Abstract
Background:
Bisphenol S (BPS) was introduced in the market as a potentially safer alternative to bisphenol A (BPA). However, there are limited studies on health effects of BPS and no epidemiologic studies on its relationship with male reproductive health outcomes, specifically semen quality.
Objective:
To investigate predictors of urinary BPS concentrations and its association with semen parameters among men attending a fertility center.
Methods:
This cross-sectional analysis included 158 men of couples seeking fertility treatment (2011-2017) contributing 338 paired semen and urine samples. At the time of sample collection, men completed a questionnaire on self-reported use of household products and food intake within the previous 24 hours. Urinary concentrations of BPA, BPS and bisphenol F were quantified using isotope-dilution tandem mass spectrometry. Semen samples were analyzed following WHO guidelines. Multivariable mixed models were used to investigate predictors of urinary BPS concentrations and to evaluate associations between urinary BPS concentrations and semen parameters, using random intercept to account for correlation in outcomes across multiple observations per man and adjusting for abstinence time, specific gravity, age, body mass index (BMI), year of sample collection and BPA concentrations. Analyses were also stratified by BMI (≥25 vs <25 kg/m2).
Results:
Median (IQR) urinary BPS concentration was 0.30 (0.20, 0.90) μg/L, and 76% of samples had detectable (>0.1 μg/L) concentrations. Self-reported fabric softener and paint/solvent use as well as intake of beef and cheese within 24 hours before urine collection were positively associated with BPS concentrations. Men with higher BPS concentrations also had significantly higher BMI. Lower semen parameters were found among men with detectable BPS concentrations, compared to men with non-detectable BPS [2.66 vs. 2.91 mL for volume (p=0.03), 30.7 vs. 38.3 mil/mL for concentration (p=0.03), 76.8 vs. 90.0 mil for total count (p=0.09), 43.7 vs. 47.0% for motility (p=0.06), and 5.42 vs. 6.77% for morphologically normal sperm (p=0.24)]. Some associations of BPS with lower semen parameters were only found among men with a BMI≥25 kg/m2.
Conclusions:
We identified dietary and lifestyle factors associated with BPS exposure, suggesting potential avenues for reducing exposures. We also observed negative associations between BPS and semen parameters, especially among overweight and obese men.
Keywords: BPS, BPA, predictors, semen quality, infertility
Introduction
Bisphenol S (BPS), a structural analog of bisphenol A (BPA), had its first reported mass commercial use in paper receipts in 2005 (Glausiusz 2014; Rochester et al. 2015). BPS was introduced in the market as a potentially safer alternative to BPA, which had demonstrated endocrine disrupting activities (Matsushima et al. 2007; De Coster et al. 2012; Bonefeld-Jörgensen et al. 2007) and shown associations with adverse health outcomes in the general population (Rochester, 2013; Rezg et al., 2014). Similar to uses for BPA, BPS can be found in canned and other pre-packaged foods, as well as thermal receipts (Clark et al. 2012; Liao et al. 2012a; Lehmler et al. 2018). BPS, along with another bisphenol analog, bisphenol F (BPF), is currently unregulated and there are no identified tolerable dose intakes (Eladak et al. 2015). Their production and utilization have increased during recent years (Liao et al. 2012; Žalmanová et al. 2016), as reflected by detection in environmental samples and human biomonitoring studies (Jin et al. 1997, CDC 2019). BPS was detected in 81% of adults from eight countries including the USA, and 78% in samples collected solely from the USA (Liao et al. 2012b; Zhou et al 2014). Another study of U.S. adults reported an increase from 19% to 74% in urinary concentrations of BPS between 2000 and 2014, with corresponding declines in BPA over the same time (Ye et al. 2015). BPS has also been commonly detected in urine samples in other regions, including the Middle -East and East Asia (Liao et al. 2012b; Zhou et al 2014). It has been reported that BPS is systematically absorbed and excreted within hours following exposure, reflecting its short half-life (Oh et al, 2017).
Given its chemical structure, BPS has not surprisingly a similar toxicological profile to BPA based on in vivo and in vitro models (Rochester et al. 2015). For example, in vivo studies of BPA have demonstrated estrogenic activity in crustaceans (Chen et al. 2002), zebrafish and rats (Ji et al 2013; Naderi et al. 2014, Yamasaki et al. 2003). In vitro studies have confirmed estrogenic properties of BPS (Grignard et al. 2012; Rosenmai et al. 2014; Vinas and Watson 2013). Specifically, animal models have demonstrated that BPS has similar endocrine disruption mechanisms to BPA, and can affect ovarian follicles, oocyte quality, and testosterone levels (Ullah et al. 2016; Nevoral et al. 2018; Žalmanová et al. 2016). Mechanisms of BPS effects have been found to be similar to those of BPA, including oxidative stress, anti-androgenic activity, genotoxicity, and mutagenicity (Usman A and Ahmad M. 2016; Fic et al. 2013, Fic et al. 2015). For example, plasma levels of both FSH and LH were diminished proportionally in rats exposed to higher levels of either BPA or BPS, indicating similar endocrine disrupting potency (Ahsan et al. 2018).
It has been observed that exposure to BPA is associated with semen quality through germ cells and spermatogenesis derangement (Phillips et al. 2008). BPA has been also linked to blood-testis barrier disruption with a subsequent immunologic insult to the testicular germ cells in utero exposure (Salian et al.2009, Toyama et al. 2004) While effects of BPA on other male reproductive outcomes have been investigated (Minguez-Alarcon et al. 2016), there is still lack of epidemiological data on the potential associations of its analogs, BPS and BPF, with testicular endpoints. We provide the first epidemiologic study examining whether urinary BPS concentrations are associated with semen quality parameters among men attending a fertility center, and investigate predictors of urinary BPS concentrations in this cross-sectional study of men attending a fertility center.
