Abstract
The ability to learn that a stimulus no longer signals danger is known as extinction. A major characteristic of extinction is that it is context-dependent, which means that fear reduction only occurs in the same context as extinction training. In other contexts, there is re-emergence of fear, known as contextual renewal. The ability to properly extinguish fear memories and generalize safety associations to multiple contexts provides therapeutic potential, but little is known about the specific neural pathways that mediate fear renewal and extinction generalization. The ventral hippocampus (VH) is thought to provide a contextual gating mechanism that determines whether fear or safety is expressed in particular contexts through its projections to areas of the fear circuit, including the infralimbic (IL) and prelimbic (PL) cortices. Moreover, VH principal cells fire in large, overlapping regions of the environment, a characteristic that is ideal to support generalization; yet it is unclear how different projection cells mediate this process. Using a pathway-specific (intersectional) chemogenetic approach, we demonstrate that selective activation of VH cells projecting to PL attenuates fear renewal without affecting fear expression. These results have implications for anxiety disorders since they uncover a neural pathway associated with extinction generalization.
Keywords: Fear Conditioning, Renewal, DREADDs, Chemogenetics, Ventral hippocampus, Prelimbic cortex
1. Introduction
The enduring quality of emotional memories can be evolutionarily advantageous because it allows an organism to remember and to avoid dangerous situations. However, under pathological conditions, traumatic memories can be repeatedly retrieved and such improper retrieval can disrupt optimal life conditions. The retrieval of fearful memories in inappropriate contexts is a trademark of several anxiety disorders, including post-traumatic stress disorder (PTSD) and phobias (Bakhi & Kalin, 2002; Kent & Rauch, 2003). Therefore, adaptive responses require that in addition to remembering fearful experiences, organisms must also be able to extinguish fear to a stimulus when it no longer signals danger.
Classical fear conditioning is the most important etiological factor underlying the pathology of anxiety disorders (Hettema, Prescott, & Kendler, 2001; Kendler, Karkowski, & Prescott, 1999; Kendler, Myers, Prescott, & Neale, 2001). In this form of learning, a neutral context or cue (conditioned stimulus, CS) is associated with an aversive outcome (unconditioned stimulus, US). This generates a learned fear response to the CS (conditioned response, CR). However, the magnitude and probability of the CR is reduced during extinction. This reduction only occurs in the context where extinction training takes place; in other contexts, there is are-emergence of the fear response, known as contextual renewal (Bouton, 2004; Bouton, Westbrook, Corcoran, & Maren, 2006; Rauhut, Thomas, & Ayres, 2001). The re-emergence of the CR following extinction has also been observed under other situations, such as spontaneous recovery and reinstatement (Bouton & Bolles, 1979; Haberlandt, Hamsher, & Kennedy, 1978; Harris, Jones, Bailey, & Westbrook, 2000; Pavlov, 1927; Quirk, 2002; Rauhut et al., 2001; Rescorla & Heth, 1975; Richardson, Duffield, Bailey, & Westbrook, 1999; Robbins, 1990; Schiller et al., 2008), supporting the idea that the original fear memory is preserved and that extinction represents the formation of a new inhibitory association rather than erasure of the original memory (Bouton et al., 2006; Haberlandt et al., 1978; Quirk, 2002; Robbins, 1990; Schiller et al., 2008). Since the expression of renewal appears to depend on several factors (Cahill & Milton, 2019; Chen, Wang, Wang, & Li, 2017; Goode & Maren, 2014), understanding how the re-emergence of fear could be controlled is of fundamental importance for anxiety disorders.
The context-dependency of extinction suggests that the hippocampus is involved in this learning process due to its established role in spatial learning and memory (Barnes, McNaughton, Mizumori, Leonard, & Lin, 1990; Maren & Holt, 2000; McNaughton, Battaglia, Jensen, Moser, & Moser, 2006; Morris, Garrud, Rawlins, & O’Keefe, 1982; O’Keefe & Conway, 1978; Olton, Wible, & Shapiro, 1986), an assumption that has been confirmed in contextual fear learning studies (Corcoran, Desmond, Frey, & Maren, 2005b; Corcoran & Maren, 2001; Hobin, Ji, & Maren, 2006; LaBar & Phelps, 2005) and extinction (Marek, Sun, & Sah, 2018). Although both the dorsal (DH) and ventral (VH) hippocampus have been implicated in these processes (Corcoran, Desmond, Frey, & Maren, 2005a; Corcoran & Maren, 2001; Hobin et al., 2006; Marek, Sun, et al., 2018; Rosas-Vidal, Do-Monte, Sotres-Bayon, & Quirk, 2014), the ventral region is in a key position to modulate the contextual expression of emotional information because this area is strongly connected with other regions involved in fear learning (Hoover & Vertes, 2007; Pikkarainen, Ronkko, Savander, Insausti, & Pitkanen, 1999; van Groen & Wyss, 1990). Additionally, the VH codes contextual information at the population (Keinath et al., 2014) and single cell (Keinath et al., 2014; Komorowski et al., 2013; Royer, Sirota, Patel, & Buzsaki, 2010) level. Moreover, we demonstrated that the large, overlapping characteristic patterns of activity of VH principal cells are ideal to support generalization (Keinath et al., 2014). However, VH cells are heterogeneous, with distinct subpopulations of cells differentially responding to emotional or contextual stimuli (Keinath et al., 2014). These findings raise the possibility that the VH controls fear or extinction generalization through distinct cells types, which route different information to areas of the fear circuit.
Ventral area CA1 has reciprocal connections with the basolateral amygdala (BLA) (Kerr, Agster, Furtak, & Burwell, 2007; Majak & Pitkanen, 2003; Petrovich, Canteras, & Swanson, 2001), a region involved in acquisition and consolidation of emotional memories (Fanselow & LeDoux, 1999; Maren, 2005; Pare, Quirk, & Ledoux, 2004). Additionally, the VH sends projections to the prelimbic (PL) and infralimbic (IL) cortices (Hoover & Vertes, 2007; Ishikawa & Nakamura, 2006; Jay & Witter, 1991), regions of the medial prefrontal cortex (mPFC) traditionally associated with fear expression and extinction, respectively (Corcoran & Quirk, 2007; Do-Monte, Manzano-Nieves, Quinones-Laracuente, Ramos-Medina, & Quirk, 2015; Laurent & Westbrook, 2009; Sierra-Mercado, Padilla-Coreano, & Quirk, 2011). Yet this functional segregation is less specific than previously thought. Recent findings have demonstrated that both IL and PL are interconnected and that the PL also plays a role during extinction (Marek, Xu, Sullivan, & Sah, 2018). These data suggest that to understand the role of the VH in contextual gating, it is critical to target specific pathways to determine how these cell types affect distinct phases of fear learning.
