Functional, pharmacological, and recent cloning studies have now established that two classes of receptors exist for cholecystokinin (CCK)/gastrin-related peptides (Table 1). One class, the CCKA receptor, has a high affinity only for the naturally occurring CCK/gastrin-related peptides that are sulfated in the seventh position from the COOH-terminus which includes all forms of CCK (CCK-58, CCK-39, CCK-33, and CCK-8), caerulein, and the invertebrate peptide cionin.1–9 In contrast, the CCKB receptor has high affinity for both CCK peptides and gastrin with sulfation in either the sixth position (cionin, gastrin-17-II) or the seventh position from the COOH-terminus (CCK peptides, caerulein, and cionin), increasing potency less than 10-fold in most studies (Table 1).2,5,8–15 Both selective agonists and antagonists were described for each of these receptors (Table 1).2,3,16–30
TABLE 1.
Characteristics of Classes of CCK Receptors
| Characteristic | CCKA Receptor | CCKB Receptor |
|---|---|---|
| Structure (human) | 428 amino acids (4) | 447 amino acids (13, 14) |
| Natural agonists | CCK-8, cionin, caerulein > > > gastrin ≈ CCK-4 | CCK-8, gastrin, cionin > CCK-4 |
| Location | CNS (limited), islets, pancreatic acini, gallbladder, neurons (GI tract), gastric mucosal cells (D-cells, chief), AR42J cells | CNS (widespread), GI smooth muscle, gastric mucosal (parietal, D-cell, chief), pancreatic acini, AR42J cells, SCLC cells |
| Selective agonists | A-71378 [0.4 nM] (19) | A-72962 [0.2 nM] (19) SNF-8702 [0.2 nM] (29) Gastrin [1 nM] (8, 10) BC-264 [0.2 nM] (18) |
| Selective antagonists | L-364,718 (devazepide) [1 nM] (22, 25, 28) Tetronothiodin [4 nM] (26) PD-140548 [10 nM] (2, 21,27) Lorglumide (CR-1409) [150 nM] (15, 20,24) SR-27897 [1 nM] (17) |
CI-988 (PD-134,308) [10 nM] (2, 21, 27) L-365,260 [2 nM] (22, 23, 30) LY262691 [30 nM] (2) |
| Transduction | IP3/Ca2+ (4, 59, 60, 65) | IP3/Ca2+ (12, 36, 52–54) |
Functional and binding studies as well as studies of the distribution of mRNA for these receptors show that they are widely distributed in the CNS and peripheral tissues (Table 1).3,31 In an increasing number of tissues such as guinea pig and dog pancreatic acinar cells, guinea pig chief cells, somatostatin-releasing cells of the stomach, gastrointestinal smooth muscle from stomach and gallbladder, in the CNS, and on the pancreatic acinar tumor cell line AR42J, both CCKA and CCKB receptors exist on the same cells.15,31–38 In other tissues such as the gastric mucosa, tissue containing both CCK/gastrin receptor subtypes exists in close proximity.39,40 Therefore, in assessing changes in biological responses frequently even in single cells, but particularly in assessing in vivo responses to CCK, it is becoming increasingly important to differentiate which effect is mediated by which receptor subtype, because CCK interacts with high affinity with both CCK receptor subtype.8,10,15 Furthermore, activation of CCKA and CCKB receptors may lead to similar responses in some tissues such as somatostatin release from isolated fundi D cells35 or increases in inositol phosphates and [Ca2+]i in AR42J cells,34 whereas in other tissues such as effects on gastric acid secretion,40 their activation in the gastric mucosa may have opposing effects. It is therefore becoming increasingly important to differentiate the ability of CCK to activate either subtype.
To differentiate these receptors in in vivo studies in humans, it is important to establish which of the various proposed CCK/gastrin receptor agonists and antagonists are selective for CCKA and CCKB receptors in humans, which agonists have full efficacy, as well as which proposed antagonists have no agonist activity. Recent studies demonstrate considerable differences for a number of these proposed agonists and antagonists in affinity in different species as well as efficacy.41–44 For example, L-365,260 has shown a high affinity and selectivity for CCKB over CCKA receptors in rat and human, but not in the dog.1,4,11,12,42 Similarly, a number of peptides function as agonists in one species and antagonists in another.43,44 It is therefore important to establish which of these various proposed antagonists function as pure antagonists in various species and in humans. In the present study we report the usefulness of some of the selective CCK receptor agonists and antagonists in establishing if both CCKA and CCKB receptors are functional in the same cell, the guinea pig chief cell.36 Also, because of the importance of establishing the potencies and efficacies of the various putative CCK receptor agonists/antagonists for human CCK receptors, we report the results of preliminary studies investigating their abilities to interact with and activate human CCKA and CCKB receptors transfected into COS-7 cells.
STUDIES ON DISPERSED GUINEA PIG CHIEF CELLS: BINDING AND INITIAL FUNCTIONAL STUDIES
As just discussed, frequently both subtypes of CCK receptors exist on the same cel115,31,32,34–38 (Table 1). In different tissues, occupation and activation of both subtypes have different effects. Both receptors can cause either similar changes in some cells such as contraction caused by CCKA and CCKB receptors on isolated gastrointestinal smooth muscle cells33 or stimulation of somatostatin release from fundic D cells.35 In other cells, occupation of either receptor has different functions such as CCKA and CCKB receptors on guinea pig pancreatic acini where only occupation of the CCKA receptor stimulates secretion and activation of phospholipase C.10 In other cells, such as rat pituitary, activation of CCKA receptors in anterior pituitary corticotrophs45 stimulates β-endorphin release, whereas activation of CCKB receptors inhibits CCK-stimulated β-endorphin release.
The role of CCKA and CCKB receptors in mediating pepsinogen release by CCK/gastrin-related peptides is a particularly good example of the difficulty in resolving the role of activation of each receptor in altering cell function. CCK and gastrin have both been shown to stimulate pepsinogen release from isolated gastric chief cells and gland.37,46–50 CCK-related peptides also have been shown to activate phospholipase C, increase cytosolic calcium, and stimulate the breakdown of phosphoinositides.36,50–54 However, in numerous cell systems, CCK and gastrin both can interact with CCKA and CCKB receptors if sufficiently high concentrations are used.8,10,15 Previous binding studies on chief cells have given variable results, with some studies suggesting a single class of receptor38,48 and another study55 suggesting heterogeneity of CCK/gastrin binding sites. Some functional studies have provided evidence for one subtype46 and others for two subtypes.37,56 It is also unclear if both subtypes are present, if they alter cell function by a similar transduction mechanism, or if the intracellular mechanisms have similar relationships.
Detailed binding studies using either 125I-BH-CCK-8 or 125I-gastrin with various selective agonists (Fig. 1)or selective antagonists (Fig. 2) failed to provide evidence for both subtypes of CCK receptors. For agonists, for both ligands the relative potencies were CCK-8 = 3 × gastrin-17-I = 30 × desulfated CCK-8 = 60 × CCK-4 (Fig. 1), which is typical for interaction with a CCKB receptor (Table 1).Similarly, with the selective antagonists (Fig. 2), for each ligand potencies were L-365,260 (IC50 = 2 nM) = 30 × L-364,718 = 300 × CBZ-CCK-27–32-NH2 = 1,000 × CR-1409 = 1,000,000 × proglumide, Bt2 cGMP. These data are similar to those reported for CCKB receptors in other cell preparation3,10,57 (Table 1). Therefore, binding studies give no evidence for CCKA receptors; however, this does not disprove their existence because they might be present in such small numbers that they are not being detected in the binding studies.
FIGURE 1.
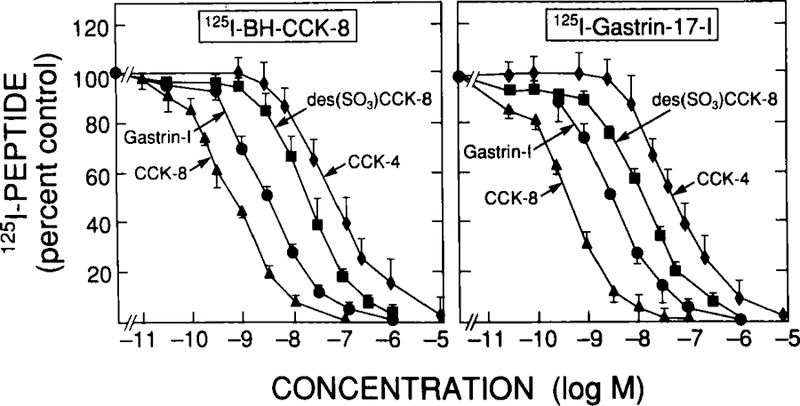
Ability of various CCK and gastrin-related peptides to inhibit binding of 125I-BH-CCK-8 or 125I-gastrin-17-I to dispersed chief cells from guinea pig pancreas. Dispersed chief cells were incubated with 50 pM 125I-BH-CCK-8 or 125I-gastrin-17-I for 30 minutes at 37°C. Results are expressed as the percentage of saturable binding in the presence of no unlabeled peptide added. Results are from three experiments, and in each experiment each point was determined in duplicate.
