Abstract
Purpose
Colorectal cancer (CRC) screening guidelines recommend increased surveillance of individuals with sessile serrated adenomas/polyps (SSA/Ps), but there is uncertainty about the risk associated with SSA/Ps. We aimed to determine the association between SSA/Ps and subsequent advanced colorectal neoplasia.
Methods
This case-control study included Kaiser Permanente Washington (KPWA) members who received an index colonoscopy between 1/1/1998 and 12/31/2007, and had hyperplastic polyps (HPs) or SSA/Ps but no conventional adenomas according to study pathologist histologic review. Subsequent pathology reports and biopsies through 1/1/2013 were reviewed for advanced colorectal neoplasia. We linked to the Seattle-Puget Sound Surveillance Epidemiology and End Results (SEER) registry to identify additional CRC cases. We used generalized estimating equations with a logit link to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for advanced colorectal neoplasia, comparing those with SSA/Ps to those with HPs.
Results
There were 161 individuals with index SSA/Ps, 548 with HPs, and 918 subsequent endoscopies included in analyses. Of those with index SSA/Ps, 19 had subsequent advanced colorectal neoplasia; 39 with HPs had subsequent advanced colorectal neoplasia. Compared to those with HPs, those with SSA/Ps were not statistically significantly more likely to have subsequent advanced colorectal neoplasia (adjusted OR 1.79; CI 0.98–3.28). Polyp size ≥ 10 mm, right colon location, and the presence of multiple serrated polyps were also not associated with advanced colorectal neoplasia.
Conclusions
Our results suggest that there is not a strong association between SSA/Ps and subsequent advanced colorectal neoplasia during the 5 years following SSA/P removal.
Keywords: Sessile serrated adenoma/polyp, Colonoscopy, Screening, Surveillance, Colorectal cancer
Introduction
Historically, colorectal polyps were categorized into two broad categories for clinical management, defined by the presence of nuclear dysplasia within the polyp. Polyps with nuclear dysplasia were considered adenomas and relevant to clinical management. Polyps without nuclear dysplasia were considered hyperplastic and of little clinical relevance. Adenomas, using the broad, historical definition of polyps with nuclear dysplasia, are established precursor lesions for colorectal cancer [1], In general, few adenomas will progress to cancer [2], but adenomas ≥ 10 mm in diameter, with villous histology, or with high-grade dysplasia are considered advanced adenomas and have a greater risk of developing into malignant tumors [1, 3]. Thus, adenomas, particularly advanced adenomas, have historically been the main targets of colorectal cancer screening [4].
Recent research suggests that in addition to advanced adenomas, some serrated polyp subtypes, such as sessile serrated adenomas/polyps (SSA/Ps), may be important precursors for colorectal cancer [5–7]. Serrated polyps include: hyperplastic polyps (HPs), SSA/Ps, and traditional serrated adenomas (TSAs) [5]. TSAs have nuclear dysplasia and have long been recognized as potential precursors for colorectal cancer [8–10]. In contrast, HPs and most SSA/Ps lack nuclear dysplasia, and recommendations for their clinical management have changed over time.
Traditionally, HPs were considered to have no malignant potential, and until recently, SSA/Ps were not distinguished histologically from HPs. Therefore, SSA/Ps were previously not considered lesions that necessitated complete endoscopic removal or increased colorectal cancer surveillance. This changed when the 2006 and 2008 guidelines from the United States Multi-Society Task Force on Colorectal Cancer and the American Cancer Society began recommending complete removal of large SSA/Ps [4, 11], In 2012, additional new guidelines for the management of patients with SSA/Ps were published, and these guidelines recommended closer endoscopic surveillance for patients with SSA/Ps [12, 13]. One of these guidelines recommended a 5 year colonoscopy surveillance regimen for patients with SSA/Ps <10 mm in diameter and 3-year colonoscopy surveillance regimen for patients with SSA/Ps ≥ 10 mm in diameter or SSA/Ps exhibiting nuclear dysplasia [12]. Other guidelines offered recommendations for the surveillance of patients with serrated polyps that varied according to histology, size, location, and number of serrated polyps [13]. However, at the time that these 2012 guidelines were published, large studies of the risk of colorectal neoplasia following a SSA/P diagnosis were absent from the literature, and these guidelines acknowledged uncertainty in the risk of colorectal cancer associated with SSA/Ps.
Recently, several studies have evaluated the association between SSA/Ps and subsequent colorectal neoplasia risk [6, 14–18], but these studies have varied results. Thus, we conducted a case-control study to test the hypothesis that SSA/Ps are associated with an increased risk of subsequent advanced colorectal neoplasia as compared to HPs. We also explored differences in the risk of advanced colorectal neoplasia according to size, site, and number of serrated polyps.
