Abstract
Background & Aims:
Parenteral control of gastric acid hypersecretion in conditions such as Zollinger–Ellison syndrome (ZES) or idiopathic gastric acid hypersecretion is necessary perioperatively or when oral medications cannot be taken for other reasons (e.g., during chemotherapy, acute upper gastrointestinal bleeding, or in intensive care unit settings).
Methods:
We evaluated the efficacy and safety of 15-minute infusions of the proton pump inhibitor pantoprazole (80–120 mg every 8–12 hours) in controlling acid output for up to 7 days. Effective control was defined as acid output >10 milliequivalents per hour (mEq/h) (<5 mEq/h in patients with prior acid-reducing surgery) for 24 hours.
Results:
The 21 patients enrolled had a mean age of 51.9 years (range, 29–75) and a mean disease duration of 8.1 years (range, <0.5–21); 13 were male, 7 had multiple endocrine neoplasia syndrome type I, 4 had undergone acid-reducing surgery, 2 had received chemotherapy, and 13 had undergone gastrinoma resections without cure. Basal acid output (mean ± SD) was 40.2 ± 27.9 mEq/h (range, 11.2–117.9). In all patients, acid output was controlled within the first hour (mean onset of effective control, 41 minutes) after an initial 80-mg intravenous pantoprazole dose. Pantoprazole, 80 mg every 12 hours, was effective in 17 of 21 patients (81%) for up to 7 days. Four patients required upward dose titration, 2 required 120 mg pantoprazole every 12 hours, and 2 required 80 mg every 8 hours. At study end, acid output remained controlled for 6 hours beyond the next expected dose in 71% of patients (n = 15); mean acid output increased to 4.0 mEq/h (range, 0–9.7). No serious or unexpected adverse events were observed.
Conclusions:
Intravenous pantoprazole, 160–240 mg/ day administered in divided doses by 15-minute infusion, rapidly and effectively controlled acid output within 1 hour and maintained control for up to 7 days in all ZES patients.
Gastrinomas are gastrin-secreting neuroendocrine tumors that generally originate in the head or neck of the pancreas or the proximal duodenum.1,2 Uncontrolled release of gastrin by these tumors leads to Zollinger– Ellison syndrome (ZES), which is characterized by the presence of a non–beta islet cell tumor of the pancreas, gastric acid hypersecretion, and severe, often fulminant, peptic ulceration.3 Gastrinomas may occur sporadically or as part of the multiple endocrine neoplasia syndrome type I (MEN-I).2 Before histamine H2-receptor antagonists (H2RAs) were available in the mid-1970s, total gastrectomy was the only effective therapy for patients with ZES, and complications resulting from gastric acid hypersecretion were the major causes of morbidity and mortality.1,4
H2RAs decrease gastric acid output by blocking the action of histamine on the parietal cell basolateral membrane.1,5 In recent years, the use of oral H2RAs has been supplanted by proton pump inhibitors (PPIs), which are more effective, easier to use, and are not associated with tachyphylaxis.1,5–8 PPIs are members of a new class of highly potent antisecretory agents that bind directly and irreversibly to parietal cell proton pumps (H+,K+-adenosine triphosphatase [ATPase]), preventing hydrogen ion exchange until new pumps are synthesized.9 All PPIs are weak bases that rapidly diffuse from the blood into parietal cells across the basolateral membrane (the serum half-lives for all PPIs are less than 3 hours). However, because PPIs are weak bases, they diffuse out of the cell into secretory canaliculi, accumulate in the acidic spaces generated by the proton pump, and undergo conversion to a cationic sulfonamide derivative.9 The specificity for the interaction of PPIs with secretory canalicular H+,K+-ATPase is explained by covalent disulfide binding between the acid-catalyzed sulfonamide product and specific cysteine residues of the H+,K+-ATPase.10 Their duration of action is thus determined more by parietal cell synthesis and proton pump turnover rates than their serum half-lives. Because PPIs block the final common step of gastric acid formation, they are effective in blocking secretion stimulated by histamine, gastrin, acetylcholine, or pituitary adenylate cyclase–activating polypeptide.11,12 With the advent of effective medical management of gastric acid hypersecretion, tumor growth and metastases are now the major determinants of long-term survival in patients with ZES.1,13
Current management of ZES includes the use of surgical resection and chemotherapy, which makes parenteral control of gastric acid hypersecretion increasingly important because patients are unable to tolerate oral intake.1,5 In addition, oral medications cannot be taken during acute illnesses such as gastroenteritis and pancreatitis, acute surgical conditions such as appendicitis, or during intensive care unit management.1,5 Inadequate management of acid hypersecretion in such situations can result in rapid and life-threatening gastrointestinal hemorrhage that requires endoscopic intervention and control of gastric acid hypersecretion.1,5 There are currently no intravenous (IV) PPIs available for general use in the United States. H2RAs have been used to suppress acid output in the absence of IV PPIs, but their use is limited by the need for continuous IV infusion of very high doses in patients with ZES.5 Moreover, this approach requires the use of continuous-infusion pumps with careful monitoring of acid output because of tachyphylaxis or other technical problems. Studies of IV H2RAs in ZES patients have shown them to be effective. For example, acid output can be safely and effectively controlled with continuous IV ranitidine at a dose of 0.5–0.9 mg · kg−1 · h−1 in 36% of patients, 1.0–1.4 mg · kg−1 · h−1 in an additional 48%, and 1.5–2.5 mg · kg−1 · h−1 in the remaining 16% for up to 11 days of therapy.14 In another continuous-infusion study, 4 mg · kg−1 · h−1 of ranitidine controlled acid output in all patients,15 whereas results from previous studies with an IV PPI indicated that acid output could be controlled in all patients with bolus injections of drugs so that continuous IV infusion was not needed.5,16–18
Recently, an IV formulation of the novel PPI pantoprazole has become available for use in the management of hypersecretory conditions in the United States. Pantoprazole is a substituted benzimidazole derivative with a chemical structure of 5-(difluoromethoxy)-2-[[(3,4dimethoxy-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole.19 In healthy male study subjects, IV pantoprazole produced a dose-dependent and long-lasting inhibition of pentagastrin-stimulated gastric acid output when administered as a single, brief (15-minute) IV infusion.20,21 We conducted a multicenter trial to evaluate the safety and efficacy of IV pantoprazole in patients with established ZES using a worst-case scenario (i.e., attempting to inhibit acid output beginning from a level of acid hypersecretion rather than controlled acid output). Another aim of this study was to identify a maintenance regimen (dose and dose frequency) for controlling acid output in all ZES patients to avoid the need for continuous acid output monitoring during use. This study provides the first evidence that IV pantoprazole rapidly and effectively controls gastric acid hypersecretion in patients with ZES.