FIGURE 2.
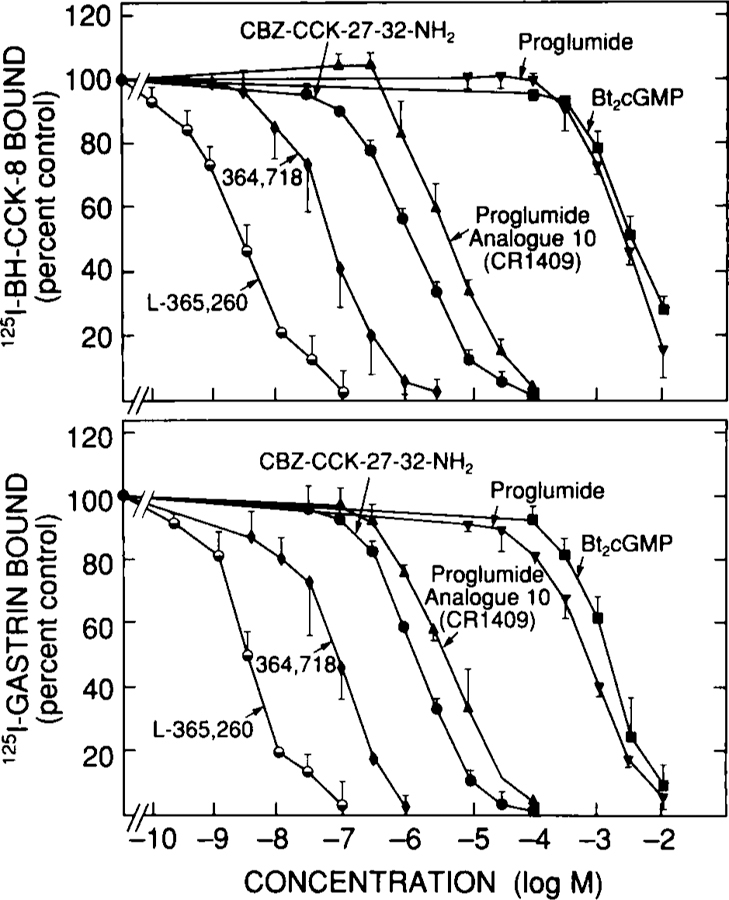
Ability of various CCKA and CCKB receptor antagonists to inhibit binding of 125I-BH-CCK-8 or 125I-gastrin-17-I to dispersed chief cells from guinea pig pancreas. Dispersed chief cells were incubated with 50 pM 125I-BH-CCK-8 or 125I-gastrin-17-I for 30 minutes at 37°C. Results are expressed as the percentage of saturable binding in the presence of no unlabeled peptide added. Results are from three experiments, and in each experiment each point was determined in duplicate.
By contrast to the binding studies, functional studies measuring pepsinogen release suggest that both CCKA and CCKB receptors might be involved in mediating pepsinogen release by CCK/gastrin peptides in guinea pig chief cells.37 CCK-8 caused detectable pepsinogen release at 0.01 nM, half-maximal release at 0.3 nM, and maximal release at 10 nM (Fig. 3). By contrast, gastrin-17-I was 180-fold less potent, desulfated CCK-8 was 900-fold less potent, and CCK-4 4,000-fold less potent. Furthermore, similar to findings in another study,37 even maximally effective concentrations of CCK-4 or gastrin-17-I were less efficacious than CCK-8 or desulfated CCK-8. These data suggest that a CCKA receptor is mediating some of the pepsinogen secretion seen with these peptides because of the marked effect of the presence of a sulfate moiety in CCK on potency. Similar to pepsinogen release, both CCK-8 and gastrin-17-I stimulated changes in [Ca2+]i and inositol phosphates (Fig. 4), with gastrin being less efficacious than CCK-8. In other cell systems, CCKA and CCKB receptors have both been coupled to activation of phospholipase C, resulting in stimulation of increases in [Ca2+]i and inositol phosphates.53,58–60 However, the fact that gastrin-17-I is less efficacious suggests that it is either a partial agonist at the CCKA receptor or is stimulating changes in cellular function interacting with a CCKB receptor. To explore this possibility further, increasing concentrations of gastrin-17-I were combined with a maximally effective concentration of CCK-8 (Fig. 5). If gastrin-17-I was a partial agonist at the CCKA receptor, it should inhibit the action of CCK-8 at this receptor with high concentrations, as shown in the figure by the dotted line which shows the predicted curve. The maximal effect of CCK-8 was not altered by increasing concentrations of gastrin-17-I, suggesting that CCK-8 and gastrin-17-I are stimulating pepsinogen release through distinct receptors, activation of which results in different efficacies (Fig. 5).
FIGURE 3.
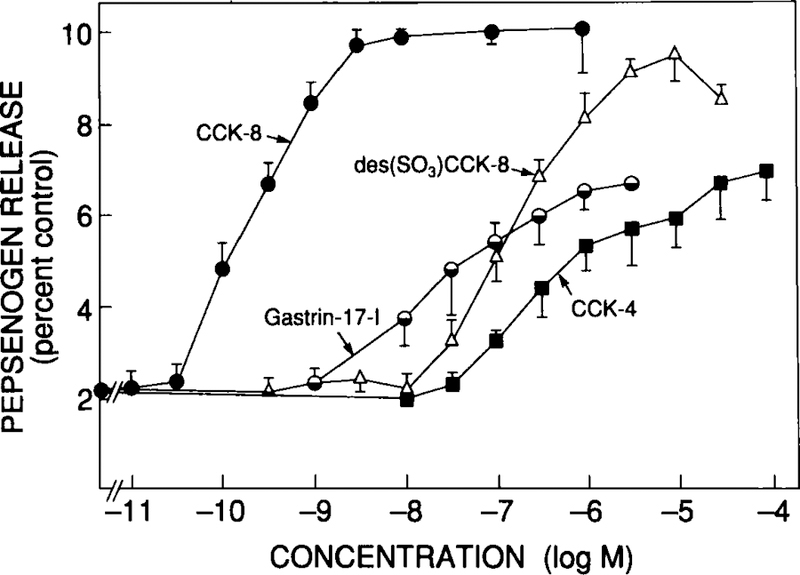
Ability of CCK and gastrin-related peptides to stimulate pepsinogen release from dispersed chief cells from guinea pig stomach. Pepsinogen release was measured after a 30-minute incubation at 37°C. Results are expressed as the percentage of cellular pepsinogen release during incubation. Data are modified from references 36 and 37.
FIGURE 4.
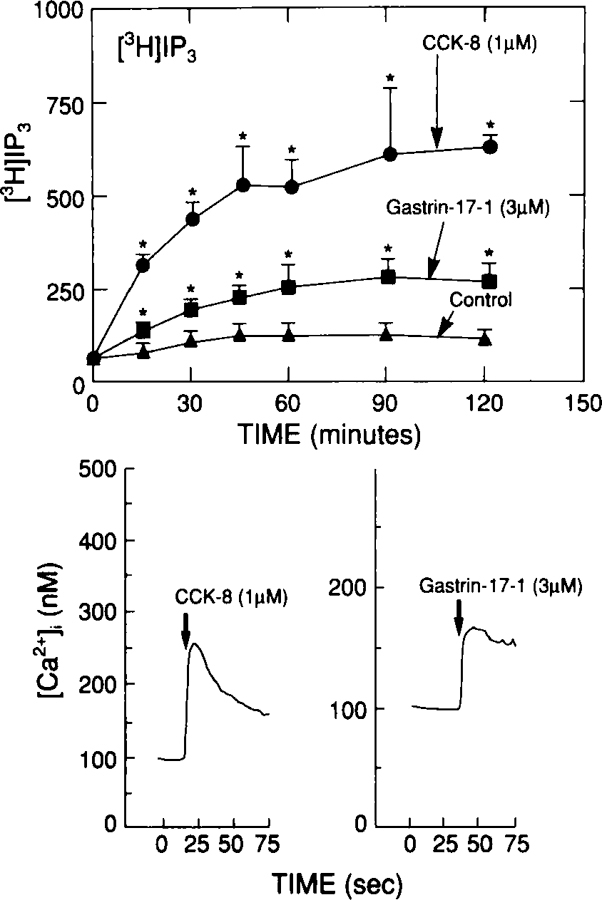
Ability of CCK and gastrin to increase [3H]IP3 (top panel) and alter cytosolic calcium [Ca2+]i levels (bottom panel) in dispersed chief cells from guinea pig stomach. Top panel shows the time course of changes in [3H]IP3 after loading dispersed chief cells with myo-[2-3H] - inositol. Data are modified from ref. 36. The bottom panel shows the change in [Ca2+]i after loading chief cells with 1 μM fura-2/AM for 30 minutes at 37°C.