In this study, we used a pathway-selective approach to express a Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to activate only cells projecting from the VH to the PL. This was achieved by injecting a retrograde virus, canine adenovirus type 2 (CAV-2-Cre), in the PL and DREADD-Gq virus in the VH. In the presence of clozapine-N-oxide (CNO), the DREADD-Gq receptors are activated producing depolarization of the VH expressing cells. Our results demonstrate that selective activation of VH cells projecting to PL prior to renewal attenuates the re-emergence of fear. This effect is not produced by reduced fear expression because activation of this neural pathway following conditioning has no effect on fear retrieval. These results indicate that VH cells projecting to PL modulate the context-specificity of extinction and their activation leads to extinction generalization.
2. Methods
2.1. Subjects
Male C57BL/6 mice (Jackson Laboratory; n = 52) aged 10–22 weeks old were housed individually on a 12 h light/dark cycle. All mice received access to food and water ad libitum. All experimental procedures were performed in accordance to NIH guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at San Antonio.
2.2. Fluorescent retrograde tracing
Animals were anesthetized with isoflurane (3%) and placed in a stereotaxic frame (David Kopf Instruments, CA, USA). Anesthesia was maintained via an inhalation nose cone affixed to the mouth bar on the frame. Under sterile conditions, a midline incision was made, and the skull exposed. The head position was adjusted until the skull was flat in both the anterior/posterior (AP) and mediolateral (ML) axis directions. 40–60 nL of undiluted red or green retrograde fluorescent latex microspheres (Lumafluor, CA, USA) were injected bilaterally into the PL (in mm: AP + 1.94, ML ± 0.30, DV −1.55) or the IL (in mm: AP + 1.92, ML ± 0.27, DV −2.80) at a rate of 60 nL/min. Coordinates were selected based on terminal pilot surgeries. For each injection, the needle was left in situ for 3–5 min both before and after the infusion of beads. When all injections were completed, the skin was cleaned and the wound closed with tissue adhesive glue (Vetbond). After 72 h the mice were given an overdose of ketamine (100 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally (1 mL/kg) and perfused with saline followed by 4% formalin. The brains were removed and stored overnight at 4 °C in 4% formalin. The brains were sectioned into 150 μm coronal slices containing the prefrontal cortex and ventral hippo-campus. Immediately after cutting, the tissue was imaged on an Olympus SZX7 microscope. Bright-field and red or green fluorescent images were taken of the sections containing the needle tracts and injection sites. Moreover, the entire region containing the ventral hippocampus (in mm: Bregma −2.92 to Bregma −3.52) was imaged at 4 × magnification with red and green fluorescent filters. For each section, three to five additional images were taken at 10X or 11.2X magnification from the regions of interest.
2.3. Viral injections
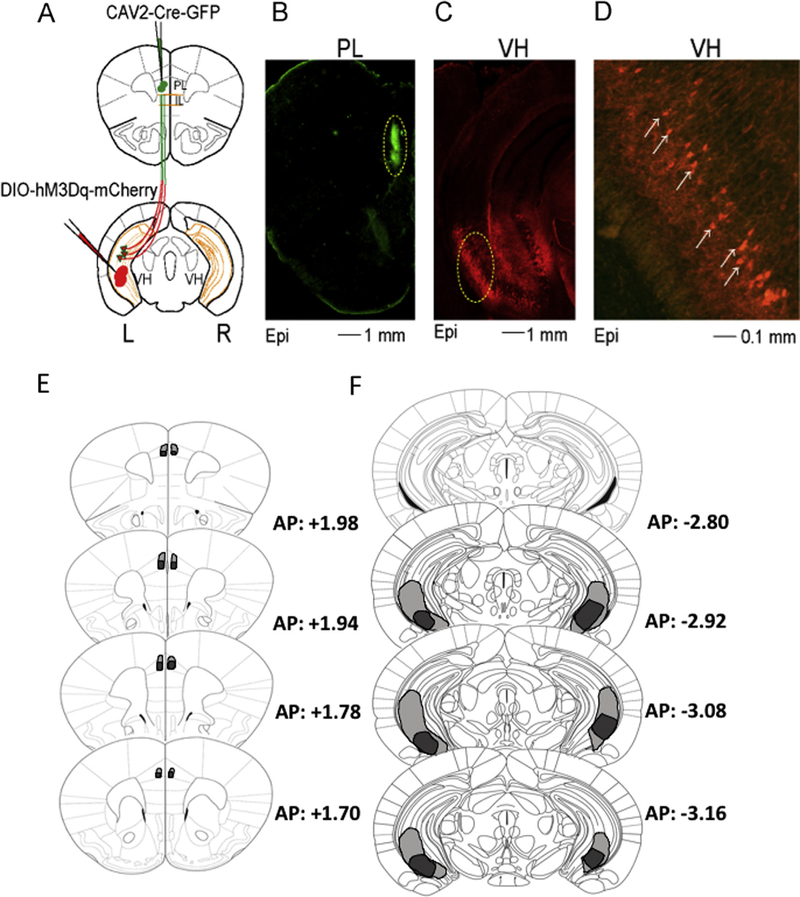
Animals were anesthetized and surgeries were conducted as described for the fluorescent microbead experiment. A group of animals received 0.4 μl bilateral infusions of CAV-2-Cre-GFP (IGMM, France) at a rate of 0.075 μl/min into the PL (in mm: AP: +1.94; ML: ± 0.30; DV: − 1.55) and 0.4 μl Gq-DREADDs (AAV-hSyn-DIO-hM3D(Gq)-mCherry, gift from Bryan Roth, Addgene viral construct #44361-AAV2; DREADD + animals) at a rate of 0.1 μl/min into the VH (in mm: AP: −2.95; ML: ± 3.6; DV: − 3.45). A control group received similar bilateral infusions of Gq-DREADDs in the VH but was injected with CAV-2-GFP in the PL (DREADD- control). Following each injection, the syringe was left in place for 10 min to allow for diffusion of virus. Mice were allowed to recover for a minimum of 4 weeks prior to the commencement of the experiments. In agreement with prior studies (Marek, Jin, et al., 2018; Parfitt et al., 2017), this time frame allowed for proper patterns of expression (Fig. 2).