Methods
Study population
Study participants were male partners of couples enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort of couples seeking fertility treatment at the Massachusetts General Hospital (MGH) Fertility Center aimed at evaluating environmental and dietary determinants of fertility (Messerlian et al. 2018). Men between the ages of 18–56 years and without a history of vasectomy were eligible to participate, and approximately 40% of those contacted by the research nurses were enrolled. This cross-sectional analysis included 158 men contributing 338 paired urine and semen samples (repeated measures); the semen sample was collected on the same day and at the same time as the urine sample for the analysis of bisphenol concentrations. Although the EARTH Study was established in 2004, urinary concentrations of the BPA analogs, BPS and BPF, were first evaluated starting in 2011. Thus, a total of 382 men were excluded because of lack of urinary BPS and BPF concentration data. After the study procedures were explained, participants signed an informed consent form. The participant’s date of birth was collected at entry, and weight and height were measured by trained study staff. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. The participants completed a staff-administered questionnaire that contained additional questions on lifestyle factors, reproductive health, and medical history. The study was approved by the Human Subject Committees of the Harvard T.H. Chan School of Public Health, Partners Healthcare, and the Centers for Disease Control and Prevention (CDC).
Quantification of urinary concentrations of bisphenols
Men provided one spot urine sample per semen sample. Urine was collected in a sterile polypropylene specimen cup. Specific gravity (SG), which was used to correct bisphenol concentrations for urine dilution, was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. The urine was divided into aliquots, frozen, and stored at −80 °C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40 °C until analysis. As previously described (Minguez-Alarcon et al. 2018b; Ye et al. 2005), online solid-phase extraction coupled with isotope dilution-high-performance liquid chromatography-tandem mass spectrometry was used to quantify the urinary concentrations of BPS, BPF and BPA. The limits of detection (LOD) were 0.1 μg/L for BPS, and 0.2 μg/L for BPF and BPA. Samples with bisphenols concentrations below the LOD were assigned a value equal to the LOD divided by the square root of 2.
At the time of the urine collection, men completed a household product use and food intake questionnaire that asked whether during the previous 24 hours they had used certain products or consumed specific foods. The percentages of use, for each group and the total cohort, were calculated as the number of times men reported having used/eaten that specific item per survey divided by the total of surveys (urines) collected.
Analysis of semen samples
Semen and urine samples were both collected at the same time with the majority of the samples collected during the morning (86%). Semen samples were collected on site at MGH in a sterile plastic specimen cup following a recommended 2-5 days abstinence period as previously described (Minguez-Alarcon et al. 2018a). Of the 158 men in the study, 69 (44%) contributed one semen sample, 51 (32%) contributed 2 samples, and 38 (24%) contributed 3 or more samples (range=1-8). Semen volume (mL) was measured by an andrologist using a graduated serological pipet. Sperm concentration (mil/mL) and motility (% motile) were assessed using a computer-aided semen analyzer (CEROS; software version 12.3; Hamilton Thorne Biosciences, 5 Beverly, MA, USA). To measure semen concentration and motility, 6 μL of semen was placed into a pre-warmed (37°C) and disposable Leja Slide (Spectrum Technologies, CA, USA). A minimum of 200 sperm cells from at least four different fields were analyzed from each specimen. Total sperm count (mil/ejaculate) was calculated by multiplying sperm concentration by semen volume. Motile spermatozoa were defined as according to the World Health Organization (WHO) four-category scheme: rapid progressive, slow progressive, non-progressive, and immotile (WHO 2010). Sperm morphology (% normal) was assessed on two slides per specimen (with a minimum of 200 cells assessed per slide) via a microscope with an oil-immersion 100× objective (Nikon, Tokyo, Japan). Strict Kruger scoring criteria was used to classify men as having normal or below normal morphology (Kruger et al. 1988). Andrologists were trained in semen analysis and participated in rigorous daily and weekly internal quality control and external monitoring of within and between observer variation as required to maintain CLIA certification and accreditation by the College of American Pathologists. Infertility diagnosis was coded according to previously described definitions of the Society for Assisted Reproductive Technology (SART) including female, male and unexplained (SART 2015).
Statistical analysis
Demographic characteristics, semen quality parameters and frequency of household products use and food intake of the men were presented using median ± interquartile ranges (IQRs) or counts (%). Due to concern regarding potential non-linear relationships between BPS and semen parameters, urinary BPS concentrations were categorized into quartiles or into two groups, below and above the LOD (e.g., detectable vs non-detectable). Associations between demographic characteristics across quartiles of urinary BPS concentrations were evaluated using Kruskal– Wallis tests for continuous variables and Chi-squared tests for categorical variables (or Fisher’s exact test where appropriate). Multivariable mixed models were used to investigate predictors of urinary BPS concentrations and to evaluate associations between urinary BPS concentrations and semen parameters, using random intercept to account for correlation in outcomes across multiple observations per man and adjusting for specific gravity and potential confounders. We evaluated the robustness of the BPS and semen parameters findings by restricting analyses to one semen sample (first sample) per man and also modeling the semen parameters as binary variables (above vs. below WHO reference limits). It may be possible that men who provided more semen samples had poorer semen quality and thus had female partners with more infertility treatment cycles (study visits).
Confounding was assessed using prior knowledge on biological relevance and descriptive statistics from our study population. The variables considered as potential confounders included factors previously related to male reproductive endpoints (Rooney and Domar 2014; Sharma et al. 2013), and factors associated with urinary BPS and semen parameters in this study. Fully adjusted models included abstinence time (days), specific gravity, age (years), BMI (kg/m2), year of sample collection (year) and log-transformed bisphenol A concentrations (μg/L), To allow for better interpretation of the results, population marginal means (Searle et al. 1980) are presented adjusting for all the covariates in the model (at the mean level for continuous variables and for categorical variables at a value weighted according to their frequencies). Stratification of associations of BPS with semen parameters by BMI (≥25 vs <25 kg/m2) was performed to evaluate modification by BMI. Statistical analyses were conducted with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). Statistical tests were two-tailed and all p-values<0.05 were regarded as statistically significant.