FIGURE 5.
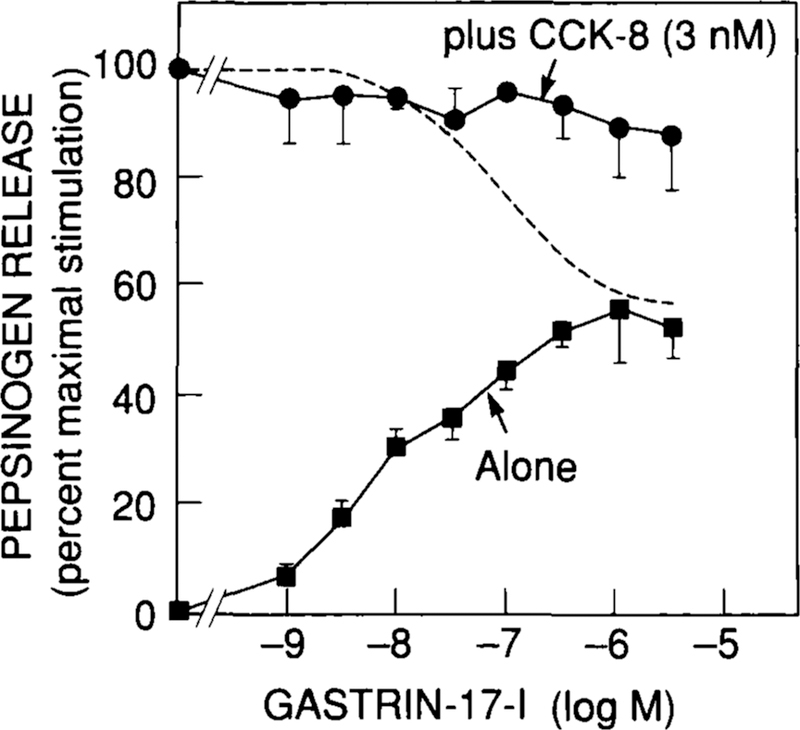
Lack of effect of gastrin-17-I on CCK-8-stimulated pepsinogen release from dispersed gastric chief cells. Pepsinogen secretion is expressed as the percentage of stimulation caused by 3 nM CCK-8 alone. Dashed line refers to hypothetical values assuming gastrin-17-I interacts with the same receptors as CCK-8 calculated using the R = [(R1C/K1) + (R2B/K2)]/[C/K1 + (B/K2) + 1], where R is the calculated response, R1 is the maximal stimulation by CCK-8 alone, R2 is the maximal stimulation by gastrin-17-I alone, C is the concentration of CCK-8, B is the concentration of gastrin-17-I. K1 is the concentration of CCK-8 that causes half-maximal stimulation of pepsinogen release, and K2 is the concentration of gastrin-17-I that causes half-maximal stimulation of pepsinogen release. Data are modified from reference 37.
In summary, the binding studies provided evidence for only a CCKB receptor subtype despite the use of highly selective CCKA and CCKB antagonists and selective agonists such as gastrin-17-I. In contrast, the functional studies suggested the presence of a functional CCKA receptor and perhaps a CCKB receptor, but the data were inconclusive. To resolve this more clearly, studies were done using the highly selective CCKA receptor agonist A-7137819 and the CCKA specific antagonist L-364,718 (Table 1).
STUDIES ON DISPERSED CHIEF CELLS: USING SELECTIVE CCKA RECEPTOR AGONIST AND ANTAGONISTS
A-71378 was equally as potent as CCK-8 in stimulating increases in [3H]IP, [Ca2+]i,or pepsinogen release; however, A-71378 was 15–20% less efficacious than CCK-836 (Figs. 6 and 7; Table 2). Furthermore, with higher concentrations of gastrin-17-I, the dose-response curves for changes in [3H]IP were clearly biphasic (Fig. 6). The data suggested that both CCK-8 and gastrin-17-I might be interacting with two different classes of receptors to stimulate changes in cellular function with activation of CCKA receptors, resulting in a significantly more efficacious response than that with activation of CCKB receptors.
FIGURE 6.
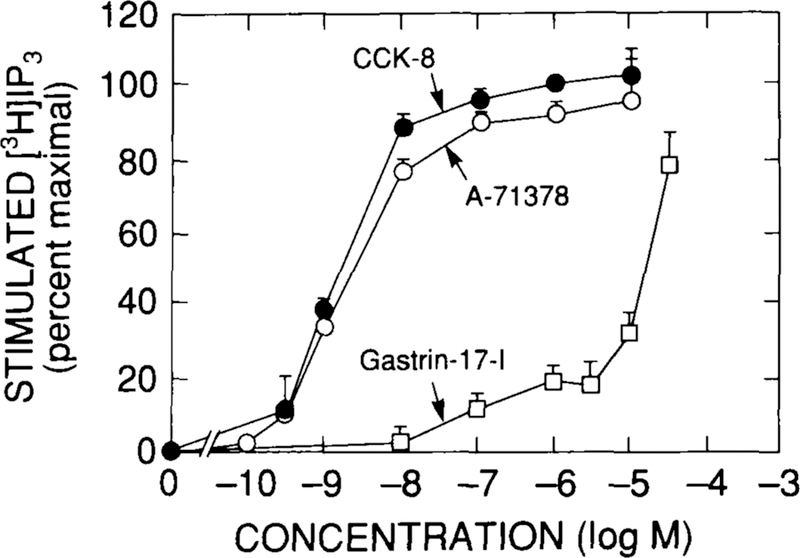
Ability of CCK-8, the selective CCKA receptor agonist A-71378, and the selective CCKB receptor agonist gastrin-17-I to stimulate [3H]inositol phosphate accumulation in dispersed gastric chief cells. Accumulation of [3H]IP3 is expressed as percentage of stimulation caused by a maximally effective concentration of CCK-8 (i.e., 1 μM).Data are modified from reference 36.
FIGURE 7.
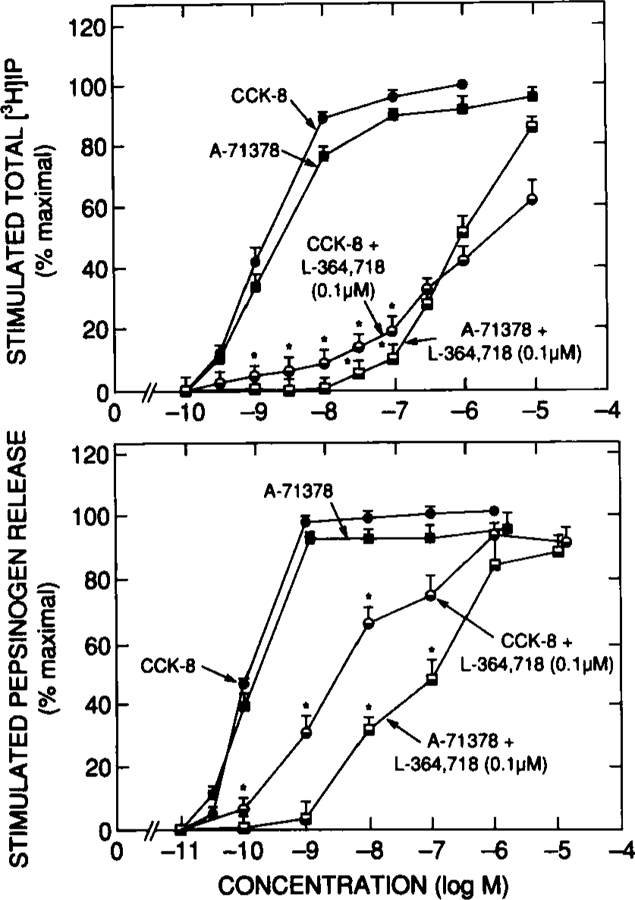
Effect of L-364,718 on CCK-8 or A-71378 stimulated increases in [3H]IP (top) or pepsinogen release (bottom). Chief cells were incubated with the indicated concentration of CCK-8 or A-71378 with 0.1 μM L-364,718 present or absent. Accumulation of [3H]IP is expressed as the percentage of stimulation caused by a maximally effective concentration of CCK-8 (i.e., 1 µM). Significant increase over incubation containing no additives. Data are modified from reference 36.
