Abstract
Purpose:
To compare the safety and efficacy of an absorbable inferior vena cava (IVC) filter and a benchmark IVC filter in a porcine model.
Materials and Methods:
A randomized controlled Good Laboratory Practice study was performed in Domestic Yorkshire cross swine. Sixteen swine were implanted with an absorbable IVC filter (test device; Adient Medical, Pearland, Texas); 8 were implanted with a benchmark metal IVC filter (control device; Cook Medical, Bloomington, Indiana). All animals underwent rotational digital subtraction pulmonary angiography and cavography (anteroposterior and lateral) before filter deployment and 5 and 32 weeks after deployment. Terminal procedures and necropsy were performed at 32 weeks. The IVC, heart, lungs, liver, and kidneys were harvested at necropsy. The reported randomized controlled GLP animal study was conducted at Synchrony Labs, Durham, North Carolina.
Results:
One animal died early in the test cohort of a recurring hemorrhage at the femoral access site resulting from a filter placement complication. All other animals remained clinically healthy throughout the study. No pulmonary embolism was detected at the 5- and 32-week follow-up visits. The absorbable filter subjects experienced less caval wall perforation (0% vs 100%) and thrombosis (0% vs 75%). The control device routinely perforated the IVC and occasionally produced collateral trauma to adjacent tissues (psoas muscle and aorta). The veins implanted with the absorbable filter were macroscopically indistinguishable from normal adjacent veins at 32 weeks except for the presence of radiopaque markers. Nontarget tissues showed no device-related changes.
Conclusions:
Implantation of the absorbable IVC filter in swine proved safe with no pulmonary emboli detected. There was complete to near-complete resorption of the filter polymer by 32 weeks with restoration of the normal appearance and structure of the IVC.
It is well established that adverse events arising from inferior vena cava (IVC) filter migration, fracture, embolization, and organ perforation increase with filter indwell (1–16). For many IVC filters, retrieval within weeks of deployment is recommended; however, a systematic review found a mean retrieval rate of only 34% at single centers (8). Even with dedicated filter retrieval programs at single centers, the filter retrieval rate is only 43%–60% (17–19). IVC filter retrieval rates in the general population tend to be in the single digits with New York at 3.5% and Florida at 6.6% (20,21).
Per American College of Chest Physicians (ACCP) guidelines for VTE prevention, maximum recommended VTE prophylaxis is 35 days (22,23). Conceptually appealing would be an IVC filter for short-term pulmonary embolism (PE) prevention (35 days) that simply vanishes following transient PE risk, thereby avoiding the retrieval procedure and long-term complications. Recently, the feasibility of absorbable IVC filters have been demonstrated in several pilot animal studies (24–27). The objective of this prospective, randomized, controlled animal study was to compare the safety and efficacy of an absorbable IVC filter (test device; Adient Medical, Pearland, Texas) and a benchmark retrievable IVC filter (control device; Cook Medical, Bloomington, Indiana) over a period of 32 weeks in porcine before clinical studies.
MATERIALS AND METHODS
Animal Model and Care
The institutional animal care and use committee approved this study. Treatment of the animals was in accordance with the US Department of Agriculture Animal Welfare and the study was conducted under good laboratory practice (GLP) regulations. Twenty-four domestic Yorkshire cross juvenile swine (Sus scrofa) weighing 35–50 kg were obtained from Palmetto Research Swine (Reevesville, South Carolina).