Materials and Methods
Patients
All participants were diagnosed with ZES according to established criteria: increased serum gastrin level (normal <100 pg/mL) in the presence of gastric acid secretion (>15 mEq/h or >10 mEq/h if the patient had previously undergone gastric acid-reducing surgery); positive provocative testing results with secretin stimulation (increase in serum gastrin >200 pg/mL after IV injection) or with calcium infusion (increase in serum gastrin >395 pg/mL); positive histological diagnosis of gastrinoma; or a combination of these criteria.1,2 Patients with both sporadic and MEN-I–associated disease were eligible. All patients were older than 18 years. Female patients were not pregnant and were using medically acceptable contraception. All patients gave written informed consent. This in-patient study was conducted in compliance with the Institutional Review Board regulations of each respective medical center and was performed in the National Institutes of Health–sponsored General Clinical Research Center at each participating institution. Each patient had a comprehensive history taken and underwent a complete physical examination. As part of the prescreening evaluation, a complete ophthalmologic examination, chest radiography, laboratory tests (including a complete blood cell count, chemistry, and liver function tests), gastrin, thyroid function tests, pregnancy test (for premenopausal women only), and urinalysis were performed. During the study, vital signs and electrocardiograms were also monitored, and laboratory evaluations were obtained at specified times. Ophthalmologic assessment was repeated on completion of the study.
Gastric Analysis
Gastric acid was collected before, during, and after IV pantoprazole administration with a nasogastric tube as described previously.1,2,22 The nasogastric tube was passed into the dependent portion of the stomach, and its position was verified by phenol red or by the water-recovery method to ensure adequate recovery of gastric juice. After aspiration of the residual gastric content for approximately 15 minutes, gastric acid was collected in 15-minute fractions using low, continuous suction. An aliquot of each sample was titrated to pH 7.0 with sodium hydroxide (0.01N NaOH), and the hydrogen ion content of each 15-minute sample was calculated. Acid output was expressed as milliequivalents per hour, calculated by determining the total titratable acid in 4 successive 15-minute gastric collections.23 The acid output was measured for 1 hour after drug washout before administration of pantoprazole (an approximation of the basal acid output [BAO]), continuously for the first 26 hours after pantoprazole administration, for study hours 47–50, and for 6 hours at the end of the study. Additional collections were also made before and after upward dose titrations of IV pantoprazole (see later).
Washout Period
All patients discontinued the use of PPI for 7 days before the first dose of pantoprazole to permit their acid output to approach BAO. To protect them from the effects of gastric acid hypersecretion during this period, high-dose ranitidine (750–1250 mg orally every 6 hours) was administered until 30 hours before the first dose of study drug and ad libitum use of antacid was permitted until 4 hours before the beginning of the study. Patients who developed symptoms of gastric acid hypersecretion during the washout period were admitted to the study site early so that a nasogastric tube could be inserted for the removal of gastric juice. To be eligible to receive IV pantoprazole, the rate of acid output had to be >15 mEq/h (>10 mEq/h in patients with previous acid-reducing surgery), thereby confirming the presence of a gastric acid hypersecretory state. If the acid output was below these threshold values, the washout period was extended for an additional 24 hours; if acid output still remained below these levels, the patient was removed from the study.
Study Drug Period
For all patients, the starting dose of IV pantoprazole was 80 mg infused over 15 minutes. This dose was based on the results of a dose-ranging study in healthy volunteers. The volunteers were rendered hypergastrinemic by continuous IV infusion of a maximally effective dose of pentagastrin21 to simulate ZES. The maximum dose of IV pantoprazole was defined a priori as 240 mg over a 24-hour period because this was the highest known daily dose previously used safely in humans.24 Effective control of acid output was defined a priori as <10 mEq/h in patients with intact stomachs (<5 mEq/h in patients with previous acid-reducing surgery).5 Based on data from gastric acid output measurements, the IV pantoprazole dose was increased to 120 mg (given over 15 minutes) every 12 hours or, later, to 80 mg every 8 hours, as necessary to maintain acid output below the target range for 24 hours, the primary study endpoint. Patients were treated with IV pantoprazole for a total of 3–6 days depending on their willingness to remain in an in-patient setting. Patients not controlled with the maximal permitted dose of 240 mg/24 h would have been considered study failures.
