Abstract
The current study was undertaken to determine the presence and distribution of PAC1-Rs within the gastric mucosa. Polyclonal antibodies to the carboxyl terminus of the rat PAC1-R were generated and shown to be specific against the PAC1-R expressed in NIH 3T3 cells. Western blot analysis using isolated (≈ 85% pure) ECL cell membranes identified a 48 kD protein consistent with the calculated molecular mass of the cloned PAC1-R. RT/PCR performed using specific primers for the PAC1-R confirmed the presence of splice variants of the rat PAC1-R, but not VPAC1-R or VPAC2-R. These data provide the first direct evidence for the existence of functional PACAP Type I receptors on ECL cells of the gastric mucosa and suggest a potential role for PACAP in the stimulation of gastric acid secretion and in the regulation of the growth of ECL cells.
Pituitary adenylate cyclase activating polypeptide (PACAP) is the most recently discovered neuropeptide in the vasoactive intestinal polypeptide (VIP)/ secretin/glucagon family of peptide hormones that was isolated in 1989 from ovine hypothalamus. PACAP has potent adenylate cyclase–stimulating activity and occurs as two amidated forms: PACAP-38, a 38–amino acid peptide; and PACAP-27, a 27–amino acid peptide.1 Radioligand binding and cloning studies have identified three major types of PACAP receptors (PACAP-R) distinguished pharmacologically by their relative affinities for PACAP, VIP, and helodermin.2–6 The PACAP-R Type 1 (PAC1) has high affinity for only PACAP; the Type 2 PACAP-R (classical VIP receptor, VPAC1) has high affinity for PACAP-38, PACAP-27, and VIP; and the Type 3 PACAP-R (VAPC2) shows high affinity for PACAP, VIP, and helodermin.5–6 The PAC1 in both rats and humans has been shown to be alternatively spliced and expressed as one of four major splice variant transcripts in a tissue-specific manner.7,8 In native cell systems, PAC1-Rs have been shown to exhibit coupling to both adenylate cyclase and phospholipase C signal transduction pathways, such as in the rat pheochromocytoma cell line, PC-12.9
Since its discovery, PACAP hormone expression has been identified in numerous tissues including the brain, gastrointestinal tract, adrenal gland, and testis by immunohistochemistry and radioimmunoassay.3,4 In the gastrointestinal tract, PACAP has been shown to inhibit pentagastrin-stimulated gastric acid secretion, reduce basal smooth muscle contraction, and stimulate intestinal secretion and pancreatic exocrine secretion.10–13
The enterochromaffin-like (ECL) cells of the gastric mucosa are responsible for the paracrine secretion of histamine in response to gastrin and other ligands and play an important role in peripheral regulation of gastric acid secretion.14–16 The exact role of PACAP and PAC1-R in the gastric mucosa have not been clearly defined. Furthermore, the existence of PAC1-R on select cells of the gastric mucosa as well as the physiological role of PACAP on these cells, such as for the gastric ECL cells, have not been previously determined. In the current study we isolated ECL cells in primary culture that were 85% pure.14,15 These cellular preparations were used for both functional assays and for immuno-cytochemical and molecular assays. The present study demonstrates that rat gastric ECL cells express high levels of the rat PAC1-R and PACAP is coupled to the release of intra-cellular calcium and to the release of histamine, suggesting a potentially important mech-anism in the regulation of gastric acid secretion and ECL growth.
MATERIAL AND METHODS
Materials
Peptides corresponding to amino acids 509–523 (YQLRMSSLPADNLAT) of the carboxyl tail of the rat PAC1-R were developed by G. Poy (National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health). This peptide was used to sensitize rabbits to develop polyclonal antibodies against the rat PAC1-R by the Antibody Core (CURE, Los Angeles, CA). Oligonucleotides for the rat PAC1-R were synthesized using a DNA synthesizer (Model 391 PCR-MATE, ABI, Perkin Elmer, Foster City, CA). All other reagents were analytical grade and were purchased from the indicated sources: Taq DNA polymerase (Stratagene, La Jolla, CA); PCR primers for β-actin (Clontech, Palo Alto, CA); BSA, acridine orange, DME-F12 medium, ITS, hydrocortisone, trypan blue, FSK, dbcAMP, gastrin, somatostatin, goat anti-rabbit fluorescein-conjugated IgG, ionomycin, TEA, EGTA (Sigma Chemical Co., St. Louis, MO); Cell-Tak (Collaborative Research); and Fura-2 (Molecular Probes, Oregon).
