Abstract
Cholecystokinin (CCK) receptors mediate pancreatic acinar secretion and gallbladder contraction. Pharmacological and functional studies in pancreas and gallbladder demonstrate a CCK-A receptor subtype in both tissues. However, some pharmacological studies and affinity cross-linking studies of CCK receptors on pancreatic acini and gallbladder suggest that these two tissues possess two different subtypes of the CCK-A receptor. We cloned these receptors in guinea pig using a cDNA clone of the CCK-A receptor from rat pancreas. The guinea pig gallbladder CCK-A receptor was cloned by hybridization screening of a gallbladder cDNA library using a cDNA probe from the rat CCK-A receptor coding region. The guinea pig pancreas CCK-A receptor cDNA was cloned via the polymerase chain reaction using primers corresponding to the guinea pig gallbladder CCK-A receptor 5′- and 3′-noncoding regions. CCK-A receptor clones from guinea pig pancreas and gallbladder had identical nucleotide sequences, which were 80% homologous to the rat CCK-A receptor cDNA sequence. The deduced amino acid sequence from guinea pig CCK-A receptors was 89% homologous to the rat CCK-A receptor sequence. Dose-inhibition binding studies of transiently expressed receptors by CCK agonists and antagonists exhibited a CCK-A receptor pharmacologically similar to the rat CCK-A receptor. These studies indicate that the CCK-A receptors in guinea pig pancreas and gallbladder are identical and do not support previous proposals that they may represent different receptor subtypes.
Keywords: cholecystokinin
TWO OF THE MAIN physiological functions of cholecystokinin (CCK) are the stimulation of pancreatic enzyme secretion and gallbladder contraction (9, 27, 28). A comparison of the ability of CCK and its analogues to stimulate pancreatic acinar secretion and gallbladder muscle contraction suggests that the receptors for CCK on these cells are each CCK-A receptors interacting with high affinity only with CCK analogues sulfated in the seventh position from the carboxy-terminal (10, 31, 32). However, a number of biochemical and pharmacological studies suggest that pancreatic and gallbladder CCK-A receptors may represent different subtypes.
CCK analogues such as Suc-(Trp229)-CCK-7 have different potencies for stimulating gallbladder contraction and pancreatic enzyme release (36). This analogue stimulates pancreatic enzyme secretion in rats with approximately equal potency to CCK-8, whereas it is less potent than CCK-8 in stimulating guinea pig gallbladder contraction. Some analogues of the CCK receptor antagonist proglumide exhibit different potencies for the inhibition of pancreatic enzyme secretion and gallbladder contraction (12, 19, 20, 36). Changes in the aliphatic chain length in two proglumide analogues caused a two-fold decrease in affinity for the pancreatic CCK receptor (12), in contrast to a 20- to 26-fold decrease in potency for inhibiting gallbladder contraction (12). Similarly, changing the 4-chloro group to a 3,4-dichloro group on the benzoyl moiety results in a 15-fold increase in affinity for the pancreatic CCK receptor with no change in the ability to inhibit CCK-8-induced gallbladder contraction (20). Physiological and pharmacological studies examining the ability of proglumide analogues to inhibit CCK-8-stimulated amylase release from pancreatic acini and gallbladder contraction demonstrated a 10- to 30-fold different affinity for pancreatic and gallbladder CCK receptors for 3 of 10 proglumide analogues examined (12, 20). Biochemical characterizations of the CCK receptor using affinity cross-linking of radiolabeled CCK analogues revealed a protein of molecular weight 85,000–95,000 in rat pancreas (23) compared with a protein of molecular weight 70,000–80,000 in the bovine smooth muscle (28–30). These differences in the pharmacological and biochemical characterizations between CCK receptors from pancreas and gallbladder suggest that they might possess different subtypes of the CCK-A receptor. Alternatively, the differences between pancreatic and gallbladder receptors may simply be explained on the basis of a species difference, differences in experimental conditions, or differences in posttranslational processing. To unequivocally address this question, we used the sequence of the recently cloned rat pancreatic CCK-A receptor cDNA (34) to clone and functionally express both the guinea pig pancreas and gallbladder CCK-A receptors.