Test and Control Articles
The Adient Absorbable Filter set contained an absorbable polymer filter (20-mm diameter, 45-mm long) preloaded within a delivery system that consisted of an introducer sheath (16.0 F, 45-cm long) and dilator. The filter was oversized for a 16-mm pig caval diameter to exert a chronic outward radial force to enhance caval apposition. The polymer filament was polydioxanone, commonly used in PDS absorbable suture. The absorbable filter was braided from a continuous PDS filament (Riverpoint Medical, Portland, Oregon) as shown in Figure 1. Because the filter is compressed radially, the filter basket section remains fixed in length while the stent section elongates, such that in a 16-mm diameter cava, the stent section is 25 mm, whereas the basket length remains fixed at 30 mm. Five radiopaque markers are attached to the filter: 1 platinum iridium cylinder at the tip of the filter and 5 stainless steel barbs attached 72° apart in a helical pattern within the stent section. The Cook Celect Filter set consisted of a metal filter (30-mm diameter, 48-mm long) preloaded on a femoral introducer, a 7.0F coaxial introducer, and dilator (Fig 1). The Cook Celect IVC filter was chosen as the control because it had the fewest complications among the most used IVC filters reported to the Manufacturer and User Facility Device Experience Database from 2000 – 2010 comprised by the US Food and Drug Administration (FDA) (8).
Figure 1.
(a) Control (metal) Cook Celect filter and (b) test (absorbable) Adient Absorbable filter.
Primary Endpoint and Sample Size
Acceptable filter complication rates have been established by the Society of International Radiology as follows (28): 1% access site thrombosis, 2% filter embolization, 5% recurrent PE, 10% IVC occlusion, and 1% death. Complication-free deployment should occur in approximately 81% (100%–19%) of deployments. Hence the primary endpoint of the study was medical success, defined as 81% IVC filter deployment without the mentioned complications.
To determine the number of animals needed to observe at least 1 complication with reasonable assurance, a binomial distribution in which P = .19 was the probability of complication (failure) in any animal considered. Assuming N independent animals, the corresponding probability of medical success for each of the N animals is (1 – p), yielding an overall success among all N animals equal to (1 – p)N. Consequently, the probability of at least 1 failure (1 complication) in the study of N animals is the complement (ie, probability of observing at least 1 complication in an N animal study = 1 – [1 – p]N ). Assuming 16 animals per cohort, it can be reasonably assured (probability 0.966) that at least 1 complication will be observed. Although 16 animals were considered for each of the cohorts, the FDA recommended reducing the control cohort to 8.
Randomization
Each animal was randomized immediately following procedure preparation whereby a blinded employee selected a test (group 1, total 16 labels) or control label (groups 2 and 3, total 8 labels) randomly from a jar with the following designation:
Group 1 (test): absorbable filter, followed 32 weeks, 16 animals
Group 2 (control): metal filter, retrieved at 5 weeks, followed 32 weeks, 4 animals
Group 3 (control): metal filter, not retrieved, followed 32 weeks, 4 animals.
The control groups were designed to reflect ideal and typical practice. Retrievable filters are ideally retrieved within 1 to 2 months, but the majority are not removed.
Treatment Procedure
Pulmonary angiography via jugular access was performed with rotational digital subtraction angiography where the C-arm was rotated from 30° left anterior oblique to 30° right anterior oblique to assess for PE using a 6F pigtail flush catheter to inject contrast (50% saline dilution) automatically at a rate of 20 mL/s for 5 seconds. Digital subtraction was used to supplement image interpretation. Then, an appropriately sized introducer was inserted percutaneously into the femoral vein using the Seldinger technique and advanced such that the distal tip was positioned at the desired infrarenal location for the filter. The filter, encased in the supplied delivery system, was then advanced into the IVC under fluoroscopic guidance and deployed. Deployment with the delivery system for the absorbable filter was a 3-step procedure: (i) unsheath the filter by pulling the forend proximal; (ii) inflate the balloon by delivering contrast solution in a syringe connected to the delivery system, hold for 10 seconds for caval apposition, then deflate; and (iii) release filter from delivery system by advancing the thumb switch proximal. At the conclusion of the procedure, hemostasis at the puncture site was obtained with manual compression. A venacavogram was obtained immediately following filter deployment to document filter positioning and potential thrombosis, and rotational pulmonary angiography was repeated for PE assessment.