Methods
Source population
We used current procedural terminology (CPT), International Classification of Disease, Ninth Revision (ICD-9), and Healthcare Common Procedure Coding System (HCPCS) codes to identify Kaiser Permanente Washington (KPWA) members who received an index colonoscopy at KPWA’s gastroenterology clinics between 1 January 1998 and 31 December 2007. Among these members, we selected all of those who were ages 20–75 years old and were clinically diagnosed as having serrated polyps at index colonoscopy. These polyps were identified using electronic text string searches of colorectal-related pathology reports followed by manual review of medical records and later confirmed through a standardized study pathology review (described below). Members with< 12 months of continuous health plan enrollment prior to their index colonoscopy, those who resided outside of the Seattle-Puget Sound Surveillance Epidemiology and End Results (SEER) cancer registry catchment area at the time of index colonoscopy, and those with CRC, inflammatory bowel disease, or colectomy at, prior to, or within 31 days after the index colonoscopy were ineligible. We also excluded those with familial colorectal cancer syndromes, such as familial adenomatous polyposis and Lynch Syndrome. To evaluate the clinical relevance of SSA/Ps in the absence of conventional adenomas (tubular, tubulovillous, or villous adenomas) and TSAs, we additionally excluded those who were diagnosed with conventional adenomas or TSAs at their index colonoscopy. Those with no CRC or no lower GI endoscopies (colonoscopy or sigmoidoscopy) occurring after the index colonoscopy were also excluded, because we could not ascertain the presence or absence of subsequent advanced colorectal neoplasia in those individuals.
In this source population, we excluded index colonoscopy exams occurring < 1 year after a prior lower gastrointestinal endoscopy, those that did not reach the cecum, and those with poor or inadequate bowel preparation. We also excluded index colonoscopies that occurred in 2001, because 2001 polyp biopsies were not available for the standardized study pathology review. Study protocols and procedures were approved by the Institutional Review Boards at KPWA and Fred Hutchinson Cancer Research Center on 17 May 2012.
Data collection
Administrative and clinical data extraction and abstraction
We collected information on the size, location, and number of HPs and SSA/Ps diagnosed at index colonoscopy through manual data abstraction of endoscopy reports and pathology reports. We ascertained medical history, demographics, and colorectal cancer risk factors, such as smoking status, family history of colorectal cancer, body mass index (BMI), and subsequent endoscopy procedures and related pathology results through a combination of electronic data extraction from administrative and clinical databases and manual abstraction of medical records. We obtained information on study participants’ subsequent endoscopy procedures, colorectal polyps, and CRC from the time of the index colonoscopy until 1 January 2013. Colorectal cancers occurring after the index colonoscopy were identified through linkage to the Seattle-Puget Sound SEER registry. Colorectal polyps diagnosed during subsequent endoscopies were ascertained through manual abstraction of medical records, followed by a standardized study pathology review (described below). CRC occurring after the index colonoscopy were identified through medication records and through linking the source population to the Seattle-Puget Sound SEER registry. For incident colorectal polyps or cancers, we also collected data on date of diagnosis and size (for polyps).
Standardized pathology review
The study pathologist (LCZ) conducted a standardized pathology review to re-review all clinical colorectal biopsies from the index colonoscopy and subsequent endoscopies. This was done to ensure standard and complete ascertainment of SSA/Ps and HPs in the study sample. A second study pathologist (MU) conducted a standardized pathology review of a random sample (n = 113) of serrated polyps to assess the reliability of SSA/P diagnoses [19]. All biopsies had previously been formalin-fixed, paraffin-embedded, cut and mounted onto slides, and stained with hematoxylin and eosin. Using established protocols and criteria [19], the pathologists classified biopsies as: (1) HPs; (2) SSA/Ps; (3) tubular adenomas; (4) tubulovillous adenomas (having ≥ 20% villous components); (5) traditional serrated adenomas; (6) other colorectal polyps; or (7) carcinomas. For all polyps, including SSA/Ps, pathologists also noted whether low-grade or high-grade dysplasia was present. If biopsy tissue from a subsequent endoscopy was unavailable for the standard pathology review, clinical pathology diagnoses were used to determine case-control status.
Data analysis
Outcomes definitions
Information collected via the standardized pathology review of biopsies from each colonoscopy or sigmoidoscopy that occurred subsequent to the index colonoscopy and through linkage to the Seattle-Puget Sound SEER registry was used to define the primary outcome of interest, advanced colorectal neoplasia, defined as CRC or advanced colorectal polyps (conventional adenomas or traditional serrated adenomas that are ≥ 10 mm in diameter, or with ≥20% villous components, or with high-grade nuclear dysplasia, or SSA/Ps with any nuclear dysplasia) [1, 3, 13]. Secondary outcomes of interest were non-advanced neoplastic polyps (tubular adenomas or TSAs< 10 mm in diameter, with <20% villous components, and without high-grade nuclear dysplasia and SSA/Ps without nuclear dysplasia), and other colorectal polyps.
