Aortic valve stenosis is the most common indication for valve replacement among older adults in the US. Surgical aortic valve replacement (SAVR) was the only treatment option for decades1, but transcatheter replacement (TAVR) volumes have now overtaken SAVR volumes in the US.2 However, the growth of TAVR volumes may not be uniformly distributed across geographic regions, raising potential concerns about access and quality of care. In this study, we assessed county-level growth of TAVR in the United States.
We included adults ≥65 years old who were hospitalized for AVR with an International Classification of Diseases, Ninth Revision (ICD-9) procedure code for TAVR (3505 or 3506) or SAVR (3521 or 3522), or Tenth Revision (ICD-10) procedure code for TAVR (02RF38H or 02RF38Z) or SAVR (02RF07Z or 02RF08Z or 02RF0JZ or 02RF0KZ) in the Centers for Medicare and Medicaid Services (CMS) fee-for-service Medicare Provider Analysis and Review files between August 25, 2011 and December 31, 2016. Since TAVR was first performed in August 25, 2011, we only included SAVR patients after this date. During the study time period, for patients with multiple procedures, only the first procedure was included. Patients with missing or inaccurately coded county data were excluded from the study. We excluded patients who received both TAVR and SAVR during the study period (n=203).
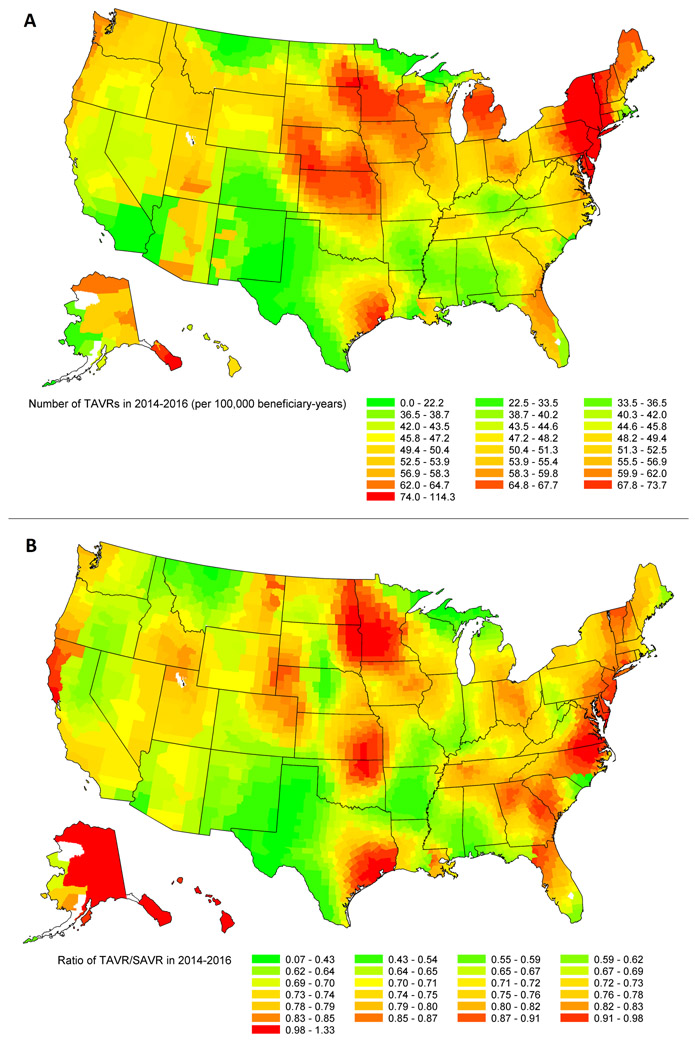
For each county in the 2014–2016 period, we calculated the observed county-specific number of TAVRs per 100,000 Medicare fee-for-service beneficiaries and mapped county level TAVR volume (per 100,000 beneficiary-years) and TAVR/SAVR rates.
The study was approved by the institutional review board of Beth Israel Deaconess Medical Center with a waiver of informed consent for retrospective data analysis.
Among the 233,363 Medicare fee-for-service beneficiaries undergoing AVR during the study period (mean age, 77.8±7.5 years (75.7± 6.5 for SAVR; 82.5±for TAVR)), 70,460 underwent TAVR and 162,903 underwent SAVR. TAVR was performed in 127 hospitals (n=671 patients) in 2011 (4 per 100,000 beneficiary-years), and expanded to 493 hospitals (n=25,494 patients, 71 per 100,000 beneficiary-years) in 2016. The ratio of TAVR/SAVR was 0.06 in 2011 and increased to 0.98 in 2016.
