory components in the homeostasis of the T-cell compartment and in the immune-defense mechanism against transformed cells [43–46]. Fas-R is widely expressed by many cell types, in which it mediates apoptotic cell death of various Fas-bearing cells, upon exposure to Fas-L [26]. Fas-L is expressed mostly by activated immune cells, including cytotoxic T cells and natural killer cells, and serves also as a mechanism used by lymphocytes in specific killing of tumor cells. Because many colon cancer cell lines have been shown to express Fas-R [23,47], its down-regulation or dysfunction has been indicated as a potential mechanism to evade anti-tumoral responses [24–26]. Therefore, we determined the expression of Fas-R on HCT8 colonic tumor cell line, and investigated PACAP’s effect on Fas-R modulation. Our data indicate that HCT8 cells express under baseline conditions Fas-R, which is down-regulated upon 24 h treatment with PACAP-38. These data support our hypothesis that PACAP is an important mediator of colon cancer cell survival and growth. These findings are consistent with other studies in which the down-regulation of Fas-R has been correlated with the growth and malignancy of cancer cells [22–25]. Hence, this may have important clinical implications in the treatment of colon cancer and needs to be investigated further.
Further support of this idea comes from our detection of intracellular PACAP hormone in HCT8 tumor cells. PACAP, released from colonic cancer cells, through an autocrine pathway, could stimulate its specific receptors on the same cells, and recruit phospholipase C, protein kinase C, and MAPK [7], causing cell proliferation as well as Fas-R down-regulation. This mechanism would not only enhance activation of immediate-early genes, c-fos, c-myc and c-jun, but also inhibit the apoptotic pathways mediated by caspases [7,49]. This is supported by our recent observations that PAC1 and PACAP are expressed in normal rat and human T lymphocytes, as well as Jurkat lymphoblast-toid cells, in which PACAP activation appears to down-regulate Fas-L [48]. This would suggests that PACAP, released by colon cancer cells, and also lymphocytes, could decrease the T-cell ability of killing colon cancer cells through a Fas-mediated mechanism, thereby enhancing the colonic cancer cell’s probability of surviving and growing in vivo. Although this hypothesis is only speculative, further studies using an in vitro system, wherein colonic cancer cells will be co-cultured with activated T lymphocytes in the presence of PACAP hormone, will test this idea.
The hypothesis that PACAP has a protective anti-apop-totic role through activation of cAMP-dependent signaling pathways has been observed in cerebellar granule cells [17]. In this study, PACAP-38 was shown to increase the survival of cerebellar granule cells by specifically decreasing the extent of apoptosis through activation of MAP kinase, via a cAMP-dependent pathway [17]. Therefore, our data reasonably suggest that PACAP may also have a role in protecting colonic tumors from Fas-R-mediated apoptosis.
From these current and previous findings, we propose a potential role for PACAP in the maintenance of human colonic tumors. PACAP would function through an autocrine mechanism to activate its specific receptors on the cancer cell membrane, transducing a signal that would lead to cell growth and survival. Moreover, PACAP would modulate tumor infiltrating T lymphocyte responses by inhibiting Fas-mediated apoptosis, through simultaneous down-regulation of Fas-R on colon cancer cell membranes, and Fas-L on the activated T-cell surface. Finally, it needs to be explored in future studies Fas-L expression and modulation by PACAP in colonic tumors. Fas-L molecules have been found to be expressed in other colonic tumor cell lines [47], and their up-regulation has been associated with malignant progression [24]. Therefore, studying PACAP effects on Fas-L expression in colonic tumors, will be important to further understand how cancer cells can escape immune responses.
In summary, our data indicate that the HCT8 tumor cell line is an ideal model for investigating native PAC1-induced signal transduction upon PACAP activation. The present study demonstrates that HCT8 tumor cell line expresses biologically active PAC1 receptors and produces PACAP, as shown by immunofluorescence, using confocal microscopy and FACS analysis. HCT8 cells show a signal transduction pathway coupled to adenylyl cyclase, and increases intracellular [Ca2 +]i, which may lead to phosphorylation of other downstream signaling molecules, resulting in the down-regulation of mediators of apoptosis and thereby increasing of cell growth. These data may be extrapolated to other tumor cell systems and prompt further studies. Our findings indicate that PACAP may function as a human colonic tumor growth inducer and T lymphocyte responses modulator. This might have important clinical implications for the development of new strategies against cancer cells.