Interim and Terminal Procedures
At 35 ± 5 days after filter deployment, the 16 animals with absorbable filters (group 1) and the 4 animals with metal filters to be left in place (group 3) were assessed for thrombus formation, filter migration, embolization, caval perforation, and caval stenosis all via venography, and PE using rotational pulmonary angiography. Five weeks was chosen as a strategic time interval to assess filter performance since the absorbable filter is indicated for short-term (5-week) PE prevention coinciding with the longest duration VTE prophylaxis per ACCP guidelines. Also, at 35 ± 5 days after filter deployment, percutaneous filter retrieval was performed on the 4 animals in the control group with IVC filter retrieval (group 2), in which an introducer was inserted into the jugular vein. Filters were then retrieved through the jugular sheath with a snare under fluoroscopic guidance.
At 32 weeks (224 ± 5 days) following IVC filter deployment, all animals were anesthetized and assessed for complications as performed at the 35-day follow up. Subsequently, animals were given an additional bolus of IV heparin which was allowed to circulate for 5 minutes. Animals were then euthanized with an overdose of intravenous potassium chloride while under deep anesthesia.
Venacavograms and pulmonary angiograms were obtained for all animals at the 5-week follow up and at 32 weeks just before necropsy. Additional Methods and Materials are available in Appendix A (available online on the article’s Supplemental Material page at www.jvir.org).
RESULTS
One animal died within 1.5 hours of the procedure from a recurring hemorrhage at the femoral access site. An immediate necropsy found no association with the test article. Most likely, death was due to inadequate compression at the access site following deployment to maintain lasting hemostasis. All other animals remained clinically healthy throughout the study. Clinical pathology results showed no clinically significant abnormalities.
The 5-Week Follow-up
Control device retrieval was attempted in the group 2 animals per protocol, however none of the devices could be retrieved due to substantial penetration through the IVC and endothelialization. In the combined control cohorts (groups 2 and 3), IVC stenosis was found in 2 of 8 animals visualized on lateral projection (sagittal plane) with the cava narrowed to 3.3–3.8 mm. Filter migration was noted in 4 of 8 animals. The range of migration was 5.7–19.9 mm, all from group 2 (retrieval attempted). Grade II perforation by the metal filter struts was present in all control animals. A representative venacavogram for the control animals (metal filter) is shown in Figure 2 with filter struts extending exterior to the IVC lumen. PE was not present in any control animals.
Figure 2.

Representative digital venacavogram frame for a control (metal) IVC filter in porcine taken at 5 weeks after filter placement. Note the filter struts have perforated the caval wall (arrows) defined by the contrast-filled IVC.
In group 1 (test) of 15 animals, no significant IVC stenosis was detected following filter placement. Filter migration in the range of 3.0–8.0 mm was found in 7 of 15 animals. No filter perforation or PE was detected. A representative venacavogram for the test animals (absorbable filter) is shown in Figure 3. Note the radiopaque markers consisting of the filter tip and 5 barbs in the stent section of the filter. The filter polymer is generally radiolucent in coronal and sagittal planes, but is radiopaque within the stent section in the axial (transverse) plane.
Figure 3.

Representative venacavogram frame for a test (absorbable) IVC filter in porcine at 5 weeks. Arrow denotes location of right renal vein influx. RO = radiopaque.
The 32-Week Follow-up
Filter migration was assessed in the group 2 (control) animals at 4.0–30.0 mm. IVC stenosis was found in 1 animal with an IVC width measuring 5.2 mm on lateral projection. No PE was detected in control animals. In group 1 (test), filter migration was found in 5 of 15 animals ranging from 7.0 to 35.0 mm. IVC stenosis occurred in 2 animals visualized at 10.2 and 5.8 mm widths (anteroposterior) on lateral projection. No PE was detected. Venacavograms at 32 weeks were similar to those obtained at 5 weeks with the exception of the radiopaque cylindrical markers at the tip of the absorbable filters that were rotated 90° and incorporated into the caval wall, as opposed to being in the center of the lumen at 5 weeks (Figs 3, 4).
Figure 4.