Methods
Isolation and Purification of ECL Cells
Rat gastric ECL cells were isolated by a combination of elutriation and density gradient centrifugation as described previously.14,15 Briefly, approximately five rat stomachs were digested to yield approximately 1 × 106 cells in the low density layer. The viability of cells was determined using trypan blue exclusion. Cellular purity was determined by immunostaining with anti-HDC antibody and anti-histamine antibody and by using the fluorescent dye acridine orange. Using these techniques, approximately 65–75% of cells in the isolated cell population were ECL cells. Isolated ECL cells were rinsed by gentle centrifugation in growth medium containing DME/F12 supplemented with 2 mg/ml bovine serum albumin (BSA), 2.5% fetal calf serum (FCS), 100 µM hydrocortisone, 1% penicillin, 1 mg/100 ml streptomycin and 5 mg/ml insulin, 5 mg/ml transferrin, and 5 µg/l sodium selenite. Enrichment of the primary culture was performed by plating 15 µl of the cell suspension onto glass coverslips pre-coated with Cell-Tak and incubated at 37°C for 45 min. Using this methodology, the ECL cell population was enriched to more than 85%.
Immunohistochemistry
Rat stomach was incised longitudinally, fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 12 h, dehydrated gradually in 70% and 100% ethanol, and embedded in paraffin. Paraffin-embedded tissue blocks were cut into 4-mm sections, deparaffinized, rehydrated, and treated with 3% hydrogen peroxide for 5 minutes. After blocking with 20% swine serum, sections were incubated with antibodies to the receptor at a 1:1,000 dilution overnight at room temperature, followed by biotinylated secondary antibodies (20 min). Streptavidin conjugated to peroxidase (20 min) and amino-ethyl carbazole-H2O2 or diaminobenzidine using the LSAB-2 kit were performed subsequently (DAKO Corp, Carpinteria, CA). All washes were done with 0.02 M Tris-HCl pH 7.2 containing 0.1% Tween-20 and 0.3 M NaCl. The slides were counter-stained with Mayer’s hematoxylin and observed under Nikon Optiphot II light microscope. For absorption of the antibodies with corresponding peptides used for immunization, the peptides were added to prediluted anti-bodies to give a final concentration of 50 mg/ml of the peptide. The antibodies were allowed to react with the peptide for 8 h prior to use.
Immunocytochemistry
Pellets of isolated cells were fixed in 4% formaldehyde in PBS for 12 h, dehydrated gradually in 70% and 100% ethanol, and then embedded in paraffin. Following blocking with 20% swine serum, the cells were incubated with antibodies to PAC1-R at a 1:1,000 dilution overnight at room temperature, followed by biotinylated secondary antibodies (20 min). Streptavidin conjugated to peroxidase (20 min) and amino-ethyl carbazole-H2O2 or diaminobenzidine using the LSAB-2 Kit were used for detection (DAKO Corp., Carpinteria, CA). All washes were performed using 0.02 M Tris-HCl, pH 7.2, containing 0.1% Tween-20 and 0.3 M NaCl. Slides were counter-stained with Mayer’s hematoxylin and observed under a Nikon Optiphot II light microscope. For absorption studies, antibodies were incubated with corresponding peptides that were used for immunization (final concentration of 50 mg/ml). The antibodies were allowed to react with the peptide for 8 h prior to use.
Western Blot Analysis
ECL cells used for Western blot analysis were isolated as described above. Following isolation, the cells were washed twice in ice-cold PBS, centrifuged (200 × g), and resuspended in homogenization buffer (HEPES 50 mM, pH 6.5, NaCl 0.1 M, MgCl2 5 mM, benzamidine 0.2 mg/ml, leupeptin 1 mg/ml, pepstatin 0.7 mg/ml, EGTA 1 mM) using a sonicator (50% power for 1 min) and subjected to a low speed centrifugation (500 × g). The supernatant was centrifuged at 25,000 × g for 20 min and the pellet resuspended in Tris-Glycine-SDS buffer (Tris HCl, pH 6.8, 63 mM glycerol 10%, SDS 2%). Following the determination of protein concentration, 10 µg of isolated protein was loaded per lane on a 10% Tris-Glycine Gel (Novex) and run at 125 V (≈ 90 min). Transfer of protein to Hybond® (Millipore) was performed using a Millipore Gel Transfer apparatus. Blotting was performed using the anit-PAC1-R antibody (1:1,000 dilution) at room temperature, and an anti-rabbit second antibody (1:10,000 dilution) at room temperature. Detection was performed using the ECL Detection Method (Amersham).