Case-control classification
Cases include those with advanced colorectal neoplasia at a subsequent endoscopy; controls were those with no advanced colorectal neoplasia at a subsequent endoscopy. Case-control status was defined at each subsequent endoscopy or at the time of incident cancer diagnoses through SEER linkages. If an individual had multiple subsequent colonoscopies, then the individual could contribute multiple observations to the analysis. However, once an individual had a subsequent “case” endoscopy, that individual could not later have a “control” endoscopy or a 2nd “case” endoscopy. For example, if an individual had two subsequent endoscopies with no advanced colorectal neoplasia at each colonoscopy, then that individual would contribute two “control” observations. If an individual had a subsequent endoscopy with advanced colorectal neoplasia, then each endoscopy for that individual that occurred after this “case” endoscopy was excluded from analyses.
Exposure definitions
The primary exposures of interest were based on polyps identified at the index colonoscopy and included: polyp histologic diagnosis (HP or SSA/P), size, location, and number of polyps. Index polyp histologic diagnosis and the presence of nuclear dysplasia were classified according to the standardized study pathology review. Polyp size, location, and number of polyps were determined from medical records abstraction.
Statistical analyses
We used generalized estimating equations with a binomial distribution, logit link, and independent correlation structure with the robust variance estimator to calculate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for advanced colorectal neoplasia, comparing those with SSP/As at the index colonoscopy to those with HPs at index. This type of model takes into account, and adjusts for, the correlation of multiple subsequent endoscopies within individuals [20], We also compared cases and controls according to size of largest serrated index polyp (< 10 mm or ≥ 10 mm in diameter), number of serrated polyps (1, 2, 3 or ≥4), and polyp location [rectum/rectosigmoid junction, left colon (including sigmoid colon, descending colon, and splenic flexure), or right colon (including transverse colon, hepatic flexure, ascending colon, and cecum)], using separate models. If serrated polyps from separate locations were identified at index colonoscopy, right-sided polyps took precedence over left-sided polyps, and left-sided polyps took precedence over rectal polyps. Analyses were also conducted restricting to those with at least one SSA/P, and we made comparisons in this subset according to size of largest SSA/P (<10 mm or ≥ 10 mm), location (rectum/rectosigmoid junction/left colon or right colon), multiplicity of SSA/Ps (1 or> 1), and number of synchronous serrated polyps (0, 1, 2, ≥ 3). ORs were adjusted for the following potential confounders a priori based on their association with polyp characteristics and risk of incident colorectal polyps or cancer, or detection of incident colorectal polyps or cancer: age at index colonoscopy, sex, body mass index (BMI) at or within 30 days of index colonoscopy, smoking status at or within 2 years of index colonoscopy, and time between index procedure and subsequent procedure. There was little racial/ethnic variability in the study cohort, because 88% of the study population was Non-Hispanic White; thus, we did not adjust for race/ethnicity in our analyses.
Data on smoking status were missing for 13%. and BMI was missing for 2%, of the study population. We used multiple imputation by chained equations (MICE) to impute these missing covariate values. [21] Imputation models were built for each covariate. We used linear regression for BMI, and logistic regression for smoking status. All the variables (outcome, exposure and covariates) in the GEE analytical model were included as independent variables in the imputation models. For BMI, we also included the following variables in the multiple imputation model: age at index colonoscopy, sex, smoking status, years between index and subsequent endoscopy, history of prior colonoscopy, sigmoidoscopy, FIT/FOBT, and barium enema, days of continuous enrollment in KPWA prior to index colonoscopy, and history of diabetes. Similarly, to impute smoking status, we included the same set of covariates in the imputation model with smoking status replaced by BMI. We performed ten rounds of imputation and inspected trace plots for each imputed variable. The diagnostics of imputation models were conducted by inspecting trace plots (Supplemental Fig. 1). Imputation models reached the convergence quickly, and the predicted values remained relatively constant.
We also conducted exploratory analyses evaluating the secondary outcomes: (1) subsequent non-advanced colorectal neoplastic polyps, and (2) any subsequent colorectal polyp or cancer. Sensitivity analyses were conducted that included those with subsequent colonoscopy exams only, rather than subsequent colonoscopy or sigmoidoscopy exams.