Representative venacavogram frame for a test (absorbable) IVC filter in porcine at 32 weeks. Note the stent and basket portions have resorbed, leaving only the radiopaque (RO) markers in the caval wall.
Necropsy
At necropsy, all control (metal filter) implant sites showed perforation of the vein wall by filter struts that were >3 mm outside the vein (Fig 5), and 2 implant sites showed struts perforating an adjacent structure (psoas muscle and/or aorta) (Fig 6). On average, the length of strut outside the IVC was about 23 mm and ranged from 5 to 33 mm. There was no visible hemorrhage or hematoma associated with the perforations and they appeared as gradual remodeling of the vein wall around the outwardly projected struts.
Figure 5.

Group 2 metal IVC filter within IVC. Arrows indicate filter struts penetrating through the wall of the vein within the perivascular tissue; V = vein proximal to the implant site, the vein is distended by the filter and remodeled around the outer most struts.
Figure 6.
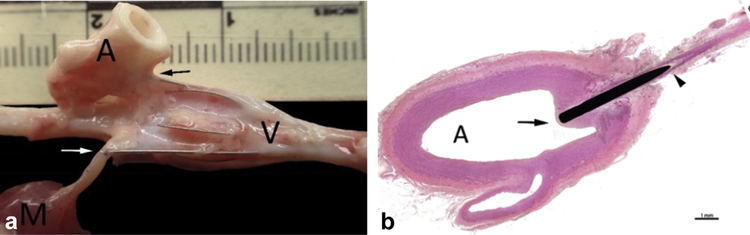
(a) Group 3 metal IVC filter within IVC. Arrows indicate filter struts penetrating through the wall into adjacent tissue (ie, skeletal muscle [M, white arrow] and aorta [A, black arrow]); V = vein proximal to the implant site where cephalic portion of the filter resides, the vein is distended by the filter and remodeled around the outer most struts. (b) Hematoxylin and eosin–prepared slide of filter strut penetrating the aorta (A). Arrow indicates cap of fibrocellular neointima covering the tip of the strut penetration within the lumen and arrowhead indicates filter strut penetrating the aorta.
All test (absorbable filter) implant sites showed no structural abnormalities in the implanted vein wall with no grossly visible residual device frame components visible and no penetration of filter through the wall. The vein was macroscopically normal, except for the presence of a small, hard nodule at the tip where the radiopaque marker embedded. Most millimeter-sized barbs were visible in the vessel wall and adventitia and were not associated with any macroscopically visible abnormalities (Fig 7).
Figure 7.

Group 1, absorbable IVC filter within IVC. The vein is normal and morphologically and structurally indistinguishable from unimplanted vein. V = vein proximal to implant site; arrow, filter tip embedded in the wall of the vein; arrowheads, filter barbs within the vein wall.
Biocompatibility
There was inconsistently and generally low-severity inflammatory response to the presence of filter elements in the vessel wall. In the test group, inflammation was almost exclusively within proximity of the barbs, characterized by dense aggregates of lymphocytes, with fewer macrophages together with foreign body giant cells. The distribution of these lympho-histiocytic aggregates was compatible with resorbing polydioxanone sutures. The associated inflammation did not result in any adverse tissue response; there was no tissue necrosis and no increase in neointima formation. There were aggregates of foamy macrophages regularly spread throughout the vein wall circumference in the test group (Fig 8). These aggregates represented the end-stage bioresorption process and were not considered to be an inflammatory response in the strictest sense.
Figure 8.
(a) Group 1 absorbable IVC filter at 32 weeks using hematoxylin and eosin. Arrow, minimal inflammation (aggregate of lymphocytes) within the vein wall; dotted lines, clusters of foamy macrophages (biomaterial phagocytosis) at the location of the PDO suture from the stent section of the filter. Black frame shows area enlarged in (b). Arrowhead = hemosiderin; V = vein.