Results
Men included in this analysis had a median (IQR) age and BMI of 35.6 (32.6–39.0) years and 26.7 (24.1–30.1) kg/m2, respectively (Table 1). Men were predominantly Caucasian (88%), highly educated (60% had a graduate degree) and 32% had ever smoked. Male factor infertility was diagnosed at enrollment among 36 men (23%). Men in the highest quartile of urinary BPS concentrations were significantly heavier compared to men in the lowest quartile of BPS (mean BMI 27.0 vs 24.9 kg/m2). No other demographic characteristics significantly differed across quartiles of urinary BPS concentrations (Table 1). For the 338 semen samples contributed by the 158 men, the median (IQR) values were 47.6 (22.4, 90.1) mil/mL for sperm concentration; 121 (58.0, 230) mil for total sperm count; 48 (27, 66) % for sperm motility; and 4 (3, 7) % for morphologically normal spermatozoa (Supplemental Table S1). Over one-third of semen samples (39%) were below the WHO 2010 lower reference limit for progressive sperm motility (> 32%) (WHO 2010). Men included in this analysis had slightly lower sperm concentration and total sperm count but were similar in their demographic characteristics and other semen parameters compared to men who were excluded from the analysis because of lack of measured urinary BPS concentrations (Supplemental Table S2).
Table 1.
Demographic and reproductive characteristics [median (IQR) or counts (%)] across quartiles of urinary bisphenol S concentrations among 158 men in the EARTH Study.
| Quartiles of urinary bisphenol S concentrations | ||||||
|---|---|---|---|---|---|---|
| Total cohort N=158 men |
Q1 N=38 men |
Q2 N=48 men |
Q3 N=34 men |
Q4 N=38 men |
p-valuea across quartiles |
|
| Age, years | 35.6 (32.6, 39.6) | 35.6 (32.6, 39.0) | 35.8 (32.7, 40.5) | 35.7 (33.4, 39.5) | 36.1 (31.8, 38.7) | 0.81 |
| Race, n (%) | 0.62 | |||||
| White/Caucasian | 139 (88) | 33 (87) | 43 (90) | 28 (82) | 35 (92) | |
| Black/Asian/Other | 19 (22) | 5 (13) | 5 (10) | 6 (18) | 3 (8) | |
| Body Mass Index, kg/m2 | 26.7 (24.1, 30.1) | 24.9 (23.5, 26.8) | 26.7 (25.3, 30.3) | 28.7 (24.3, 31.6) | 27.0 (24.2, 29.4) | 0.008 |
| Ever smoker, n (%) | 51 (32) | 13 (34) | 16 (33) | 10 (29) | 12 (32) | 0.97 |
| Education, n (%) | 0.48 | |||||
| High school/some college | 15 (10) | 3 (8) | 4 (8) | 3 (9) | 5 (13) | |
| College graduate | 49 (31) | 7 (18) | 17 (36) | 11 (32) | 14 (37) | |
| Graduate degree | 94 (60) | 28 (74) | 27 (56) | 20 (59) | 19 (50) | |
| Total physical activity (hrs/week) | 4.02 (0, 10.7) | 3.6 (0, 7.9) | 4.7 (0, 8.4) | 3.7 (0, 11.7) | 6.9 (0.8, 12.5) | 0.48 |
| Infertility diagnosis, n (%) | 0.82 | |||||
| Male | 36 (23) | 8 (21) | 9 (19) | 8 (24) | 11 (29) | |
| Female | 57 (36) | 12 (32) | 19 (40) | 11 (32) | 15 (39) | |
| Unexplained | 65 (41) | 18 (47) | 20 (42) | 15 (44) | 12 (32) | |
In the 338 urine samples collected from the 158 men in the EARTH Study between 2011 and 2017, detection frequency was 76% (BPS) and 88% (BPA) (Table 2). We excluded BPF from further analysis because its detection frequency was 25%. The geometric mean (GM) BPS and BPA urinary concentrations were 0.37 and 0.77 μg/L, respectively. BPS concentrations did not significantly differ in samples collected between 2015 and 2017, compared to those collected between 2011 and 2014 (medians=0.40 vs. 0.30 μg/L, respectively). Urinary concentrations of BPA significantly decreased during the second part of the study (2015-2017) compared to those collected in earlier years (2011-2014) (medians=0.60 vs. 1.00 μg/L, respectively). The Spearman correlation for urinary concentrations of BPS and BPA was 0.45.
Table 2.
Distribution of urinary concentrations (μg/L) of bisphenol S, bisphenol F and bisphenol A among 158 men contributing 338 semen samples in the EARTH Study.
| Entire study period, 2011-2017 N=338 |
2011-2014 N=186 |
2015-2017 N=152 |
p-value comparing both periods |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N urines |
Detection Frequency % |
GM (SD) | 25th | 50th | 75th | 25th | 50th | 75th | 25th | 50th | 75th | ||
| Bisphenol S | 338 | 76 | 0.37 (0.03) | 0.20 | 0.30 | 0.90 | 0.20 | 0.30 | 0.80 | <LOD | 0.40 | 0.90 | 0.92 |
| Bisphenol F | 181 | 25 | <LOD | <LOD | <LOD | 0.30 | <LOD | <LOD | 0.40 | <LOD | <LOD | 0.30 | - |
| Bisphenol A | 338 | 88 | 0.77 (0.05) | 0.40 | 0.80 | 1.60 | 0.50 | 1.00 | 1.80 | 0.30 | 0.60 | 1.15 | .0001 |
Note: The limit of detection (LOD) for bisphenol S is 0.1 μg/L. and bisphenol F and bisphenol A are 0.2 μg/L.