Study Drug Washout Period
After the last infusion of pantoprazole, the recovery of acid output was subsequently measured starting from 1 hour before the next normally scheduled dose (to confirm control) and continued for the next 5 hours or until acid output reached either 50% of the previously measured approximate BAO or a rate of ≥10 mEq/h. Thereafter, the patients reinstituted their previous maintenance antisecretory regimens and were discharged from the General Clinical Research Center.
Administration of IV Pantoprazole
Pantoprazole was supplied by Wyeth-Ayerst Research (Radnor, PA) as a lyophilized powder. It was reconstituted with 10 mL of sterile normal saline (0.9% NaCl), added to an infusion bag, and infused in a piggyback fashion over 15 minutes.
Ophthalmologic Evaluations
All participating subjects underwent a mydriatic ophthalmologic examination by an independent, board-certified ophthalmologist at each of the participating institutions. Examinations consisted of external examination, fundoscopy, tonometry, visual acuity, color vision, slit lamp examination, and stereo fundus photographs. Retinal photographs were obtained before study, at discharge from study, and 2 weeks after completion of the study. These were also reviewed by 2 independent ophthalmologists (Dr. S. Feman, Vanderbilt University, Nashville, TN; Dr. S. S. Hayreh, University of Iowa, Iowa City, IA).
Results
The clinical and demographic characteristics of the study cohort are shown in Table 1. Twenty-one patients with ZES (13 men, 8 women) with a mean age of 51.9 years (range, 29–75 years) were enrolled. Fourteen patients had sporadic ZES (67% of patients) and 7 had ZES associated with MEN-I syndrome (33% of patients). None of the MEN-I patients had active hyperparathyroidism when studied. The mean duration of disease was 8.1 years (range, <0.5–21 years). Although none of the study participants were cured of ZES at the time of the study, 13 had previously undergone surgical resection and 2 had received chemotherapy. The mean fasting serum gastrin was 930 pg/mL (range, 250–5270 pg/mL). Four patients had previously undergone acid-reducing surgery (3 patients with sporadic ZES and 1 with MEN-I syndrome). After the washout period, the mean immediate prestudy BAO approximation was 40.2 mEq/h (range, 11.2–117.9 mEq/h). Overall, this study population is representative of the average ZES population except for a slightly higher proportion of male subjects.6,8,13,17
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Sporadic ZES (n = 14) | ZES + MEN-I (n = 7) | All patients (n = 21) |
|---|---|---|---|
| Age (yr) | |||
| Mean (SD) | 56.4 (13.0) | 43.0 (7.9) | 51.0 (13.0) |
| Range | 29–75 | 31–52 | 29–75 |
| Sex (n [%]) | |||
| Male | 8 (57.1) | 5 (71.4) | 13 (61.9) |
| Female | 6 (42.9) | 2 (28.6) | 8 (38.1) |
| Years since ZES diagnosed | |||
| <5 | 8 | 2 | 10 |
| ≥5 to <15 | 4 | 2 | 6 |
| ≥15 | 2 | 3 | 5 |
| Range | <1–21 | <0.5–17 | <0.5–21 |
| Previous ZES therapy (n [%]) | |||
| Surgical resection | 7 (50) | 6 (85.7) | 13 (61.9) |
| Prior chemotherapy | 2 (14.3) | 0 | 2 (9.5) |
| Prior acid-reducing surgery (n [%]) | 3 (21.5) | 1 (14.3) | 4 (19.0) |
| Serum gastrin (pg/mL) | |||
| Mean (SD) | 1034 (1335) | 687 (290) | 930 (1126) |
| Range | 250–5270 | 387–1054 | 250–5270 |
| Basal acid output (mEq/h) | |||
| Mean (SD) | 49.2 (30.1) | 22.1 (7.7) | 40.2 (27.9) |
| Range | 15.2–117.9 | 11.2–33.1 | 11.2–117.9 |
The pharmacodynamics of the initial IV pantoprazole dose are summarized in Table 2 and Figure 1. The onset of action of pantoprazole was rapid in all patients studied. Acid output was reduced within 15 minutes and completely controlled in all 21 patients within 60 minutes of drug administration, and the mean onset of effective control (<10 mEq/h) was 41 minutes from the start of the infusion (Figure 1). The mean duration of controlled acid output of the initial dose of 80 mg of pantoprazole was 10.9 hours (Table 2). These responses did not differ significantly between patients with sporadic ZES and those with MEN-I–associated ZES (Table 2). Once control was achieved, the 80-mg starting dose of IV pantoprazole maintained control for 12 hours in 17 patients (81%) whose maintenance regimen was therefore 80 mg every 12 hours (Table 2). This dose maintained control of acid output in an additional 2 patients (9.5%) for more than 8 hours, but less than 12 hours, resulting in a maintenance regimen of 120 mg every 12 hours (Table 2). Finally, control of acid output was lost rapidly in the 2 last patients (9.5%) whose maintenance regimen was thus established as 80 mg every 8 hours (Table 2).
Table 2.