cDNA Synthesis and RT-PCR Analysis
RNA was extracted from 1 g of purified ECL cell preparation using the Fast Track RNA Purification kit (Invitrogen, La Jolla, CA). Total RNA (5 µg) from enriched rat ECL cells was used to synthesize cDNA by reverse transcriptase with oligo-dT15 primers (Boerhinger-Mannheim). PCR was performed in low salt Taq+ DNA polymerase buffer and 5 U Taq+ DNA polymerase (Stratagene, La Jolla, CA) in the presence of oligonucleotide primers under the following conditions: the initial step (one cycle) was 94°C for 2 min, 57°C for 1 min, and 72°C for 2 min; followed by 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min (30 cycles); and a final extension step (one cycle) at 94°C for 1 min, 57°C for 1 min, and 72°C for 15 min. The sense primers used were: (SENSE 1) 5′ CGAGTGGACAGTGGCAGGCGGTGA 3′ (52–77); and (SENSE 2): 5′GCTCTCCCTGACTGCTCTCCTGCTG 3′ (145–170). The antisense primers used were: (ANTISENSE 1) 5′ CAGTAGTGAGGGTGGCGAGGGAAGT 3′ (611–636); and (ANTISENSE 2) 5′ CAGTAGGTGTCCCCCAGCCGATGAT 3′ (935–960). To standardize the amount of total RNA used for RT-PCR, duplicate samples were analyzed for β-actin as controls. The sense primer for β-actin was 5′ TTGTAACCAACTGGGACGATATGG (1552–1575) and antisense primer sequence was 5′ GATCTTGATCTTCATGGTGCTAGG (2991–2844). The rat PAC1-R PCR products amplified from ECL cell cDNA were purified from 0.7% agarose gels using the Quiex Gel Extraction Kit, and subcloned into plasmid pCR-Script Amp SK(+)(Stratagene, La Jolla, CA). DNA sequence analysis was performed on 500 fmol of extracted DNA product using a DNA Autoanalyzer (ABT).
RESULTS
Validation of the Rat PAC1-R Antibody
Polyclonal antibodies were generated against specific amino acids of the deduced amino acid sequence of the rat PAC1-R as described in the Methods.5 Specificity of this antibody was determined using both Western blot analysis and immunocytochemistry in NIH/3T3 cells stably expressing the rat PAC1-R (Fig. 1A). Immunocytochemistry confirmed the expression of PACAP receptors on these NIH/3T3 cells expressing rPAC1-Rs but not on the NIH/3T3 cells expressing the VPAC1-R (Fig. 1B) or VPAC2-Rs (data not shown). These results demonstrated that the polyclonal antibodies to the PACAP Type 1 receptor are specific.
FIGURE 1.