Results
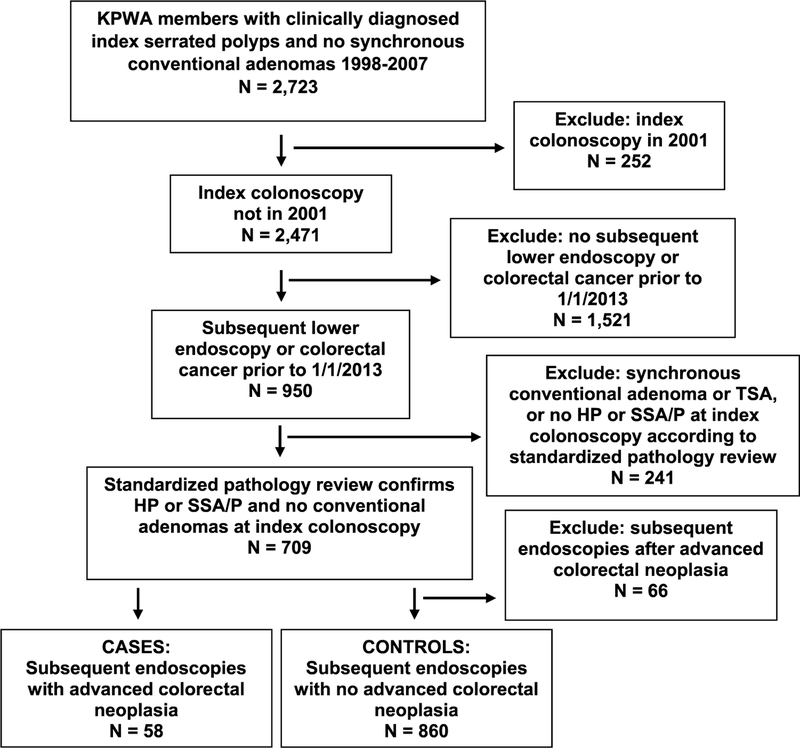
There were 2,723 KPWA members with clinically diagnosed index serrated polyps and no synchronous conventional adenomas from 1998 to 2007. Among these, 252 individuals had an index colonoscopy in 2001; these were excluded, because biopsies from 2001 were not available for the standardized study pathology review. Of the 2,471 study participants remaining, 950 individuals had ≥ 1 subsequent colonoscopy or sigmoidoscopy, or a CRC diagnosis through SEER prior to 1 January 2013. After the standardized study pathology review of index colonoscopy biopsies, 241 individuals were excluded, because they were determined to have no SSA/Ps or HPs at their index colonoscopy (n = 189), or they were found to have synchronous conventional adenomas (n = 42) or traditional serrated adenomas (n = 10). Thus, 709 individuals having a total of 984 lower gastrointestinal endoscopies after the index colonoscopy were eligible for analyses. Of these 709 individuals, 161 had ≥ 1 SSA/P, and 548 had HPs with no synchronous SSA/Ps at index colonoscopy. When the second study pathologist conducted an independent pathology review of the H&E slides, she agreed with the primary study pathologist’s SSA/P diagnosis 92% of the time.
Of the 984 subsequent endoscopies, 58 had advanced colorectal neoplasia, including five CRCs, and were considered cases; 860 were controls with no advanced colorectal neoplasia at subsequent endoscopy, and 66 were excluded because they occurred after a subsequent endoscopy with advanced colorectal neoplasia (Fig. 1).
Fig. 1.
Study population flow diagram
Of the 161 individuals with SSA/Ps at index colonoscopy, only three had nuclear dysplasia, and none of those carrying SSA/Ps with nuclear dysplasia developed CRC or had a subsequent endoscopy with an advanced colorectal neoplasia diagnosis. Among the 158 individuals with SSA/Ps without nuclear dysplasia, 19 had subsequent procedures with an advanced colorectal neoplasia diagnosis, and two of these were CRCs diagnosed 5.3 and 7.2 years after index colonoscopy. Of the 548 individuals with HPs, 39 had subsequent endoscopies with an advanced colorectal neoplasia diagnosis, including three with CRCs diagnosed 4.6, 9.5, and 14.2 years after index colonoscopy.
Compared to those without advanced colorectal neoplasia at subsequent endoscopy, those who had subsequent endoscopies with advanced colorectal neoplasia were more likely to be > 50 years old, male, and to have BMI ≥ 30 kg/m2. Subsequent endoscopies with advanced colorectal neoplasia were also more likely to be > 5 years after the index colonoscopy (Table 1).
Table 1.
Characteristics of advanced colorectal neoplasia cases and controls at the procedure level (n=918)
| Case n = 58 n (%) |
Control n = 860 n (%) |
|
|---|---|---|
| Age at index colonoscopy, in years | ||
| 20–49 | 6 (10) | 139 (16) |
| 50–64 | 35 (60) | 500 (58) |
| 65–75 | 17 (29) | 221 (26) |
| Sex | ||
| Female | 28 (48) | 474 (55) |
| Male | 30 (52) | 386 (45) |
| Body mass index at index, in kg/m2 | ||
| <25 | 9(16) | 256 (30) |
| 25–29.99 | 27 (47) | 323 (38) |
| 30 + | 22 (38) | 265(31) |
| Missing | 0 | 16 (2) |
| Race/ethnicity | ||
| Non-white | 2 (3) | 68 (8) |
| White | 52 (90) | 746 (87) |
| Missing | 4 (7) | 46 (5) |
| Smoking status at index | ||
| Ever | 29 (50) | 414 (48) |
| Never | 22 (38) | 330 (38) |
| Missing | 7 (12) | 116 (13) |
| Time between index and subsequent colonoscopy | ||
| <1 year | 0 | 22 (3) |
| 1–2 years | 1 (2) | 70 (8) |
| 3–5 years | 11 (19) | 155 (18) |
| > 5 years | 46 (79) | 613 (71) |
In comparing those with SSA/Ps at index colonoscopy to those with only HPs, SSA/P histology was associated with an increase in subsequent advanced colorectal neoplasia, but the association was not statistically significant (OR 1.79; CI 0.98–3.28). Subsequent advanced neoplasia was also not associated with index serrated polyp size (OR for≥ 10 mm vs. < 10 mm = 0.95; CI 0.38–2.34), location (OR for left colon vs. rectum/rectosigmoid = 1.62; CI 0.73002D3.60 and OR for right colon vs. rectum/rectosigmoid = 1.64; Cl 0.75–3.58), or number of serrated polyps (OR for four or more vs. one serrated polyp = 1.46; CI 0.34–6.32) (Table 2).