Self-reported use of fabric softener or paints/solvents during the 24 hours preceding urine collection was positively associated with urinary BPS concentrations (Table 3). Specifically, all six men who reported having used fabric softener in the 24 hours prior to urine collection had detectable urinary BPS concentrations (p-value=0.002). Similarly, a total of 10 men reported having used paints or solvents during the previous 24 hours to urine collection, and nine of those 10 men had detectable BPS concentrations in urine, compared to one man who had non-detectable concentrations (p-value=0.05). In addition, a higher percentage of men with detectable BPS had self-reported beef or cheese intake during the 24 hours prior to urine collection than those with non-detectable BPS (41% vs 19% with beef intake, 72% vs 59% with cheese intake) (Table 3). No other personal household products or food queried were significantly related to urinary concentrations of BPS.
Table 3.
Household product use [n (%)] and food consumption [n (%)] within the previous 24 hours of urine collection by urinary bisphenol S concentrations among 158 men in the EARTH Study.
| Quartiles of urinary bisphenol S concentrations, μg/L (range) |
||||||||
|---|---|---|---|---|---|---|---|---|
| N urines | Total cohort | Q1 (<LOD) |
Q2 (0.20, 0.30) |
Q3 (0.40, 0.80) |
Q4 (0.90, 52.0) |
Q2-4 (>LOD) |
p-value Q1 vs. Q2-4 |
|
| Fabric softener | 239 | 6 (3) | 0 (0) | 1 (2) | 3 (5) | 2 (3) | 6 (3) | 0.002 |
| Laundry detergent | 238 | 33 (14) | 6 (11) | 8 (13) | 10 (18) | 9 (14) | 27 (15) | 0.87 |
| Dishwashing liquid | 238 | 150 (63) | 34 (63) | 38 (60) | 35 (63) | 43 (66) | 116 (63) | 0.68 |
| Paints/solvents | 239 | 10 (4) | 1 (2) | 3 (5) | 2 (4) | 4 (6) | 9 (5) | 0.05 |
| Gum | 238 | 34 (14) | 10 (19) | 12 (19) | 9 (16) | 3 (5) | 24 (10) | 0.42 |
| Mints | 234 | 30 (13) | 6 (11) | 10 (16) | 6 (11) | 8 (13) | 24 (13) | 0.23 |
| Had eaten in stored/heated plastic container | 238 | 98 (41) | 18 (33) | 26 (42) | 27 (47) | 27 (42) | 80 (44) | 0.28 |
| Canned beverages | 238 | 20 (8) | 4 (7) | 6 (10) | 4 (7) | 6 (9) | 16 (9) | 0.81 |
| Canned food | 239 | 62 (26) | 13 (24) | 16 (25) | 15 (26) | 18 (28) | 49 (27) | 0.76 |
| Beef | 239 | 94 (40) | 10 (19) | 33 (52) | 22 (39) | 29 (45) | 84 (41) | 0.0006 |
| Lamb | 239 | 9 (4) | 1 (2) | 2 (3) | 1 (2) | 5 (8) | 8 (4) | 0.18 |
| Pork | 239 | 71 (30) | 14 (26) | 17 (27) | 18 (32) | 22 (34) | 57 (31) | 0.17 |
| Chicken | 239 | 112 (47) | 22 (41) | 32 (51) | 29 (51) | 29 (45) | 90 (49) | 0.59 |
| Fish | 239 | 41 (17) | 11 (20) | 5 (8) | 16 (28) | 9 (14) | 30 (16) | 0.26 |
| Other poultry | 239 | 30 (13) | 4 (7) | 10 (16) | 6 (11) | 10 (15) | 26 (14) | 0.12 |
| Cold cuts | 238 | 49 (21) | 9 (17) | 14 (22) | 9 (16) | 17 (26) | 40 (22) | 0.60 |
| Hot dogs | 238 | 11 (5) | 1 (2) | 3 (5) | 1 (2) | 6 (9) | 10 (5) | 0.23 |
| Milk | 238 | 142 (60) | 27 (51) | 38 (60) | 39 (68) | 38 (58) | 115 (62) | 0.20 |
| Sausage | 238 | 28 (12) | 6 (11) | 7 (11) | 9 (16) | 6 (9) | 22 (12) | 0.20 |
| Cheese | 238 | 164 (69) | 31 (59) | 43 (68) | 44 (77) | 46 (71) | 133 (72) | 0.05 |
| Ice-cream | 238 | 44 (19) | 13 (25) | 13 (21) | 6 (11) | 12 (19) | 31 (17) | 0.45 |
Note: Floor wax, car wax, pesticides, flea/tick prevention products, laundry starch, vinyl boots, vinyl gloves, vinyl raincoat, grooming products, mothballs and intake of veal have been excluded from analysis because of low frequency (<3%). The limit of detection (LOD) was 0.1 μg/L.
Lower semen quality parameters with higher urinary BPS concentrations were observed in models adjusted for abstinence time and specific gravity, and in those further adjusted for age, BMI and year of sample collection after controlling for urinary BPA concentrations (Table 4). For example, men in the second, third and fourth quartile of urinary BPS concentrations had, respectively, 18% (p=0.05), 24% (p=0.03) and 20% (p=0.09) lower sperm concentration, compared to men in the first quartile of BPS, in the fully adjusted models. Similarly, 8% (p=0.06), 4% (0.33) and 9% (p=0.09) lower motility was found among men in quartiles 2, 3, and 4, respectively, compared to men in the lowest quartile of urinary BPS concentrations. Differences were also observed when comparing men with detectable urinary BPS concentrations to those with non-detectable BPS [2.66 vs. 2.91 mL for volume (p=0.03), 30.7 vs. 38.3 mil/mL for concentration (p=0.03), 76.8 vs. 90.0 mil for total count (p=0.09), 43.7 vs. 47.0% for motility (p=0.06), and 5.42 vs. 6.77% for morphologically normal sperm (p=0.24)] (Table 4). Similar differences in semen quality parameters by urinary BPS concentrations were found when analyses were restricted to the first semen sample per man (Supplemental Table 3). However, results did not reach significant because of the smaller sample size (N=158). In addition, significantly higher probabilities of having low sperm concentration (<15 mil/mL) and motility (<40 %) were observed among men with detectable urinary BPS concentrations compared to those with non-detectable BPS (Supplemental Table S4).