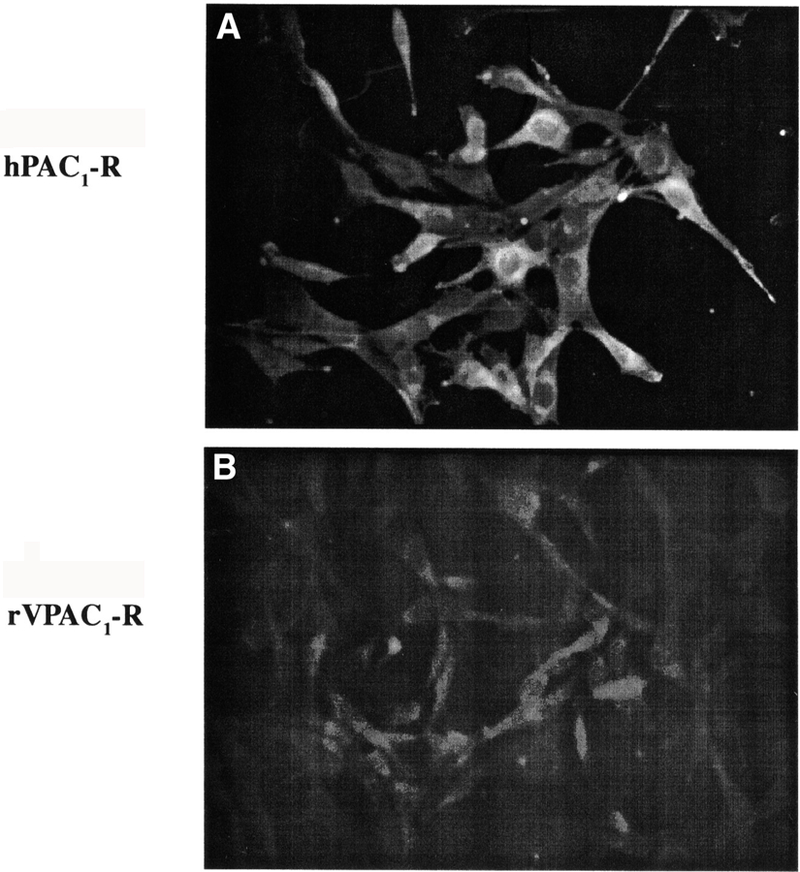
Immunocytochemistry using the anti-hPAC1-R Ab on NIH/3T3 cells expressing the hPAC1-R (A) or VPAC1-R (B). Positive staining is present against the human PAC1-R expressing cells. Preincubation of cells with the immunization peptides was negative (data not shown).
Western Blot Analysis
To confirm the expression of PAC1-Rs on ECL cells, membrane preparations from ECL cells and Western blot analysis using the polyclonal antibodies to the PAC1-Rs (described above) were performed as shown in Figure 2. Western blot analysis demonstrated a single protein band with a molecular weight of ≈ 46 kD (indicated by the arrow) expressed on ECL cells. As a positive control, membranes from AR4–2J cells were used and showed a similar protein size (Fig. 2). No expression of the PACAP receptor was observed in the HEK293 cells (data not shown).
FIGURE 2.
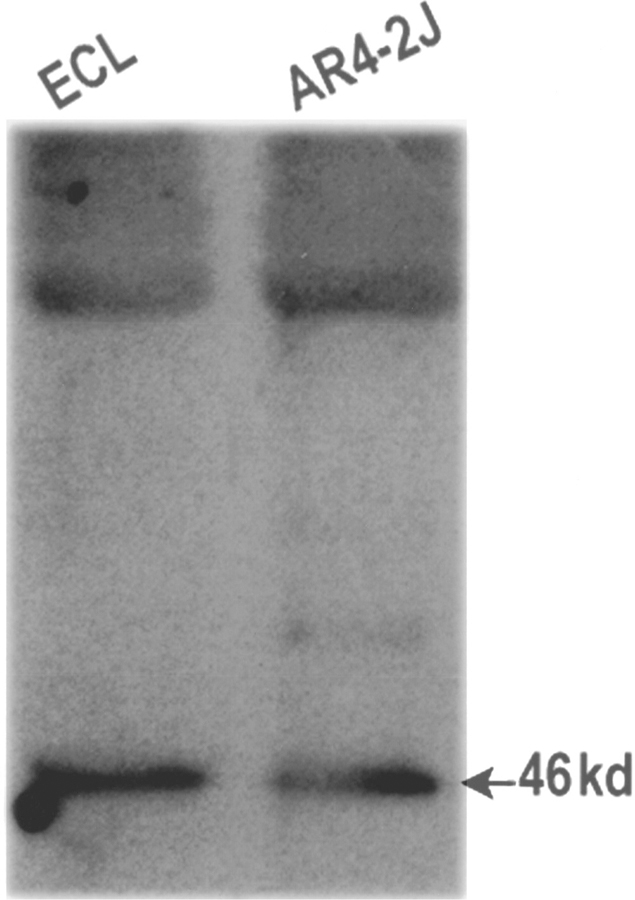
Western blot analysis using polyclonal antibodies to the rat PAC1-R. Receptor expression is detected in membrane preparations from gastric enterochromaffin-like cells (ECL) (left lane). The antibody detects receptor expression corresponding to 46 kD (arrow). Similar expression was detected in the rat pancreatic cancer cell line, AR4–2J (right lane).