MATERIALS AND METHODS
Tissue procurement and mRNA isolation.
Male Hartley guinea pigs (150–175 g) were obtained (Small Animal Section, Veterinary Resources Branch, National Institutes of Health) and killed. Guinea pig pancreases and gallbladders were dissected and immediately frozen in liquid nitrogen. Total RNA was extracted using a low-temperature guanidine isothiocyanate-guanidine hydrochloride method (7). Poly (A)+ mRNA was isolated using oligo(dT) cellulose (Bethesda Research Laboratory, Gaithersburg, MD).
Construction of a guinea pig gallbladder cDNA library and isolation of cDNA clones.
Oligo(dT)-primed cDNA >2 kb was size selected by agarose gel electrophoresis, electroeluted, adapted with EcoR I (New England Biolabs, Beverly, MA), ligated into the phage vector λgt10, and in vitro packaged using established methods (4). Approximately 8 × 105 plaques were screened with a 32P-labeled randomly primed probe generated from the rat CCK-A receptor coding region. Duplicate filters were washed once at room temperature for 5 min in 2× standard saline citrate (SSC; 2× SSC = 300 mM NaCl and 3 mM sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) and three times at 55°C for 20 min in 0.1× SSC and 0.1% SDS, dried, and examined by autoradiography after 2 days using methods previously described (4). Positive clones were plaque purified, and the cDNA insert was excised with Not 1 (New England Biolabs) and subcloned into the vector pCDNA-1 (Invitrogen, San Diego, CA) and pCDL-SRα (32) at the Not 1 restriction site.
CCK-A receptor cDNA cloning from guinea pig pancreas.
Approximately 5 ng of single-stranded cDNA was reverse transcribed from oligo(dT)-primed poly (A)+ mRNA and amplified using 0.5-μM sense (5′-GTTGCCTTTAGGAATGGCTGC-3′)and antisense (5′-CAGTACAGCAGGAAGGGGCC-3′) primers corresponding to the 5′- and 3′-untranslated sequence of the guinea pig gallbladder CCK-A receptor cDNA under standard polymerase chain reaction (PCR; Perkin-Elmer, Norwalk, CT) conditions with the following cycle times and temperatures: 36 cycles of denaturation at 94°C for 45 s, annealing at 59°C for 25 s, extension at 72°C for 2 min, and a final cycle with an extension duration of 15 min.
DNA sequencing.
Two cDNA clones isolated from the guinea pig gallbladder cDNA library were sequenced by the dideoxy-chain termination method of Sanger et al. (26) with Sequenase version 2.0 (United States Biochemical, Cleveland, OH). The double-stranded DNA product obtained by PCR cloning from guinea pig pancreas was cycle sequenced using the dsDNA cycle sequencing system (Bethesda Research Laboratory).
Northern blot analysis of mRNAs.
Poly (A)+ mRNA was isolated from guinea pig gallbladder, pancreas, kidney, muscle, and liver as previously described (7). Poly (A)+ mRNA (4 μg/lane) was electrophoretically separated on a 1.4% agarose-formaldehyde gel before capillary transfer onto Nytran membrane (Schleicher and Schuell, Keene, NH), hybridized with the rat CCK-A receptor probe, labeled with 32P by random priming, washed under high-stringency conditions (3 × 20 min at 55°C in 0.2× SSC and 0.1% SDS), and examined by autoradiography using a phosphorimager (Molecular Dynamics, Sunnyvale, CA) after 24 h exposure (4).
Expression of CCK-A receptor cDNAs in mammalian cells.