TABLE 2.
Ability of Various CCK/Gastrin-Related Peptides to Stimulate Pepsinogen Release, Increase [Ca2+]i,and Stimulate Accumulation of [3H]IP3 in Dispersed Chief Cells from Guinea Pig Stomach
| Agent | EC50 (nM) |
||
|---|---|---|---|
| Pepsinogen Release | Increase in [Ca2+]i | Increase in [3H]IP3 | |
| A-71378 | 0.2 ± 0.1 | 1.4 ± 0.5 | 2.0 ± 0.1 |
| CCK-8 | 0.3 ± 0.1 | 1.8 ± 0.3 | 1.7 ± 0.3 |
| des(SO3)CCK-8 | 280 ± 65 | 1,300 ± 120 | 420 ± 70 |
| Gastrin-17-I | 55 ± 20 | 3,600 ± 70 | > 5,000 |
| Gastrin-17-I + L-364,718 (0.1 μM) | 12 ± 5 | 15 ± 9 | 40 ± 23 |
Note: Data are mean ± 1 SEM from at least four separate experiments. Data are modified from reference 36.
To explore this possibility further, the effect of a selective CCKA (A-71378) or CCKB (gastrin-17-I) agonist on cell function in the presence of the CCKA receptor antagonist L-364,718 was determined36(Figs. 7 and 8; Table 2). This concentration of L-364,718 was used because in previous studies it caused 90% inhibition of binding to CCKA receptors with no effect on interaction with CCKB receptors.10,15 L-364,718 (0.1 μM) completely inhibited the ability of A-71378 up to concentrations of 10 nM for increases in [3H]IP or 1 nM A-71378 for pepsinogen release (Fig. 7), A-71378 concentrations that caused maximal stimulation when L-364,718 was not present (Fig. 7). In contrast, CCK-8 continued to cause 10–20% maximal stimulation over this concentration range (Fig. 7), demonstrating that this proportion of its stimulation was due to occupation of CCKB receptors by CCK-8. Similarly, with gastrin-17-I stimulated increases in [3H]IP or pepsinogen release (Fig. 8), all stimulation caused by gastrin-17-I concentrations < 1 μM was unaffected by 0.1 pM L-364,718, whereas further stimulation by gastrin-17-I concentrations > 1 μM was inhibited to the extent seen with a 1 WMgastrin-17-I concentration (Fig. 8). These data demonstrate that gastrin-17–1causes stimulation through the CCKB receptor at low concentrations and through the CCKA receptor at high concentrations. Approximately 15% of the maximal stimulation of pepsinogen release by CCK-8 at low concentrations is due to occupation of CCKB receptors and the remaining 85% to occupation of CCKA receptors. These results are in close agreement with results of another recent study56 using primarily guinea pig gastric glands and a series of selective CCKA and CCKB receptor agonists and the CCKA selective antagonist L-364,718 and the CCKB selective antagonist (21–988. Similar to the present study, a close correlation was found between the ability of various agonists to stimulate pepsinogen release and cause PI hydrolysis 56. In this gastric gland preparation, 30–40% of the gastrin-stimulated increase in pepsinogen release was not inhibited by L-364,718, but was inhibited by the CCKB antagonist CI-988.56 This study56 concluded that 60–70% of the stimulation caused by CCK-8 was due to occupation of CCKA receptors and 30–40% to occupation of CCKB receptors.
FIGURE 8.
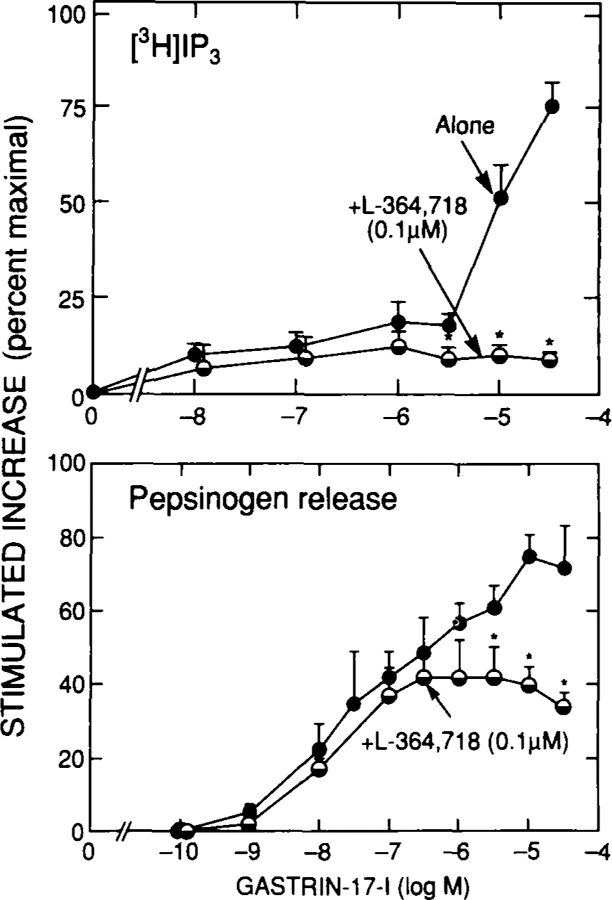
Effect of L-364,718 on the ability of various concentrations of gastrin-17-I to stimulation and accumulation of [3H]IP3 (top panel) or pepsinogen release (bottom panel) in dispersed chief cells. Chief cells were incubated with the indicated concentration of gastrin-17-I with or without 0.1 μM L-364,718. Accumulation of [3H]IP3 and pepsinogen release are expressed as percentage of stimulation caused by a maximally effective concentration of CCK-8 (i.e., 1 μM).*Significantly different (p < 0.05) from incubation containing no L-364,718. Data are modified from reference 36.
In addition to clearly establishing the ability of occupation of CCKA and CCKB receptors on guinea pig chief cells to cause pepsinogen release, because of the selectivity of the CCKA and CCKB receptor agonists and antagonists, it was possible to compare the stoichiometric relationships between increases in [Ca2+]i,[3H]IP, and pepsinogen release with each receptor.36 Because the ability of A-71378 to increase [3H]IP or [Ca2+]i up to a concentration of 10 nM could be completely inhibited by L-364,718, this represented only stimulation by occupation of the CCKA receptor (Fig. 7). In contrast, because the CCKA-specific antagonist L-364,718 did not inhibit stimulation of the initial component of the biphasic dose-response curve with gastrin-17-I for any changes in cellular function, this represented activation entirely of the CCKB receptor (Fig. 8). CCKA receptor activation resulted in superimposible dose-response curves for changes in [Ca2+]i and [3H]IP3 (Fig. 9, bottom) with half-maximal effects at 1–2 nM, whereas the dose-response curve for pepsinogen release was to the left with a half-maximal effect at 0.2 nM (Fig. 9, bottom). Therefore, maximal stimulation of pepsinogen release was seen with a 30% maximal increase in the intracellular mediators. For gastrin-17–1, the dose-response curves for changes in [3H]IP3, [Ca2+]i, and pepsinogen release were almost superimposible; therefore, maximal changes in pepsinogen release by CCKB receptor activation occurred with maximal stimulation of intracellular mediators (Fig. 9, top). These data demonstrate that amplification of the calcium/IP3 signal differs markedly for activation of CCKA and CCKB receptors in the same cell.36 The results with the CCKB receptor on chief cells are similar to those reported for changes in inositol phosphates and acid secretion in rabbit parietal cells which possess only CCKB receptors61–63 and for the ability of muscarinic cholinergic agents to stimulate glycoprotein release and changes in [Ca2+]i and inositol phosphates in isolated gastric mucous cells.64 The results with activation of the CCKA receptor on chief cells are both similar and different from those for activation of CCKA receptors on pancreatic acini.3,65 Similar to chief cells with activation of CCKA receptors in pancreatic acini, both the dose-response curve for changes in [Ca2+]i and [3H]IP are to the right of the enzyme release curve; however, the [3H]IP curve is further to the right than are the changes in [Ca2+]i, demonstrating that intracellular amplification with activation of this receptor differs in different cell types or could possibly represent species differences.
FIGURE 9.