Table 2.
Odds ratios of the association between index serrated polyps (n = 918) and advanced colorectal neoplasia according to histology, size, location, and number of serrated polyps
| Cases n (%) | Controls n (%) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Histology | |||
| HPs only | 39 (67) | 662 (77) | 1.00 (Ref) |
| SSA/Ps | 19 (33) | 198 (23) | 1.79(0.98–3.28) |
| Size | |||
| All < 10mm | 43 (74) | 632 (73) | 1.00 (Ref) |
| Any 10 +mm | 6 (10) | 87 (10) | 0.95 (0.38–2.34) |
| Location | |||
| Rectum/Rectosig | 11(19) | 235 (27) | 1.00 (Ref) |
| Left | 17 (29) | 192 (22) | 1.62(0.73–3.60) |
| Right | 24 (41) | 334 (39) | 1.64(0.75–3.58) |
| Number of serrated polyps | |||
| 1 | 40 (69) | 650 (76) | 1.00 (Ref) |
| 2 | 13 (22) | 138 (16) | 1.49 (0.78–2.87) |
| 3 | 3 (5) | 46 (5) | 1.19 (0.35–4.02) |
| 4 + | 2 (3) | 26 (3) | 1.46 (0.34–6.32) |
Adjusted for age at index (continuous), sex, BMI (continuous with multiple imputation), smoking status (never vs. ever with multiple imputation), and year between index and subsequent procedure
Table 3 displays associations between polyp characteristics and subsequent advanced colorectal neoplasia, restricted to those with one or more SSA/P at index colonoscopy. There was no variation in the odds of subsequent advanced colorectal neoplasia by SSA/P size (OR for – 10 mm vs. < 10 mm = 1.22; CI 0.29–5.10), location (OR for right colon vs. rectum/rectosigmoid/left colon = 0.67; CI 0.20–2.22), multiplicity (OR for 1 SSA/P vs. >1 SSA/P =1.35; CI 0.46–3.93), or number of synchronous serrated polyps (OR for SSA/P + 3 or more synchronous serrated polyps vs. only 1 SSA/P = 1.60; CI 0.30–8.50).
Table 3.
Odds ratios of the association between index serrated polyps and advanced colorectal neoplasia according to size, location, and the number of serrated polyps among those with index SSA/Ps (n = 217)
| Cases n (%) | Controls n (%) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Size | |||
| All < 10 mm | 12 (63) | 136 (69) | 1.00 (Ref) |
| Any 10 +mm | 3 (16) | 46 (23) | 1.22 (0.29–5.10) |
| Location | |||
| Rectum/RSG/Left | 5 (26) | 44 (22) | 1.00 (Ref) |
| Right | 13 (68) | 149 (75) | 0.67 (0.20–2.22) |
| Presence of multiple SSA/Ps | |||
| No | 14 (74) | 156 (79) | 1.00 (Ref) |
| Yes | 5 (26) | 42 (21) | 1.35 (0.46–3.93) |
| Number of synchronous serrated polyps | |||
| 1 SSA/P only | 10 (53) | 109 (55) | 1.00 (Ref) |
| SSA/P+1 HP or SSA/P | 5 (26) | 52 (26) | 0.80 (0.22–2.89) |
| SSA/P+ 2 HP or SSA/P | 2 (11) | 18 (9) | 1.04 (0.26–4.12) |
| SSA/P + 3 or more HP or SSA/P | 2 (11) | 19 (10) | 1.60 (0.30–8.50) |
Adjusted for age at index colonoscopy (continuous), sex, BMI (continuous with multiple imputation), smoking status (never vs. ever with multiple imputation), and year between index and subsequent procedure
Exploratory analyses evaluating associations between the secondary outcomes: (1) non-advanced colorectal neoplasia, and (2) any polyp or CRC also suggested no statistically significant association between serrated polyp characteristics and these outcomes (data not shown). Sensitivity analyses restricting to subsequent endoscopy type to only colonoscopies did not meaningfully affect the analysis results (data not shown).