pCDL-SRα, (2 μg) containing the CCK-A receptor cDNA coding region insert subcloned at the Not I site in the sense orientation was transfected into a near-confluent 100-mm tissue culture plate containing ~1 × 106 COS-7 cells using a diethylaminoethyl-dextran method previously described (3). Approximately 48 h posttransfection, the cells were washed twice at 4°C with phosphate-buffered saline (pH 7.4) containing bovine serum albumin (BSA; 1 mg/ml) at 4°C, scraped from the plate in 4°C Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY) containing BSA at 1 mg/ml, centrifuged (400 g), and resuspended in the same medium at 4°C (~3 × 105 cells/ml). Resuspended cells (500 ~1) were incubated for 60 min at 37°C with 50 pM of the radiolabeled hormone, 1251-Bolton-Hunter CCK-8 (1251-BH-CCK-8; 2,200 Ci/mmol; New England Nuclear, Boston, MA) either with or without varying concentrations of unlabeled agonist or antagonist. Cells were subsequently washed three times at 4°C with 2 ml phosphate-buffered saline containing BSA (1 mg/ml) by filtration on glass-fiber filters (Whatman GF/C, Maidstone, UK) using a suction manifold (Millipore, Bedford, MA). Filters were assayed for gamma radioactivity (Auto-gamma, Packard, Downers Grove, IL).
DNA and protein sequence analysis.
Nucleotide and amino acid sequences were analyzed by the Wisconsin Genetics Computer Group software package using the “Pileup” program (5).
RESULTS
Cloning of the CCK-A receptor from gallbladder and pancreas.
Screening of an oligo(dT)-primed cDNA library constructed in the phage vector λgt10 with a 32P-labeled random-primed probe corresponding to the rat pancreatic CCK-A receptor cDNA coding: region under high-stringency conditions identified 150 strongly hybridizing clones. Eighteen clones were randomly selected and isolated as single clones after three rounds of plaque purification. Two clones with the longest cDNA insert (2.9 kb) were selected for further structural and functional characterization. The 1,621-base pair nucleotide sequence common to both clones and the deduced amino acid sequence are shown in Fig. 1. The first in frame ATG consistent with a consensus translation initiation site represents the start codon (17) of a single long open reading frame encoding a 450-amino acid protein with a calculated molecular weight of 48,200. The sequence allows for three potential N-linked glycosylation sites in the amino-terminal and one in the second extracellular loop (residues 11, 14, 34, and 190; Fig. 1). There are nine potential sites for protein kinase A and protein kinase C phosphorylation (residues 248, 274, 411, 414, 416, and 419; Fig. 1) (14).
Fig. 1.
Nucleotide and deduced amino acid sequence of the cholecystokinin A (CCK-A) receptor in guinea pig gallbladder and pancreas. Lines labeled with Roman numerals delineate putative 7 transmembrane domains predicted by Kyte and Doolittle (18) criteria and homology with other G protein-coupled receptor superfamily members. Triangles indicate potential sites for N-linked glycosylation. Underlines indicate potential sites for serine and threonine phosphorylation.
Hydropathy analysis of the predicted amino acid sequence using the criteria of Kyte and Doolittle (18) reveals seven regions of hydrophobic residues corresponding to transmembrane spanning domains. Using single-stranded cDNA reverse transcribed from poly (A)+ mRNA from guinea pig pancreas as a template and primers flanking the coding region of the rat pancreas CCK-A receptor cDNA (34), we used the PCR to generate a single ~1.6-kilobase product (data not shown). PCR in the presence of cDNA from tissue not known to possess CCK-A receptors or without target DNA did not result in any amplified products. Cycle sequencing of this product revealed an identical nucleotide sequence with both of the clones isolated from the guinea pig gallbladder cDNA library. A comparison of the nucleotide and amino acid sequence between the guinea pig CCK-A receptor and the rat CCK-A receptor using the Gap sequence analysis program (5) shows the expected high homology of 80% (data not shown) for the nucleotide sequence and 89% for the amino acid sequence (Fig. 2).
Fig. 2.
Alignment of guinea pig CCK-A receptor (GPCCK-AR) and rat CCK-A receptor (RTCCK-AR) deduced protein sequences. By use of Gap program sequence analysis package of Genetics Research Group (5), guinea pig CCK-A receptor deduced protein sequence was aligned for maximal homology with rat CCK-A receptor deduced protein sequence (33). Solid lines denote amino acid identity, and dotted lines denote relative degree of conservative substitutions. Solid lines labeled with Roman numerals indicate 7 putative transmembrane domains.
Northern blot hybridization.