Table 4.
Semen quality parameters (adjusted mean, 95% CI) by urinary bisphenol S concentrations among 158 men contributing 338 semen samples in the EARTH Study.
| Ejaculate volume (mL) |
Sperm concentration (mil/mL) |
Total sperm count (mil) |
Total motility (%) |
Total motile count (mil/ejaculate) |
Normal Morphologya (%) |
Normal morphology counta (mil/ejaculate) |
|
|---|---|---|---|---|---|---|---|
| Adjusted for abstinence time and specific gravity | |||||||
| Q1 | 2.67 (2.41, 2.94) | 38.5 (27.9, 53.1) | 91.3 (66.2, 126) | 47.0 (42.7, 51.3) | 26.5 (14.3, 49.1) | 6.90 (6.09, 7.72) | 6.85 (5.05, 9.29) |
| Q2 | 2.99 (2.75, 3.25)* | 31.5 (22.9, 43.4)ǂ | 83.4 (60.8, 114) | 43.2 (39.0, 47.4)ǂ | 21.0 (11.2, 39.3) | 6.18 (5.59, 6.78) | 5.80 (4.43, 7.58) |
| Q3 | 2.88 (2.60, 3.15) | 29.0 (20.4, 41.2)* | 71.2 (49.7, 102)ǂ | 44.8 (39.8, 49.8) | 19.0 (9.6, 37.9) | 6.75 (6.01, 7.49) | 5.02 (3.40, 7.41)ǂ |
| Q4 | 2.82 (2.55, 3.10) | 30.6 (21.7, 43.1)ǂ | 73.4 (51.5, 105)ǂ | 43.2 (38.6, 47.7)ǂ | 17.4 (8.8, 34.5) | 6.69 (5.87,7.50) | 5.23 (3.71, 7.39) |
| Adjusted for abstinence time, specific gravity, age, BMI and year of sample collection | |||||||
| Q1 | 2.68 (2.41, 2.94) | 38.5 (28.0, 52.8) | 91.0 (66.2, 125) | 47.0 (42.7, 51.2) | 26.3 (14.2, 48.8) | 6.91 (6.08, 7.75) | 6.84 (5.05, 9.28) |
| Q2 | 3.00 (2.76, 3.26)* | 31.4 (22.8, 43.1)ǂ | 82.7 (60.0, 114) | 43.2 (39.0, 49.8)ǂ | 20.6 (10.9, 39.0) | 6.12 (5.52, 6.71)ǂ | 5.72 (4.34, 7.53) |
| Q3 | 2.89 (2.62, 3.15) | 29.1 (20.6, 41.1)* | 71.7 (50.3, 102)ǂ | 44.8 (40.0, 49.8) | 19.2 (9.80, 37.7) | 6.78 (6.06, 7.50) | 5.15 (3.57, 7.45)ǂ |
| Q4 | 2.82 (2.56, 3.08) | 30.8 (22.0, 43.0)ǂ | 74.1 (52.5, 105)ǂ | 43.2 (38.7, 47.7)ǂ | 17.7 (9.10, 34.7) | 6.71 (5.92, 7.51) | 5.31 (3.80, 7.43) |
| Adjusted for abstinence time, specific gravity, age, BMI, year of sample collection and log-bisphenol A concentrations | |||||||
| Q1 | 2.68 (2.41, 2.95) | 38.4 (28.0, 52.7) | 91.1 (66.4, 125) | 46.9 (42.6, 51.3) | 26.3 (14.3, 48.7) | 6.91 (6.09, 7.73) | 6.84 (5.05, 9.26) |
| Q2 | 3.00 (2.75, 3.25)* | 31.4 (22.8, 43.3)ǂ | 82.5 (59.8, 114) | 43.2 (39.0, 47.5)ǂ | 20.6 (10.9, 39.0) | 6.11 (5.51, 6.71)ǂ | 5.69 (4.30, 7.53) |
| Q3 | 2.87 (2.61, 3.14) | 29.2 (20.6, 41.5)* | 71.4 (49.9, 102)ǂ | 44.9 (39.9, 49.9) | 19.2 (9.70, 38.0) | 6.77 (6.06, 7.49) | 5.12 (3.53, 7.43)ǂ |
| Q4 | 2.81 (2.55, 3.08) | 30.9 (22.0, 43.4)ǂ | 74.0 (52.2, 105)ǂ | 43.3 (38.7, 47.8)ǂ | 17.7 (9.00, 34.8) | 6.71 (5.91, 7.51) | 5.29 (3.78, 7.41) |
| <LODb | 2.91 (2.69, 3.12) | 38.3 (28.0, 52.4) | 90.0 (65.7, 123) | 47.0 (42.6, 41.3) | 25.9 (14.1, 47.7) | 6.99 (6.16, 7.82) | 6.77 (4.99, 9.19) |
| >LOD | 2.66 (2.39, 2.92)* | 30.7 (22.4, 42.0)* | 76.8 (56.2, 105)ǂ | 43.7 (40.0, 47.4)ǂ | 19.3 (10.4, 35.7) | 6.46 (5.94, 7.00) | 5.42 (4.16, 7.06) |
A total of 10 semen samples (3%) had missing data for normal sperm morphology and thus total normal morphology count, resulting in N=328 semen samples.
The limit of detection (LOD) was 0.1 μg/L.
p-value <0.05 when compared that group with the lowest group of exposure.
p-value <0.10 when compared that group with the lowest group of exposure.