Discussion
Our results suggest that the risk of CRC within 5 years of an SSA/P or HP diagnosis is low. Among 161 individuals with SSA/Ps and 548 with HPs at index colonoscopy, only one developed CRC within 5 years of the index colonoscopy Our results also suggest that the odds of subsequent advanced colorectal neoplasia did not differ significantly between those with SSA/Ps and those with HPs at index colonoscopy. Serrated polyp size, location, or multiplicity were not associated with advanced colorectal neoplasia risk. These results do not support prior guidelines recommending increased colonoscopy surveillance intervals of 3 or 5 years in patients with SSA/Ps [12, 13]. Because protocols at KPWA during the time period for this study aimed for complete excision of polyps identified at endoscopy, our results should be interpreted in the context of SSA/Ps that have been removed at endoscopy.
Prior cross-sectional studies of molecular markers in SSA/Ps and HPs support the thesis that serrated polyps, particularly SSA/Ps, are precursors to the subset of CRCs that are BRAF-mutant and CpG Island methylator phenotype (CIMP)-high [7, 22–28]. These studies reported that BRAF mutations are found in as many as 50–80% of serrated polyps, and that BRAF mutations are rare or absent in tubular and tubulovillous adenomas [7, 22–24, 26, 27]. SSA/Ps and other serrated polyps are also commonly CIMP-high. [25, 27]. Other cross-sectional studies report an association between large serrated polyps and synchronous colorectal neoplasia [29–31], Additional evidence supporting SSA/Ps as precursors to colorectal cancer includes a histological study of eight SSA/P polypectomies containing focal invasive adenocarcinoma or high-grade dysplasia [32], Despite consistency in these cross-sectional studies linking SSA/Ps to colorectal cancer, research on the association between SSA/Ps and subsequent colorectal neoplasia is mixed, and the results vary depending on length of follow-up and the presence or absence of dysplasia within SSA/Ps [6, 14–18, 33].
One of the first longitudinal studies of patients with SSA/Ps included 40 individuals and reported that 12.5% developed CRC an average of 8.3 years after the SSA/P diagnosis [6]. Another study of 103 Scandinavian patients with large serrated polyps identified via flexible sigmoidoscopy had a median follow-up of 10.9 years, and this study reported that large serrated polyps were associated with CRC risk (OR 3.3; 95% Cl 1.3–2.9) (18). A separate nested case-control study of CRC among a cohort of more than 272,000 Danish individuals receiving colonoscopy reported that association between SSA/Ps and subsequent CRC was stronger in those with ≥ 10 years of follow-up time than it was in those with <5 years of follow-up (OR 5.5; 95% CI 1.9–16.2 after 10 years of follow-up and OR 2.5; 95% CI 1.5–4.3 for< 5 years of follow-up) [16]. Another study of SSA/Ps with dysplasia, reported that the risk of CRC was particularly high in patients with SSA/Ps exhibiting dysplasia, in which 23% developed CRC [33], In contrast to these retrospective studies with long-term follow-up, a recent study by Park et al. evaluated the utility of regular surveillance endoscopy in 152 patients with SSA/Ps [17]. After the 4th surveillance endoscopy, none of the patients had developed CRC, and the authors concluded that annual colonoscopy was not necessary for patients with SSA/Ps. In the present study, we also reported a very low risk of CRC in the first 5 years after an SSA/P or HP diagnosis.
A possible explanation for the differences in subsequent colorectal neoplasia risk associated with SSA/Ps in analyses with long-term follow-up versus short-term follow-up is that SSA/Ps may have a long dwell time. A recent study by Bettington et al. supports the hypothesis that SSA/Ps may take a long time to develop into CRC [34]. This study reported that the frequency of SSA/Ps did not significantly differ between older and young patients, but the frequency of the CRC-associated mutation, BRAF mutation, within SSA/Ps did vary by age. Only 3.8% of SSA/Ps in patients who were < 50 years old carried BRAF mutations; this increased to 9.3% of SSA/Ps in those who were < 60 years old and to 39.8% in those who were > 80 years old. The increasing frequency of BRAF-mutation with increasing age supports the thesis that SSA/Ps may take many years to accumulation cancer-related molecular changes and thus have a long dwell time.
Our study has several strengths, including: a large population of serrated polyps, standardized study pathology review to confirm SSA/P diagnoses, and complete ascertainment of advanced colorectal neoplasia through review of subsequent endoscopy reports and linking the study population to the SEER cancer registry. Despite these strengths, our results should be interpreted with consideration of study limitations. First, although all patients generally had at least 5 years of follow-up after serrated polyp removal, most participants had <10 years of follow-up. This would bias our results toward the null, if SSA/Ps tend to have a long dwell time. However, our results are still relevant to informing SSA/P surveillance guidelines, which currently recommend 1, 3, or 5-year surveillance colonoscopy for patients with SSA/Ps [12, 13]. Another limitation of our study is that our population included only three SSA/Ps with nuclear dysplasia; thus, we were unable to fully evaluate this important subtype of SSA/Ps, and the interpretation of our results should be limited to patients with SSA/Ps without dysplasia. Furthermore, we were missing data on some potential confounders, including physical activity and sedentary behaviors. However, we did have data on BMI and were able to adjust for BMI which is correlated with physical activity and sedentary behaviors.