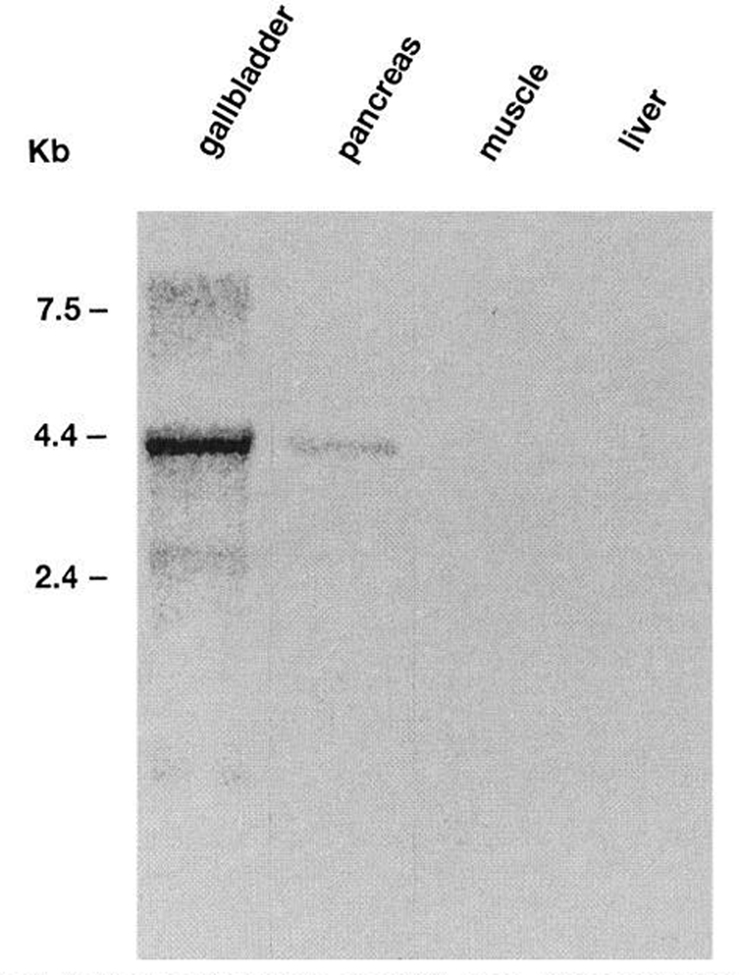
High-stringency Northern blot analysis of organ and tissue specific polyadenylated mRNA from guinea pig gallbladder, pancreas, kidney, muscle, and liver using the CCK-A receptor cDNA probe reveals a 4.4-kilobase hybridizing transcript in guinea pig gallbladder and pancreas. No hybridization was observed in the guinea pig kidney, muscle, or liver (Fig. 3).
Fig. 3.
Northern blot analysis of mRNA from guinea pig tissues. A radiolabeled probe of rat CCK-A receptor coding region was hybridized to 4 μg of poly (A)+ mRNA from each tissue per lane and washed under conditions of high stringency. A 4.4-kilobase hybridizing transcript can be identified in mRNA from guinea pig gallbladder and pancreas. No hybridizing mRNA could be identified in mRNA from guinea pig muscle and liver.
Pharmacological characterization of transfected receptors.
To identify the pharmacological profile of the cloned CCK-A receptor cDNA sequence common to both gallbladder and pancreas, the full-length cDNA insert from one of the gallbladder clones was subcloned into the mammalian vector pCDL-SRα at the Not I site and transiently expressed in COS-7 cells using diethylaminoethyldextran (3). The transfected COS-7 cells were incubated with the radiolabeled ligand 125I-BH-CCK-8 alone or in the presence of increasing concentrations of unlabeled CCK receptor agonists CCK-8 and gastrin-17-I and antagonists L-364,718 and L-365,260 for 60 min at 37°C.