Overall, the test article revealed very advanced resorption of the absorbable filter material characterized by complete effacement of the polymer struts and the presence of small and circumferentially distributed aggregates of foamy macrophages in the neointima and/or media. Some of these aggregates included a few larger macrophages and giant cells whose cytoplasm showed small, clear rhomboid inclusions interpreted as small unresorbed, semicrystalline polymer particles.
There was a very low inflammatory response to the components of the control filter, as demonstrated in Figure 9.
Figure 9.
(a) Group 2 metal IVC filter at 32 weeks using hematoxylin and eosin. Arrow, minimal focal para-strut inflammation. Black frame shows area detailed in (b). Arrowheads = filter struts embedded in the mature fibrocellular neointima; V = vein lumen.
Complications
Complications associated with the implants are shown in Table 1. Device migration was assessed by the location of the filter relative to adjacent vertebrae. True filter migration was difficult to quantitate due to the growth
Table 1.
Device-Related Complications in Test (Absorbable Filter) and Control (Metal Filter) Cohorts
| Complication | Test Device |
Control Device |
|---|---|---|
| (Absorbable) | (Metal) | |
| Number of Subjects | 15 | 8 |
| Migration | ||
| 5 wk | ||
| No. subjects (%) | 7 (47) | 4 (50) |
| Distance, mm | 3.0–8.0 | 5.7–19.9 |
| 32 weeks | ||
| No. subjects (%) | 5 (33) | 4 (50) |
| Distance, mm | 7.0–35.0 | 4.0–30.0 |
| Stenosis (lateral) | ||
| 5 weeks, N (%) | 2 (13) | 2 (25) |
| 32 weeks, N (%) | 2 (13) | 1 (13) |
| Perforation grade, N (%) | ||
| No perforation | 15 (100) | 0 |
| 1: <3 mm | 0 | 0 |
| 2: >3 mm | 0 | 6 (75) |
| 3: contacting adjacent structure | 0 | 0 |
| 4: perforating adjacent structure | 0 | 2 (25) |
| Pulmonary embolism | 0 | 0 |
Note–The absorbable and metal filters had similar migration, caval stenosis, and embolization outcomes; however, the absorbable filter did not perforate the IVC as evident with the metal IVC.
IVC = IVC
of the vertebral bodies (2×) during rapid animal growth. The majority of the migration can be attributed to the changing length of the vertebral bodies since the greatest “migration” was evident between 5 and 32 weeks when the absorbable filter stent sections were completely endothelialized and the metal filters had 5 perforated struts with barbs residing outside the cava rendering true migration unlikely in both cohorts. An IVC filter can move in the craniocaudal direction up to 10 mm with respiration. Additional results are available in Appendix A (available online on the article’s Supplemental Material page at www.jvir.org).
DISCUSSION
The primary endpoint of medical success rate was successfully met for the absorbable IVC filter test group in a GLP randomized controlled study. No pulmonary emboli were observed at any time, and no evidence of IVC thrombosis, filter perforation, permanent IVC stenosis, or death from the device. One animal died of a recurring hemorrhage at the femoral access secondary to a procedural complication during deployment in the test group.
The control benchmark metal IVC filter routinely perforated the vein wall and produced occasional collateral trauma to adjacent tissues (ie, psoas muscle and aorta), which were not seen with the test filter. Although the 100% IVC perforation rate appears high in the control group, it is comparable to the 93% reported from a 2-year retrospective clinical study at the University of California San Francisco and Penn State (13) as well as the 86% reported from a 5-year clinical study at Cleveland Clinic (31). The moderately higher perforation rate for the Celect filter in the animals versus humans may be due to the moderately increased radial force exerted on the caval wall as the diameter is decreased. The Cook Celect IVC filter is indicated for IVC diameters ranging from 15 to 30 mm, wherein the radial force varies from 1.5 N at 16.3 mm (average anteroposterior IVC projection for porcine at study initiation) to 1.3 N at 19 mm (the average adult human IVC diameter) as measured with a radial force testing machine (Machine Solutions Inc. RX 650, Arizona). However, the primary reason for the high perforation rate for the Cook Celect filter (100%) versus the absorbable filter (0%) is that the Cook Celect filter distributes its inherent radial force to 4 submillimeter barb-tipped struts (each 0.44-mm diameter) for a total surface area of 2.4 mm2, whereas the absorbable filter distributes its comparable inherent radial force throughout the braided stent section of 0.35-mm diameter suture, which constitutes a surface area of approximately 905 mm2. Hence the pressure at the sharp, rigid barbed contact points of the Cook Celect filter against the caval wall is more than 300× the pressure of the dull contact points of the flexible absorbable filter. Local biocompatibility of the control and test filters was considered optimal. Nontarget tissues showed no implant-related changes.