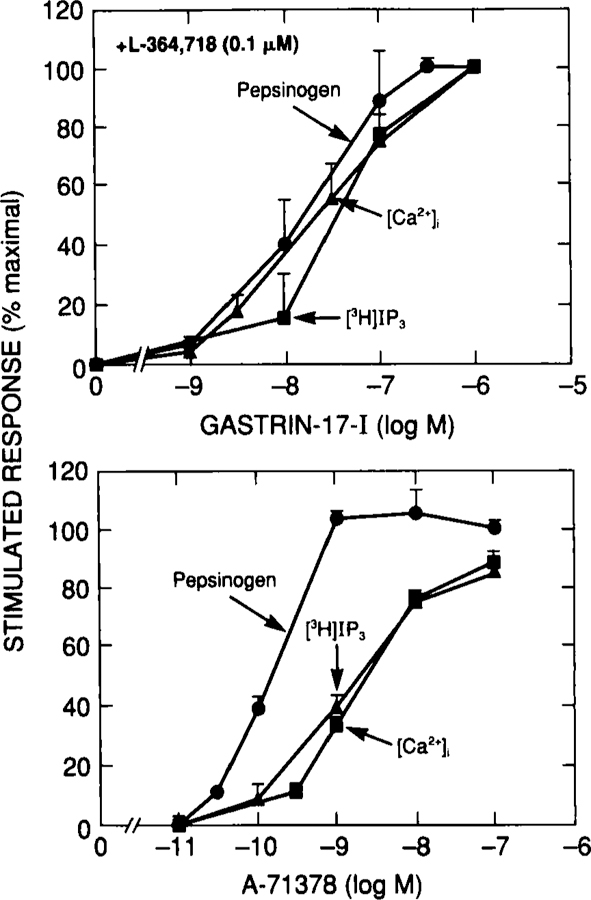
Comparison of the ability to stimulate pepsinogen release changes in [Ca2+]i and accumulation of [3H]IP3 by gastrin-17-I or CCK-8. Stimulation was determined after incubation of chief cells with the indicated concentration of gastrin-17-I or A-71378 with 0.1 μM L-364,718. Data with gastrin-17-I are expressed as percentage of maximal stimulation caused by 1 μM gastrin-17-I, and data with A-71378 are expressed as the percentage of maximal stimulation caused by A-71378. Data are modified from reference 36.
In conclusion, by the use of highly selective agonists and antagonists for CCKA and CCKB receptors, this study demonstrates that it is possible, even when both receptors are present on the same cell, altering similar intracellular mediators and both causing a similar change in cellular function (i.e., pepsinogen release), to determine the consequences of activation of either receptor. Similar results should be obtainable in vivo when both receptor subtypes may also be involved in causing the changes studied, if the agonists and antagonists are sufficiently stable in vivo and can penetrate the areas of interest. However, in human studies as in other species, it will be important to establish which CCK receptor antagonists/agonists are selective and have either no agonist activity for an antagonist or full agonist activity for an agonist. This may be particularly true for CCKA/CCKB receptors, because recent studies demonstrate that some synthetic analogs such as CCK-JMV-180can have almost no agonist activity and function as an antagonist in the guinea pig pancreas, as a partial agonist in rat, and a full agonist in mouse.43,65,66 Similarly, the antagonist L-365,260 has high selectivity for CCKB receptors in some species (rat, guinea pig, and man), but not in the dog.11–14,42 Therefore, to address this issue, we have begun to assess the ability of reported CCKA and CCKB receptor selective agonists and antagonists to interact with and activate human CCKA and CCKB receptors transfected into COS-7 cells.
STUDIES ON TRANSFECTED HUMAN CCKA AND CCKB RECEPTORS
Preliminary data from binding studies to human CCKA and CCKB receptors transfected in COS-7 cells are presented in Figures 10 and 11 and for changes in [3H]IP with human CCKB receptors in Figure 12. To first establish the ability of naturally occurring agonists to interact with human CCKA and human CCKB receptors, their ability to inhibit binding of 125I-BH-CCK-8to each receptor transiently transfected into COS-7 cells was determined and compared with results with the highly selective CCKA agonist A-71378 (Fig. 10; Table 3). 125I-BH-CCK-8 was used as the ligand because it has high affinity for both CCKA and CCKB receptors (Tables 1 and 3), and similar results are obtained with CCKB receptors in animal studies (Figs. 1 and 2) whether 125I-gastrin-17-I or 125I-BH-CCK-8 is used. CCK-8, similar to that previously reported with CCKA and CCKB receptors in guinea pig (Table 3), had a high affinity for both human CCKA and CCKB receptors (IC50 – 2 nM). In contrast, the selective CCKA agonist A-71378 had an equally high affinity for CCK-8 for human CCKA receptors, but a 1,600-fold lower affinity for human CCKB receptors. These data, similar to studies in guinea pig (Table 3), demonstrate that this CCK analog has marked selectivity for human CCKA receptors and thus should be a useful selective CCKA agonist in human studies. In contrast, gastrin-17-I had high affinity for human CCKB receptors (Table 3; IC50 – 6 nM) and had a 260-fold higher affinity for human CCKB than CCKA receptors. These data, similar to previous studies in guinea pig (Table 3) and other species,6 demonstrate that gastrin-17-I is a relatively specific natural ligand for CCKB receptors. For human CCK, receptors the relative order of potency was CCK-8 >> des(SO3)CCK-8 = gastrin-17-I >> CCK-4 with absolute potencies of 1:608:7,138. This relative order is, in general, close to that reported previously in guinea pig pancreas (Table 3). For human CCKB receptors the relative order was CCK-8 > gastrin-17-I > des(SO3)CCK-8 > CCK-4 with absolute potencies of 1:3:51:582. This relative order of potencies for these agonists is, in general, very close to that previously reported for CCKB receptors in guinea pig pancreas and in other species.8,10
FIGURE 10.
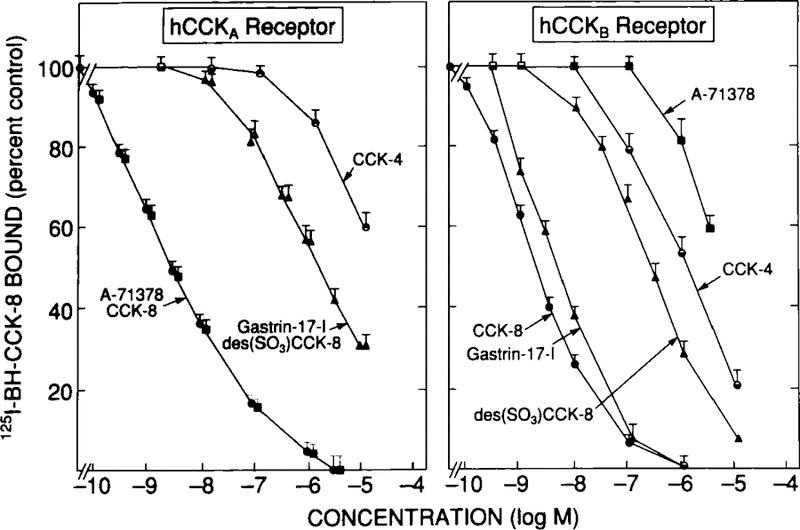
Ability of CCK-8, gastrin-17-I, and related peptides to inhibit binding of 125I-BH-CCK-8 to COS-7 cells transfected with human CCKA or human CCKB receptors. COS-7 cells were transiently transfected with a full-length human CCKA4 or CCKB receptor clone13 using DEAE/Dextran as described previously.4,13 Binding was determined using 50 pM 125I-BH-CCK-8 and was for 30 minutes at 37°C. Results are expressed as the percentage of the saturable binding seen with no unlabeled peptide present. Data are mean ± 1 SEM of four separate experiments.
FIGURE 11.
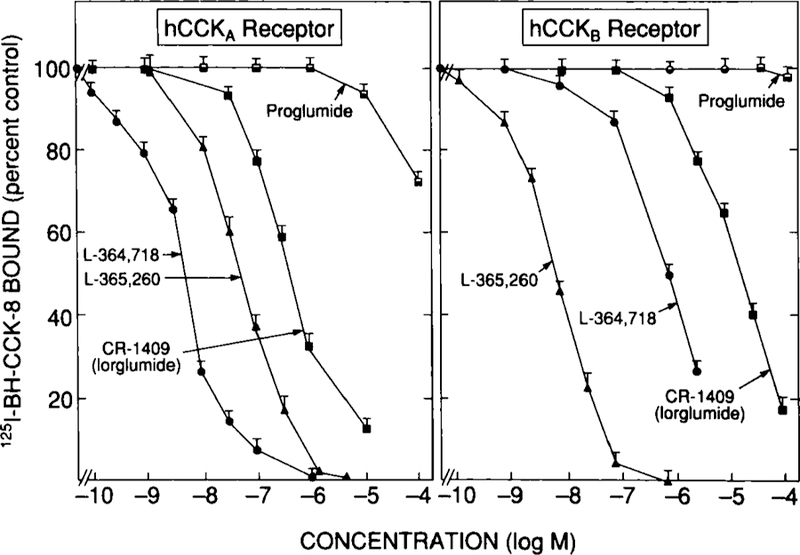
Ability of various CCKA and CCKB receptor antagonists to inhibit binding of 125I-BH-CCK-8 to COS-7 cells transfected with human CCKA or human CCKB receptors. COS-7 cells were transiently transfected with a full-length human CCKA4 or CCKB receptor clone13 using DEAE/Dextran as described previously.4,13 Binding was determined using 50 pM 125I-BH-CCK-8 and was for 30 minutes at 37°C. Results are expressed as the percentage of the saturable binding seen with no unlabeled peptide present. Data are mean ± 1 SEM of four separate experiments.