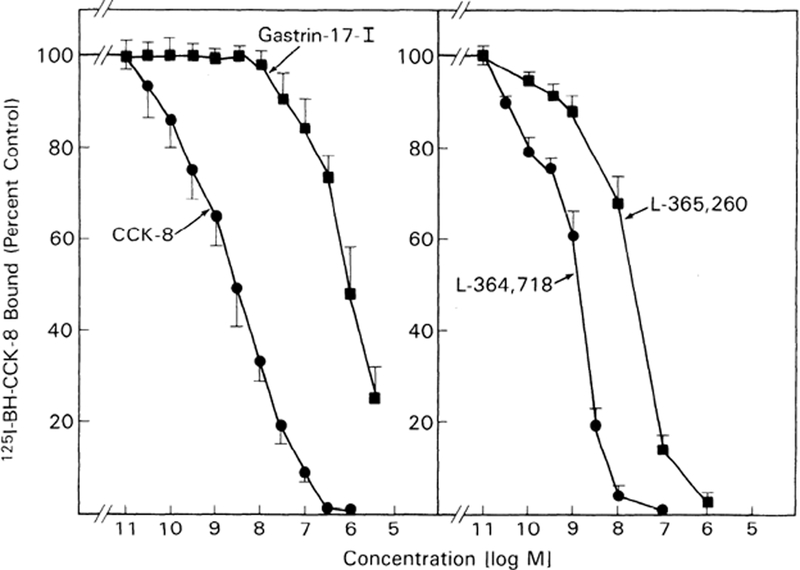
Inhibition of binding of 125I-BH-CCK-8 by CCK-8 was detectable at 30 pM, half maximal at 3.3 nM, and complete by 0.3 μM (Fig. 4). Gastrin-17-I was 1,000-fold less potent than CCK-8 with detectable inhibition at 30 nM and half-maximal inhibition at 7.3 μM (Fig. 4). The CCK-A receptor specific antagonist, L-364,718, was equally potent to CCK-8 and 100-fold more potent than the CCK-B receptor specific antagonist, L-365,260. Inhibition by L-364,718 was detectable at 30 pM, half maximal at 1.65 nM, and complete by 100 nM (Fig. 4). For L-365,260, inhibition was detectable at 0.1 nm, half maximal at 29 nM, and complete by 1 μM (Fig. 4). No binding activity was detected in untransfected COS-7 cells, and nonsaturable binding was always <20% of saturable binding.
Fig. 4.
Ability of CCK receptor agonists and antagonists to inhibit binding of 125I-Bolton-Hunter (BH) CCK-8 to COS-7 cells transfected with guinea pig gallbladder CCK-A receptor cDNA. COS-7 cells were transfected with expression vector pCDL-SRα containing CCK-A receptor cDNA sequence. 125I-BH-CCK-8 (50 PM) was incubated either alone or with increasing concentrations of agonists (CCK-8 and gastrin-17-I) (left) or antagonists (L-364,718 and L-365,260; right). Data are presented as percent of control saturable binding (total binding in presence of radio-labeled hormone alone minus binding in presence of 1 μM CCK-8). Results are means ± SE from at least 3 experiments performed in duplicate.
DISCUSSION
We have cloned a cDNA encoding a CCK-A receptor from a guinea pig gallbladder cDNA library using a radiolabeled full-length coding region probe of the recently cloned CCK-A receptor from rat pancreas (34). Using single-stranded cDNA from guinea pig pancreas as a template and primers flanking the coding region of the guinea pig gallbladder CCK-A receptor cDNA, we PCR cloned a cDNA having an identical nucleotide sequence to clones isolated from the gallbladder cDNA library.
A comparison between the nucleotide and amino acid sequences between the guinea pig and rat CCK-A receptors indicates 80 and 89% homology, respectively. This high degree of sequence homology is in the range expected for the same receptor in two different species (24).
The second in-frame ATG was chosen as the start codon of the single long open reading frame for two reasons. First, it is surrounded by an appropriate consensus sequence for an initiation site (17). Second, the alignment between the amino acid sequence of guinea pig and rat CCK-A receptors shown in Fig. 2 indicates a consistent high degree of sequence homology beginning at the second in-frame ATG of both the rat and guinea pig CCK-A receptors. This same rationale suggests that the second ATG with a consensus initiation sequence within the cDNA sequence of the CCK-A receptor of the rat pancreas should be the actual start codon. The CCK-A receptor from rat pancreas would then have a length of 429 amino acids rather than the 444 amino acids previously reported when the first ATG with a consensus initiation sequence was proposed as the start codon (34). This would make the 430-amino acid guinea pig CCK-A receptor closer in size to the rat CCK-A receptor as expected for the same receptor in two closely related species (33).