In comparing absorbable filter venacavograms from 5 and 32 weeks (filtering to fully resorbed states), the radiopaque platinum-iridium cylindrical marker at the filter tip was observed to rotate 90° and incorporate into the caval wall (compare Figs 3, 4) as confirmed via necropsy. It is hypothesized that as certain filaments in the filter basket break sequentially during resorption (beginning approximately 13 weeks postdeployment), the balance of the basket flops to the caval wall and becomes endothelialized as the filter tip rotates. Consequently, operation of the filter can be assessed by radiography; if the filter tip appears as an open ring (Fig 4) instead of a rectangle (Fig 3), then the device is no longer filtering. Finally, because the entire stent section of the filter and about half of the basket section (including the tip) is endothelialized and ultimately reduced to carbon dioxide and water, only a volume of 0.029 mL of polydioxanone (about one-half of an eye drop) is released in circulation during 13–32 weeks that embolizes and ultimately resorbs asymptomatically in lung parenchyma.
Additional discussion regarding inflammatory response and comparison to other IVC filters with absorbable components is available in Appendix A (available online on the article’s Supplemental Material page at www.jvir.org).
Limitations of the study include: (i) lack of an additional control cohort consisting of animals without IVC filters that could have been used for qualitative vascular analysis comparison; (ii) smaller porcine IVC diameter than average adult human, potentially contributing to moderately higher filter perforation rates; (iii) lack of an absolute measurement reference to accurately determine filter migration (rather than relying on vertebral bodies that grew 2× in length), and realizing that an IVC filter can move up to 10 mm in the craniocaudal direction due to respiration; and (iv) inability to determine the origin of the trapped thrombus within the filters detected by venography and pathology. Another limitation could perhaps be the choice of the Cook Celect for the control filter. The Celect was chosen because it had the fewest complications reported in FDA Manufacturer and User Facility Device Experience from 2000 through 2010. Some may argue that nitinol filters would have fared better, although Deso, Idakoji, and Kuo (32), in their evidence-based systematic review of IVC filter complications in 2016 reported that the Simon Nitinol filters experienced 25%–95% perforation and the Bard nitinol filters with many years of performance data experienced perforation rates of 18%–44% in addition to upward of 38% fracture at 60 months. Limited data exist for newer nitinol filters such as the Bard Denali. In general, Deso et al (32) concluded that purely conical metal filters were associated with the highest caval penetration rates (90%–100%), whereas filters with cylindrical elements were associated with highest risk of IVC thrombosis (30%–50%), and conical Bard filters were associated with the highest reported fracture risk (40%).
In conclusion, 15 absorbable IVC filters were successfully deployed and assessed during a 32-week period in porcine. Primary and secondary endpoints were successfully met, showing an optimal safety profile with complete to near complete resorption of the filter material with restoration of the normal appearance and structural properties of the IVC in the porcine model.
Supplementary Material
Acknowledgments
M.E. is a paid employee of, receives royalties from, and is a shareholder in Adient Medical (Pearland, Texas). S.R., M.U., R.A., A.W., B.J., and C.G. receive research grants from Adient Medical. S.D. is a paid employee of and owns stock in Adient Medical. S.H. and J.S. own stock in Adient Medical.