Mean Onset and Duration of Action of the Initial Pantoprazole Dose (80 mg)
| Population | Onset of actiona (min) |
Duration of actionb (h) |
||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| All patients by diagnosis (n = 21) | ||||
| Sporadic ZES (n = 14) | 43 (14) | 15–60 | 11.1 (2.6) | 2–12.0 |
| ZES + MEN-I (n = 7) | 39 (8) | 30–45 | 10.6 (2.8) | 4–12.0 |
| Total (n = 21) | 41 (12) | 15–60 | 10.9 (2.6) | 2–12.0 |
| All patients by regimen at efficacyc (n = 21) | ||||
| 80 mg every 12 h (n = 17) | 43 (12) | 30–60 | 12.0 (0) | 12.0–12.0 |
| 120 mg every 12 h (n = 2) | 30 (21) | 15–45 | 9.0 (0.4) | 8.75–9.25 |
| 80 mg every 8 h (n = 2) | 38 (±11) | 30–45 | 3.6 (1.6) | 2.5–4.75 |
| Total (n = 21) | 41 (12) | 15–60 | 10.9 (2.6) | 2.5–12.0 |
Onset is defined as the point at which acid output decreases to <10 mEq/h for patients who had not undergone prior acid-reducing surgery or to <5 mEq/h for patients who had undergone such surgery.
Duration of action is defined as the duration acid output remained under control after administration of IV pantoprazole with a maximal duration of 12 hours (the time at which a second IV dose was mandatory).
Efficacy is defined as control of acid output for any 24-hour period over the course of active therapy, at <10 mEq/h for patients who had not undergone prior acid-reducing surgery or <5 mEq/h for patients who had undergone such surgery.
Figure 1.
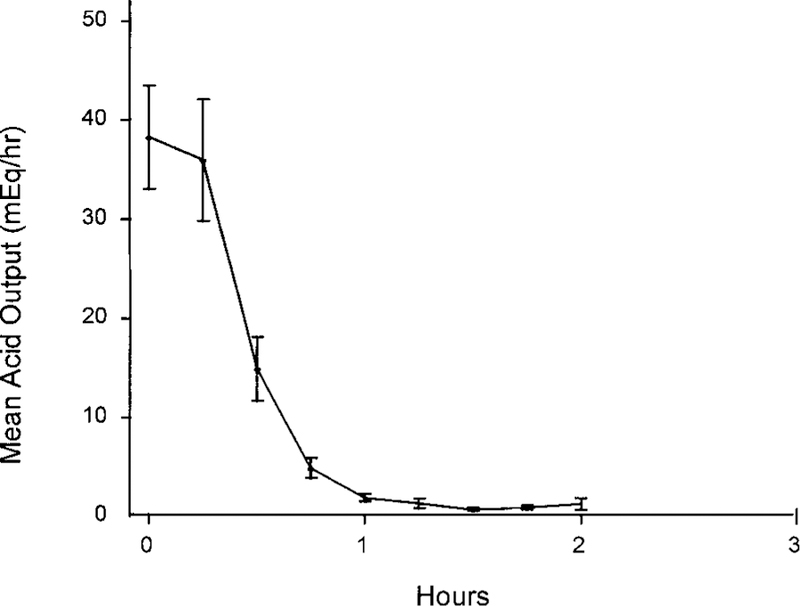
Inhibition of mean gastric acid output over the first 2 hours after the initial IV administration of 80 mg pantoprazole. Collection periods were 15 minutes long. Baseline value for these data is based on a 15-minute collection period, whereas data in Table 3 for initial acid output are based on a 1-hour collection period.
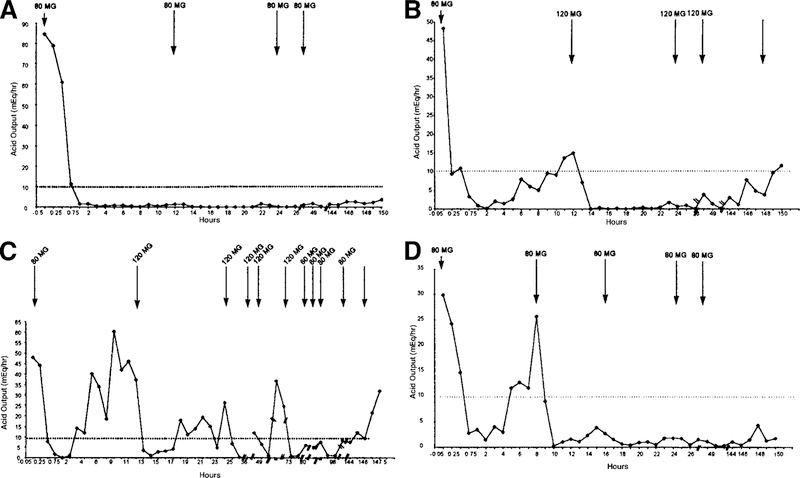
Dose requirements for maintenance of controlled acid inhibition throughout the study are summarized in Table 3. Of the 21 patients enrolled, 81% (17) were effectively controlled at a dose of 80 mg every 12 hours. This pattern of control is illustrated in Figure 2A. Two patients (9.5%), a 56-year-old white man with sporadic ZES and a 51-year-old white man with MEN-I and ZES, required 120 mg every 12 hours (Figure 2B). Two other patients (9.5%) required 80 mg every 8 hours (Figure 2C and D). The first of these 2 patients (a 52-year-old white woman with sporadic ZES) had gastric acid output that was extremely difficult to manage (Figure 2C). However, control was achieved in this patient when the dose interval was reduced to 8 hours while the previously defined maximum daily dose of 240 mg was maintained. The second of these 2 patients (a 49-year-old white man with MEN-I and ZES) was titrated up to 80 mg every 8 hours at the first opportunity, and control was achieved easily (Figure 2D). Overall, once achieved, control of acid secretion was maintained with 240 mg of IV pantoprazole per 24 hours for at least 24 hours in all patients. The degree of acid suppression achieved exceeded the study endpoint requirement in all cases (Table 3). The mean onset time of the initial 80 mg dose of IV pantoprazole was similar for all 3 treatment regimens (Table 2).