Hydropathy analysis of the predicted amino acid sequence using the criteria of Kyte and Doolittle (18) reveals seven regions of hydrophobic residues corresponding to transmembrane-spanning domains similar to the rat CCK-A receptor and suggests its membership in the superfamily of G protein-coupled receptors. Like the rat, the guinea pig CCK-A receptor has the same number of potential glycosylation sites and conserved cysteines in the first and second extracellular loop for potential disulfide bond formation (6, 8, 14) and in the carboxy-terminal for potential membrane-anchoring palmitoylation (21, 22). Similar to the rat CCK-A receptor, there are also a number of potential phosphorylation sites in the third intracellular loop and carboxy-terminal (14), some of which may become phosphorylated with receptor stimulation (15, 16). These areas may also represent potential sites for receptor regulation.
High-stringency Northern blot analysis of organ- and tissue-specific polyadenylated RNA using the rat CCK-A receptor cDNA probe reveals a 4.4kilobase hybridizing transcript in guinea pig gallbladder and pancreas consistent with the sequence identity between these two organs. As expected, no signal was observed in tissues like skeletal muscle and liver. The size of the hybridizing transcript is consistent with the cloned cDNA size and stands in contrast to the 2.7-kilobase hybridizing mRNA identified for the CCK-A receptor in rat pancreas (34). Repeat Northern blot analysis under low-stringency conditions did not identify additional hybridizing transcripts and suggests that the predominant pancreatic receptor is the same CCK-A receptor also found in the gallbladder. However, one cannot rule out the possibility of another CCK receptor subtype either present in too small a quantity to detect or whose sequence is sufficiently different from the CCK-A receptor to detect by hybridization even under low-stringency conditions.
Pharmacological characterization of the guinea pig gallbladder and pancreas CCK-A receptor cDNA in COS-7 cells was consistent with a CCK-A receptor sub-type having 1,000-fold greater affinity for the sulfated agonist CCK-8 and 100-fold greater affinity for the CCK-A receptor specific antagonist L-364,718 (10, 11, 27). In addition, the 50% inhibitory concentrations of the transfected receptor for both the agonists (CCK-8 3.3 ± 0.9 nM and gastrin-17-I 7.3 ± 1.65 μM) and the antagonists (L-364,718 1.65 ± 0.2 nM and L-365,260 29 ± 1.7 nM) are in close agreement with binding studies performed on isolated gallbladder smooth muscle cells and gallbladder tissue sections in guinea pig (25, 33). These data support the classification of these receptors cloned from guinea pig gallbladder and pancreas as a CCK-A receptor subtype.
Gallbladder and pancreatic CCK receptors have been classified as a CCK-A receptor subtype on the basis of similar pharmacological and physiological responses to the CCK family of peptides. Several studies have demonstrated that CCK acts directly on pancreatic acini and smooth muscle cells of the gallbladder (2, 10, 25, 31, 36). Because of the technical difficulty in obtaining CCK-A receptor-enriched membranes from gallbladder smooth muscle in small animals, characterization of the CCK receptor by chemical cross-linking has been reported in species other than the guinea pig (cow), making it difficult to compare the receptor types within the same species. Therefore it cannot be ruled out that the differences in molecular weight reported for bovine gallbladder CCK-A receptor, molecular weight 70,000–80,000 (29) and rat pancreas CCK-A receptor, molecular weight 85,000–95,000 (23), were probably due to species differences. This possibility is supported by binding and localization studies performed under identical experimental conditions in the same species that did not reveal significant differences in affinity for various CCK agonists and antagonists in guinea pig gallbladder and pancreas (33).
Observed differences in affinity of proglumide antagonists between the gallbladder and pancreatic CCK-A receptors (11, 17) could not be demonstrated by others (33) but may be explained by different experimental conditions that make these comparisons difficult. Our study demonstrating that CCK receptors independently cloned from gallbladder and pancreas within the same species have identical cDNA sequences does not support any differences between gallbladder and pancreatic CCK-A receptors previously reported using proglumide analogues (11, 17). It cannot, however, be ruled out that tissue or cell specific posttranslational processes leading to differences in structure of the gene product may explain these observed differences.