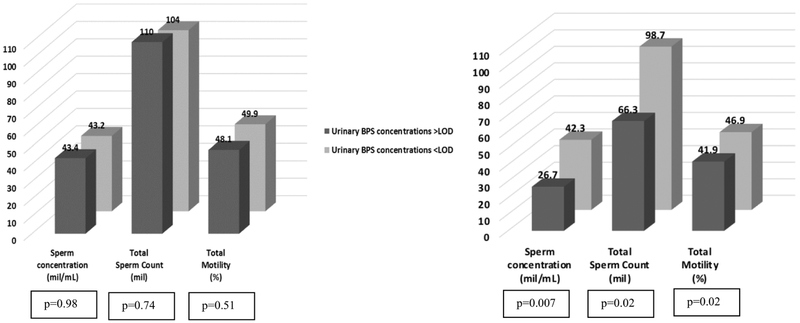
Some of these inverse associations of BPS with semen parameters were only observed among overweight or obese men, but not among normal weight men (Figure 1). Specifically, among men with BMI>25 kg/m2 (106 men contributing 225 semen samples), those with detectable concentrations of urinary BPS had significantly lower sperm concentration, total count and motility [26.7 vs. 42.3 mil/mL (p=0.007), 66.3 vs. 98.7 mil (p=0.02), and 41.9 vs. 46.9% (p=0.02), respectively] compared to men with non-detectable concentrations of urinary BPS. No significant associations of BPS with ejaculate volume or morphologically normal sperm were observed among overweight/obese men (data not shown). Among leaner men (<25 kg/m2, 52 men contributing 113 semen samples), no significant differences in semen parameters were found when comparing men with detectable BPS concentrations versus men with non-detectable BPS [43.4 vs. 43.2 mil/mL for concentration (p=0.98), 110 vs. 104 mil for total count (p=0.74), and 48.1 vs. 49.9% for motility (p=0.51)].
Figure 1.
Models are adjusted for abstinence time, specific gravity, age, BMI, year of sample collection and log-bisphenol A concentrations. P-interactions: 0.001 for sperm concentration, 0.05 for total sperm count and 0.15 for total motility. The limit of detection (LOD) was 0.1 μg/L. Medians (IQRs) of urinary BPS (μg/L) for leaner and obese/overweight men with concentrations above LOD were 0.50 (0.30, 1.00) and 0.50 (0.30, 1.10), respectively.
Discussion
To our knowledge, this is the first study to investigate potential associations between urinary BPS concentrations and semen parameters among a group of men. We detected BPS in 76% of men, and found associations of urinary BPS concentrations with lower ejaculate volume, sperm concentration, total count and motility, in adjusted models and models further adjusted for urinary BPA concentrations. Some of these associations were only observed among overweight and obese men, but not among leaner men. In addition, urinary BPS concentrations were positively associated to use of fabric softener and paint/solvent as well as intake of beef and cheese within 24 hours before urine collection. Our results indicate that men are exposed to BPS and that BPS concentrations are associated with poorer semen quality in this study population of men who presented to the MGH Fertility Center.
Our results are in agreement with animal studies showing detrimental effects of BPS on the male reproductive system (Ji et al. 2013; Ullah et al. 2016). For example, BPS exposure has been associated with cellular oxidative stress and antiandrogenic activities (Fic et al. 2015; Fic et al. 2013). In an in vitro study, Eladak et al observed harmful effects of BPS exposure, similar to BPA exposure, on the physiologic function of human and mouse testes tissue (Eladak et al. 2015). They used a culture system of fetal testis assay and measured testosterone secretion in a dose-response curve for exposure of different bisphenol analogues at varying concentrations. A study by Kitamura et al. observed toxicological effects of bisphenol analogs on androgen activity alteration (Kitamura et al. 2004). It examined the role of BPA, BPS, and BPF on the androgen receptors, and found that BPS exhibited anti-androgenic activities at concentrations between 1×10−6 to 1×10−4 M. These results are in agreement with another study indicating that all analogs for BPA have potentially toxic effects on human reproduction through the alteration of the endocrine activity (Rosenmai et al. 2014). Specifically, BPS showed an inhibition of testosterone secretion in human fetal testis and was found to have a more inhibitory potential effect on mouse fetal testis compared to BPA. Further cortisol and aldosterone secretion inhibition were shown to occur with all bisphenol analogs, including BPS.
Some of the inverse associations of urinary BPS concentrations with semen parameters were only observed among overweight and obese men. One possible hypothesis for this interesting finding is that overweight/obese men may be more sensitive to BPS exposure given that they are simultaneously exposed to a hyperestrogenic environment, since obesity increases circulating estrogen levels in men (Schneider et al, 1979), and an antiandrogenic signal, since BPS has antiandrogenic activity (Usman A & Ahmad M. 2016). Similar interpretation was given when we previously reported that the negative association between soy intake and semen parameters was stronger among overweight/obese men from the same study (Chavarro et al. 2008). This is particularly relevant since overweight and obesity have become a major public health concern worldwide especially among the U.S. adult population (Kelly et al. 2005; Wang et al. 2008; Flegal et al. 2012). Further studies are needed to corroborate this hypothesis.
Fabric softener and paint/solvent use, as well as consumption of beef and cheese within 24 hours before urine collection were associated with urinary BPS concentrations in the current study. In a study investigating bisphenols in consumer products collected in New York (USA), BPS was mainly detected in meat products (Liao and Kannan. 2013). However, urinary BPS concentrations were not associated with any self-reported product use (cleaning, personal care and pet products) among pregnant women in Northern Puerto Rico even though BPS was detected in 90% of urine samples (LOD=0.1 μg/L) with a median concentration of 0.5 μg/L (Ashrap et al. 2018). Because BPS is one of the replacements for BPA, co-exposure in humans is expected, as the Spearman correlation results between both bisphenols in our analysis indicated. We also found a trend of higher BPS concentration and lower BPA concentrations in recent years of the study (2015-2017), compared to earlier years (2011-2014). Other studies reported similar trends. For example, BPS concentrations in urine samples from U.S. adults have been increasing between 2000 and 2014 (Ye et al. 2015). Nevertheless, although BPA concentrations show a downward trend (CDC 2019; Ye et al. 2015; Ashrap et al. 2018), BPA concentrations are still higher compared to BPS (CDC 2019; Lehmler et al. 2018; Wu et al. 2018). Despite the negative associations between urinary BPS and certain semen parameters among men in this study, GM for urinary concentrations of BPS and BPA in this study were lower than those reported for males of all ages in the U.S. general population in 2013-2014: 0.46 μg/L for BPS and 1.43 μg/L for BPA (CDC 2019).