Conclusions
Overall, our results do not support guidelines recommending aggressive colonoscopy surveillance in patients that have SSA/Ps without dysplasia removed at endoscopy. In the present study, even patients with large and proximal SSA/Ps did not develop CRC within 5 years of SSA/P removal. Despite our findings indicating that patients that have SSA/Ps removed at endoscopy have a low-risk of CRC within 5 years, the body of evidence around SSA/Ps does support the thesis that SSA/Ps are precursors to a subset of CRC. Thus, additional research is needed to allow for better risk stratification of patients with SSA/Ps. In particular, research should be conducted to determine if molecular characterization of SSA/Ps can better inform the clinical management of patients with SSA/Ps.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health. National Cancer Institute (Grant No. R01 CA168338).
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552-019-01205-y) contains supplementary material, which is available to authorized users.
Ethical approval This study was conducted in accordance with the ethical standards of the institutional review boards at Kaiser Permanente Washington, the Fred Hutchinson Cancer Research Center, and with the 1964 Helsinki declaration and its later amendments.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Winawer SJ, Zauber AG, Ho MN, O ‘Brien MJ, Gottlieb LS, Sternberg SS, W aye JD, Schapiro M, Bond JH, Panish JF et al. (1993) Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 329(27): 1977–1981. 10.1056/nejm199312303292701 [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M (2007) Case-control study supports extension of surveillance interval after colonoscopic polypectomy to at least 5 yr. Am J Gastroenterol 102(8): 1739–1744 [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, Bond JH, Nelson DB. Triadafilopoulos G. Ramirez FC, Collins JF, Johnston TK McQuaid KR, Garewal H, Sampliner RE Esquivel R Robertson D (2007) Five-year colon surveillance after screening colonoscopy. Gastroenterology 133(4):1077–1085. https://doi.Org/10.1053/j.gastro.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD. Levin TR, Pickhardt PJ, Rex DK, Smith RA Thorson A, Winawer SJ (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US M ulti-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134(5): 1570–1595. https://doi.Org/10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Jass JR (2007) Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50(1): 113–130. 10.1111/j.1365-2559.2006.02549.x [DOI] [PubMed] [Google Scholar]
- 6.Lu FI, van de Niekerk W, Owen D, Tha SP, Turbin DA, Webber DL (2010) Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol 34(7):927–934. 10.1097/pas.0b013e3181e4f256 [DOI] [PubMed] [Google Scholar]
- 7.Vaughn CP, Wilson AR, Samowitz WS (2010) Quantitative evaluation of CpG island methylation in hyperplastic polyps. Mod Pathol 23(1):151–156. 10.1038/modpathol.2009.150 [DOI] [PubMed] [Google Scholar]
- 8.Longacre TA, Fenoglio-Preiser CM (1990) Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 14(6):524–537 [DOI] [PubMed] [Google Scholar]
- 9.Makinen MJ, George SM, Jernvall P, Makela J, Vihko P, Kart-tunen TJ (2001) Colorectal carcinoma associated with serrated adenoma-prevalence, histological features, and prognosis. J Pathol 193(3):286–294 [DOI] [PubMed] [Google Scholar]
- 10.Jass JR (2003) Hyperplastic-like polyps as precursors of microsatellite-unstable colorectal cancer. Am J Clin Pathol 119(6):773–775. 10.1309/UYN7-0N9W-2DVN-9ART [DOI] [PubMed] [Google Scholar]
- 11.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O ‘Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH. Brooks D, Byers T, Hyman N, Kirk L, Thorson A, Simmang C, Johnson D, Rex DK (2006) Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130(6): 1872–1885. 10.1053/j.gastro.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, United States Multi-Society Task Force on Colorectal C (2012) Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 143(3):844–857. https://doi.Org/10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Michael JO, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 107(9): 1315–1329. 10.1038/ajg.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oono Y, Fu K, Nakamura H, Iriguchi Y, Yamamura A, Tom-ino Y, Oda J, M izutani M, Takayanagi S, Kishi D, Shinohara T. Yamada K, Matumoto J, Imamura K (2009) Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci 54(4):906–909. 10.1007/si0620-008-0407-7 [DOI] [PubMed] [Google Scholar]