From our results, we conclude that guinea pig gallbladder and pancreas express the same CCK-A receptor sub-type. This finding has major implications that must be considered in pharmacological strategies aimed at selectively altering either gallbladder of pancreatic function. The cloning of the CCK receptor in guinea pig will provide a new tool to examine the physiological role of CCK-A receptors in gallbladder and pancreatic function.
REFERENCES
- 1.Amer MS Studies with cholecystokinin II. Cholecystokinetic potency of porcine gastrins I and II and related peptide in three systems. Endocrinology 84: 1277–1281, 1969. [DOI] [PubMed] [Google Scholar]
- 2.Chovdhury J, Berkowitz JM, Praissman M, and Fara JW. Effects of sulfated and nonsulfated gastrin and octapeptide cholecystokinin on cat gallbladder in vitro. Experientia Basel 32: 1173–1175, 1975. [DOI] [PubMed] [Google Scholar]
- 3.Cullen BR Molecular genetics of mammalian cells. Methods Enzymol. 152: 684–704, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Davis L, Dibner MD, and Battey JF. Basic Methods in Molecular Biology. New York: Elsevier, 1986, p. 1–388. [Google Scholar]
- 5.Devereux J, Haeberli P, and Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12: 387–395, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon RA, Sigal IS, Candelore MR, Register RB, Rands E, and Strader CD. Structural features required for ligand binding to the beta adrenergic receptor. EMBO J. 6: 3269–3275, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han JH, Stratowa C, and Rutter WJ. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry 26: 1617–1625, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Hulme EC, Birdsall NJ, and Buckley NJ. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 30: 633–673, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Jensen RT, Lemp GF, and Gardner JD. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 77: 2079–2083, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen RT, Lemp GF, and Gardner JD. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J. Biol. Chem. 257: 5554–5559, 1982. [PubMed] [Google Scholar]
- 11.Jensen RT, von Schrenck T, Yu D-H, Wank SA, and Gardner JD. The Neuropeptide Cholecystokinin (CCK). Anatomy and Biochemistry Receptors, Pharmacology and Physiology London, UK: Ellis Horwood, 1989, p. 150–162. [Google Scholar]
- 12.Jensen RT, Zhou Z-H, Murphy RB, Jones SW, Setnikar I, Rovati LA, and Gardner JD. Structural features of various proglumide-related cholecystokinin receptor antagonists. Am. J. Physiol. 251 (Gustrointest. Liver Physiol. 14): G839–G846, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Karnik SS, Sackmann JP, Chen HA, and Khorana G. Cysteine residues 180–187 are essential for the formation of the correct structure in bovine rhodopsin. Proc. Natl. Acad. Sci. USA 85: 8459–8463, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenelly PJ, and Krebs GE. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266: 15555–15558, 1991. [PubMed] [Google Scholar]
- 15.Kishimoto A, Nishijama K, Nakanishi H, Uratsuji Y, Nomura H, Takeyama Y, and Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3′,5′-monophosphate dependent protein kinase. J. Biol. Chem. 260: 12492–12499, 1985. [PubMed] [Google Scholar]
- 16.Klueppelberg UG, Gates LK, Gorelick FS, and Miller LJ. Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J. Biol. Chem. 266: 2403–2408, 1991. [PubMed] [Google Scholar]
- 17.Kozak M Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 266: 19867–19870, 1991. [PubMed] [Google Scholar]
- 18.Kyte J, and Doolittle RE. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157: 105–132, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Macovec F, Christie R, Bani M, Pacini M, Setnikar I, and Rovati LA. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arznein-Borsch. 36: 1048–1051, 1985. [PubMed] [Google Scholar]
- 20.Macovec F, Christie R, Bani M, Revel L, Setnicar I, and Rovati AL. New glutaramic and aspartic derivates with potent CCK-antagonistic activity. Eur. J . Med. Chem. 21: 9–20, 1986. [Google Scholar]