The current study has several limitations. First, it is uncertain whether our findings can be generalized to men in the general population and in non-Western countries. However, men in our study tended to have good semen quality compared to international reference standards (WHO 2010) and also fertile men (Levine et al. 2017). Second, exposure misclassification is possible given the short biological half-lives of target bisphenols and the likely episodic nature of the exposures (Braun et al. 2012). However, 56% of the participants contributed more than one urine sample which would partially reduce exposure misclassification. Third, the cross-sectional design of this particular analysis limits our ability to infer causality. Last, some of the positive associations between urinary BPS and product use and food intake may be due to chance because of the low frequency of use for some items. Further studies are needed to corroborate these novel findings. The biggest strength of this study is the comprehensive adjustment for other demographic, reproductive and lifestyle factors that could result in residual confounding, such as co-exposure to BPA. Another important strength included the use of data on product use and food intake questionnaire
In conclusion, we identified some household products and foods as predictors of BPS exposure. Our findings also showed, for the first time, negative associations between urinary BPS concentrations and semen parameters, especially among overweight and obese men. Further studies are needed to replicate our findings.
Supplementary Material
Highlights.
BPS was detected in 76% of the urine samples.
Urinary BPS was positively associated to use of fabric softener and paint/solvent as well as intake of beef and cheese within 24 hours before urine collection.
Urinary BPS was associated with lower semen parameters, and some associations were only observed among overweight and obese men.
Acknowledgments and grant information:
The project was funded by grants R01ES022955, R01ES009718 and P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS). The authors gratefully acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research staff Myra Keller, Ramace Dadd and Alex Azevedo, physicians and staff at Massachusetts General Hospital fertility center. We also gratefully acknowledge Xiaoliu Zhou, Tao Jia, and the late Xiaoyun Ye (CDC, Atlanta, GA) for technical assistance in measuring the urinary concentrations of bisphenols. A special thank you to all of the study participants.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the US Government, the Department of Health and Human Services (DHHS) or the National Institutes of Health (NIH). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: None of the authors has any conflicts of interest to declare.
References
- Ahsan N, Ullah H, Ullah W, & Jahan S. 2018. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere,197, 336–343. doi: 10.1016/j.chemosphere.2017.12.118 [DOI] [PubMed] [Google Scholar]
- Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC 2015. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ.Int 85, 352e379 10.1016/j.envint.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, Meeker JD. 2018. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environment International, 121, 990–1002. doi: 10.1016/j.envint.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. 2007. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect.115 Suppl 1:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, Ahmed S, Atlas E. 2016. Bisphenol S Induces Adipogenesis in Primary Human Preadipocytes From Female Donors. Endocrinology. 2016 April;157(4):1397–407. doi: 10.1210/en.2015-1872. Epub 2016 Mar [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. 2012. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environmental health perspectives 120:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences. Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables, January, 2019).
- Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences. Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables, February, 2009).
- Chavarro JE, Toth TL, Sadio SM, & Hauser R 2008. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Human Reproduction,23(11), 2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Ike M, Fujita M. 2002. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol. 2002;17:80–86. [DOI] [PubMed] [Google Scholar]
- Clark E In: Kirk-Othmer Encyclopedia of Chemical Technology. New York, NY:John Wiley & Sons; 2012.Sulfolane and sulfones. [Google Scholar]
- De Coster S, Van Larebeke N. 2012. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J. Environ. Public. Health 713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn, et al. 2015. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril 103 (1), 11e21. [DOI] [PubMed] [Google Scholar]
- Fic A, Mlakar SJ, Juvan P, Mlakar V, Marc J, Dolenc MS, Broberg K, Masic LP. 2015. Genome-wide gene expression profiling of low-dose, long-term exposure of human osteosarcoma cells to bisphenol A and its analogs bisphenols AF and S. Toxicol. Invit 29, 1060e1069. [DOI] [PubMed] [Google Scholar]
- Fic A, Zegura B, Sollner Dolenc M, Filipic M, Peterlin Masic L. 2013. Mutage- nicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells. Arh. Hig. Rada Toksikol 64, 189e200. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. 2012. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999-2010. Jama 307, 491. [DOI] [PubMed] [Google Scholar]
- Glausiusz J 2014. Toxicology: the plastics puzzle. Nature 508, 306e308. [DOI] [PubMed] [Google Scholar]
- Grignard E, Lapenna S, Bremer S. Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol In Vitro. 2012;26:727–731. [DOI] [PubMed] [Google Scholar]
- Héliès-Toussaint C, Peyre L, Costanzo C, Chagnon M, & Rahmani R. 2014. Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicology and Applied Pharmacology, 280(2), 224–235. [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol. 2013;47:8793–8800 [DOI] [PubMed] [Google Scholar]
- Jin FR, Zhao ZS. 1997. The production and application of the diphenol sulfone. Hua. Gong. Shi. Kan 11, 21. [Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. 2008. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity 32, 1431–1437. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K et al. 2005. Comparative Study of the Endocrine-Disrupting Activity of Bisphenol A and 19 Related Compounds, Toxicological Sciences, Volume 84, Issue 2,1 April 2005, Pages 249–259. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. 1988. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertility and sterility 49:112–117. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Liu B, Gadogbe M, Bao W. 2018. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. 2017. Temporal trends in sperm count: A systematic review and meta-regression analysis. Human reproduction update 23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Liu F, Kannan K. 2012a. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol;46(12):6515–22. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K. 2012b. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ. Sci. Technol. 46, 6860e6866. [DOI] [PubMed] [Google Scholar]