Table 3.
Maintenance Dose Requirements
| Variable | Sporadic ZES (n = 14) | ZES + MEN-I (n = 7) | Total (n = 21) |
|---|---|---|---|
| Initial acid output (mEq/h) | |||
| Mean (SD) | 49.2 (30.1) | 22.1 (7.7) | 40.2 (27.9) |
| Range | 15.2–117.9 | 11.2–33.1 | 11.2–117.9 |
| Acid output at evaluabilitya (mEq/h) | |||
| Mean (SD) | 1.9 (2.3) | 1.6 (1.2) | 1.8 (2.0) |
| Range | 0.0–7.4 | 0.6–4.1 | 0.0–7.4 |
| Patients controlled (n) | |||
| 80 mg every 12 h | 12 | 5 | 17 |
| 120 mg every 12 h | 1 | 1 | 2 |
| 80 mg every 8 h | 1 | 1 | 2 |
| Total patients controlled (N) | 14 | 7 | 21 |
Evaluability is defined as the time point at which acid output had been controlled for the previous 24 hours, i.e., the time point at which efficacy was reached.
Figure 2.
Gastric acid output curves after IV pantoprazole in 4 representative ZES patients. For 17 of 21 patients (81% of the study cohort), acid secretion was controlled with 80 mg IV pantoprazole every 12 hours (a representative example of this pattern of control is shown in A). Two patients (9.5%) required 120 mg IV pantoprazole every 12 hours (a representative example of this pattern of control is shown in B). (Cand D) Two patients (9.5%) required 80 mg IV pantoprazole every 8 hours. (C)The patient’s condition was uncontrolled with twice daily therapy, but after the study protocol was amended to permit dosing every 8 hours, control was achieved rapidly. (D) The dose interval was shortened to 8 hours in a patient after the first control measurement showing loss of control with immediate results.
Seventeen patients were maintained on study drug for an additional 5 days (a total of 7 days from the time of the first dose); 4 patients, including 1 who withdrew because of a headache, elected to discontinue treatment with the study medication after achieving the primary study endpoint. Acid recovery was assessed in all 21 patients at withdrawal (Table 4). The mean acid output in the last hour before the next normally scheduled dose of medication (12 hours in 19 patients and 8 hours in 2 patients) was 2.6 mEq/h (range, 0–7.9 mEq/h). One hour after the next expected dose would have been given, the mean acid output was 2.8 mEq/h (range, 0–12 mEq/h) and 2 patients (9.5%), a 52-year-old white woman and the 51-year-old white man, both with sporadic ZES, lost acid control rapidly after discontinuation (Table 4). Three hours after missing the last dose, 2 additional patients (a 52-year-old white woman and a 75-year-old black man, both with sporadic ZES) no longer had their acid output controlled (Figure 2B and C). Five hours after missing the last dose, the mean acid output had increased to 4.0 mEq/h (range, 0–9.7 mEq/h), and an additional 2 patients (29% in total), a 56-year-old white man and a 29-year-old black woman, both with sporadic ZES (Figure 2B and C), were no longer effectively controlled (Table 4). None of these proportional values differed between sporadic and MEN-I–associated patients. (Acid output was controlled in both patients who were maintained with pantoprazole every 8 hours, 5 hours after missing their first dose.)
Table 4.
IV Pantoprazole Offset
| Population | Acid output (mEq/h) |
|||
|---|---|---|---|---|
| At end of last dose interval | 1 hour later | 3 hours later | 5 hours later | |
| Sporadic ZES (n) | 13 | 13 | 12 | 9 |
| Mean (SD) | 2.6 (2.9) | 3.0 (3.7) | 5.2 (6.5) | 3.4 (3.7) |
| Range | 0.0–7.9 | 0.0–12.0 | 0.0–21.5 | 0.0–9.7 |
| ZES + MEN-I (n) | 6 | 6 | 6 | 6 |
| Mean (SD) | 2.6 (2.6) | 2.6 (2.4) | 4.1 (3.4) | 5.0 (3.5) |
| Range | 0.1–7.4 | 0.2–6.5 | 0.0–10.1 | 0.0–8.3 |
| Total (n) | 19 | 19 | 18 | 15 |
| Mean (SD) | 2.6 (2.7) | 2.8 (3.3) | 4.9 (5.6) | 4.0 (3.6) |
| Range | 0.0–7.9 | 0.0–12.0 | 0.0–21.5 | 0.0–9.7 |
NOTE. After the last infusion of pantoprazole, acid output was measured at the end of the last dose interval, starting from 1 hour before the next normally scheduled dose, and continued for the next 5 hours or until acid output reached either 50% of the previously measured approximate BAO or a rate ≥10 mEq/h.
Overall, IV pantoprazole was well tolerated in all patients and there were no serious adverse events or any significant hematologic or biochemical toxicity (including changes in liver-associated enzymes). Serial ophthalmologic examinations including retinal evaluations and photographs (which were evaluated by an independent retinal specialist) showed no drug-induced changes in any patients. Only 1 patient withdrew from the study because of headaches after receiving 5 doses of therapy. Other minor side effects noted in more than 10% of patients included headache, insomnia, and injection site, and nasogastric tube reactions, but no other patients withdrew from the study because of these effects (data not shown). Most of these effects were mild and transient in nature, and were not considered related to therapy with pantoprazole.