Acknowledgements
This work was supported by a VA Career Development Award and VA Merit Review grant (J.R. Pisegna), and a NATO Collaborative Research Grant, CRG 970129 (C. McArdle and J.R. Pisegna).
References
- [1].Miyata A, Arimura A, Dahl RR, Uehara A, Jiang L, Culler MD, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989;164:567–74. [DOI] [PubMed] [Google Scholar]
- [2].Miyata A, Jiang L, Dahl RR, Kitada C, Kubo K, Fujino M, et al. Isolation of a neropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP-38). Biochem Biophys Res Commun 1990;170:643–8. [DOI] [PubMed] [Google Scholar]
- [3].Said SI, Mutt V. Polypeptide with broad biological activity: isolation from the small intestine. Science 1970;69:1217–8. [DOI] [PubMed] [Google Scholar]
- [4].Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International Union of Pharmacology: XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 1998;50(2):265–70. [PMC free article] [PubMed] [Google Scholar]
- [5].Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 2000;52(2):269–324. [PubMed] [Google Scholar]
- [6].Cauvin A, Buscail L, Gourlet P, De Neef P, Gossen D, Arimura A, et al. The novel VIP-like hypothalamic polypeptide PACAP interacts with high affinity receptors in the human neuroblastoma cell line NB-OK. Peptides 1990;11(4):773–7. [DOI] [PubMed] [Google Scholar]
- [7].Zia F, Fagarasan M, Bitar K, Coy DH, Pisegna JR, Wank SA, et al. Pituitary adenylate cyclase activating peptide receptors regulate the growth of non-small cell lung cancer cells. Cancer Res 1995;55(21): 4886–91. [PMC free article] [PubMed] [Google Scholar]
- [8].Moody TW, Zia F, Makheja A. Pituitary adenylate cyclase activating polypeptide receptors are present on small cell lung cancer cells. Peptides 1993;14(2):241–6. [DOI] [PubMed] [Google Scholar]
- [9].Buscail L, Cambillau C, Seva C, Scemama JL, De Neef P, Robberecht P, et al. Stimulation of rat pancreatic tumoral AR4–2J cell proliferation by pituitary adenylate cyclase-activating peptide. Gastroenterology 1992;103(3):1002–8. [DOI] [PubMed] [Google Scholar]
- [10].Leyton J, Gozes Y, Pisegna JR, Coy D, Purdom S, Casibang M, et al. PACAP (6–38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat 1999;56:177–86. [DOI] [PubMed] [Google Scholar]
- [11].Zeng N, Kang T, Wong H, Walsh JH, Sachs GA, Pisegna JR. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 1999;104:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pisegna JR, Leyton J, Coelho T, Hida T, Jakolew S, Birrer S, et al. Differential activation of immediate-early gene expression by four splice variants of the human pituitary activating polypeptide receptor: evidence for activation by PACAP hybrid and the phospholipase C inhibitor U73122. Life Sci 1997;61(6):631–9.9250719 [Google Scholar]
- [13].Lu N, Zhou R, DiCicco-Bloom E. Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J Neurosci Res 1998;53:651–62. [DOI] [PubMed] [Google Scholar]
- [14].Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci U S A 1997;94:3357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E. PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci 2001;4(2):123–4. [DOI] [PubMed] [Google Scholar]
- [16].Gonzalez BJ, Basille M, Mei YA, Vaudry D, Fournier A, Cazin L, et al. Ontogeny of PACAP and PACAP receptors in the rat brain: role of PACAP in the cerebellum during development. Ann NY Acad Sci 1996;805:302–14. [DOI] [PubMed] [Google Scholar]
- [17].Journot L, Villalba M, Boachaert J. PACAP-38 protects cerebellar granule cells from apoptosis. Ann NY Acad Sci 1998;865:100–10. [DOI] [PubMed] [Google Scholar]
- [18].Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H. Neuro-trophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc Natl Acad Sci 1999;96:9415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 1999;104(10):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lauffer JM, Modlin IM, Hinoue T, Kidd M, Zhang T, Schmid SW, et al. Pituitary adenylate cyclase-activating polypeptide modulates gastric enterochromaffin-like cell proliferation in rats. Gastroenterology 1999; 116(3):623–35. [DOI] [PubMed] [Google Scholar]
- [21].Pisegna JR, Ohning GV, Athmann C, Zeng N, Walsh JH, Sachs G. Role of PACAP1 receptor in regulation of ECL cells and gastric acid secretion by pituitary adenylate cyclase activating peptide. Ann NY Acad Sci 2000;921:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miampamba M, Germano PM, Arli S, Wong HH, Scott D, Tache Y, et al. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type I receptor in the rat gastric and colonic myenteric neurons. Regul Pept 2002. (May 30);105(3):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moller P, Koretz K, Leithauser F, Bruderlein S, Henne C, Quentmeier A, et al. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 1994. (May 1);57(3):371–7. [DOI] [PubMed] [Google Scholar]