Extensive geographic variation in the rate of TAVR and the ratio of TAVR/SAVR was observed. In 2014–2016, the county-specific number of TAVR ranged from 0 to 114 per 100,000 beneficiary-years (Figure 1, top panel) and the ratio of TAVR/SAVR ranged from 0.07 to 1.33 (Figure 1, bottom panel). Population rates of TAVR in the West, Southwest, and Southeast regions were significantly lower compared to the Midwest and Northeast, even after adjusting for county-specific characteristics. Similar geographic variation patterns were also observed in TAVR/SAVR ratio suggesting lower availability for TAVR in these areas.
FIGURE. Geographic variation in the number of TAVRs per 100,000 beneficiary-years (top panel) and ratio of TAVR/SAVR (bottom panel) in 2014-2016.
SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
In this study of Medicare beneficiaries who underwent TAVR in the United States, we observed considerable geographic variation in the growth of TAVR that persisted over time.
Following marketing approval of TAVR in 2011, TAVR volumes have increased significantly. To date, more than 80,000 commercial procedures have been performed in the United States in patients with intermediate, high, and prohibitive surgical risk.3 The total number of TAVR-eligible patients will continue to increase as the population ages and the approved indications for TAVR expand to include patients at lower surgical risk.
While the observed geographic differences in TAVR growth may reflect differences in regional practice patterns or patient case mix, they may also reflect the differential availability and access to a new and potentially life-saving therapy. Differential geographic availability of cardiac procedures has been previously observed for angiography and percutaneous coronary intervention without improving access to the least-serviced areas.4 Regulators evaluating strict volume requirements to begin and maintain TAVR programs will need to balance the goals of favorable procedural outcomes and reasonable access for all eligible aortic stenosis patients. Previously, we demonstrated that historical SAVR outcomes at a given hospital predict subsequent outcomes of TAVR, and could be considered as an additional measure of quality, in addition to procedural volume.5 However, overly burdensome volume benchmarks for either SAVR or TAVR may have the regressive effect of propagating entrenched disparities in healthcare access.
This analysis is limited by inclusion of only Medicare fee-for-service beneficiaries. Variables used in this study were also taken from administrative claims and may be subject to inaccuracies in coding. Additionally, the population being treated was limited before the expansion of TAVR to intermediate- and low-risk patients, with restrictive requirements for new programs. Thus, our findings may not accurately represent the second wave of expansion with intermediate- and low-risk patients.
In summary, TAVR volumes have risen nationally since 2011, and the TAVR/SAVR ratio has dramatically increased, but shows considerable geographic variation. The West, Southwest, and Southeast regions of the United States had lower rates of TAVR compared to the Midwest and Northeast. Regulators should include geographic location and patient access as key factors in developing coverage decisions applicable to patients with aortic stenosis.
Acknowledgments
Sources of Funding: Members of the study team are supported by funding from the National Heart, Lung, and Blood Institute (1R01HL136708–01 [RWY]).
Footnotes
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Disclosures:
The following authors have no conflicts of interest to declare: HK, KFF, YW, RKW, LRV, and DBK. Dr. Popma reports grants from Medtronic, Abbott Vascular, and Direct Flow Medical and personal fees from Boston Scientific, Cordis, and Direct Flow Medical, outside the submitted work. Dr. Yeh reports investigator-initiated grant funding from Abiomed, grant support from Boston Scientific, and consulting from Abbott, Medtronic, and Teleflex, outside the submitted work.
REFERENCES
- 1).Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2).Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G, Bavaria JE, Tuzcu EM, Peterson ED, Fitzgerald S, Kourtis M, Michaels J, Christensen B, Seward WF, Hewitt K, Holmes DR Jr, STS/ACC TVT Registry. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy registry. J Am Coll Cardiol. 2017;69:1215–1230. [DOI] [PubMed] [Google Scholar]
- 3).Brennan JM, Thomas L, Cohen DJ, Shahian D, Wang A, Mack MJ, Holmes DR, Edwards FH, Frankel NZ, Baron SJ, Carroll J, Thourani V, Tuzcu EM, Arnold SV, Cohn R, Maser T, Schawe B, Strong S, Stickfort A, Patrick-Lake E, Graham FL, Dai D, Li F, Matsouaka RA, O’Brien S, Li F, Pencina MJ, Peterson ED. Transcatheter versus surgical aortic valve replacement: a propensity-matched analysis from two United States registries. J Am Coll Cardiol. 2017;70:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Matlock DD, Groeneveld PW, Sidney S, Shetterly S, Goodrich G, Glenn K, Xu S, Yang L, Farmer SA, Reynolds K, Cassidy-Bushrow AE, Lieu T, Boudreau DM, Greenlee RT, Tom J, Vupputuri S, Adams KF, Smith DH, Gunter MJ, Go AS, Magid DJ. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 310(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Kundi H, Popma JJ, Khabbaz KR, Chu LM, Strom JB, Valsdottir LR, Shen C, Yeh RW. Association of hospital surgical aortic valve replacement quality with 30-day and 1-year mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2019;4:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]