ABBREVIATIONS
- GLP
good laboratory practice
- IVC
inferior vena cava
- QVA
qualitative vascular analysis
Footnotes
Appendix A, Figures E1 and E2, and Tables E1 and E2 can be found by accessing the online version of this article on www.jvir.org and clicking on the Supplemental Material tab.
Contributor Information
Mitchell Eggers, Adient Medical, 2315 Delta Bridge Dr, Pearland, TX 77854; University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Serge Rousselle, Alizee Pathology, Thurmont, Maryland.
Mark Urtz, Synchrony Labs, Durham, North Carolina.
Rhonda Albright, Synchrony Labs, Durham, North Carolina.
Alice Will, Synchrony Labs, Durham, North Carolina.
Bettina Jourden, Synchrony Labs, Durham, North Carolina.
Cynthia Godshalk, East Coast Veterinary Imaging, Stuart, Florida.
Stephen Dria, Adient Medical, 2315 Delta Bridge Dr, Pearland, TX 77854.
Steven Huang, University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Joseph Steele, University of Texas M.D. Anderson Cancer Center, Houston, Texas.
REFERENCES
- 1.Lee JK, So YH, Choi YH, et al. Clinical course and predictive factors for complication of inferior vena cava filters. Thromb Res 2014; 133:538–543. [DOI] [PubMed] [Google Scholar]
- 2.Cipolla J, Weger NS, Sharma R, et al. Complications of vena cava filters: a comprehensive clinical review. OPUS 12 Sci 2008; 2:11–24. [Google Scholar]
- 3.Stein PD, Alnas M, Skaf E, et al. Outcome and complications of retrievable inferior vena cava filters. Am J Cardiol 2004; 94:1090–1093. [DOI] [PubMed] [Google Scholar]
- 4.Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med 2013; 173:513–517. [DOI] [PubMed] [Google Scholar]
- 5.Joels CS, Sing RF, Heniford BT. Complications of inferior vena cava filters. Am Surgeon 2003; 69:654–659. [PubMed] [Google Scholar]
- 6.Nazzal M, Chan E, Nazzal M, et al. Complications related to inferior vena cava filters: a single-center experience. Ann Vasc Surg 2010; 24: 480–486. [DOI] [PubMed] [Google Scholar]
- 7.Jia Z, Wu A, Tam M, Spain J, McKinney JM, Wang W. Caval penetration by inferior vena cava filters: a systematic literature review of clinical significance and management. Circulation 2015; 132:944–952. [DOI] [PubMed] [Google Scholar]
- 8.Angel LF, Tapson V, Galgon RE, Restrepo MI, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol 2011; 22:1522–1530. [DOI] [PubMed] [Google Scholar]
- 9.Rajasekhar A, Streiff MB. Vena cava filters for management of venous thromboembolism: a clinical review. Blood Rev 2013; 27(5):225–241. [DOI] [PubMed] [Google Scholar]
- 10.Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26:23–28. [DOI] [PubMed] [Google Scholar]
- 11.Shang EK, Nathan DP, Carpenter JP, Fairman RM, Jackson BM. Delayed complications of inferior vena cava filters: case report and literature review. Vasc Endovascular Surg 2011; 45:290–294. [DOI] [PubMed] [Google Scholar]
- 12.Wada H, Sakakura K, Kubo N, et al. Complications of temporary vena cava filter placement. J Cardiol 2012; 60:306–309. [DOI] [PubMed] [Google Scholar]
- 13.Durack JC, Westphalen AC, Kekulawela S, et al. Perforation of the IVC: rule rather than the exception after longer indwelling times for the Gunther Tulip and Celect retrievable filters. Cardiovasc Interv Radiol 2012; 35:299–308. [DOI] [PubMed] [Google Scholar]
- 14.Harvey JJ, Hopkins J, McCafferty IJ, Jones RG. Inferior vena cava filters: what radiologists need to know. Clin Radiol 2013; 68(7):721–732. [DOI] [PubMed] [Google Scholar]
- 15.Dabbagh O, Nagam N, Chitima-Matsiga R, Bearelly S, Bearelly D. Retrievable inferior vena cava filters are not getting retrieved. Where is the gap? Thrombosis Res 2010; 126:493–497. [DOI] [PubMed] [Google Scholar]