Reverse Transcriptase/PCR Confirmation
To further demonstrate that rat ECL cells express the PAC1-R, reverse transcriptase–PCR, was performed using RNA extracted from isolated ECL cells. Amplification of the cDNA was performed with two different rat PAC1-R primers selected because they span intron/exon junctions of the rat PACAP receptor gene (unpublished data). As demonstrated in Figure 3, specific DNA products were observed using separate PCR primer combinations.
FIGURE 3.
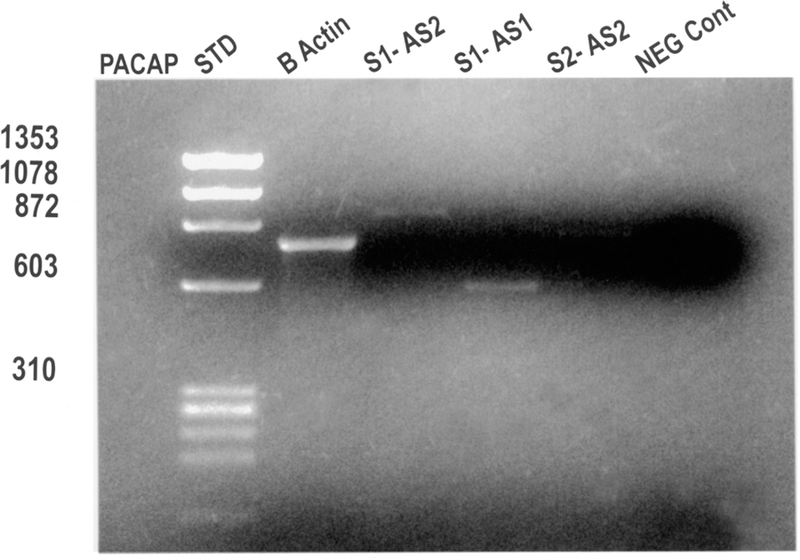
Reverse transcriptase/polymerase chain reaction (RT-PCR) demonstrating rat PAC1-R expression in isolated rat gastric ECL cells. Rat gastric ECL cells were isolated as described previously.14–16 Lane 1 indicates DNA standards at the sizes indicated on the left margin. Lane 2 demonstrates β-actin expression as a positive control. Lanes 3–5 indicate the expression of rat PAC1-Rs using different primer pairs derived from the cloned rPAC1-R cDNA.5 Specific bands are identified for all three primer combinations and are the sizes expected from the primers used. The last lane demonstrates that no bands are detected when the enzme, reverse transcriptase, is omitted from the reaction mixture (negative control).
One primer combination, using Sense 1 and Antisense 2 primers, yielded a band at approx-imately 900 kbp with a predicted size of 883 kbp. A second primer combination using S1 and AS1 yielded a PCR product of approximately 600 kbp with a predicted size of 559 kbp. Amplification of reverse-transcribed RNA from ECL cells using primers specific for either the VPAC1 or VPAC2 receptor was negative (data not shown). Experiments using rat null, hop, hip, and hiphop primers based on the data by Spengler and coworkers7 confirmed the expression of all four splice variants (data not shown).
Tissue Localization by Immunohistochemistry
To further confirm the results obtained by Western blot and RT–PCR, immunohisto-chemisty was performed using the anti-rat PAC1-R antibody (dilution of 1:1,000). At low magnifications, the majority of the immunostaining is noted at the base of the oxynic gland with little or no receptor expression observed at the isthmus or luminal regions of the gland. At higher magnifications, staining is present on ECL cells at the base of the gastric gland (data not shown).
Immunocytochemistry
The specific polyclonal antibodies generated against carboxl-terminal amino acids of the rat PAC1-R were used for immunocytochemistry (Fig. 4) using isolated cell preparations of ECL cells isolated from the rat gastric mucosa. The cell composition was determined using antibodies to either histidine decarboxylase (ECL cells), gastrin (G cells), or somatostatin (D cells) in order to confirm that the cells were pure (data not shown). Intense staining on the surface of the ECL cells but not of the G cells was observed using the anti PAC1-R antibody (Fig. 4) and was specific compared to cells stained in the presence of pre-immune serum (data not shown).
FIGURE 4.