- [24].Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat Med 1996. (December);2(12):1361–6. [DOI] [PubMed] [Google Scholar]
- [25].French LE, Tschoop J. Defective death receptor signaling as a cause of tumor immune escape. Semin Cancer Biol 2002;12(1):51–5. [DOI] [PubMed] [Google Scholar]
- [26].Nagata S, Golstein P. The Fas death factor. Science 1995; 267(5203): 1449–56. [DOI] [PubMed] [Google Scholar]
- [27].Cleveland JL, Ihle JN. Contenders in FasL/TNF death signaling. Cell 1995;81(4):479–82. [DOI] [PubMed] [Google Scholar]
- [28].Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1994. (August);1(5):365–71. [DOI] [PubMed] [Google Scholar]
- [29].Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, School-ey KA, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 1995;181(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995; 373(6513):441–4. [DOI] [PubMed] [Google Scholar]
- [31].Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Auto-crine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995. (February 2);373(6513):438–41. [DOI] [PubMed] [Google Scholar]
- [32].Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem 1996; 271: 17267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Germano PM, Stalter J, Le SV, Wu M, Yamaguchi DJ, Scott D, et al. Characterization of the pharmacology, signal transduction and internalization of the fluorescent PACAP ligand, fluor-PACAP, on NIH/ 3T3 cells expressing PAC1. Peptides 2001;22(6):861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem 1974;58:541–8. [DOI] [PubMed] [Google Scholar]
- [35].McArdle CA, Schomerus E, Groener I, Poch A. Estradiol regulates gonadotropin-releasing hormone receptor number, growth and inositol phosphate production in T3–1, gonadotrope-derived cell line. Mol Cell Neurosci 1992;3:124–32. [DOI] [PubMed] [Google Scholar]
- [36].Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2 + indicator with greatly improved fluorescence properties. J Biol Chem 1995;260:3440–50. [PubMed] [Google Scholar]
- [37].Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase activating polypeptide (PACAP): characterization and molecular identification. Endocrinology 1990;127:272–7. [DOI] [PubMed] [Google Scholar]
- [38].Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature (Lond) 1993;365:170–5. [DOI] [PubMed] [Google Scholar]
- [39].Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type 1 receptor. Proc Natl Acad SciUSA 1993;90:6345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Svoboda M, Tastenoy M, Ciccarelli E, Stievenart M, Christopher J. Cloning of a splice variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor. Biochem Biophys Res Commun 1993;195:881–8. [DOI] [PubMed] [Google Scholar]
- [41].Lyu R, Germano PM, Choi JK, Le SV, Pisegna JR. Identification of an essential amino acid motif within the C-terminus of the pituitary adenylate cyclase-activating polypeptide type 1 receptor that is critical for signal truncation but not for receptor internalization. J Biol Chem 2000;275(46):36134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moody TW, Leyton J, Casibang M, Pisegna JR, Jensen RT. PACAP-27 tyrosine phosphorylates mitogen activated protein kinase and increases VEGF mRNAs in human lung cancer cells. Regul Pept 2002. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991;66(2):233–43. [DOI] [PubMed] [Google Scholar]
- [44].Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993;75(6):1169–78. [DOI] [PubMed] [Google Scholar]
- [45].Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today 1995;16(12): 569–74. [DOI] [PubMed] [Google Scholar]
- [46].Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today 1995;16(1):39–43. [DOI] [PubMed] [Google Scholar]
- [47].O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996;184:1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Germano P, Wu M, Yamaguchi DJ, Tachiki K, Pisegna JR. PACAP hormone effects on PAC1-posititve Jurkat cells. Regul Pept 2001; 102:55 [Abstract]. [Google Scholar]
- [49].Wallach D, Varfolomeev EE, Malinin NL, Goltsev TV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 1999;17:331–67. [DOI] [PubMed] [Google Scholar]