Fig. 2.
Schematic illustrating design, epifluorescent (Epi) patterns of expression, and extent of transfection. A. Schematic of intersectional chemogenetic viral approach. Green circles indicate injection sites of CAV2-Cre-GFP in the PL cortex and red circles injection sites of DREADD-Gq in the VH. Red cells surrounded by the green color represent recombination leading to DREAD-Gq expression. B-C. CAV2-Cre-GFP injection sites in the PL (B) and DREADD-Gq expression in the VH (C). D. Amplification of area surrounded by a yellow dashed line in (B) shows expression restricted to pyramidal ventral cells. E. Extend of diffusion of the CAV-2-cre-GFP virus in PL. F. Extent of transfection in the VH.
2.4. Cued fear conditioning
On day 1, mice were placed in a rectangular training context (30 × 40 cm; Context A) for a total period of 340 sec. The first 180 sec were given to familiarize the animals to the context (contextual pre exposure). After this period, a CS tone was presented (2 kHz at 80 dB; 20 sec) followed by a foot shock (US, 2 sec; 0.35 mA) that co-terminated with the CS. After 60 sec, a second tone-shock pairing was presented. The mice remained in the chamber for 60 sec before being removed from the context.
2.5. Cued fear extinction
On day 2, mice were placed in a novel context with different textured and colored walls (30 × 40 cm; Context B) for a total period of 1780. After 180 sec, mice received 20 presentations of the auditory CS alone, spaced 60 sec apart. Freezing during the first tone presentation was used as a measure of fear memory.
2.6. Extinction memory test and renewal
On day 3, mice were returned to both Context B and Context A for an extinction memory retrieval test, which lasted 340 sec. Order of testing in each context was counterbalanced in all groups. Following 180 sec, the mice received two tone presentations spaced 60 sec apart; an identical procedure to day 1 but without concurrent shock presentations. Tests in each context were conducted 30min apart.
2.7. Fear analysis
All behavioral procedures were video recorded by an overhead camera. Freezing was defined as total immobility, with the exception of respiratory movements. Freezing was scored as a percentage of overall recorded time using Freeze Frame 4 (Actimetrics). During cued fear conditioning, freezing was measured during presentation of the tone stimulus. Freezing behavior was further corroborated through video analysis by an observer blind to the experimental condition who scored randomly selected behavioral sessions based on previously published criteria (Phillips & LeDoux, 1992; Wang et al., 2012). The Pearson r correlation coefficient between the observer and software was 0.99.
2.8. Groups included in the study
The DREADD+ experimental animals were divided into two groups dependent on when animals received injections of clozapine-N-oxide (CNO), a ligand that selectively activates DREADDs. One group received CNO injections prior to renewal in context A on day 3, whereas another group received CNO injections prior to retrieval on day 2. Mice were then removed from the context for 3 h prior to resuming extinction. Three control groups were included in the study: (1) Mice injected with DREADD-Gq in the VH and CAV2-GFP in the PL receiving CNO injections prior to the renewal test. This group controlled for the effect of viruses and constructs in the absence of recombination (DREADD-control). (2) Mice not undergoing surgery, but receiving CNO prior to the renewal test. This group assessed potential effects of the drug by itself (CNO only control). (3) M ice not undergoing surgery, but receiving a saline injection either prior to the renewal or the fear retrieval tests (saline controls).
2.9. Drugs
CNO (Sigma-Aldrich) was dissolved in 5% dimethyl sulfoxide (DMSO) and administered i.p. at a dose of 1 mg/kg. This concentration has been shown to be effective under similar chemogenetic manipulations (Boender et al., 2014), and was effective in inducing activation in VH slices (see electrophysiology section). CNO was administered 20 min prior to the start of the behavioral tests.
2.10. Histology
Following the conclusion of behavioral testing, mice were overdosed under isoflurane anesthesia and transcardially perfused with phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA). After extraction, brains were post-fixed in 4% PFA for 24 h and then transferred to 20% sucrose with sodium azide at 4 °C for another 24 h. Frozen brains were serially sectioned into 60 μm coronal slices that were mounted onto Superfrost Plus microscope slides.
2.11. Immunohistochemistry
Immunohistochemistry was performed on sectioned mounted tissue. Prior to wash, slides were outlined with a hydrophilic border (Immunopen; EMD Millipore). Tissue was washed three times in 5% BSA (Bovine Serum Albumin) in phosphate-buffered saline (PBS) and then incubated in 5% BSA in PBS containing 0.2% Triton-X100 (PBST) for 60 min. The tissue was then directly incubated in primary antibody solution in 5% BSA in PBST [VH slices: mouse anti-mCherry 1:100; mPFC slices: rabbit anti-GFP 1:500, (Abcam, Cambridge, MA)] for 24 h. The tissue was washed five times in PBST before incubation in secondary antibody in 5% BSA in PBST [VH slices: chicken anti-mouse IgG Alexa Fluor 594 1: 200; mPFC slices: goat anti-rabbit IgG Alexa Fluor 488 1:200, (Abcam, Cambridge, MA)] for 2h. Finally, the tissue was washed in PBS five times and cover-slipped with Fluoromount for imaging. Fluorescence was visualized using an Olympus SZX7 fluorescent microscope to confirm viral infection and extent of expression.