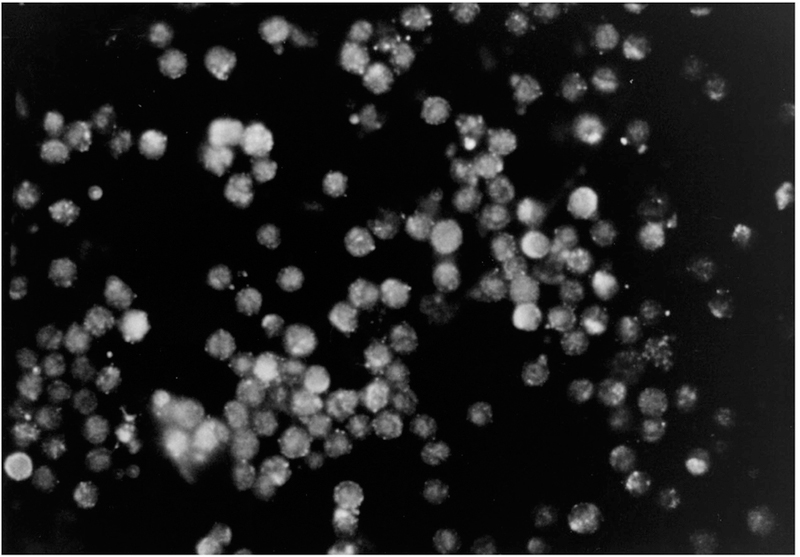
Localization of PACAP-Rs in isolated ECL cells by immunocytochemistry (50 × magnification). Immunocytochemistry was preformed on elutriated ECL cells (≈90% pure) with the specific anti-PACAP-R antibody showing the expression of PACAP-Rs on the cell surface. Immunocytochemistry on isolated ECL cells with pre-incubation of the anti-PACAP-R antibody with preimmune rabbit serum revealed no staining (data not shown).
DISCUSSION
It has been demonstrated by both pharmacological and molecular studies that three classes of PACAP receptors exist: PAC1 (PACAP Type 1), VPAC1 (VIP1/PACAP), and VPAC2 (VIP2/PACAP).5,6 However, much confusion exists as to their localization and physiological significance. Although PACAP hormone expression has been shown to occur in numerous peripheral tissues, their role has not been completely understood because PACAP-27 and PACAP-38 have high affinity for all three types of receptors. The current study is significant because it is the first study to identify the specific expression of PAC1-Rs in the stomach. The current study also demonstrates that PAC1-Rs are expressed on gastric ECL cells, suggesting that PACAP may regulate gastric acid secretion.
The cloning of the rat PAC1-R afforded the generation of molecular probes and the construction of immunogenic peptides for the localization of the PAC1-R. The rat PAC1-R shares several structural features that are similar to the other members of the VIP/secretin/glucagon receptor family, such as a long amino terminus ( >120 amino acids), which may be important for ligand recognition; five potential N-linked glycosylation sites (ASN-X-SER/THR) in the amino terminus, which may be important for high affinity ligand; and seven cysteine residues, which may be necessary for agonist binding conformation.5 The rat PACAP receptor expressed on ECL cells (Fig. 2) with a molecular mass of ≈ 46 kD is similar in size to the calculated molecular mass of 51 kD and is in close agreement to the 55 kD size previously published for the rat PACAP-R using crosslinking studies.5
PACAP hormone expression has been identified in numerous tissues including the brain, gastrointestinal tract, adrenal gland, and testis by immunohistochemistry and radioimmunoassay. The highest density of PACAP binding sites occurs in the brain cortex, olfactory bulb, hippocampus, and hypothalamus.19,20 Therefore, PACAP appears to play an important role in the growth and development of the brain. In the gastrointestinal tract, PACAP-immunoreactive nerve fibers have been previously identified in the esophagus, stomach, duodenum, and small and large intestines.21–23 In addition, PACAP receptors have been identified in the liver and pancreatic islets and on the rat pancreatic carcinoma cell line, AR-42J.24 On smooth muscle cells of the gastrointestinal tract, PACAP acts as a potent non-adrenergic, non-cholinergic inhibitory neurotransmitter that directly relaxes intestinal smooth muscle cells, an action that is probably mediated by apamin-sensitive potassium channels or by the activation of nitric oxide.25–27 Characterization of PACAP or VIP receptor subtypes mediating these effects on smooth muscles has not been determined previously. Immunohistochemistry performed in the current study shows that the PAC1-R is expressed in high densities in the gastric mucosa. Examination of the gastric fundus demonstrates expression on ECL cell using ECL cell isolation techniques. No receptor expression was demonstrated on the mucous neck cells or on parietal cells of the stomach. Similarly, no receptor expression was demonstrated on the circular or longitudinal smooth muscles of the stomach. This is surprising given the potential role of PACAP on gastrointestinal motility previously reported.25 These results also suggest that the role of PACAP in gastric motility may be mediated by another receptor subtype as has been shown recently in gallbladder smooth muscle.27