Discussion
This multicenter study was designed to evaluate the safety and efficacy of IV pantoprazole in controlling gastric acid hypersecretion in patients with ZES and to identify a dosing regimen that is effective and safe in all patients, thus obviating the need for continuous monitoring of gastric acid output. ZES patients are especially suitable for IV pantoprazole therapy because, in contrast to other patients, they have high ongoing baseline acid secretion. This is the first such study in patients with ZES conducted anywhere with IV pantoprazole. We show that 80 mg of IV pantoprazole had a remarkably rapid onset of action (within 15–60 minutes) in patients with ZES and profoundly inhibited acid output to levels that were low and safe. This inhibition was maintained in all patients for an additional 6 days with a maintenance dose of up to 240 mg/day. Although an IV omeprazole formulation has been evaluated in ZES patients in 2 prior studies from a single center,16,17 IV omeprazole is not being further developed in the United States. The results with pantoprazole are similar to the level of gastric acid control achieved in earlier IV PPI studies.17
We found that IV pantoprazole administered twice daily controlled acid output in more than 90% of patients, whereas the remaining patients were controlled with 3 times daily administration. Thus, our data suggest that administration of up to 240 mg/day will achieve the goal of complete control of acid output in all ZES patients. There is precedent in the literature showing that more effective inhibition of acid output can be achieved by increasing the frequency with which PPIs are administered instead of giving a higher dose less often.25 This may occur because parietal cell synthesis and proton pump turnover are continuous phenomena and PPIs have a short half-life in the bloodstream. In normal volunteers, IV pantoprazole had a mean serum half-life of less than 2 hours.26 This is similar to the mean half-lives in ZES patients16 for IV and oral omeprazole (2.3 ± 0.4 vs. 2.4 ± 0.5 hours). In patients without ZES the oral bioavailability of pantoprazole is 77%.27 The oral bioavailability of pantoprazole in ZES patients has not been determined.
Two major findings emerged from this study. First, 80 mg of IV pantoprazole effectively controlled acid output within 1 hour in all patients, even when treatment started from a baseline hypersecretory state. Second, 240 mg of IV pantoprazole administered in divided doses over 24 hours maintained control of gastric acid secretion in all patients for at least 24 hours and for up to 6 days. It is notable that 6 days represents a reasonable period of time in keeping with requirements for most patients who undergo emergency abdominal surgery. We also showed that once control of acid output was achieved, acid output was reduced far below the threshold required to prevent acid peptic complications and permit mucosal healing in all patients.5,15,28,29 The starting dose of 80 mg IV twice daily was based on previous studies with IV pantoprazole that were carried out in healthy men rendered hypergastrinemic with a continuous infusion of a maximally effective dose of pentagastrin.20,21 In these studies, healthy subjects attained gastric acid output levels similar to those observed in hypersecretory conditions such as ZES, and 80 mg of pantoprazole reduced the maximal secretory rate to less than 10 mEq/h. In a real-world application for IV pantoprazole it is likely that patients would already be receiving oral acid suppression with a PPI, and thus have lowered levels of acid secretion at the time they become eligible for IV PPI therapy. Two studies have reported that there is equivalency in dosing between oral and IV pantoprazole.22,30 Because the 80-mg dose of IV pantoprazole was so effective in both the current study in which patients started from a hypersecretory state as well as in the normal volunteers rendered hypergastrinemic by pentagastrin, it can safely be assumed that an initial IV dose of 80 mg will work as well, if not better, in ZES patients already receiving oral antisecretory medication.22
It can be argued that we exposed the patient volunteers in the current study to unnecessary risk by administering IV pantoprazole after a maintenance PPI washout period. In the normal course of events there should be no need to discontinue previously administered antisecretory therapy before commencing IV pantoprazole. However, one cannot evaluate whether an agent is capable of controlling gastric acid output in hypersecretory states without allowing the study subjects to hypersecrete acid. Furthermore, the current study was carefully designed to subject patients to the briefest possible period in which their acid output was uncontrolled. This is a well-established previously validated model to evaluate antisecretory therapy in hypersecretory states.5,8,14–18,31,32
The magnitude of the dose of IV pantoprazole required to effectively control acid output in all ZES patients is only important to the degree that it needs to be within an acceptable nontoxic range. In the current study, IV pantoprazole was found to be remarkably free of medication-associated side effects. The drug was well tolerated with only minor complaints. One patient withdrew because of headache. Of specific importance was the absence of any ophthalmologic problems relating to the continued use of high-dose IV pantoprazole for up to 6 days. This is important because concerns were raised regarding the safety of IV omeprazole because of the development of anterior ischemic optic neuropathy in severely ill patients in the intensive care unit in Germany.33,34 This was initially believed to possibly be related to the rate with which omeprazole was administered by the IV route. However, it is currently believed that the ocular changes observed in the German study were caused by severe underlying illnesses rather than to the rapid bolus injection of IV omeprazole therapy.33,34 Nonetheless, in the current study, we initially chose to administer pantoprazole by brief IV infusion over 15 minutes to limit potential side effects that may occur with rapid bolus injections. A short, 15-minute IV infusion can either be accomplished with an infusion pump (now standard equipment in most U.S. hospitals), or it can be done by allowing a minibag to infuse rapidly by gravity. Thus, our study found a universally effective regimen for IV pantoprazole in patients with ZES that was without significant side effects. This regimen can therefore be applied to other ZES patients without the need for continuous monitoring of acid output or a constant infusion pump.