2.12. In vitro recordings
Whole-cell recordings were performed in ACSF at 31–33 °C. Borosilicate glass pipettes (Warner Instruments, Hamden, CT) were pulled on a vertical pipette puller (Narishige, PC-10). The pipettes displayed 3–5 MΩ resistance when filled with a potassium-based intracellular solution. Intrinsic properties were recorded in current-clamp configuration using a potassium-based intracellular solution at 31–33 °C. The solution contained (in mM): 120 potassium gluconate, 20 KCl, 10 HEPES, 10 phosphocreatine, 4 ATP, 0.3 GTP, 0.2 EGTA, and 0.3–0.5% biocytin. The cells were maintained under patch clamp for at least 20 min to allow diffusion of biocytin. The slices were then fixed with formalin at 4 °C, washed 6 times in PBS, and incubated overnight in a PBS solution containing 4% streptavidin (Alexa Fluor 488 or 594; Life Technologies, Waltham, MA) with 0.3% Triton X-100. The next day slices were washed 6 times in PBS and mounted with Fluoromount-G on glass microscope slides. Confocal images were taken with a Zeiss LSM-710 microscope. Image adjustment for brightness/contrast corrections and pseudocoloring was performed in ImageJ. Using this method, 4 animals were excluded because they either had unilateral or no expression.
2.13. Statistics
Two way repeated measures ANOVAs were used to compare the percentage of overall freezing across groups during baseline and retrieval, early- and late-extinction, and extinction test and renewal. Extinction was confirmed by comparing average freezing during the first two extinction trials (after fear retrieval) and last two extinction trials. Bonferroni multiple comparisons were used to determine which groups were statistically different using a p < 0.05.
3. Results
3.1. VH cells projecting to PL and IL differentially expand along the rostro-caudal axis.
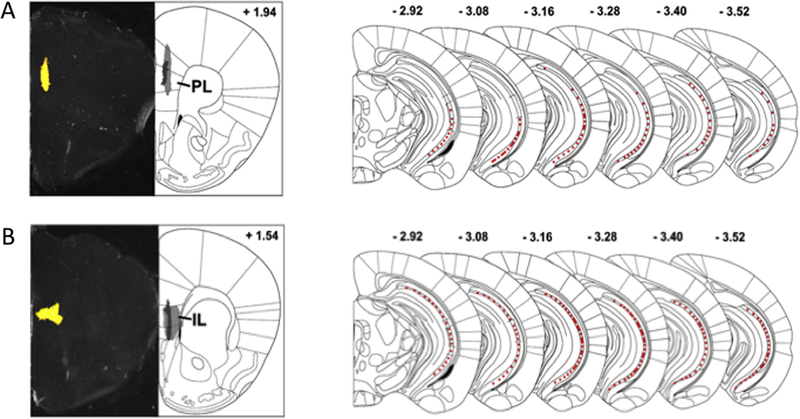
We first examined the location of the VH cells projecting to PL and IL by injecting fluorescent retrobeads, a retrograde tracers, in the target areas. We found that animals injected with retrobeads in the PL cortex (n = 4) displayed fluorescence in the most ventro-caudal portions of the VH (Fig. 1A), whereas animals injected with retrobeads in the IL cortex (n = 4) displayed fluorescent retrograde labelled cells along the whole dorso-ventral extent of the posterior hippocampus (Fig. 1B). These results corroborate previous findings indicating that VH cells projecting to IL cortex are more numerous than those projecting to PL cortex (Hoover & Vertes, 2007; Marek, Jin, et al., 2018), and further extend these observations by showing that the majority of the VH projections to PL are localized in the most ventro-caudal portions of the hippocampus.
Fig. 1.
Fluorescent retrograde labeling showing spread of VH projections to PL and IL. A-B. Bright-field showing retrobead injection site (left) and spread of the injection (right) in the PL (A) and IL (B). Light grey: largest injection, dark grey: smallest injection. C-D. Schematics showing the extent of fluorescent labeling in the VH from the IL (C) and PL (D). VH: ventral hippocampus, PL: prelimbic, IL: infralimbic.
3.2. Activation of VH to PL projections attenuates fear renewal after extinction
Recent studies have shed light into the functional roles of VH projections to IL (Marek, Jin, et al., 2018) and BLA (Xu et al., 2016). In this study, we used an intersectional DREADD approach to assess the role of the VH to PL projections in fear learning (Fig. 2A). Histological analysis revealed that the CAV-2-Cre-GFP injected in the PL was restricted to this area (Fig. 2B and E). Additionally, expression of DREADD-Gq, which happened following recombination of the DIO-hM3D(Gq)-mCherry construct, was most evident in the ventro-caudal portions of the VH (Fig. 2C–F).
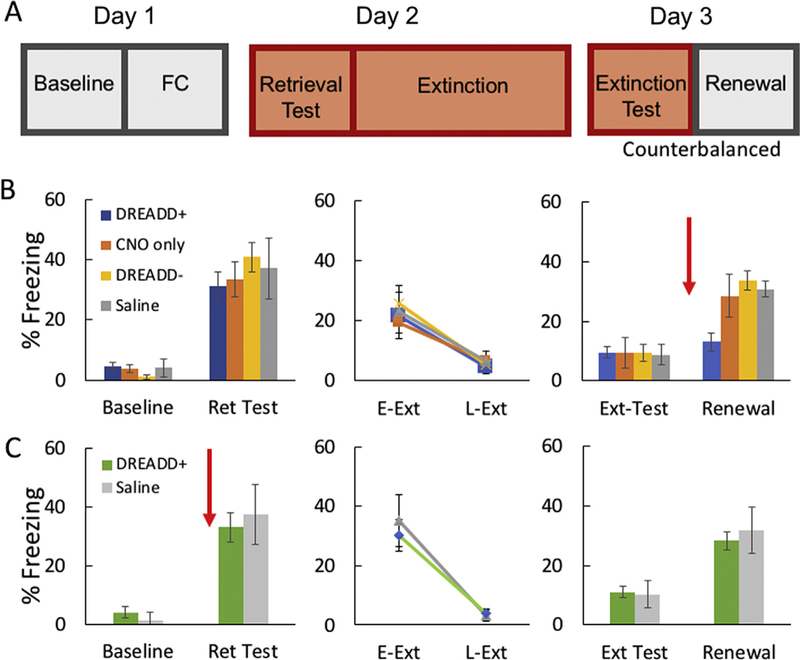
All experimental and control animals were fear conditioned, extinguished, and tested following the timeline shown in Fig. 3A. Mice expressing DREADD-Gq in VH cells projecting to the PL (n = 11) displayed levels of conditioned fear in response to the auditory tone CS that were similar to those observed in DREADD- (n = 8), saline (n = 7), or CNO only (n = 9) controls (Fig. 3B, left panel). A two-way repeated measures ANOVA revealed that mice showed significantly increased freezing during the tone CS retrieval test relative to baseline [main effect of session (baseline vs. retrieval: F(1,31) = 95.50, p < 0.001]. Bonferroni multiple comparisons revealed that the increase in freezing during retrieval was similar in all groups (p < 0.05).