- 16.Morales Jose Pablo, Li X, Irony TZ, Ibrahim NG, Moynahan M, Cavanaugh J. Decision analysis of retrievable inferior vena cava filter in patients without pulmonary embolism. J Vasc Surg 2013; 1:376–384. [DOI] [PubMed] [Google Scholar]
- 17.Charlton-Ouw KW, Leake SS, Sola CN, et al. Technical and financial feasibility of an inferior vena cava filter retrieval program at a level one trauma center. Ann Vasc Surg 2015; 29:84–89. [DOI] [PubMed] [Google Scholar]
- 18.Minocha J, Idakoji I, Riaz A, et al. Improving inferior vena cava filter retrieval rates: impact of a dedicated inferior vena cava filter clinic. J Vasc Interv Radiol 2010; 21:1847–1851. [DOI] [PubMed] [Google Scholar]
- 19.Sutphin PD, Reis SP, McKune A, et al. Improving inferior vena cava filter retrieval rates with the define, measure, analyze, improve, control methodology. J Vasc Interv Radiol 2015; 26:491–498. [DOI] [PubMed] [Google Scholar]
- 20.Charalel RA, Durack JC, Mao J, Ross JS, Meltzer AJ, Sedrakyan A. A statewide inferior van cava filter placement, complications, and retrievals; epidemiology and recent trends. Med Care 2018; 56:260–265. [DOI] [PubMed] [Google Scholar]
- 21.Mohapatra A, Liang NL, Chaer RA, Tzeng E. Persistently low inferior vena cava filter retrieval rates in a population cohort. J Vasc Surg 2019; 7: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133:381S–453S. [DOI] [PubMed] [Google Scholar]
- 23.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in non-orthopedic surgical patients: American College of Chest Physicians evidence-based clinical practice guidelines (9th edition). Chest 2012; 141:227S–240S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggers M, McArthur M, Figueira T, et al. Pilot in-vivo study of an absorbable polydioxanone vena cava filter. J Vasc Surg 2015; 3:409–420. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Eggers M, McArthur M, Dixon K, Dria S, Steele J, Wallace M. Safety and efficacy of an absorbable IVC filter for the prevention of pulmonary embolism in swine. Radiology 2017; 285:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thors A, Muck P. Resorbable inferior vena cava filters: trial in an in-vivo porcine model. J Vasc Interv Radiol 2011; 22:330–335. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Li H, Liang G, Zhang H. Development and evaluation of a new biodegradable vena cava filter in a canine model. Asian J Surg 2017; 40: 12–16. [DOI] [PubMed] [Google Scholar]
- 28.Grassi C, Swan T, Cardella J, et al. Quality improvement guidelines for percutaneous permanent inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol 2003; 14:S271–S275. [PubMed] [Google Scholar]
- 29.Rouselle S, Dillon K, Rouselle-Sabiac T, Brady D, Tunev S, Tellez A. Historical incidence of spontaneous lesions in kidney from naïve swine utilized in interventional renal denervation studies. J Cardiovasc Trans Rsch 2016; 9:360–367. [DOI] [PubMed] [Google Scholar]
- 30.Dincer Z The minipig. In: Gad S, editor. Animal Models in Toxicology Boca Raton: CRC/Taylor & Francis; 2007. p. 739–760. [Google Scholar]
- 31.Zhou D, Spain J, Moon E, Mclennan G, Sans M, Wang W. Retrospective review of 120 Celect inferior vena cava filter retrievals: experience at a single institution. J Vasc Interv Radiol 2012; 23:1557–1563. [DOI] [PubMed] [Google Scholar]
- 32.Deso SE, Idakoji IA, Kuo W. Evidence-based evaluation of inferior vena cava filter complications based on filter type. Semin Intervent Radiol 2016; 33:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.