- 21.O’Dowd B, Hnatowich M, Caron MB, Lefkowitz RJ, and Bouvier M. Site directed mutagenesis of the cytoplasmatic domains of the human beta-2 adrenergic receptor. Localisation of regions involved in G-protein-receptor coupling. J. Biol. Chem. 264: 7564–7569, 1988. [Google Scholar]
- 22.Ovchinikov YA, Ablulajew NG, and Bogachuck AS. Two adjacent cysteine residues in the C-terminal cytoplasmatic fragment of bovine rhodopsin are palmitoylated. FEBS Lett. 230: 1–5, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Pearson RK, Miller LJ, Hadac EM, and Powers SP. Analysis of the carbohydrate composition of the pancreatic plasmalemmal glycoprotein affinity labeled by short probes for the cholecystokinin receptor. J. Biol. Chem. 262: 13850–13856, 1987. [PubMed] [Google Scholar]
- 24.Pisegna JR, de Weerth A, and Wank SA. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem. Biophys. Res. Commun. 189: 296–303, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin B, and Engel SL. Some biological characteristics of cholecystokinin (CCK-PZ) and synthetic analogues Nobel symposium XVI. In: Frontiers in Gastrointestinal Hormone Research; Stockholm: Almquist & Wiksell, 1973, p. 41–56. [Google Scholar]
- 26.Sanger F, Nicklen S, and Coulsen AR. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74: 5463–5467, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sankaran H, Goldfine ID, Deveney WC, Wonga KY, and Williams JA. Binding of cholecystokinin to high affinity receptors on rat pancreas. J. Biol. Chem. 255: 1849–1853, 1980. [PubMed] [Google Scholar]
- 28.Schjoldager B, Molero X, and Miller LJ. Functional and biochemical characterization of the human gallbladder muscularis cholecystokinin receptor. Gastroenterology 96: 119–125, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Schjoldager B, Shaw MJ, Powers SA, Schmalz PH, Szurszewski J, and Miller LJ. Bovine gallbladder muscularis: source of a myogenic receptor for cholecystokinin. Am. J. Physiol. 254 (Gastrointest. Liver Physiol. 17): G294–G299, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Shaw MJ, Hadac EM, and Miller LJ. Preparation of enriched plasma membranes from bovine gallbladder muscularisfor characterization of cholecystokinin receptors. J. Biol. Chem. 262: 313–318, 1987. [PubMed] [Google Scholar]
- 31.Steigerwald RW, Goldfine ID, and Williams JA. Characterization of cholecystokinin receptors on bovine gallbladder. Am. J. Physiol. 247 (Gastrointest. Liver Physiol. 10): G709–G714, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Takebe Y, Seiki M, Fujisava J-I, Hoy P, Yokota K, Arai K-I, Yoshida M, and Arai N. SR promotor: an efficient and versatile mammillian cDNA expression system composed of the simian virus 40 early promotor and the RU-5 segment of human T-cell leukemia virus type long terminal repeat. Mol. Cell. Biol. 32: 466–472, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Schrenck T, Moran TH, Heinz-Erian P, Gardner JD, and Jensen RT. Cholecystokinin receptors on gallbladder muscle and on pancreatic acinar cells: a comparative study. Am. J. Physiol. 255 (Gastrointest. Liver Physiol. 18): G512–G521, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Wank SA, Harkins R, Jensen RT, Shapira H, de Weerth A, and Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc. Natl. Acad. Sci. USA 89: 3125–3129, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wank SA, Pisegna JR, and de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc. Natl. Acad. Sci. USA 89: 8912–8917, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanaihara C, Sigura N, Kashimoto K, Kondo M, Kawamura M, Naruse S, Yasui A, and Yanaihara N. Dissociation of pancreozymin (PZ) activity from cholecystokinin (CCK) activity by Na-carboxyacyl CCK-7 and CCK-8 analogues with substituted glycine. Biomed. Res. 6: 111–115, 1985. [Google Scholar]
- 37.Yau WM, Macklouf GM, Edwards LE, and Farrar JT. The mode of action of cholecystokinin and related peptides on gallbladder muscle. Gastroenterology 65: 451–456, 1973. [PubMed] [Google Scholar]