FIGURE 12.
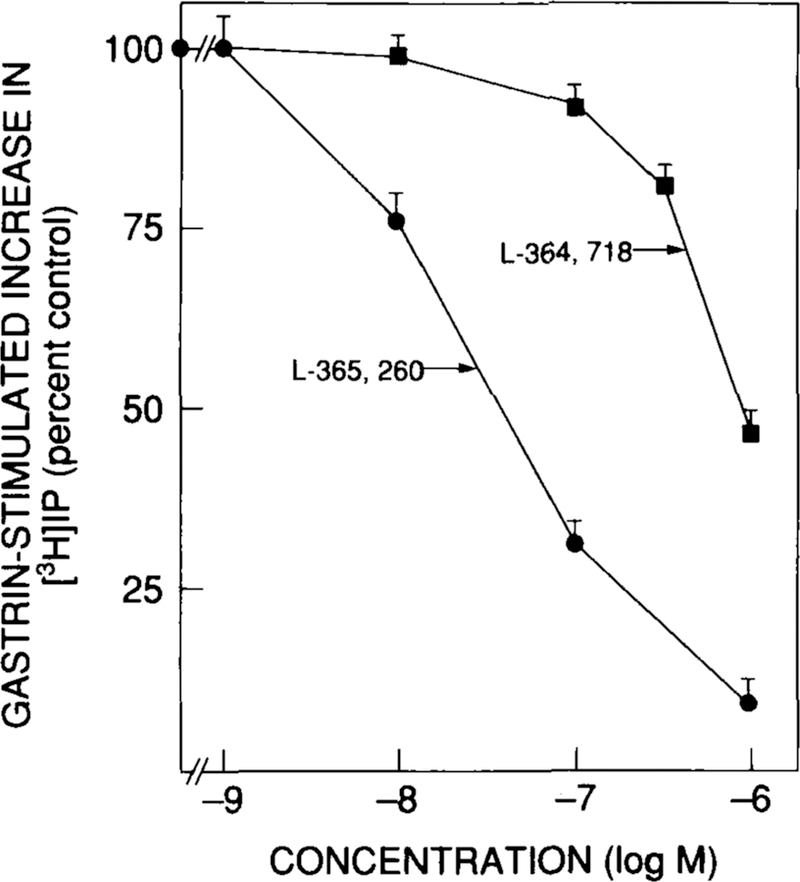
Ability of various CCK receptor antagonists to inhibit gastrin-17-I-stimulated increases in [3H]IP in COS-7 cells transfected with a full-length human CCKA4 or CCKB13 receptor clone using DEAE/Dextran. [3H]IP was measured as described previously.4,65 [3H]IP is expressed as the percentage of stimulation caused by 10 nM gastrin-17-I alone. Data are means ± 1 SEM from four separate experiments.
TABLE 3.
Comparison of the Affinities of Various CCK Receptor Agonists and Antagonists for Human CCKA and CCKB Receptors Transfected into COS-7 Cells and for CCKA and CCKB Receptors in Guinea Pig Pancreas
| IC50 (nM) |
||||
|---|---|---|---|---|
| CCKA Receptor |
CCKB Receptor |
|||
| Human | Guinea Pig Pancreatic Acini | Human | Guinea Pig Pancreatic Acini | |
| Antagonists | ||||
| L-364,718 | 5 ± 2 | 4 ± 1 | 890 ± 150 | 500 ± 100 |
| CR-1409 (lorglumide) | 520 ± 120 | 200 ± 10 | 12,800 ± 1,200 | 30,200 ± 12,900 |
| Proglumide | 660,000 ± 120,000 | 3,000,000 ± 1,500,000 | 4,130,000 ± 1,350,000 | ND |
| L-365,260 | 55 ± 15 | 570 ± 50 | 10 ± 1 | 7 ± 1 |
| Agonists | ||||
| A-71378 | 2.6 ± 1.2 | 0.4 ± 0.1 | 4,260 ± 456 | 300 ± 45 |
| CCK-8 | 2.8 ± 1.2 | 0.7 ± 0.1 | 5.7 ± 0.6 | 1.5 ± 1.0 |
| Gastrin-17-I | 1,580 ± 490 | 1,000 ± 100 | 1,280 ± 217 | 508 ± 155 |
| CCK-4 | 18,600 ± 2,000 | 29,000 ± 3,100 | 112 ± 46 | 28 ± 6 |
| des(SO3)CCK-8 | 1,580 ± 559 | 352 ± 36 | 2.2 ± 0.2 | 0.4 ± 0.01 |
For the reported CCKB receptor antagonists, preliminary studies suggest that the affinities will be similar to those reported on guinea pig pancreatic acinar cells (Table 3). L-364,718 had a high affinity for human CCKA receptors (IC50 - 5 nM) and had a 200-fold lower affinity for human CCKB receptors (Fig. 11). For human CCKB receptors, L-365,260 had a similar affinity to that found on guinea pig pancreatic acini (IC50 - 10 nM)and was 90-fold more potent than L-364,718 (Table 3. These data demonstrate that similar to recent reports, the human CCKB receptor resembles that reported in rat, guinea pig, and mouse in having a higher affinity for L-365,260 than L-364,718 and differs from the canine CCKB receptor which has a higher affinity for L-364,718 than L-365,260.4,11–14,42 The comparative affinity data of L-364,718 and L-365,260 for human CCK receptors in Table 3 suggest that L-364,718 will be a highly selective CCKA receptor antagonist and useful for human studies. However, L-365,260 has only a fivefold greater affinity for human CCKB than CCKA receptors and in another only a twofold greater affinity; therefore, its selectivity for the human CCKB receptor is sufficiently low that it will likely not be useful for in vivo human studies.
To determine if the proposed antagonists actually function as CCK receptor antagonists in human CCK receptors, studies on the ability of the various antagonists to inhibit stimulated increases in [3H]IP were started. Preliminary data from studies with human CCKB receptors are shown in Figure 12. Neither L-364,718 nor L-365,260 had activity at concentrations up to 1 μM. However, each inhibited gastrin-17-I stimulated increases in [3H]IP (Fig. 12). L-365,260 was 50-fold more expressed as the percentage of stimulation caused by 10 nM gastrin-17-I alone. Data are means ± 1 SEM from four separate experiments. potent than L-364,718 in inhibiting 10 nM gastrin-17-I-stimulated [3H]IP, and these data are in close agreement with the results of the binding studies (Table 3).
These preliminary data suggest that, using this transfected cell system, it will be possible to obtain good pharmacological data about the relative affinities of the different CCK receptor agonists and antagonists for human CCKA and CCKB receptors. Furthermore, it will be possible to determine if they have full or partial agonist activity or behave as pure antagonists. Therefore, it should be possible to identify which compounds should be useful to distinguish CCKA and CCKB receptors in humans even when present on the same cell.