Fig. 3.
A. Schematic showing behavioral timeline for fear conditioning, extinction, and testing. B-C. Freezing behavior during baseline, retrieval, extinction, extinction retrieval, and renewal for animals receiving CNO or saline prior to fear renewal testing (B) or prior to fear retrieval (C). Red arrow indicates time of CNO or saline administration. Asterisk denotes significant difference at p < 0.05. DREAD D+: experimental animals expressing DREADD-Gq, DREADD –: viral control animals with no recombination. Ret: retention, E-Ext: early extinction, L-Ext: late extinction. Ext: extinction.
Following the retrieval test, mice underwent extinction training. A two way repeated measures ANOVA showed a significant effect of time of testing during extinction [main effect of time (early- vs. late-extinction): F(1,31) = 38.95, p < 0.001]. Bonferroni multiple comparisons showed that freezing during late-extinction (last 2 tone presentations) was significantly lower than freezing during early-extinction (first 2 tone presentations) with no differences between groups, p < 0.05 (Fig. 3B, center panel). The next day (24 h following extinction), all mice received an extinction retrieval test in the extinction context B and a renewal test in context A, counterbalanced across animals. 20 min prior to the fear renewal test, the DREADD+, CNO only, and DREADD- groups received CNO (1 mg/kg, i.p.) and the saline controls received a saline injection. Two way repeated measures ANOVA showed that the groups displayed a significant effect of session [main effect of session (extinction test vs. renewal): F(1,31) = 81.66, p < 0.001] and an interaction [group and session interaction: F (3,31) = 6.58, p < 0.01]. Bonferroni multiple comparisons indicated that DREADD+ mice displayed attenuated fear renewal in the presence of CNO, which was evident in similar freezing levels between the extinction retrieval test and renewal test within that group (p < 0.005). Moreover, the three control groups (CNO only, DREADD-, or saline groups) displayed significantly more freezing during renewal than the DREADD+ experimental group (p < 0.05) [Fig. 3B, right panel]. These results indicate that selective activation of VH cells projecting to the PL blocked fear renewal in mice, suggesting that this pathway may play a role in extinction generalization.
3.3. Activation of VH to PL projections does not affect fear expression before extinction
Previous studies showed that the PL cortex plays a role in fear expression (Maren & Holt, 2004; Sierra-Mercado et al., 2011; Zhu et al., 2014). Therefore, we wanted to determine if the attenuation in fear renewal observed following CNO injections in DREADD+ animals was produced by a non-specific decrease in fear mediated by the VH to PL pathway. To test this possibility, one day after conditioning, a different group of DREAD+ animals (n = 9) received CNO (1 mg/kg; i.p.) and a control group (n = 8) a saline injection prior to the cue retrieval test. Two w ay repeated measures AN O VA indicated that these groups displayed significantly more freezing during retrieval than baseline [main effect of session (baseline vs. retrieval): F(1,15) = 45.92, p < 0.001]. Bonferroni multiple comparisons indicated that freezing levels were similar between the groups (p > 0.05; Fig. 3C, left panel). Similarly, both groups displayed normal extinction [main effect of time of testing (early- vs. late-extinction): F(1,15) = 73.74, p < 0.001], with no significant differences between the groups (p > 0.05; Fig. 3C, middle panel). Finally, both groups displayed significantly more freezing during renewal than the extinction test [main effect of session (extinction test vs. renewal): F(1,15) = 5.02, p < 0.042], with no differences between the groups (p > 0.05). [Fig. 3C, right panel]. Overall, these results indicate that activation of VH to PL projection cells plays a role in modulating context-dependent fear renewal without affecting the long-term expression of fear or extinction.
3.4. Administration of CNO increases excitability of VH neurons expressing DREADDs
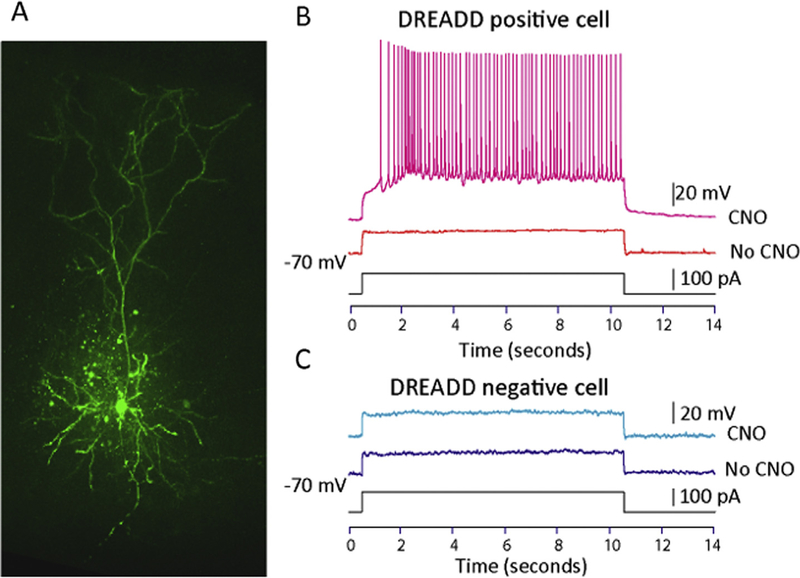
DREADDs produce effects through G-protein coupled receptors, which have diverse effects in different brain areas (Chang, Todd, Bucci, & Smith, 2015; Vazey & Aston-Jones, 2014). Therefore, we tested the specific effect of the intersectional viral approach used in this study on VH to PL projection cells. Using a whole-cell patch-clamp technique, we showed that cells expressing DREADD-Gq displayed increased excitability in response to a current injection in the presence of CNO (2 animals, 5 cells), but the same current failed to elicit action potentials in the absence of the drug (Fig. 4B, top panel). Furthermore, CNO did not have an effect in DREADD- cells from the same animals (Fig. 4B, bottom panel, 5 cells). These results indicate that the intersectional DREADDs approach used in this study reliably produced an increase in excitability specifically in VH cells expressing the construct. During whole recordings, neurons were filled with a solution containing 0.3–0.5% biocytin. After the experiment was finished, biocytin-filled DREADD+ cells were stained and confocal imaging was conducted to verify the experiments were carried out in pyramidal cells (Fig. 4A).