- 15.Salaria SN. Streppel MM, Lee LA, Iacobuzio-Donahue CA, Montgomery EA (2012) Sessile serrated adenomas: high-risk lesions? Hum Pathol 43(11): 1808–1814. https://doi.org/10.1016/j.humpath.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erichsen R, Baron JA, Hamilton-Dutoit SJ, Snover DC, Torlakovic EE, Pedersen L, Froslev T, Vyberg M, Hamilton SR, Sorensen HT (2016) Increased Risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 150(4):895–902. https://doi.Org/10.1053/j.gastro.2015.ll.046 [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Yoon H, Jung IS, Shin CM, Park YS, Kim NY, Lee DH (2018) Clinical outcomes of surveillance colonoscopy for patients with sessile serrated adenoma. Intest Res 16(1): 134–141. 10.5217/ir.2018.16.1.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holme O, Bretthauer M, Eide TJ, Loberg EM, Grzyb K, Loberg M, Kalager M, Adami HO, Kjellevold O, Hoff G (2015) Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 64(6):929–936. 10.1136/gutjnl-2014-307793 [DOI] [PubMed] [Google Scholar]
- 19.Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu LC, Potter JD, Newcomb PA (2013) Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol 177(7):625–637. 10.1093/aje/kws282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley JA, Negassa A, Edwardes MDd, Forrester JE (2003) Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 157(4):364–375. 10.1093/aje/kwf215 [DOI] [PubMed] [Google Scholar]
- 21.Azur MJ, Stuart EA, Frangakis C, Leaf PJ (2011) Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 20(1):40–19. 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA (2006) Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 30(12): 1491–1501 [DOI] [PubMed] [Google Scholar]
- 23.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW. Apple-yard M, Hewett D, Togashi K, Jass JR, Leggett BA (2006) High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 131(5):1400–1407. https://doi.Org/10.1053/j.gastro.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Kakar S, Cun L, Deng G, Kim YS (2008) Distinct CpG island methylation profiles and BRAF mutation status in serrated and adenomatous colorectal polyps. Int J Cancer 123(11):2587–2593. 10.1002/ijc.23840 [DOI] [PubMed] [Google Scholar]
- 25.Velho S, Moutinho C, Cirnes L, Albuquerque C, Hamelin R, Schmitt F, Carneiro F, Oliveira C, Seruca R (2008) BRAF, KRAS and PIK3CA mutations in colorectal serrated polyps and cancer: primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer 8:255 10.1186/1471-2407-8-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehall VL, Rickman C, Bond CE, Ramsnes I, Greco SA, Umapathy A, McKeone D, Faleiro RJ, Buttenshaw RL, Worthley DL, Nayler S, Zhao ZZ, Montgomery GW, Mallitt KA. Jass JR, Matsubara N, Notohara K, Ishii T, Leggett BA (2012) Oncogenic PIK3CA mutations in colorectal cancers and polyps. Int J Cancer 131(4):813–820. 10.1002/ijc.26440 [DOI] [PubMed] [Google Scholar]
- 27.Burnett-Hartman AN, Newcomb PA, Potter JD, Passarelli MN, Phipps AI, Wurscher MA, Grady WM, Zhu LC, Upton MP, Makar KW (2013) Genomic aberrations occurring in subsets of serrated colorectal lesions but not conventional adenomas. Cancer Res 73(9):2863–2872. 10.1158/0008-5472.CAN-12-3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernando WC, Miranda MS, Worthley DL, Togashi K, Watters DJ, Leggett BA, Spring KJ (2014) The CIMP phenotype in BRAF mutant serrated polyps from a prospective colonoscopy patient cohort. Gastroenterol Res Pract 2014:374926 10.1155/2014/374926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiner MA, Weiss DG, Lieberman DA (2010) Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 139(5): 1497–1502. https://doi.Org/10.1053/j.gastro.2010.06.074 [DOI] [PubMed] [Google Scholar]
- 30.Li D, Jin C, McCulloch C, Kakar S, Berger BM, Imperiale TF, Terdiman JP (2009) Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 104(3):695–702. 10.1038/ajg.2008.166 [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka S, Kato J, Fujiki S, Kaji E, Morikawa T, M urakami T, Nawa T, Kuriyama M, Uraoka T, Ohara N, Yamamoto K (2010) The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology 139(5): 1503–1510. 10.1053/j.gastro.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 32.Goldstein NS (2006) The gray area between heritable and cancer somatic (tumor phenotype) molecular genetic testing of colorectal adenocarcinomas. Am J Clin Pathol 125(6):813–814. 10.1309/JX4Q-8FJT-PJ03-3KGE [DOI] [PubMed] [Google Scholar]
- 33.Cenaj O, Gibson J, Odze RD (2017) Clinicopathologic and outcome study of sessile serrated adenomas/polyps with serrated versus intestinal dysplasia. Mod Pathol, 10.1038/modpathol.2017.169 [DOI] [PubMed] [Google Scholar]
- 34.Bettington M, Brown I, Rosty C, Walker N, Liu C, Croese J, Rahman T, Pearson SA, McKeone D, Leggett B, Whitehall V (2018) Sessile serrated adenomas in young patients may have limited risk of malignant progression. J Clin Gastroenterol. 10.1097/mcg.0000000000001014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.