All oral PPIs have a long duration of action with an offset time (i.e., the time taken for acid output to reach half BAO) in ZES patients of about 24 hours.31,32 Drug offset is a reflection of proton pump and parietal cell turnover after achievement of steady-state inhibition with repeated doses. Because the bioavailability of oral pantoprazole is 77% (among the highest of all PPIs),26 and because all PPIs have such short serum half-lives,27 this measurement should be unaffected by the route of administration (i.e., IV vs. oral therapy). In the current study, control of acid output was lost in 25% of ZES patients within 5 hours of missing their first dose. That is, control was lost by 13 hours after the final dose in the 2 patients receiving 8-hour therapy and by 17 hours after the final dose in the 19 patients receiving 12-hour therapy. In fact, acid output control was lost in 10% of patients within 2 hours of missing their first dose. These results suggest that proton pump and parietal cell turnover rates are rapid in hypergastrinemic ZES patients. Thus, it is essential to ensure that ZES patients on IV pantoprazole receive all scheduled doses because gastric acid hypersecretion can return rapidly with serious consequences.
Our current study design was weighted in favor of a high dose of IV pantoprazole to maintain control of acid output. The primary concern was to identify a dose of IV pantoprazole that controlled acid output rapidly and effectively in all patients given a worst-case scenario (i.e., starting from near BAO). In practice, most ZES patients who need to switch from oral to IV therapy will do so in the presence of some oral drug-induced acid suppression to start with and lower doses may be effective. Studies to determine the efficacy of switching dosing forms are currently under way.22 Moreover, because the effect of PPIs initially improves with increasing duration of therapy,35 ultimate dose requirements may also be lower. Formal studies will be needed to answer this question, but, for practical purposes, the results of this study indicate that a dose of 80 mg of IV pantoprazole every 8 hours is recommended for safe and effective control of gastric acid secretion in patients with ZES or idiopathic gastric acid hypersecretion until these formal dose-reduction studies have been performed. In conclusion, this study identifies an effective and safe regimen for the use of IV pantoprazole for patients with gastric acid hypersecretory conditions such as ZES. This regimen rapidly controlled acid output and maintained control for 8–12 hours without dose increases in most patients and without the need for continuous infusion.
Abbreviations used in this paper:
- BAO
basal acid output
- H2RA
H2-receptor antagonist
- MEN-I
multiple endocrine neoplasia syndrome type I
- PPI
proton pump inhibitor
- ZES
Zollinger–Ellison syndrome
References
- 1.Metz DC, Jensen RT. Endocrine tumors of the pancreas. In: Haubrich WB, Schaffner F, Berk JE, eds. Bockus gastroenterology 5th ed. Volume 4 Philadelphia: Saunders, 1994:3002–3034. [Google Scholar]
- 2.Metz DC. Peptic ulcer disease; diagnosis and treatment. In: DiMarino AJ, Benjamin SB, eds. Gastrointestinal disease: an endoscopic approach Volume 1 Cambridge: Blackwell Science, 1997:285–304. [Google Scholar]
- 3.Zollinger RM, Ellison EC. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 1955;142:709–728. [PMC free article] [PubMed] [Google Scholar]
- 4.Zollinger RM, Ellison EC, OíDorisio TM, Sparks J. Thirty years experience with gastrinoma. World J Surg 1984;8:427–435. [DOI] [PubMed] [Google Scholar]
- 5.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RV. The control of gastric acid hypersecretion in the management of patients with Zollinger–Ellison syndrome. In: Progress symposium on the treatment of islet cell tumors. World J Surg 1993;17:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of omeprazole in Zollinger–Ellison syndrome: a prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther 1993;7:597–610. [DOI] [PubMed] [Google Scholar]
- 7.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjostrand SE, Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+/K+)ATPase. Nature 1981;290:159–161. [DOI] [PubMed] [Google Scholar]
- 8.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RT. Currently used doses of omeprazole in Zollinger–Ellison syndrome are too high. Gastroenterology 1992;103:1498–1508. [DOI] [PubMed] [Google Scholar]
- 9.Sachs G Proton pump inhibitors and acid-related diseases. Pharmacotherapy 1997;17:22–37. [PubMed] [Google Scholar]
- 10.Shin JM, Besancon M, Bamberg K, Sachs G. Structural aspects of the gastric H,K ATPase. Ann NY Acad Sci 1997;834:65–76. [DOI] [PubMed] [Google Scholar]
- 11.Hirschowitz BI, Keeling D, Lewin M, Okabe S, Parsons M, Sewing K, Wallmark B, Sachs G. Pharmacological aspects of acid secretion. Dig Dis Sci 1995;40(suppl 2):3S–23S. [DOI] [PubMed] [Google Scholar]
- 12.Zeng N, Athmann C, Kong T, Lyu RM, Walsh JH, Ohning GV, Sachs G, Pisegna JR. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 1999;104:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber HC, Venzon DJ, Lin J-T, Fishbein VA, Orbuch M, Strader DB,Gibril F, Metz DC, Fraker DL, Norton JA, Jensen RT. Determinants of metastatic rate and survival in Zollinger–Ellison syndrome: a prospective long-term study. Gastroenterology 1995;108:1637–1649. [DOI] [PubMed] [Google Scholar]