Fig. 4.
Electrophysiological effects of CNO on VH pyramidal cells. A. Confocal image of a patched VH cell injected with biocytin corroborating the morphological properties of pyramidal cells. B. Recordings from a D READD + cell. Before CNO application a small current step (100pA) does not elicit action potentials; however, the same current elicits robust firing after drug is applied to the bath. C. Recordings from a DREADD- cell. Before and after CNO application the cell does not respond to a small current (100 pA).
4. Discussion
In this study, we used a pathway-specific DREADD viral approach to selectively target VH to PL neurons during fear retrieval and renewal following extinction. Our findings indicate that selective activation of this projection is important for modulating fear renewal but has no effect on fear expression. We corroborated that the construct used in this study increased the excitability of VH cells projecting to PL. These data suggest that the VH to PL projection underlies context-dependent extinction generalization.
Although we have concentrated on the role of the VH in renewal, it is important to mention that several previous studies have investigated the contribution of the DH in this process. However, these studies found contradictory results. In some instances, lesions or temporal inactivation of the dorsal area reduced freezing during renewal (Corcoran, Desmond, Frey, & Maren, 2005a; Corcoran & Maren, 2001; Ji & Maren, 2005), but other studies found that renewal was not affected after the dorsal region was compromised (Campese & Delamater, 2013; Frohardt, Guarraci, & Bouton, 2000; Todd, Jiang, DeAngeli, & Bucci, 2017; Wilson, Brooks, & Bouton, 1995). One possibility for these mixed results is that the VH, rather than the DH, is the area necessary for providing contextual information during renewal. Since the VH receives spatial information via intrahippocampal connections (Amaral & Witter, 1989; Steffenach, Witter, Moser, & Moser, 2005), and indirectly through the entorhinal cortex (Dolorfo & Amaral, 1998), lesions that spare some of these inputs may still allow the VH to generate context-dependent renewal. A corollary of this idea is that lesions of the entorhinal cortex, an area that provides spatial information to both the DH and VH, should eliminate renewal. Indeed, this prediction was corroborated in a study (Ji & Maren, 2008). Interestingly, the effect of dorsal lesions on renewal depends on the time when the lesions are produced (Zelikowsky, Pham, & Fanselow, 2012). Post-extinction lesions result in overall reduction of renewal, whereas pre-training lesions do not. These data suggest that context-specific renewal can occur when the DH is compromised as long as pathways carrying contextual information are engaged during learning, which may allow the VH to process this information. Conversely, studies using a variety of approaches including pharmacological inactivation (Hobin et al., 2006; Sierra-Mercado et al., 2011; Sotres-Bayon, Sierra-Mercado, Pardilla-Delgado, & Quirk, 2012), cFos expression (Jin & Maren, 2015; Kim & Cho, 2017; Orsini, Kim, Knapska, & Maren, 2011; Wang, Jin, & Maren, 2016), and chemogenetic approaches (Marek, Jin, et al., 2018; Zhu et al., 2014) consistently found that the VH is involved in renewal, providing support for the idea that this region is critical for contextual fear relapse.
Previous studies suggested that the VH gates fear relapse through its projections to the mPFC by modulating activity in the PL (Sotres-Bayon et al., 2012). Specifically, it was found that inactivation of the VH decreases activity of PL inhibitory neurons, resulting in increased spontaneous activity of PL pyramidal cells. This disinhibition of PL pyramidal cells was shown to produce enhanced fear renewal in response to an extinguished conditioned tone. Our findings extend these results by showing that selective chemogenetic activation of VH cells projecting to PL results in decreased renewal. Interestingly, a recent study showed a similar mechanism as the one proposed by Sotres-Bayon et al. in VH cells projecting to IL (Marek, Jin, et al., 2018). Specifically, it was shown that activation of VH projecting pyramidal cells excite GABAergic interneurons in the IL cortex, which results in feed-forward inhibition of IL principal cells. At the behavioral level, inhibition of VH cells projecting to IL attenuates fear renewal. Together, these data suggest that VH projections to PL and IL cortices have opposite effects on fear renewal through the modulation of GABAergic interneurons.
An intriguing remaining question in our study is why selective activation of the VH cells projecting to PL do not affect fear expression. This result differs slightly from previous findings showing that inactivation of VH following conditioning reduces fear expression. It is important to note that in these studies VH cells were inactivated using either the GABA agonist muscimol (Sierra-Mercado et al., 2011; Sotres-Bayon et al., 2012) or DREADD-Gi overexpression in glutamatergic neurons (Zhu et al., 2014). Therefore, it is likely that these manipulations resulted in the silencing of all output projections from the VH. Together, these data, and our current finings, suggest that while VH projections to other brain regions may be responsible for mediating fear expression prior to extinction, selective VH projections to the PL mediate renewal.
Two previous studies (Hoover & Vertes, 2007; Marek, Jin, et al., 2018), in addition to our current observations, reported that VH projections to the IL are more numerous than those to the PL. Interestingly, these observations suggest that VH projections to IL extend from the dorsal to the ventral portions of area CA1, whereas the vast majority of the VH projections to PL are concentrated in the most ventro-caudal portions of the hippocampus. Hoover and Vertes (2007) also described a very small, segregated projection from the anterior DH to the PL, which has been corroborated in a recent study (Ye, Kapeller-Libermann, Travaglia, Inda, & Alberini, 2017). Collectively, these data suggest that while VH projections to IL continuously expand from dorsal to ventral locations, VH projections to PL are more compartmentalized. Nevertheless, these conclusions should be taken cautiously because one limtation of retrograde labeling/viral approaches is that level of expression depends on the volume of virus/retrograde tracer injected, the stereotaxic coordinates used in the experiment, the level of transfection, and the species tested. Therefore, without standardized methods across experiments and species, it is too early to determine with precision if there are significant differences among VH projections to the medial prefrontal cortex.