- Liao C and Kannan K. 2013. Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure J. Agric. Food Chem, 61 (2013), pp. 4655–4662 [DOI] [PubMed] [Google Scholar]
- Liu B, Lehmler HJ, Sun Y, Xu Guifeng, Liu Yuewei, Zong Geng, Sun Qi, Hu Frank B., Wallace Robert B., Bao Wei. Bisphenol A substitutes and obesity in US adults: analysis of a population-based, cross-sectional study..Lancet Planet Health. 2017. June; 1(3): e114–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, et al. 2007. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR. J. Biochem. 142, 517–524. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. 2010. Semen quality and sperm DNA damage in relation to urinary bisphenol a among men from an infertility clinic. Reproductive toxicology (Elmsford, NY) 30:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R, et al. 2018. The environment and reproductive health (earth) study: A prospective preconception cohort. Human reproduction open 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Hauser R, Gaskins AJ. 2016. Effects of bisphenol a on male and couple reproductive health: A review. Fertility and sterility 106:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Gaskins AJ, Chiu YH, Messerlian C, Williams PL, Ford JB, et al. 2018a. Type of underwear worn and markers of testicular function among men attending a fertility center. Human reproduction (Oxford, England) 33:1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Williams PL, Chiu YH, Gaskins AJ, Nassan FL, Dadd R, et al. 2018b. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: Identifying potential predictors. Environment international 121:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moral LI, Corre LL, Poirier H, Niot I, Truntzer T, Merlin J, Chagnon M. 2016. Obesogen effects after perinatal exposure of 4,4′-sulfonyldiphenol (Bisphenol S) in C57BL/6 mice. Toxicology, 357–358, 11–20. [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MY, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203 [DOI] [PubMed] [Google Scholar]
- Nevoral J, Kolinko Y, Moravec J, Žalmanová T, Hošková K, Prokešová Š, Klein P, Ghaibour K, Hošek P, Štiavnická M, et al. Reproduction. 2018. July; 156(1):47–57. Epub 2018 May 10. [DOI] [PubMed] [Google Scholar]
- Oh J, Choi JW, Ahn Y, Kim S. 2018. Pharmacokinetics of bisphenol S in humans after single oral administration. Environment International,112, 127–133. [DOI] [PubMed] [Google Scholar]
- Phillips KP, et al. J Toxicol Environ Health B Crit Rev, part B 2008;11:188–220 [DOI] [PubMed] [Google Scholar]
- Rezg R, El-Fazaa S, Gharbi N, Mornagui B. 2014. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ. Int 64, 83e90. [DOI] [PubMed] [Google Scholar]
- Rochester JR. 2013. Bisphenol A and human health: a review of the literature. Reprod. Toxicol 42, 132e155. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. 2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environmental Health Perspectives, 123(7), 643–650. doi: 10.1289/ehp.1408989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney KL, Domar AD. 2014. The impact of lifestyle behaviors on infertility treatment outcome. Current opinion in obstetrics & gynecology 26:181–185. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, et al. Are structural analogues to bisphenol A safe alternatives? Toxicol Sci. 2014;139:35–47. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to bisphenol A impairs fertility and expression of Sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. [DOI] [PubMed] [Google Scholar]
- SART. 2015. Preliminary sart clinic summary report: Sart (societry for assisted reproductive technologies), (vol 2017). [Google Scholar]
- Searle SR, Speed FM, Milliken GA. 1980. Population marginal means in the linear model: An alternative to leasts quare means. AmStat 34:216–221. [Google Scholar]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A. 2013. Lifestyle factors and reproductive health: Taking control of your fertility. Reproductive biology and endocrinology : RB&E 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. 1979. Increased Estrogen Production in Obese Men. The Journal of Clinical Endocrinology & Metabolism, 48(4), 633–638. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Yuasa S. Effects of neonatal administration of 17β-estradiol, β-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reproductive Toxicology. 2004;19:181–188. [DOI] [PubMed] [Google Scholar]
- Ullah H, Jahan S, Ain QU, Shaheen G, Ahsan N. 2016. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere. June; 152:383–91. Epub 2016 Mar 17. [DOI] [PubMed] [Google Scholar]
- Usman A & Ahmad M. 2016. From BPA to its analogues: Is it a safe journey? Chemosphere,158, 131–142. doi: 10.1016/j.chemosphere.2016.05.070 [DOI] [PubMed] [Google Scholar]
- Viñas R, Watson CS. 2013aBisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 121352–358.; 10.1289/ehp.1205826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. 2008. Will All Americans Become Overweight or Obese? Estimating the Progression and Cost of the US Obesity Epidemic. Obesity 16, 2323–2330. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Wang Z, Qin J, Wang W, Tian H. and Ru S. 2018. “Bisphenol S induces obesogenic effects through deregulating lipid metabolism in zebrafish ( Danio rerio ) larvae”, Chemosphere, Vol. 199, pp. 286–296. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Qin J, Wei P, Jia Y, Wang J, Ru S. 2019. Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring. Chemosphere. 2019 January 11;221:500–510. [DOI] [PubMed] [Google Scholar]
- WHO. 2010. World health organization. Laboratory manual for the examination and processing of human semen, 5th edn. Geneva, Switzerland: WHO. [Google Scholar]
- Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, Guo Y, Zeng EY. 2018. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Science of The Total Environment 615, 87–98. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K, Takatsuki M. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology. 2003;183:93–115. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong L, Kramer J, Zhou X, Jia T, & Calafat AM. 2015. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environmental Science & Technology, 49(19), 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Automated on-line column-switching hplc-ms/ms method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry 77:5407–5413. [DOI] [PubMed] [Google Scholar]
- Žalmanová T, Hošková K, Nevoral J, Prokešová Š, Zámostná K, Kott T, & Petr J. 2016. Bisphenol S instead of bisphenol A: A story of reproductive disruption by regretable substitution – a review. Czech Journal of Animal Science,61(No. 10), 433–449. [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X, Xia Y. 2019. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environment International 123, 325–336. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. 2014. Automated on-line column- switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci 944, 152e156. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jiang G, Wei P, Wang H, Ru S. 2018. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio). Ecotoxicology and Environmental Safety 147, 794–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.