REFERENCES
- 1.WANK SA, HARKINS R, JENSEN T, SHAPIRA H, DEWEERTH DE & SLATTERY TS. 1992. Purification, molecular cloning and functional expression of the CCK receptor from rat pancreas. Proc. Natl. Acad. Sci. USA 89: 3125–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RECEPTOR NOMENCLATURE SUPPLEMENT TRENDS. PHARMACOL. SCI, Jan. 1991. [PubMed]
- 3.JENSEN RT, WANK SA, ROWLEY WH, SATO S & GARDNER JD 1989. Interactions of cholecystokinin with pancreatic acinar cells: A well-studied model of a peripheral action of CCK. Trends Pharmacol. Sci 10: 418–423. [DOI] [PubMed] [Google Scholar]
- 4.DeWeerth A, Pisegna JR & Wank SA. 1993. Gallbladder and pancreas possess identical CCKA receptor subtypes: Receptor cloning and expression. Am. J. Physiol, in press. [DOI] [PMC free article] [PubMed]
- 5.SCHJOLDAGER B, PARK J, JOHNSEN AH, YAMADA T & REHFELD JF. 1991. Cionin, a protochordean hybrid of cholecystokinin and gastrin: Biological activity in mammalian systems. Am. J. Physiol 260: G977–G982. [DOI] [PubMed] [Google Scholar]
- 6.SANKARAN H, GOLDFINE ID, DEVENEY C,W, WONG KY & WILLIAMS JA. 1980. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J. Biol. Chem 255: 1849–1853. [PubMed] [Google Scholar]
- 7.JENSEN RT, LEMP GF & GARDNER JD 1980. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 77: 2079–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HUANG SC, YU D-H, WANK SA, MANTEY SA, GARDNER JD & JENSEN RT. 1989. Importance of sulfation of gastrin or cholecystokinin (CCK) in determining affinity for gastrin and CCK receptors. Peptides 10: 785–789. [DOI] [PubMed] [Google Scholar]
- 9.JORPES JE & MUTT V, Eds. 1973. Handbook of Experimental Pharmacology. Secretin, Cholecystokinin, Pancreozymin: 1–376. Springer-Verlag; Berlin, Heidelberg. [Google Scholar]
- 10.Yu D-H, NOGUCHI M, ZHOU Z-C, VILLANUEVA ML, GARDNER JD & JENSEN RT. 1987. Characterization of gastrin receptors on guinea pig pancreatic acini. Am. J. Physiol. (Gastrointest. Liver Physiol. 16) 253: G793–G801. [DOI] [PubMed] [Google Scholar]
- 11.WANK SA, PISEGNA JR & DEWEERTH A 1992. Brain and gastrointestinal cholecystokinin receptor family: Structure and functional expression. Proc. Natl. Acad. Sci. USA 89: 8691–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KOPIN AS, LEE YM, MCBRIDE EW, MILLER LJ, LU M, LIN HY, KOLAKOWSKI LF JR. & BEINBORN M. 1992. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. USA 89: 3605–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PISEGNA JR, DEWEERTH A, HUPPI K & WANK SA 1992. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem. Biophys. Res. Comm 189: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LEE YM, BEINBORN M, MCBRIDE EW, LU M, KOLAKOWSKI LF JR. & KOPIN AS. 1993. The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J. Biol. Chem 268: 8164–8169. [PubMed] [Google Scholar]
- 15.Yu D-H, HUANG S-C, WANK SA, MANTEY SA, GARDNER JD & JENSEN RT. 1990. Pancreatic receptors for cholecystokinin: Evidence for interaction with 3 receptor classes. Am. J. Physiol 258 (GLP 21): G86–G95. [DOI] [PubMed] [Google Scholar]
- 16.JENSEN RT & GARDNER JD 1991. Cholecystokinin receptor antagonists in vivo. In Cholecystokinin Antagonists in Gastroenterology: Basic and Clinical Studies Adler G & Beglinger C, eds.: 93–111. Springer-Verlag GmbH and Co; Heidelberg, Germany. [Google Scholar]
- 17.GULLY D, FREHEL D, MARCY C, SPINAZZE A, LESPY L, NELIAT G, MAFFRAND JP & LE FUR G 1993. Peripheral biological activity of SR 27897: A new potent non-peptide antagonist of CCKA receptors. Eur. J. Pharmacol 232: 13–19. [DOI] [PubMed] [Google Scholar]
- 18.ROQUES BP, WENTIR B, MARSEIGN I, et al. 1989. Development of CCK-related compounds as probes for biochemical and pharamcological characteristics of CCK binding site heterogeneity. In The Neuropeptide Cholecystokinin Hughes J, Dockery G & Woodruff G, eds.: 133–142. Ellis Horwood Limited; Chichester, UK. [Google Scholar]
- 19.LIN CW, HOLLADAY MW, WITTE DG, MILLER R, WOLFRAM CAW, BIANCHI BR, BENNETT MJ & NADZAN AM 1990. A71378: A CCK agonist with high potency and selectivity for CCK-A receptors. Am. J. Physiol 258: G648–G651. [DOI] [PubMed] [Google Scholar]
- 20.MAKOVEC F, CHRISTE R, BANI M, PACINI MA, SETNIKAR I & ROVATI LA 1985. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung 35: 1048–1051. [PubMed] [Google Scholar]
- 21.HUGHES J, BODEN P, COSTALL B, DOMENEY A, KELLY E, HORWELL DC, HUNTER JC, PIMNOCK RD & WOODRUFF GN 1990. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc. Natl. Acad. Sci. USA 87: 6728–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HUANG SC, ZHANG L, WANK SA, MATON PN, GARDNER JD & JENSEN RT 1989. Comparison of benzodiazepine analogues L-365,260 and L-364,718 as gastrin and pancreatic CCK receptor antagonists. Am. J. Physiol 257 (GLP 20): G169–G174. [DOI] [PubMed] [Google Scholar]
- 23.BOCK MG, DIGARDO RM, EVANS BE, RITTLE KE, WHITTER WE, VEBER DF, ANDERSON PS & FREIDINGER RM 1989. Benzodiazepine gastrin and cholecystokinin receptor ligands: L-365 260. J. Med. Chem 32: 13–16. [DOI] [PubMed] [Google Scholar]
- 24.NIEDERAU C, NIEDERAU M, WILLIAMS JA & GRENDELL JH 1986. New proglumide-analogue CCK receptor antagonists: Very potent and selective for peripheral tissues. Am. J. Physiol 251: G856–G860. [DOI] [PubMed] [Google Scholar]
- 25.LOUIE DS, LIANG J-P & OWYANG C 1988. Characterization of a new antagonist, L-364,718 in vitro and in vivo studies. Am. J. Physiol 255: G261–G266. [DOI] [PubMed] [Google Scholar]
- 26.OHTSUKA T, KOTAKI H, NAKAYAMA N, ITEZONO Y, SHIMMA N, KUDOH T, KUWAHARA T, ARISAWA M, YOKOSE K & SETO H. 1993. Tetronothiodin, a novel cholecystokinin type-B receptor antagonist produced by Srreptomyces sp. NR0489. II. Isolation, characterization and biological activities, J. Antibiot. (Tokyo) 46: 11–17. [DOI] [PubMed] [Google Scholar]
- 27.HOWELL DC 1991. Development of CCK-B antagonists. Neuropeptides 19(suppl): 57–64. [DOI] [PubMed] [Google Scholar]
- 28.CHANG RSL & LOTTI VJ. 1986. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokonin antagonist. Proc. Natl. Acad. Sci. USA 83: 4923–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KNAPP RJ, VAUGHN LK, FANG SN, BOGERT CL, YAMAMURA MS, HRUBY VJ & YAMAMURA HI. 1990. A new, highly selective CCK-B receptor radioligand ([3H][N-methyl-NIe28,31]CCK26–33):Evidence for CCK-B receptor heterogeneity. J. Pharmacol. Exp. Ther 255: 1278–1286. [PubMed] [Google Scholar]
- 30.LOTTI VJ & CHANG RSL.1989. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365 260. Eur. J. Pharmacol 162: 273–280. [DOI] [PubMed] [Google Scholar]
- 31.HONDA T, WADA E, BATTEY JF & WANK SA. 1993. Differential gene expression of CCKA and CCKB receptors in the rat brain. Mol. Cell. Neurosci 4: 143–154. [DOI] [PubMed] [Google Scholar]
- 32.FOURMY D, ZAHIDI A, FABRE R, GUIDET M, PRADAYROL L & RIBET A. 1987. Receptors for cholecystokinin and gastrin peptides display specific binding properties and are structurally different in guinea-pig and dog pancreas. Eur. J. Biochem 165: 683–692. [DOI] [PubMed] [Google Scholar]
- 33.GRIDER JR & MAKHLOUF GM. 1990. Distinct receptors for cholecystokinin and gastrin on muscle cells of stomach and gallbladder. Ann. N. Y. Acad. Sci 259: G184–G190. [DOI] [PubMed] [Google Scholar]
- 34.LAMBERT M, BUI ND & CHRISTOPHE J. 1991. Functional and molecular characterization of CCK receptors in the rat pancreatic acinar cell line AR 4–2J. Regul. Pept 32: 151–167. [DOI] [PubMed] [Google Scholar]