- 14.Vinayek R, Hahne WF, Euler AR, Norton JA, Jensen RT. Parenteral control of gastric acid hypersecretion in patients with Zollinger–Ellison syndrome. Dig Dis Sci 1993;38:1857–1865. [DOI] [PubMed] [Google Scholar]
- 15.Maton PN. Zollinger–Ellison syndrome. Recognition and management of acid hypersecretion. Drugs 1996;52:33–44. [DOI] [PubMed] [Google Scholar]
- 16.Vinayek R, Amantea MA, Maton PN, Frucht H, Gardner JD, Jensen RT. Pharmacokinetics of oral and intravenous omeprazole in patients with the Zollinger–Ellison syndrome. Gastroenterology 1991;101:138–147. [DOI] [PubMed] [Google Scholar]
- 17.Vinayek R, Frucht H, London JF, Miller LS, Stark HA, Norton JA, Cederberg C, Jensen RT, Gardner JD, Maton PN. Intravenous omeprazole in patients with Zollinger–Ellison syndrome undergoing surgery. Gastroenterology 1990;99:10–16. [DOI] [PubMed] [Google Scholar]
- 18.Blanchi A, Delchier JC, Soule JC, Payen D, Bader JP. Control of acute Zollinger–Ellison syndrome with intravenous omeprazole. Lancet 1982;2:1223–1224. [DOI] [PubMed] [Google Scholar]
- 19.Kohl B, Sturm E, Senn-Bilfinger J, Simon WA, Kruger U, Schaefer H, Rainer G, Figala V, Klemm K. (H+,K+)-ATPase inhibiting 2-[(2pyridylmethyl)sulfinyl]benzimidazoles. 4. A novel series of dimethoxypyridyl-substituted inhibitors with enhanced selectivity. The selection of pantoprazole as a clinical candidate. J Med Chem 1992;35:1049–1057. [DOI] [PubMed] [Google Scholar]
- 20.Simon B, Muller P, Bliesath H, Luhmann R, Hartmann M, Huber R, Wurst W. Single intravenous administration of the H1,K(1)ATPase inhibitor BY 1023/SK&F 96022—inhibition of pentagastrin-stimulated gastric acid secretion and pharmacokinetics in man. Aliment Pharmacol Ther 1990;4:239–245. [DOI] [PubMed] [Google Scholar]
- 21.Pisegna JR, Martin P, McKeand W, Ohning G, Walsh JH, Paul J. Inhibition of pentagastrin-induced gastric acid secretion by intravenous pantoprazole: a dose response study. Am J Gastroenterol 1999;10:2874–2880. [DOI] [PubMed] [Google Scholar]
- 22.Metz DC, Forsmark CE, Soffer E, Bochenek W, Beg M, Pisegna J. Zollinger–Ellison syndrome (ZES) patients can replace oral proton pump inhibitors (PPIs) with intravenous (IV) pantoprazole (PANTO) without losing control of acid output (AO) (abstr). Gastroenterology 1999;116:A252. [Google Scholar]
- 23.Metz DC, Jensen RT. The Zollinger-Ellison syndrome. In: Snape WJ Jr, ed. Consultations in gastroenterology Philadelphia: Saunders, 1995:322–334. [Google Scholar]
- 24.Gugler R, Hartmann M, Rudi J, Brod I, Huber R, Steinijans VW, Bliesath H, Wurst W, Klotz V. Lack of pharmacokinetic interaction of pantoprazole with diazepam in man. Br J Clin Pharmacol 1996;42:249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue M, Nakamura M. Studies of various administration methods for lansoprazole injection using 24-hour intragastric pH monitoring. J Clin Gastroenterol 1995;20(suppl 2):S17–21. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Hartmann M, Bliesath H, Luhmann R, Steinijans VW, Zech K. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther 1996;34(suppl):S7–S16. [PubMed] [Google Scholar]
- 27.Kromer W. Similarities and differences in the properties of substituted benzimidazoles: a comparison between pantoprazole and related compounds. Digestion 1995;56:443–454. [DOI] [PubMed] [Google Scholar]
- 28.Maton PN, Frucht H, Vinayek R, Wank SA, Gardner JD, Jensen RT. Medical management of patients with Zollinger–Ellison syndrome who have had previous gastric surgery: a prospective study. Gastroenterology 1988;94:294–299. [DOI] [PubMed] [Google Scholar]
- 29.Frucht H, Maton PN, Jensen RT. Use of omeprazole in patientswith Zollinger–Ellison syndrome. Dig Dis Sci 1991;36:394–404. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann M, Ehrlich A, Fuder H, Luhmann R, Emerklibas S,Timmer W, Wurst W, Lucker PW. Equipotent inhibition of gastric acid secretion by equal doses of oral or intravenous pantoprazole. Aliment Pharmacol Ther 1998;12:1027–1032. [DOI] [PubMed] [Google Scholar]
- 31.Metz DC, Pisegna JR, Ringham GL, Feigenbaum K, Koviack PD, Maton PN, Gardner JD, Jensen RT. Prospective study of efficacy and safety of lansoprazole in Zollinger–Ellison syndrome. Dig Dis Sci 1993;38:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur KE, Collen MJ, Maton PN, Cherner JA, Howard JM, Ciarleglio CA, Cornelius MJ, Jensen RT, Gardner JD. Omeprazole: effective, convenient therapy for Zollinger–Ellison syndrome. Gastroenterology 1985;88:939–944. [DOI] [PubMed] [Google Scholar]
- 33.Schonhofer PS, Werner B, Troger U. Ocular damage associatedwith proton pump inhibitors. Br Med J 1997;314:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachs G. Omeprazole and ocular damage. Lack of causality holdstrue. Br Med J 1998;316:67–68. [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen JB, Lundberg P, Baak LC, Greve J, Ohman M, Stover C,Rohss K, Lamers CB. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut 1988;29:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]