As previously shown in native cells such as in somatotrophs and gonadotrophs, PACAP stimulates intracellular Ca2+ and hormone secretion.28 This study demonstrates that, like the pituitary gland, the PAC1-R expressed on ECL cells may also be coupled to the stimulation of intracellular Ca2+ and the release of hormone. The specificity of this response is demonstrated by localization of PAC1-Rs on ECL cells by immunohistochemical staining and RT-PCR. The dose response of intracellular calcium release following PACAP-38 or PACAP-27 is similar to that previously demonstrated for the cloned rat and human PAC1-R (data not shown).5,7,30 No comparable stimulation was observed following exposure to VIP suggesting the specificity of this response (data not shown). Because PAC1-Rs have been shown to be expressed as splice variants in both rats and humans, studies were conducted to determine whether particular splice variants were expressed on the ECL cells. Using primer combinations based on published cDNA sequences,7 RT-PCR demonstrated the expression of all of the rPACAP-R splice variants. Although the RT-PCR was performed on cells that were over 80% homogeneous, the possibility that contamination from other cells in the preparation occurred cannot be overlooked. The expression of rPACAP-R splice variants is important to understand in light of recent data demonstrating that particular splice variants (i.e., hop) in human cells are associated with an increased efficacy for phospholipase C coupling, immediate-early gene expression, and influence growth.8,30
The development of ECL cell neoplasia in both humans and rats has become of significant interest for the understanding of the development of tumors in general. Profound and long-term inhibition of gastric acid secretion has been considered to be one of the major mechanisms underlying the development of ECL tumors in rodents and humans. The mechanisms responsible for the development of ECL tumors are unclear. The suggestion that PACAP stimulates intracellular calcium and histamine release by ECL cells and the specific expression of PAC1 on ECL cells may be of importance in understanding those mechanisms regulating ECL tumor growth in addition to the potential effects on gastric acid secretion. Evidence exists for suggesting that histamine secreted by ECL cells influences growth of ECL cells by an autocrine mechanism.31 These observations and the observations that PACAP stimulates the growth of certain established tumor cell lines suggest a potential role for PACAP in influencing the growth of native cells.32 It is unclear at the present time whether PACAP stimulation of histamine secretion or perhaps a direct growth effect of PACAP on ECL growth, as has been shown on certain lung cancer cell lines,30 may be important for the genesis of gastric carcinoid tumors. Studies aimed at elucidating these complex interactions in the gastric mucosa deserve careful investigation.
REFERENCES
- 1.MIYATA A, ARIMURA A, DAHL RR, MINAMINO N, VEHARA A, JIANG L, CULLER MD & COY DH 1989. Biochem. Biophys. Res. Commun 164: 567–574. [DOI] [PubMed] [Google Scholar]