Anxiety disorders, such as post-traumatic stress disorder (PTSD) and phobias, are characterized by the retrieval of fearful memories in inappropriate contexts (Levy-Gigi, Szabo, Richter-Levin, & Keri, 2015). A major hurdle facing the treatment of these disorders is the difficulty in facilitating extinction (i.e. the formation of inhibitory associations to fearful memories) across multiple contexts (VanElzakker, Dahlgren, Davis, Dubois, & Shin, 2014). Here, we show that activation of the VH to PL pathway specifically attenuates fear renewal. This has important implications for the treatment of anxiety disorders as it uncovers a specific neural pathway associated with extinction generalization.
Acknowledgements
We thank Matthew Lopez, and Maria Garza for comments on previous versions of the manuscript.
Funding
This work was supported by the National Science Foundation [NSF CAREER Award 1565410 (I.A.M)], National Institutes of Health [NIH, NIGMS, grant GM122645 (A.J.A.), and UTSA MARC Program GM007717 (J.H.V.)].
Abbreviations:
- PL
prelimbic cortex
- IL
infralimbic cortex
- VH
ventral hippocampus
- mPFC
medial prefrontal cortex
- BLA
basolateral amygdala
References
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31 (3), 571–591 0306–4522(89)90424–7 [pii]. [DOI] [PubMed] [Google Scholar]
- Bakhi VP, & Kalin NH (2002). Animal models and anxiety endophenotypes and anxiety and stress disorders. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, & Lin LH (1990).Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Progress in Brain Research, 83, 287–300. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G,& Adan RA (2014). Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One, 9(4), e95392 10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2004). Context and behavioral processes in extinction. Learning &Memory, 11(5), 485–494 11/5/485 [pii]10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Role of Conditioned Contextual Stimuli in Reinstatement of Extinguished Fear. Journal of Experimental Psychology: Animal Behavior Processes, 5(4), 11. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, & Maren S (2006). Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry, 60(4), 352–360 S0006–3223(06)00099–0 [pii] 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Cahill EN, & Milton AL (2019). Neurochemical and molecular mechanisms underlying the retrieval-extinction effect. Psychopharmacology (Berl). 10.1007/s00213-018-5121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese V, & Delamater AR (2013). ABA and ABC renewal of conditioned magazine approach are not impaired by dorsal hippocampus inactivation or lesions. Behavioural Brain Research, 248, 62–73. 10.1016/j.bbr.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, & Smith KS (2015). Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. European Journal of Neuroscience, 42(12), 3105–3116. 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang Y, Wang X, & Li H (2017). Neural circuits involved in the renewal of extinguished fear. IUBMB Life, 69(7), 470–478. 10.1002/iub.1636. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, & Maren S (2005a). Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. The Journal of Neuroscience, 25(39), 8978–8987. 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, & Maren S (2005b). Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. The Journal of Neuroscience, 25(39), 10 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Maren S (2001). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. The Journal of Neuroscience, 21(5), 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Quirk GJ (2007). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. The Journal of Neuroscience, 27(4), 840–844. 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, & Quirk GJ (2015). Revisiting the role of infralimbic cortex in fear extinction with optogenetics. The Journal of Neuroscience, 35(8), 3607–3615. 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, & Amaral DG (1998). Entorhinal cortex of the rat: Organization of intrinsic connections. The Journal of Comparative Neurology, 398(1), 49–82 10.1002/(SICI)1096–9861(19980817)398:1 < 49::AID-CNE4 > 3.0.CO;2–9 [pii]. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & LeDoux JE (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron, 23(2), 229–232. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, & Bouton ME (2000). The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behavioral Neuroscience, 114(2), 227–240. [DOI] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2014). Animal models of fear relapse. ILAR Journal, 55(2), 246–258. 10.1093/ilar/ilu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt K, Hamsher K, & Kennedy AW (1978). Spontaneous recovery in rabbit eyelid conditioning. The Journal of General Psychology, 98(2d Half), 241–244. 10.1080/00221309.1978.9920877. [DOI] [PubMed] [Google Scholar]
- Harris JA, Jones ML, Bailey GK, & Westbrook RF (2000). Contextual control over conditioned responding in an extinction paradigm. Journal of Experimental Psychology: Animal Behavior Processes, 26(2), 12. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, & Kendler KS (2001). A population-based twin study of generalized anxiety disorder in men and women. The Journal of Nervous and Mental Disease, 189(7), 413–420. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, & Maren S (2006). Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus, 16, 9. [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function, 212, 31 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, & Nakamura S (2006). Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. Journal of Neurophysiology, 96(4), 2134–2138 00069.2006 [pii]10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Jay TM, & Witter MP (1991). Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology, 313(4), 574–586. [DOI] [PubMed] [Google Scholar]
- Ji J, & Maren S (2005). Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning & Memory, 12(3), 270–276. 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008). Lesions of the entorhinal cortex or fornix disrupt the context-dependence of fear extinction in rats. Behavioural Brain Research, 194(2), 201–206. 10.1016/j.bbr.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, & Maren S (2015). Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Scientific Reports, 5, 8388 10.1038/srep08388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath AT, Wang ME, Wann EG, Yuan RK, Dudman JT, & Muzzio IA (2014). Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus, 24(12), 1533–1548. 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, & Prescott CA (1999). Fears and phobias: Reliability and heritability. Psychological Medicine, 29(3), 539–553. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA, & Neale MC (2001). The genetic epidemiology of irrational fears and phobias in men. Archives of General Psychiatry, 58(3), 257–265 yoa20158 [pii]. [DOI] [PubMed] [Google Scholar]
- Kent JM, & Rauch SL (2003). Neurocircuitry of anxiety disorders. Current Psychiatry Reports, 5(4), 266–273. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus, 17(9), 697–708. 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kim WB, & Cho JH (2017). Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. The Journal of Neuroscience, 37(19), 4868–4882. 10.1523/JNEUROSCI.3579-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, & Eichenbaum H (2013). Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. The Journal of Neuroscience, 33(18), 8079–8087. 10.1523/JNEUROSCI.5458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, & Phelps EA (2005). Reinstatement of Conditioned Fear in Humans Is Context Dependent and Impaired in Amnesia. Behavioral Neuroscience, 119(3), 10 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Laurent V, & Westbrook RF (2009). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learning & Memory, 16(9), 520–529. 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Levy-Gigi E, Szabo C, Richter-Levin G, & Keri S (2015). Reduced hippocampal volume is associated with overgeneralization of negative context in individuals with PTSD. Neuropsychology, 29(1), 151–161. 10.1037/neu0000131. [DOI] [PubMed] [Google Scholar]