- 35.DELVALLE J, CHIBA T, PARK J & YAMADA T. 1993. Distinct receptors for cholecystokinin and gastrin on canine fundic D-cells. Am. J. Physiol 264: G811–G815. [DOI] [PubMed] [Google Scholar]
- 36.QIAN J-M, ROWLEY WH & JENSEN RT 1993. Gastrin and CCK activate phospholipase C and stimulate pepsinogen release by interacting with two distinct receptors. Am. J. Physiol. (Gastrointest. Liver Physiol. #27) 264: G718–G727. [DOI] [PubMed] [Google Scholar]
- 37.CHERNER JA, SUTLIFF VEI, GRYBOWSKI DA, JENSEN RT & GARDNER JD. 1988. Functionally distinct receptors for cholecystokinin and gastrin on dispersed chief cells from guinea pig stomach. Am. J. Physiol. (Gastrointest. Liver Physiol. 17) 254: G151–G155. [DOI] [PubMed] [Google Scholar]
- 38.SUTLIFF VEI, CHERNER JA, JENSEN RT & GARDNER JD 1990. Binding of 125I-CCK and 125I-gastrin to dispersed chief cells from guinea pig stomach. Biochem. Biophys. Acta 1052: 9–16. [DOI] [PubMed] [Google Scholar]
- 39.LLOYD DC, RAYBOULD HE & WALSH JH 1992. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am. J. Physiol 263 (3 Pt 1): G287–G292. [DOI] [PubMed] [Google Scholar]
- 40.WALSH JH 1992. Gastrin physiology. Regul. Pept. Lett IV: 21–26. [Google Scholar]
- 41.ADLER G & BEGLINGER C, Eds. 1991. Cholecystokinin antagonists in gastroenterology: 1–233. Springer-Verlag; Berlin, Heidelberg. [Google Scholar]
- 42.BEINBORN M, LEE YM, MCBRIDE EW, QUINN SM & KOPIN AS. 1993. A single amino acid of the cholecystokinin-B/gastrin receptor determines specificity for non-peptide antagonists. Nature 362: 348–350. [DOI] [PubMed] [Google Scholar]
- 43.MATOZAKI T, GOKE B, TSUNODA Y, RODRIGUEZ M, MARTINEZ J & WILLIAMS JA. 1990. Two functionally distinct cholecystokinin receptors show different modes of action on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. J. Biol. Chem 265: 62474–254. [PubMed] [Google Scholar]
- 44.VINAYEK R, JENSEN RT & GARDNER JD 1987. The role of the sulfate ester in influencing the biologic activity of cholecystokinin-related peptides. Am. J. Physiol. (Gastrointest. Liver Physiol. 15) 252: G178–GI81. [DOI] [PubMed] [Google Scholar]
- 45.MILLINGTON WR, MUELLER GP & LAVIGNE GJ. 1992. Cholecystokinin type A and type B receptor antagonists produce opposing effects on cholecystokinin-stimulated beta-endorphin secretion from the rat pituitary. J. Pharmacol. Exp. Ther 261: 454–461. [PubMed] [Google Scholar]
- 46.HERSEY SJ, MAY D & SCHYBERG D. 1983. Stimulation of pepsinogen release from isolated gastric glands by cholecystokinin-like peptides. Am. J. Physiol 244: G192–G197. [DOI] [PubMed] [Google Scholar]
- 47.KASBEKAR DK, JENSEN RT & GARDNER JD. 1983. Pepsinogen secretion from dispersed glands from rabbit stomach. Am. J. Physiol. (Gastrointest. Liver Physiol. 7) 244: G392–G396. [DOI] [PubMed] [Google Scholar]
- 48.NOGUCHI M, ADACHI H, SATO S, HONDA T, OHNISHI S, AOKI E & TORIZUKA K. 1987. Cholecystokinin-stimulated pepsinogen secretion and cholecystokinin receptors on gastric chief cells in guinea pigs. Endocrinol. Jpn 34: 727–736. [DOI] [PubMed] [Google Scholar]
- 49.RAUFMAN J-P, SUTLIFF VE, KASBEKAR DK, JENSEN RT & GARDNER JD. 1984. Pepsinogen secretion from dispersed chief cells from guinea pig stomach. Am. J. Physiol. (Gastrointest. Liver Physiol. 10) 247: G95–Gl04. [DOI] [PubMed] [Google Scholar]
- 50.SAKAMOTO C, MATOZAKI T, NISHISAKI H, KONDA Y, NAGAO M & NAKANO O. 1990. Effects of CCK-receptor antagonists on CCK-stimulated pepsinogen secretion and calcium increase in isolated guinea pig gastric chief cells. Dig. Dis. Sci 35: 873–878. [DOI] [PubMed] [Google Scholar]
- 51.CHEW CS & BROWN MR 1986. Release of intracellular Ca2+and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim. Biophys. Acta 888: 116–125. [DOI] [PubMed] [Google Scholar]
- 52.TSUNODA Y, TAKEDA H, OTAKI T, ASAKA M, NAKAGAKI I & SASAKI S. 1988. A role for Ca2+in mediating hormone-induced biphasic pepsinogen secretion from the chief cell determined by luminescent and fluorescent probes and X-ray microprobe. Biochim. Biophys. Acta 941: 83–101. [DOI] [PubMed] [Google Scholar]
- 53.TSUNODA Y, WILLIAMS JA & DELVALLE J 1991. Secretagogue-induced Ca2+ oscillations in isolated canine gastric chief cells. Biochim. Biophys. Acta 1091: 251–254. [DOI] [PubMed] [Google Scholar]
- 54.CHANG RS, LOTTI VJ & CHEN TB. 1985. Cholecystokinin receptor mediated hydrolysis of inositol phospholipids in guinea pig gastric glands. Life Sci 36: 965–971. [DOI] [PubMed] [Google Scholar]
- 55.MATOZAKI T, SAKAMOTO C,NAGAO M,NISHISAKI H, KONDA Y, NAKANO O, MATSUDA K, WADA K, SUZUKI T & KASUGA M. 1991. Characterization of cholecystokinin receptors on guinea pig gastric chief cell membranes. Biochem. Biophys. Res. Commun 174: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 56.LIN CW, BIANCHI BR, MILLER TR, WITTE DG & WOLFRAM CA 1992. Both CCK-A and CCK-B/gastrin receptors mediate pepsinogen release in guinea pig gastric glands. Am. J. Physiol 262: G1113–G1120. [DOI] [PubMed] [Google Scholar]
- 57.JENSEN RT, HUANG SC, VON SCHRENCK T, WANK SA & GARDNER JD 1990. Cholecystokinin receptor antagonists: Ability to distinguish various cholecystokinin receutor classes. In Gastrointestinal Endocrinolom: Receutors and Postreceutor Mechanisms Thompson JC, Townsend CM Jr., Greeley GA Jr., et al. : 95–114. Academic Press, Inc; New York, NY. [Google Scholar]
- 58.STALEY J, JENSEN RT & MOODY TW. 1990. CCK antagonists interact with CCK-B receptors on human small cell lung cancer cells. Peptides 11: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 59.JENSEN RT 1993. Receptors on pancreatic acinar cells. In Physiology of the Gastrointestinal Tract 3rd ed. Johnson LR, Jacobsen ED, Christensen J, Alpers DH & Walsh JH. Raven Press Publishing Co; New York, NY, in press. [Google Scholar]
- 60.WILLIAMS JA & YULE DI.1993. Stimulus-secretion coupling in pancreatic acinar cells. In The Pancreas. Biology, Pathobiology and Disease 2nd ed. Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA & Scheele GA.: 167–189. Raven Press; New York. [Google Scholar]
- 61.MAGOUS R, GALLEYRAND JC & BALI JP. 1989. Common or distinct receptors for gastrin and cholecystokinin in gastric mucosa? Biochim. Biophys. Acta 1010: 357–362. [DOI] [PubMed] [Google Scholar]
- 62.ROCHE S, BALI JP, GALLEYRAND JC & MAGOUS R 1991. Characterization of a gastrin-type receptor on rabbit gastric parietal cells using L365, 260 and L364,718. Am J. Physiol 260: G182–G188. [DOI] [PubMed] [Google Scholar]
- 63.ROCHE S & MAGOUS R. 1989. Gastrin and CCK-8 induce inositol 1,4,5-trisphosphate formation in rabbit gastric parietal cells. Biochim. Biophys. Acta 1014: 313–318. [DOI] [PubMed] [Google Scholar]
- 64.SEIDLER U & PFEIFFER A. 1991. Inositol phosphate formation and [Ca2+]i in secretagogue-stimulated rabbit gastric mucous cells. Am. J. Physiol 260: G133–G141. [DOI] [PubMed] [Google Scholar]
- 65.ROWLEY WH, SATO S, HUANG S-C, COLLADO-ESCOBAR DM, BEAVEN MA, WANG L-H, MARTINEZ J, GARDNER JD & JENSEN RT 1990. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am. J. Physiol. (Gastrointest. Liver Physiol. 22) 259: G655–G665. [DOI] [PubMed] [Google Scholar]
- 66.SATO S, STARK HA, MARTINEZ J, BEAVEN MA, JENSEN RT & GARDNER JD. 1989. Receptor occupation, calcium mobilization and amylase release in pancreatic acini: effect of CCK-JMV-180. Am. J. Physiol. (Gastrointest. Liver Physiol. 20) 257: G202–G209. [DOI] [PubMed] [Google Scholar]