- 2.LAM H-C, TAKAHASHI K, GHATEI MA, KANSE SM, POLAK JM & BLOOM SR 1990. Eur. J. Biochem 193: 725–729. [DOI] [PubMed] [Google Scholar]
- 3.SHIVERS BD, GORCS TJ, GOTTSCHALL PE & ARIMURA A 1991. Endocrinology 128: 3055–3065. [DOI] [PubMed] [Google Scholar]
- 4.ISHIHARA T, SHIGEMOTO R, MORI K, TAKAHASHI K & NAGATA S 1992. Neuron 8: 811–819. [DOI] [PubMed] [Google Scholar]
- 5.PISEGNA JR & WANK SA 1993. Proc. Natl. Acad. Sci. USA 90: 6345–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LUTZ EM, SHEWARD WJ, WEST KM, MORROW JA, FINK G & HARMAR AJ 1993. FEBS Lett 334: 3–8. [DOI] [PubMed] [Google Scholar]
- 7.SPENGLER D, WAEBER C, PANTALONI C, HOLSBOER F, BOCKAERT J, SEEBURG PH & JOURNOT L 1993. Nature 365: 170–175. [DOI] [PubMed] [Google Scholar]
- 8.PISEGNA JR & WANK SA 1996. J. Biol. Chem 271:17267–17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DEUTSCH PJ & SUN Y 1992. J. Biol. Chem 267: 5108–5113. [PubMed] [Google Scholar]
- 10.SUDA K, SMITH DM, GHATEI MA, MURPHY JK & BLOOM SR 1991. J. Clin. Endocrinol. Methodol 72: 958–964. [DOI] [PubMed] [Google Scholar]
- 11.CHRISTOPHE J 1993. Biochim. Biophys. Acta 1154: 183–199. [DOI] [PubMed] [Google Scholar]
- 12.SHEN Z, LARSSON LT, MALMFORS G, ABSOOD A, HAKANSON R & SUNDLER F 1992. Cell Tissue Res 269: 369–374. [DOI] [PubMed] [Google Scholar]
- 13.MOODY TW, ZIA F & MAKHEJA L 1993. Peptides 14: 241–246. [DOI] [PubMed] [Google Scholar]
- 14.PRINZ C, KAJIMURA M, SCOTT DR et al. 1993. Gastroenterology 105: 449–461. [DOI] [PubMed] [Google Scholar]
- 15.PRINZ C, SCOTT DR, HURWITZ D et al. 1994. Am. J. Physiol 267: G663–G675. [DOI] [PubMed] [Google Scholar]
- 16.MODLIN IM, LAWTON GP, TANG LH, GEIBEL J, ABRAHAM R & DARR U 1994. Digestion 55:31–37. [DOI] [PubMed] [Google Scholar]
- 17.SANDVIK A, BRENNA E, ALDUM H 1993. Am. J. Physiol 27: G51–G56. [DOI] [PubMed] [Google Scholar]
- 18.ZENG N, WALSH JH, KANG T, HELANDER K, HELANDER HF & SACHS G 1996. Gastroenterology 110: 1835–1846. [DOI] [PubMed] [Google Scholar]
- 19.ARIMURA A, SOMOGYVARI-VIGH A, MIYATA A, MIZUNO K, COY DH & KITADA C 1991. Endocrinology 129: 2787–2789. [DOI] [PubMed] [Google Scholar]
- 20.TATSUNO I, SOMOGYVARI-VIGH A & ARIMURA A 1994. Peptides 15: 55–60. [DOI] [PubMed] [Google Scholar]
- 21.NGUYEN TD, HEINTZ GG & COHN JA 1992. Gastroenterology 103: 539–544. [DOI] [PubMed] [Google Scholar]
- 22.MUNGAN Z, ARIMURA A, ERTAN A, ROSSOWSKI WJ & COY DH 1992. Scand. J. Gastroenterol 27: 375–380. [DOI] [PubMed] [Google Scholar]
- 23.Cox HM 1992. Br. J. Pharmacol 106: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BUSCAIL L, GOURLET P, CAUVIN P et al. 1990. FEBS Lett 262: 77–81. [DOI] [PubMed] [Google Scholar]
- 25.SCHWORER H, KATSOULIS S, CREUTZFELDT W & SCHMIDT WE 1992. Naunyn-Schmiedeberg’s Arch. Pharmacol 346: 511–515. [DOI] [PubMed] [Google Scholar]
- 26.YADA T et al. 1994. J. Biol. Chem 269: 1290–1293. [PubMed] [Google Scholar]
- 27.MURTHY KS & MAKHLOUF GM 1994. J. Biol. Chem 269: 15977–15980. [PubMed] [Google Scholar]
- 28.SCHOMERUS E, POCH A, BUNTING R, MASON WT & MCARDLE CA 1994. [DOI] [PubMed]
- 29.SHEN Z, LARSSON LT, MALMFORS G, ABSOOD A, HAKANSON R & SUNDLER F 1992. Cell Tissue Res 269: 369–374. [DOI] [PubMed] [Google Scholar]
- 30.PISEGNA JR, LEYTON J, COELHO et al. 1997. Life Sci 61: 631–639. [DOI] [PubMed] [Google Scholar]
- 31.MODLIN IM, KUMAR RR, SOROKA CJ et al. 1994. Dig. Dis. Sci 39: 1446–53. [DOI] [PubMed] [Google Scholar]
- 32.ZIA F, FAGARASAN M, BITAR K, COY DH, PISEGNA JR, WANK SA & MOODY TW 1996. Cancer Res 55: 4886–4891. [PMC free article] [PubMed] [Google Scholar]