- Majak K, & Pitkanen A (2003). Projections from the periamygdaloid cortex to the amygdaloid complex, the hippocampal formation, and the parahippocampal region: A PHA-L study in the rat. Hippocampus, 13(8), 922–942. [DOI] [PubMed] [Google Scholar]
- Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, … Sah P (2018). Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nature Neuroscience, 21(3), 384–392. 10.1038/s41593-018-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Sun Y, & Sah P (2018). Neural circuits for a top-down control of fear and extinction. Psychopharmacology (Berl). 10.1007/s00213-018-5033-2. [DOI] [PubMed] [Google Scholar]
- Marek R, Xu L, Sullivan RKP, & Sah P (2018). Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nature Neuroscience, 21(5), 654–658. 10.1038/s41593-018-0137-x. [DOI] [PubMed] [Google Scholar]
- Maren S (2005). Synaptic mechanisms of associative memory in the amygdala. Neuron, 47(6), 783–786. 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, & Holt W (2000). The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural Brain Research, 110(1–2), 97–108 S0166432899001886 [pii]. [DOI] [PubMed] [Google Scholar]
- Maren S, & Holt WG (2004). Hippocampus and Pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience, 118(1), 97–110. 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, & Moser MB (2006). Path integration and the neural basis of the ‘cognitive map’. Nature Reviews Neuroscience, 7(8), 663–678 nrn1932 [pii]10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, & O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681–683. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Conway DH (1978). Hippocampal place units in the freely moving rat: Why they fire where they fire. Experimental Brain Research, 31 (4), 573–590. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wible CG, & Shapiro ML (1986). Mnemonic theories of hippocampal function. Behavioral Neuroscience, 100(6), 852–855. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, & Maren S (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. The Journal of Neuroscience, 31(47), 17269–17277 31/47/17269 [pii] 10.1523/JNEUROSCI.4095–11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, & Ledoux JE (2004). New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology, 92(1), 1–9. 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, … Kim JC (2017). Bidirectional control of anxiety-related behaviors in mice: Role of inputs arising from the ventral hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology, 42(8), 1715–1728. 10.1038/npp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP (1927). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London: Oxford Univ. Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research. Brain Research Reviews, 38(1–2), 247–289 S0165017301000807 [pii]. [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, & Pitkanen A (1999). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippo-campal formation in rat. The Journal of Comparative Neurology, 403(2), 229–260 10.1002/(SICI)1096–9861(19990111)403:2 < 229::AID-CNE7 > 3.0.CO;2-P [p ii]. [PubMed] [Google Scholar]
- Quirk GJ (2002). Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning & Memory, 9(6), 402–407. 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Thomas BL, & Ayres JJB (2001). Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: Implications for treating human phobias. Journal of Experimental Psychology: Animal Behavior Processes, 27(2), 16. [PubMed] [Google Scholar]
- Rescorla RA, & Heth CD (1975). Reinstatement of Fear to an Extinguished Conditioned Stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 104(1), 9. [PubMed] [Google Scholar]
- Richardson R, Duffield TQ, Bailey GK, & Westbrook RF (1999). Reinstatement of fear to an extinguished conditioned context. Learning & Behavior, 27(4), 17. [Google Scholar]
- Robbins SJ (1990). Mechanisms underlying spontaneous recovery in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes, 16(3), 15 10.1037/0097-7403.16.3.235. [DOI] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, & Quirk GJ (2014). Hippocampal-prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology, 39(9), 2161–2169. 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, & Buzsaki G (2010). Distinct representations and theta dynamics in dorsal and ventral hippocampus. The Journal of Neuroscience, 30(5), 1777–1787 30/5/1777 [pii]10.1523/JNEUROSCI.4681–09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, LeDoux JE, & Phelps EA (2008). Evidence for recovery of fear following immediate extinction in rats and humans. Learning & Memory, 15, 9 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, & Quirk GJ (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36(2), 529–538 npp2010184 [pii]10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, & Quirk GJ (2012). Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron, 76(4), 804–812. 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, & Moser EI (2005). Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron, 45(2), 301–313. [DOI] [PubMed] [Google Scholar]
- Todd TP, Jiang MY, DeAngeli NE, & Bucci DJ (2017). Intact renewal after extinction of conditioned suppression with lesions of either the retrosplenial cortex or dorsal hippocampus. Behavioural Brain Research, 320, 143–153. 10.1016/j.bbr.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, & Wyss JM (1990). Extrinsic projections from area CA1 of the rat hippocampus: Olfactory, cortical, subcortical, and bilateral hippocampal formation projections. The Journal of Comparative Neurology, 302(3), 515–528. 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, & Shin LM (2014). From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem, 113, 3–18. https://doi.org/10.10167j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, & Aston-Jones G (2014). Designer receptors: Therapeutic adjuncts to cell replacement therapy in Parkinson’s disease. Journal of Clinical Investigation, 124(7), 2858–2860. 10.1172/JCI76833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ME, Wann EG, Yuan RK, Ramos Alvarez MM, Stead SM, & Muzzio IA (2012). Long-term stabilization of place cell remapping produced by a fearful experience. Journal of Neuroscience, 32, 15802–15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Jin J, & Maren S (2016). Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiology of Learning and Memory, 134 Pt A, 38–43. 10.1016/j.nlm.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, & Bouton ME (1995). The role of the rat hippocampal system in several effects of context in extinction. Behavioral Neuroscience, 109(5), 828–836. [DOI] [PubMed] [Google Scholar]
- Xu C, Krabbe S, Grundemann J, Botta P, Fadok JP, Osakada F, … Luthi A (2016). Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell, 167(4), e916 10.1016/j.cell.2016.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, & Alberini CM (2017). Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nature Neuroscience, 20(1), 52–61. 10.1038/nn.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Pham DL, & Fanselow MS (2012). Temporal factors control hippocampal contributions tofear renewal after extinction. Hippocampus, 22(5), 1096–1106. 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, & Roth BL (2014).Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory. Neuropsychopharmacology, 39(8), 1880–1892. 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]